Synthesis and Characterization of Ciprofloxacin Loaded Star-Shaped Polycaprolactone–Polyethylene Glycol Hydrogels for Oral Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Optimization of Star Polymers PCL-PEG
2.3. Characterization of Star Polymers PCL and Star Polymers PCL-PEG
2.4. Preparation of Ciprofloxacin-PCL-PEG Star Polymer-Based Hydrogel
2.5. Characterization of Hydrogels
2.6. Drug Entrapment Efficiency
- W: Amount of drug used in formulation
- w: Amount of drug found in the solution
2.7. In Vitro Drug Release
2.8. Antimicrobial Assay (Well Diffusion)
2.9. Cell Culture and MTT Cell Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical and Physical Characterization of Star-Shaped PCL and PCL-PEG
3.2. Ciprofloxacin Loaded Star Polymers PCL-PEG
3.3. Encapsulation Efficiency
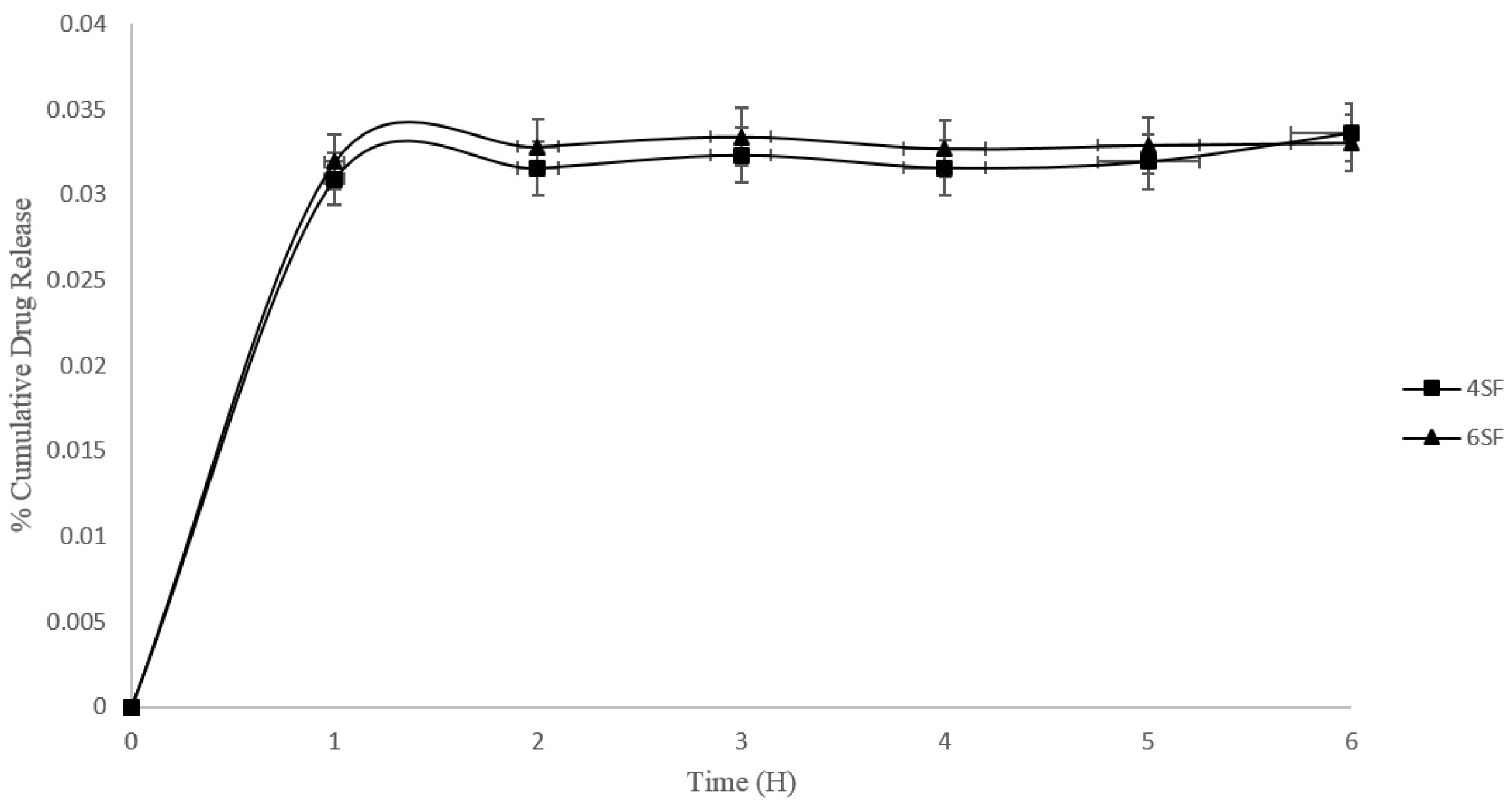
3.4. Drug Release
3.5. Kinetic Model
3.6. Antimicrobial Activity
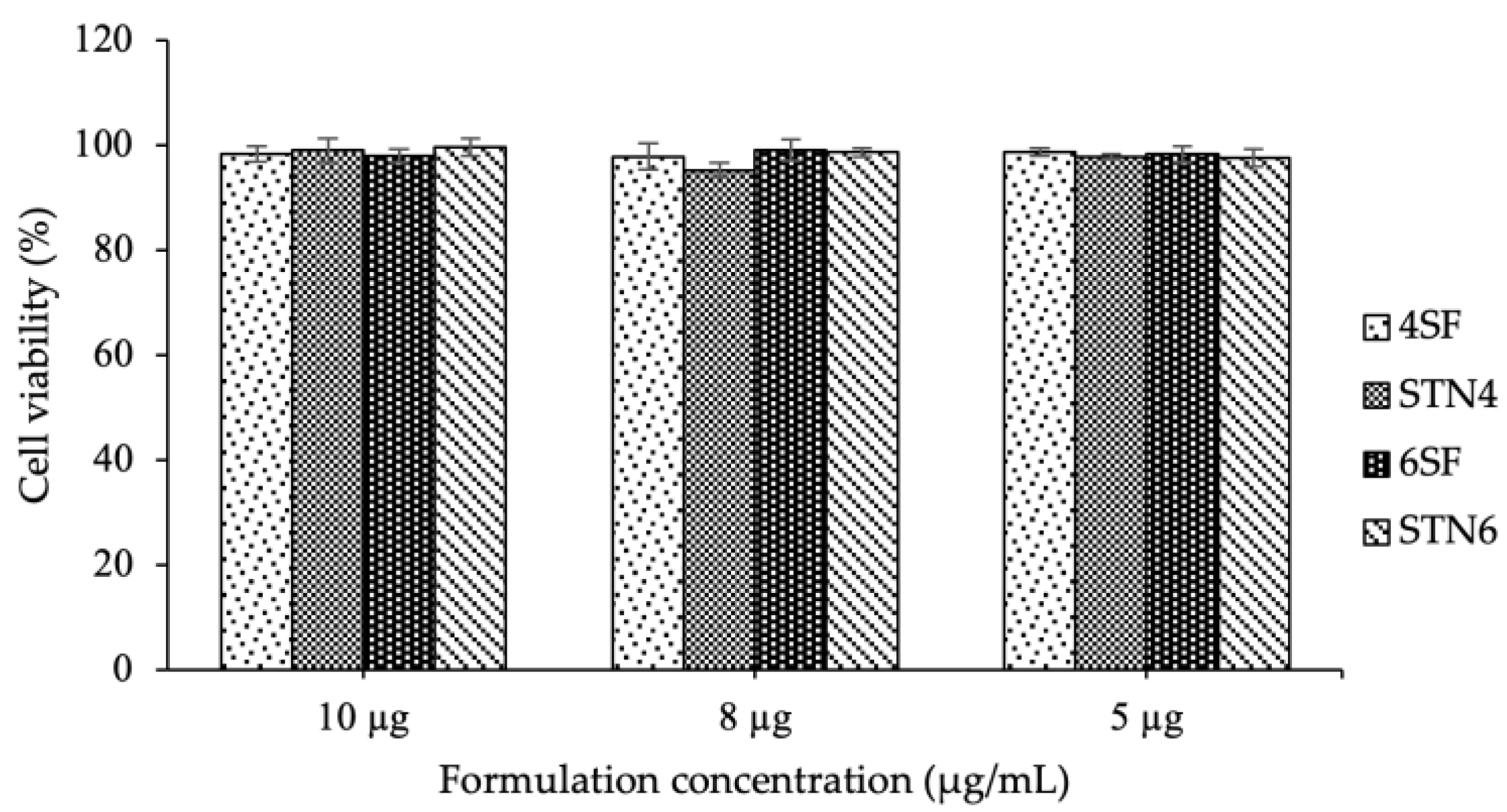
3.7. Cell Biocompatibility Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boyd, B.J.; Bergström, C.A.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. X 2020, 2, 100050. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Khodir, W.K.W.A.; Ambrosio, L. Biodegradable Microparticles and Nanoparticles by Electrospraying Techniques. J. Appl. Biomater. Funct. Mater. 2012, 10, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Fasolino, I.; Guarino, V.; Cirillo, V.; Ambrosio, L. 5-Azacytidine-mediated hMSC behavior on electrospun scaffolds for skeletal muscle regeneration. J. Biomed. Mater. Res. Part A 2017, 105, 2551–2561. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abrahamsson, B.; Artursson, P.; Batchelor, H.; Berben, P.; Bernkop-Schnürch, A.; Butler, J.; Ceulemans, J.; Davies, N.; Dupont, D.; et al. Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network. Adv. Drug Deliv. Rev. 2021, 171, 289–331. [Google Scholar] [CrossRef]
- Uhljar, L.; Kan, S.Y.; Radacsi, N.; Koutsos, V.; Szabó-Révész, P.; Ambrus, R. In Vitro Drug Release, Permeability, and Structural Test of Ciprofloxacin-Loaded Nanofibers. Pharmaceutics 2021, 13, 556. [Google Scholar] [CrossRef]
