Natural Solid-State Hydrogel Electrolytes Based on 3D Pure Cotton/Graphene for Supercapacitor Application
Abstract
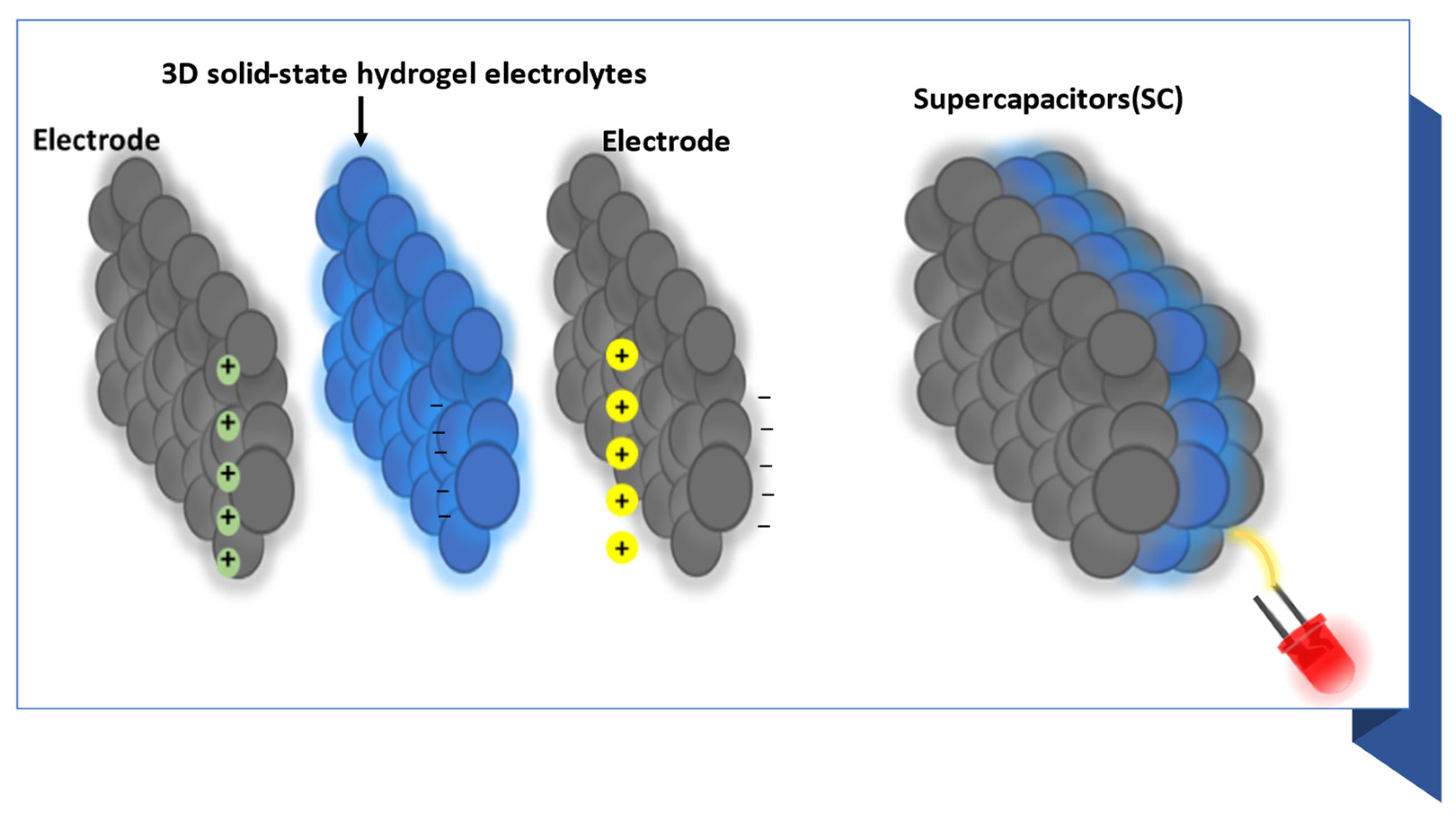
:1. Introduction
2. Materials and Preparation
2.1. Raw Materials
2.2. Material Preparation
2.3. Fabrication Supercapacitors
2.4. Characterization
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Analysis
3.2. FTIR Analysis
3.3. Morphological Studies
3.4. Electrochemical Impedance Performance of Hydrogel Electrolytes
3.5. Cyclic Voltammetry (CV)
3.6. Tensile Strain
3.7. Proof of Concept
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Zhang, X.; Sun, X.; Ma, Y. Matter Conducting polymer hydrogel materials for high-performance flexible solid-state supercapacitors. Sci. China Mater. 2016, 59, 412–420. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Qu, K.; Shi, C.; Sun, Z.; Huang, Z.; Li, J.; Dong, M.; Guo, Z. Binder-free CuS/ZnS/sodium alginate/rGO nanocomposite hydrogel electrodes for enhanced performance supercapacitors. Int. J. Biol. Macromol. 2020, 162, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Shao, J.J. Graphene-based flexible all-solid-state supercapacitors. Mater. Chem. Front. 2021, 5, 557–583. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, D.H.; Lee, W.J.; Kim, S.O. Tailored Assembly of Carbon Nanostructures: Tailored Assembly of Carbon Nanotubes and Graphene (Adv. Funct. Mater. 8/2011). Adv. Funct. Mater. 2011, 21, 1338–1354. [Google Scholar] [CrossRef]
- Bhauriyal, P.; Mahata, A.; Pathak, B. Graphene-like Carbon-Nitride Monolayer: A Potential Anode Material for Na- and K-Ion Batteries. J. Phys. Chem. C 2018, 122, 2481–2489. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Ren, W.; Zhao, L.; Li, X.; Wang, M.; Lin, Y. Natural Biomass Hydrogel Based on Cotton Fibers/PVA for Acid Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 9144–9153. [Google Scholar] [CrossRef]
- Jia, M.; Li, Y.; Cui, L.; An, Y.; Pan, C.; Jin, X. An anthraquinone-decorated graphene hydrogel based on carbonized cotton fibers for flexible and high-performance supercapacitors. Sustain. Energy Fuels 2021, 5, 862–873. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Yousaf, M.; Lu, Y.; Mahmoud, M.Z.; Iqbal, J.; Khan, M.A.; Khallidoon, M.U.; Ullah, S.; Hussien, M. Magnetic, structural, optical band alignment and conductive analysis of graphene-based REs (Yb, Gd, and Sm) doped NiFe2O4 nanocomposites for emerging technological applications. Synth. Met. 2022, 284, 116994. [Google Scholar] [CrossRef]