- Muhammad Sarfraz, R.; Bashir, S.; Mahmood, A.; Ahsan, H.; Riaz, H.; Raza, H.; Rashid, Z.; Atif Raza, S.; Asad Abrar, M.; Abbas, K.; et al. Application of Various Polymers and Polymers Based Techniques Used to Improve Solubility of Poorly Water Soluble Drugs: A Review. Acta Pol. Pharm. Drug Res. 2017, 74, 347–356. [Google Scholar]
- Alkattan, N.S.; Alasmael, N.; Ladelta, V.; Khashab, N.M.; Hadjichristidis, N. Poly(2-oxazoline)-based core cross-linked star polymers: Synthesis and drug delivery applications. Nanoscale Adv. 2023, 5, 2794–2803. [Google Scholar] [CrossRef]
- Lotocki, V.; Kakkar, A. Miktoarm Star Polymers: Branched Architectures in Drug Delivery. Pharmaceutics 2020, 12, 827. [Google Scholar] [CrossRef]
- Somszor, K.; Allison-Logan, S.; Karimi, F.; McKenzie, T.; Fu, Q.; O’connor, A.; Qiao, G.; Heath, D. Amphiphilic Core Cross-Linked Star Polymers for the Delivery of Hydrophilic Drugs from Hydrophobic Matrices. Biomacromolecules 2021, 22, 2554–2562. [Google Scholar] [CrossRef]
- Wakaskar, R.R. General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J. Drug Target. 2018, 26, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Bayat, N.; McOrist, N.; Ariotti, N.; Lai, M.; Sia, K.C.; Li, Y.; Grace, J.L.; Quinn, J.F.; Whittaker, M.R.; Kavallaris, M.; et al. Thiol-Reactive Star Polymers Functionalized with Short Ethoxy-Containing Moieties Exhibit Enhanced Uptake in Acute Lymphoblastic Leukemia Cells. Int. J. Nanomed. 2019, 14, 9795–9808. [Google Scholar] [CrossRef] [PubMed]
- Somers, K.; Wen, V.W.; Middlemiss, S.M.C.; Osborne, B.; Forgham, H.; Jung, M.; Karsa, M.; Clifton, M.; Bongers, A.; Gao, J.; et al. A novel small molecule that kills a subset of MLL-rearranged leukemia cells by inducing mitochondrial dysfunction. Oncogene 2019, 38, 3824–3842. [Google Scholar] [CrossRef] [PubMed]
- Sahranavard, M.; Shahriari, M.; Abnous, K.; Hadizadeh, F.; Taghdisi, S.M.; Zolfaghari, R.; Ramezani, M.; Alibolandi, M. Design and synthesis of targeted star-shaped micelle for guided delivery of camptothecin: In vitro and in vivo evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112529. [Google Scholar] [CrossRef] [PubMed]
- Braunová, A.; Chytil, P.; Laga, R.; Šírová, M.; Machová, D.; Parnica, J.; Říhová, B.; Janoušková, O.; Etrych, T. Polymer nanomedicines based on micelle-forming amphiphilic or water-soluble polymer-doxorubicin conjugates: Comparative study of in vitro and in vivo properties related to the polymer carrier structure, composition, and hydrodynamic properties. J. Control Release 2020, 321, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Ladewig, K.; Klimak, M.; Haylock, D.; McLean, K.M.; O’Connor, A.J.; Qiao, G.G. Amphiphilic core cross-linked star polymers as water-soluble, biocompatible and biodegradable unimolecular carriers for hydrophobic drugs. Polym. Chem. 2015, 6, 6475–6487. [Google Scholar] [CrossRef]
- Oliveira, A.S.R.; Mendonça, P.V.; Simões, S.; Serra, A.C.; Coelho, J.F.J. Amphiphilic well-defined degradable star block copolymers by combination of ring-opening polymerization and atom transfer radical polymerization: Synthesis and application as drug delivery carriers. J. Polym. Sci. 2021, 59, 211–229. [Google Scholar] [CrossRef]
- Omura, T.; Imagawa, K.; Kono, K.; Suzuki, T.; Minami, H. Encapsulation of Either Hydrophilic or Hydrophobic Substances in Spongy Cellulose Particles. ACS Appl. Mater. Interfaces 2017, 9, 944–949. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Jana, U.; Manna, P.K.; Mohanta, G.P. Synthesis and evaluation of MePEG-PCL diblock copolymers: Surface properties and controlled release behavior. Prog. Biomater. 2015, 4, 89–100. [Google Scholar] [CrossRef]
- Bolourchian, N.; Mahboobian, M.M.; Dadashzadeh, S. The Effect of PEG Molecular Weights on Dissolution Behavior of Simvastatin in Solid Dispersions. Iran. J. Pharm. Res. 2013, 12, 11–20. [Google Scholar] [CrossRef]
- Guarino, V.; Galizia, M.; Alvarez-Perez, M.; Mensitieri, G.; Ambrosio, l. Improving surface and transport properties of macroporous hydrogels for bone regeneration. J. Biomed. Mater. Res. A 2015, 103, 1095–1105. [Google Scholar] [CrossRef]