- Qamar, S.; Akhtar, M.N.; Aleem, W.; Rehman Z ur Khan, A.H.; Ahmad, A.; Batoo, K.M.; Aamir, M. Graphene anchored Ce doped spinel ferrites for practical and technological applications. Ceram. Int. 2020, 46, 7081–7088. [Google Scholar] [CrossRef]
- Qamar, S.; Yasin, S.; Ramzan, N.; Umer, A.; Akhtar, M.N. Structural, morphological and magnetic characterization of synthesized Co-Ce doped Ni ferrite/Graphene/BNO12 nanocomposites for practical applications. Chin. J. Phys. 2020, 65, 82–92. [Google Scholar] [CrossRef]
- Du, P.; Liu, H.C.; Yi, C.; Wang, K.; Gong, X. Polyaniline-Modified Oriented Graphene Hydrogel Film as the Free-Standing Electrode for Flexible Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 23932–23940. [Google Scholar] [CrossRef]
- Denisa, H.J.; Puziy, A.M.; Poddubnaya, O.I.; Fabian, S.G.; Tascón JM, D.; Lu, G.Q. Highly stable performance of supercapacitors from phosphorus-enriched carbons. J. Am. Chem. Soc. 2009, 131, 5026–5027. [Google Scholar] [CrossRef]
- Nujud Badawi, M.; Bhatia, M.; Ramesh, S.; Ramesh, K.; Khan, M.; Adil, S.F. Enhancement of the Performance Properties of Pure Cotton Fabric by Incorporating Conducting Polymer (PEDOT:PSS) for Flexible and Foldable Electrochemical Applications. J. Electron. Mater. 2023, 52, 2201–2215. [Google Scholar] [CrossRef]
- Wang, F.; Sun, S.; Xu, Y.; Wang, T.; Yu, R.; Li, H. High-performance asymmetric supercapacitor based on Cobalt Nickle Iron-layered double hydroxide/carbon nanofibres and activated carbon. Sci. Rep. 2017, 7, 4707. [Google Scholar] [CrossRef] [Green Version]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Kadir, M.; Aspanut, Z.; Majid, S.; Arof, A. FTIR Studies of Plasticized Poly (vinyl alcohol)–Chitosan Blend Doped with NH4NO3 Polymer Electrolyte Membrane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1068–1074. [Google Scholar] [CrossRef]
- Su’Ait, M.S.; Ahmad, A.; Rahman, M.Y.A. Ionic conductivity studies of 49% poly (methyl methacrylate)-grafted natural rubber-based solid polymer electrolytes. Springer 2009, 15, 497–500. [Google Scholar] [CrossRef]
- Tomiyasu, H.; Shikata, H.; Takao, K.; Asanuma, N.; Taruta, S.; Park, Y.Y. An aqueous electrolyte of the widest potential window and its superior capability for capacitors. Sci. Rep. 2017, 7, srep45048. [Google Scholar] [CrossRef] [Green Version]
- Tai, Z.; Yan, X.; Xue, Q. Three-Dimensional Graphene/Polyaniline Composite Hydrogel as Supercapacitor Electrode. J. Electrochem. Soc. 2012, 159, A1702–A1709. [Google Scholar] [CrossRef]
- Gürünlü, B.; Taşdelen-Yücedağ, Ç.; Bayramoğlu, M. Graphene Synthesis by Ultrasound Energy-Assisted Exfoliation of Graphite in Various Solvents. Crystals 2020, 10, 1037. [Google Scholar] [CrossRef]
- Ajalli, N.; Alizadeh, M.; Hasanzadeh, A.; Khataee, A.; Azamat, J. A theoretical investigation into the effects of functionalized graphene nanosheets on dimethyl sulfoxide separation. Chemosphere 2022, 297, 134183. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lang, J.; Yan, X. Effect of surface area and heteroatom of porous carbon materials on electrochemical capacitance in aqueous and organic electrolytes. Sci. China Chem. 2014, 57, 1570–1578. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Yin, L.; Koratkar, N.; Li, F.; Cheng, H.M. Stabilizing sulfur cathodes using nitrogen-doped graphene as a chemical immobilizer for Li [Formula presented] S batteries. Carbon 2016, 108, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Badawi, N.M.; Batoo, K.M. Conductive Nanocomposite Cotton Thread Strands for Wire and Industrial Applications. J. Electron. Mater. 2020, 49, 6483–6491. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, S.Y.; Xiao, R.; Yin, J.; Wu, Z.L.; Zheng, Q.; Qian, J. Constitutive behaviours of tough physical hydrogels with dynamic metal-coordinated bonds. J. Mech. Phys. Solids 2020, 139, 103935. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Jiang, B.; Chen, J. A review of the enzymatic, physical, and chemical modification techniques of xanthan gum. Int. J. Biol. Macromol. 2021, 186, 472–489. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, Y.; Li, Y.; Xu, J.; Fu, X.; Gao, X.; Mao, X.; Li, Z. UV-Fully physically crosslinked pectin-based hydrogel with high stretchability and toughness for biomedical application shielding alginate films crosslinked with Fe3+ containing EDTA. Carbohydr. Polym. 2020, 239, 115480. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.; Qin, Z.; Che, P.; Yi, X.; Yu, Q.; Zhang, H.; Sun, X.; Yao, F.; Li, J. Fully physically crosslinked pectin-based hydrogel with high stretchability and toughness for biomedical application. Int. J. Biol. Macromol. 2020, 149, 707–716. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Conway, B.E.; Pell, W.G. Double-layer and pseudocapacitance types of electrochemical capacitors and their applications to the development of hybrid devices. J. Solid State Electrochem. 2003, 7, 637–644. [Google Scholar] [CrossRef]
- Influence of the Surface—Chemistry of Modified Mesoporous Carbon on the Electrochemical Behavior of Solid-State Supercapacitors|Francesco Lufrano—Academia.edu. Available online: https://www.academia.edu/21596941/Influence_of_the_Surface_Chemistry_of_Modified_Mesoporous_Carbon_on_the_Electrochemical_Behavior_of_Solid_State_Supercapacitors (accessed on 7 April 2022).