- Lust, S.T.; Hoogland, D.; Norman, M.D.A.; Kerins, C.; Omar, J.; Jowett, G.M.; Yu, T.T.L.; Yan, Z.; Xu, J.Z.; Marciano, D.; et al. Selectively Cross-Linked Tetra-PEG Hydrogels Provide Control over Mechanical Strength with Minimal Impact on Diffusivity. ACS Biomater. Sci. Eng. 2021, 7, 4293–4304. [Google Scholar] [CrossRef]
- Kumar, A.C.; Erothu, H. Synthetic Polymer Hydrogels. In Biomedical Applications of Polymeric Materials and Composites; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 141–162. [Google Scholar]
- Heng, P.W.S. Controlled release drug delivery systems. Pharm. Dev. Technol. 2018, 23, 833. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Brambilla, E.; Locarno, S.; Gallo, S.; Orsini, F.; Pini, C.; Farronato, M.; Thomaz, D.V.; Lenardi, C.; Piazzoni, M.; Tartaglia, G. Poloxamer-Based Hydrogel as Drug Delivery System: How Polymeric Excipients Influence the Chemical-Physical Properties. Polymers 2022, 14, 3624. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khodir, W.; Abdul Razak, A.; Ng, M.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Han, S.D.; Shin, B.S.; Park, J.B. Development of an in vitro dissolution test method for hydrogel-based drug delivery systems. J. Pharm. Investig. 2016, 46, 275–285. [Google Scholar]
- Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Marques, M.; Rytting, H.; Shaw, S.; Thakker, K.; et al. Topical and Transdermal Drug Products. Pharmacop. Forum 2009, 35, 750–764. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Güney, A.; Gardiner, C.; McCormack, A.; Malda, J.; Grijpma, D.W. Thermoplastic PCL-b-PEG-b-PCL and HDI Polyurethanes for Extrusion-Based 3D-Printing of Tough Hydrogels. Bioengineering 2018, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Nabid, M.R.; Tabatabaei Rezaei, S.J.; Sedghi, R.; Niknejad, H.; Entezami, A.A.; Oskooie, H.A.; Heravi, M.M. Self-assembled micelles of well-defined pentaerythritol-centered amphiphilic A4B8 star-block copolymers based on PCL and PEG for hydrophobic drug delivery. Polymer 2011, 52, 2799–2809. [Google Scholar] [CrossRef]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and Evaluation of a Star Amphiphilic Block Copolymer from Poly(ε-caprolactone) and Poly(ethylene glycol) as a Potential Drug Delivery Carrier. Bioconjugate Chem. 2005, 16, 397–405. [Google Scholar] [CrossRef]
- Letchford, K.; Zastre, J.; Liggins, R.; Burt, H. Synthesis and micellar characterization of short block length methoxy poly(ethylene glycol)-block-poly(caprolactone) diblock copolymers. Colloids Surf. B Biointerfaces 2004, 35, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Faisal, K.S.; Clulow, A.J.; MacWilliams, S.V.; Gillam, T.A.; Austin, A.; Krasowska, M.; Blencowe, A. Microstructure—Thermal Property Relationships of Poly (Ethylene Glycol-b-Caprolactone) Copolymers and Their Micelles. Polymers 2022, 14, 4365. [Google Scholar] [CrossRef]
- Castillo, R.V.; Müller, A.J.; Raquez, J.-M.; Dubois, P. Crystallization Kinetics and Morphology of Biodegradable Double Crystalline PLLA-b-PCL Diblock Copolymers. Macromolecules 2010, 43, 4149–4160. [Google Scholar] [CrossRef]
- Hua, C.; Dong, C.-M. Synthesis, characterization, effect of architecture on crystallization of biodegradable poly(ɛ-caprolactone)-b-poly(ethylene oxide) copolymers with different arms and nanoparticles thereof. J. Biomed. Mater. Res. A 2007, 82A, 689–700. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef]
- Colinet, I.; Dulong, V.; Mocanu, G.; Picton, L.; Le Cerf, D. New amphiphilic and pH-sensitive hydrogel for controlled release of a model poorly water-soluble drug. Eur. J. Pharm. Biopharm. 2009, 73, 345–350. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Tsay, R.-Y. Drug Release from a Spherical Matrix: Theoretical Analysis for a Finite Dissolution Rate Affected by Geometric Shape of Dispersed Drugs. Pharmaceutics 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, A.; Sakaino, M.; Yuguchi, Y.; Yamane, C. Relaxation phenomenon and swelling behavior of regenerated cellulose fibers affected by water. Carbohydr. Polym. 2019, 231, 115663. [Google Scholar] [CrossRef]
- Mishra, G.P.; Tamboli, V.; Mitra, A.K. Effect of hydrophobic and hydrophilic additives on sol–gel transition and release behavior of timolol maleate from polycaprolactone-based hydrogel. Colloid Polym. Sci. 2011, 289, 1553–1562. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.; Peterchristoper, G. Release Kinetics-Concepts and Applications. Int. J. Pharm. Res. Technol. 2019, 8, 12–20. [Google Scholar] [CrossRef]