- Larciprete, R.; Gardonio, S.; Petaccia, L.; Lizzit, S. Atomic oxygen functionalization of double-walled C nanotubes. Carbon 2009, 47, 2579–2589. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yoo, J.J.; Kim, Y.I.; Yoon, J.K.; Yoon, H.N.; Kim, J.-H.; Park, S.B. Oxygen functional groups and electrochemical capacitive behavior of incompletely reduced graphene oxides as a thin-film electrode of supercapacitor. Electrochim. Acta 2014, 116, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.-W.; Yan, X.-B.; Liu, W.-W.; Wang, R.-T.; Xue, Q.-J. Influence of nitric acid modification of ordered mesoporous carbon materials on their capacitive performances in different aqueous electrolytes. J. Power Sources 2012, 204, 220–229. [Google Scholar] [CrossRef]
- Arof, A.K.; Amirudin, S.; Yusof, S.Z.; Noor, I.M. A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 1856–1867. [Google Scholar] [CrossRef] [Green Version]
- Deng, F.; Wang, X.; He, D.; Hu, J.; Gong, C.; Ye, Y.S.; Xie, X.; Xue, Z. Microporous polymer electrolyte based on PVDF/PEO star polymer blends for lithium-ion batteries. J. Membr. Sci. 2015, 491, 82–89. [Google Scholar] [CrossRef]
- Xiao, Q.; Deng, C.; Wang, Q.; Zhang, Q.; Yue, Y.; Ren, S. In Situ Cross-Linked Gel Polymer Electrolyte Membranes with Excellent Thermal Stability for Lithium-Ion Batteries. ACS Omega 2019, 4, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Sun, G.; Xie, L.; Su, F.; Li, X.; Liu, Z.; Kong, Q.; Lu, C.; Li, K.; Chen, C. A high energy density asymmetric supercapacitor based on a CoNi-layered double hydroxide and activated carbon. Carbon 2016, 100, 710. [Google Scholar] [CrossRef]
- Alipoori, S.; Torkzadeh, M.M.; Moghadam MH, M.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Graphene oxide: An effective ionic conductivity promoter for phosphoric acid-doped poly (vinyl alcohol) gel electrolytes. Polymer 2019, 184, 121908. [Google Scholar] [CrossRef]
- Khan, M.; Assal, M.E.; Tahir, M.N.; Khan, M.; Ashraf, M.; Hatshan, M.R.; Khan, M.; Varala, R.; Badawi, N.M.; Adil, S.F. Graphene/inorganic nanocomposites: Evolving photocatalysts for solar energy conversion for environmental remediation. J. Saudi Chem. Soc. 2022, 26, 101544. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, G.Z. New precursors derived activated carbon and graphene for aqueous supercapacitors with unequal electrode capacitances. WuliHuaxueXuebao/Acta Phys. Chim. 2019, 36, 1904025. [Google Scholar] [CrossRef]
- Boonpakdee, D.; Guajardo Yévenes, C.F.; Surareungchai, W.; La-O-Vorakiat, C. Exploring non-linearities of carbon-based microsupercapacitors from an equivalent circuit perspective. J. Mater. Chem. A 2018, 6, 7162–7167. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Yue, Z.; Shu, K.; Wallace, G.G. Intrinsically stretchable supercapacitors composed of polypyrrole electrodes and highly stretchable gel electrolytes. ACS Appl. Mater. Interfaces 2013, 5, 9008–9014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azman, N.H.N.; Mamat Mat Nazir, M.S.; Ngee, L.H.; Sulaiman, Y. Graphene-based ternary composites for supercapacitors. Int. J. Energy Res. 2018, 42, 2104–2116. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhang, B.L.; Yu, Z.L.; Qu, M.Z. Manganese oxide/MWNTs composite electrodes for supercapacitors. Solid State Ion. 2005, 176, 1169–1174. [Google Scholar] [CrossRef]
- Yu, M.; Ji, X.; Ran, F. Chemically building interpenetrating polymeric networks of Bi-crosslinked hydrogel macromolecules for membrane supercapacitors. Carbohydr. Polym. 2021, 255, 117346. [Google Scholar] [CrossRef]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose hydrogels: Green and sustainable soft biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Fonseca, R.G.; Kuster, A.; Fernandes, P.P.; Tavakoli, M.; Pereira, P.; Fernandes, J.R.; de Bon, F.; Serra, A.C.; Fonseca, A.C.; Coelho, J.F.J. Facile Synthesis of Highly Stretchable, Tough, and Photodegradable Hydrogels. Adv. Healthc. Mater. 2023, e2300918. [Google Scholar] [CrossRef]
- Zhao, X.; Ran, F.; Shen, K.; Yang, Y.; Wu, J.; Niu, X.; Kong, L.; Kang, L.; Chen, S. Facile fabrication of ultrathin hybrid membrane for highly flexible supercapacitors via in-situ phase separation of polyethersulfone. J. Power Sources 2016, 329, 104–114. [Google Scholar] [CrossRef]
- Badawi, N.M.; Bhatia, M.; Ramesh, S.; Ramesh, K.; Kuniyil, M.; Shaik, M.R.; Khan, M.; Shaik, B.; Adil, S.F. Self-Healing, Flexible and Smart 3D Hydrogel Electrolytes Based on Alginate/PEDOT:PSS for Supercapacitor Applications. Polymers 2023, 15. [Google Scholar] [CrossRef]
- Zhao, L.; Ran, F. Electrolyte-philicity of electrode materials. Chem. Commun. 2023, 59, 6969–6986. [Google Scholar] [CrossRef]
- Wirthl, D.; Pichler, R.; Drack, M.; Kettlguber, G.; Moser, R.; Gerstmayr, R.; Hartmann, F.; Bradt, E.; Kaltseis, R.; Siket, C.M.; et al. Instant tough bonding of hydrogels for soft machines and electronics. Sci. Adv. 2017, 3, e1700053. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Singh, B.; Vishavnath. Designing Starch-Alginate Hydrogels for Controlled Delivery of Fungicide for the Alleviation of Environmental Pollution. ACS Agric. Sci. Technol. 2022, 2, 1239–1250. [Google Scholar] [CrossRef]
- Kumar, S.S.A.; Batoo, K.M.; Ma, I.A.W.; Ramesh, K.; Ramesh, S.; Shah, M.A. Fabrication and characterization of graphene oxide based polymer nanocomposite coatings, improved stability and hydrophobicity. Sci. Rep. 2023, 13, 8946. [Google Scholar] [CrossRef]














| System | |
|---|---|
| CGH1 | Cotton (0.8 g) + graphene (0.2 g) + 5 mL hydrogel solution + 4 mL H2SO4. |
| CGH2 | Cotton (0.8 g) + graphene (0.4 g) + 5 mL hydrogel solution + 4 mL H2SO4. |
| CGH3 | Cotton (0.8 g) + graphene (0.2 g) + 5 mL hydrogel solution + 4 mL KCl. |
| CGH4 | Cotton (0.8g) + graphene (0.4 g) + 5 mL hydrogel solution + 4 mL KCl. |
| Cell | Specific Capacitance (F/g) | Power Density (W/Kg) | Energy Density (Wh/Kg) |
|---|---|---|---|
| CGH1 | 312.88 | 478.50 | 48.79 |
| CGH2 | 390.75 | 499.99 | 50.99 |
| CGH3 | 250.30 | 100.00 | 35.18 |
| CGH4 | 333.50 | 100.10 | 52.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, N.B.; Batoo, K.M.; Hussain, S.; Subramaniam, R.; Kasi, R.; Bhuyan, M.; Imran, A.; Muthuramamoorthy, M. Natural Solid-State Hydrogel Electrolytes Based on 3D Pure Cotton/Graphene for Supercapacitor Application. Micromachines 2023, 14, 1379. https://doi.org/10.3390/mi14071379
Mohammed NB, Batoo KM, Hussain S, Subramaniam R, Kasi R, Bhuyan M, Imran A, Muthuramamoorthy M. Natural Solid-State Hydrogel Electrolytes Based on 3D Pure Cotton/Graphene for Supercapacitor Application. Micromachines. 2023; 14(7):1379. https://doi.org/10.3390/mi14071379
Chicago/Turabian StyleMohammed, Nujud Badawi, Khalid Mujasam Batoo, Sajjad Hussain, Ramesh Subramaniam, Ramesh Kasi, Mrutunjaya Bhuyan, Ahamad Imran, and Muthumareeswaran Muthuramamoorthy. 2023. "Natural Solid-State Hydrogel Electrolytes Based on 3D Pure Cotton/Graphene for Supercapacitor Application" Micromachines 14, no. 7: 1379. https://doi.org/10.3390/mi14071379