- Padinjarathil, H.; Mudradi, S.; Balasubramanian, R.; Drago, C.; Dattilo, S.; Kothurkar, N.K.; Ramani, P. Design of an Antibiotic-Releasing Polymer: Physicochemical Characterization and Drug Release Patterns. Membranes 2023, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.; Gu, J.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control Release 2020, 320, 337–346. [Google Scholar] [CrossRef]
- Caccavo, D. An overview on the mathematical modeling of hydrogels’ behavior for drug delivery systems. Int. J. Pharm. 2019, 560, 175–190. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Hydrogels: Experimental characterization and mathematical modelling of their mechanical and diffusive behaviour. Chem. Soc. Rev. 2018, 47, 2357–2373. [Google Scholar] [CrossRef]
- Crovetto, S.I.; Moreno, E.; Dib, A.L.; Espigares, M.; Espigares, E. Bacterial toxicity testing and antibacterial activity of parabens. Toxicol. Environ. Chem. 2017, 99, 858–868. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K.; Grenda, A.; Jakubczyk, A.; Kiersnowska, K.; Bik-Małodzińska, M. Natural Compounds with Antimicrobial Properties in Cosmetics. Pathogens 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Lincho, J.; Martins, R.C.; Gomes, J. Paraben Compounds—Part I: An Overview of Their Characteristics, Detection, and Impacts. Appl. Sci. 2021, 11, 2307. [Google Scholar] [CrossRef]
- Asghar, A.A.; Akhlaq, M.; Jalil, A.; Azad, A.K.; Asghar, J.; Adeel, M.; Albadrani, G.M.; Al-Doaiss, A.A.; Kamel, M.; Altyar, A.E.; et al. Formulation of ciprofloxacin-loaded oral self-emulsifying drug delivery system to improve the pharmacokinetics and antibacterial activity. Front. Pharmacol. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kloskowski, T.; Gurtowska, N.; Nowak, M.; Joachimiak, R.; Bajek, A.; Olkowska, J.; Drewa, T. The influence of ciprofloxacin on viability of A549, HepG2, A375.S2, B16 and C6 cell lines in vitro. Acta Pol. Pharm. 2011, 68, 859–865. [Google Scholar] [CrossRef]



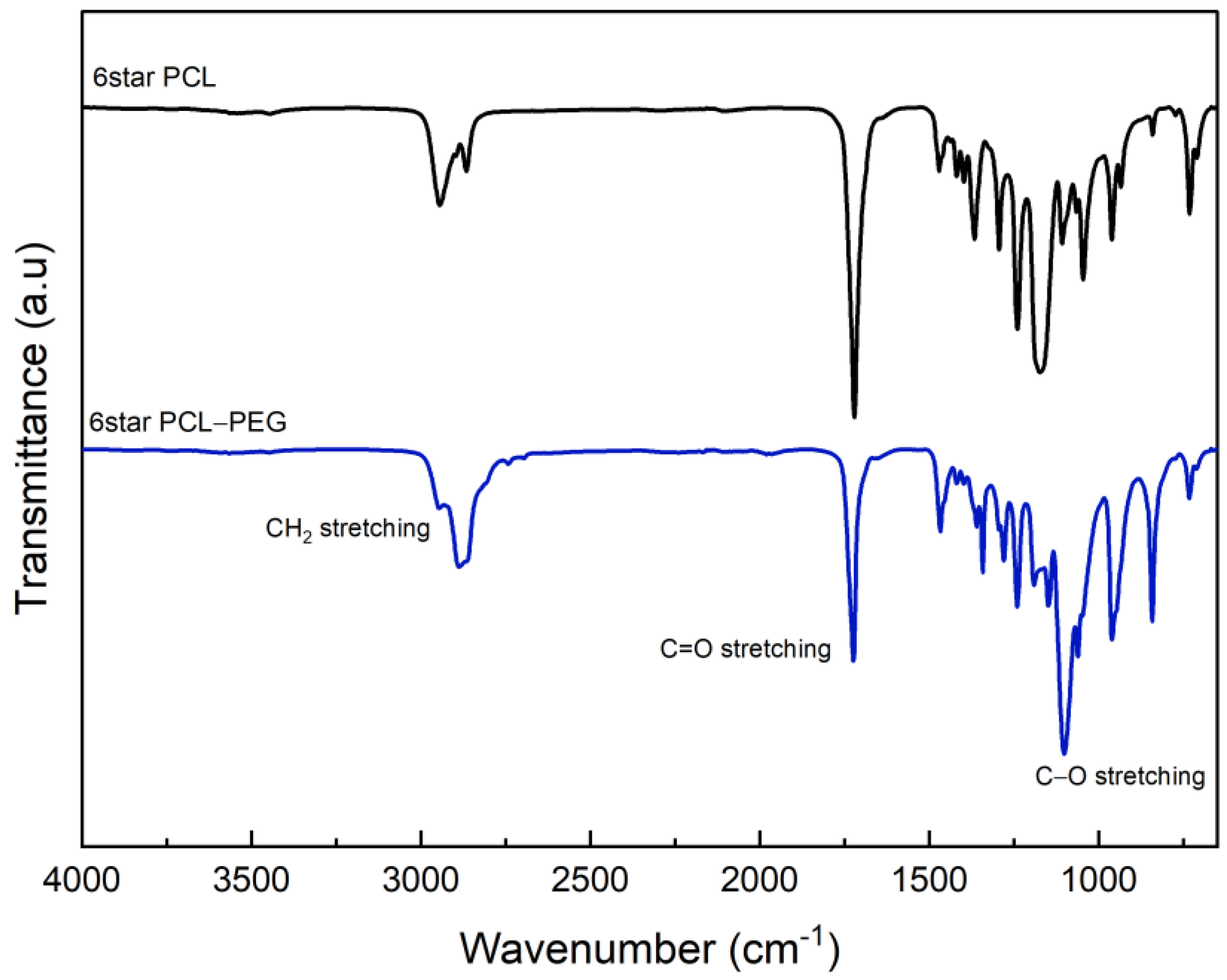
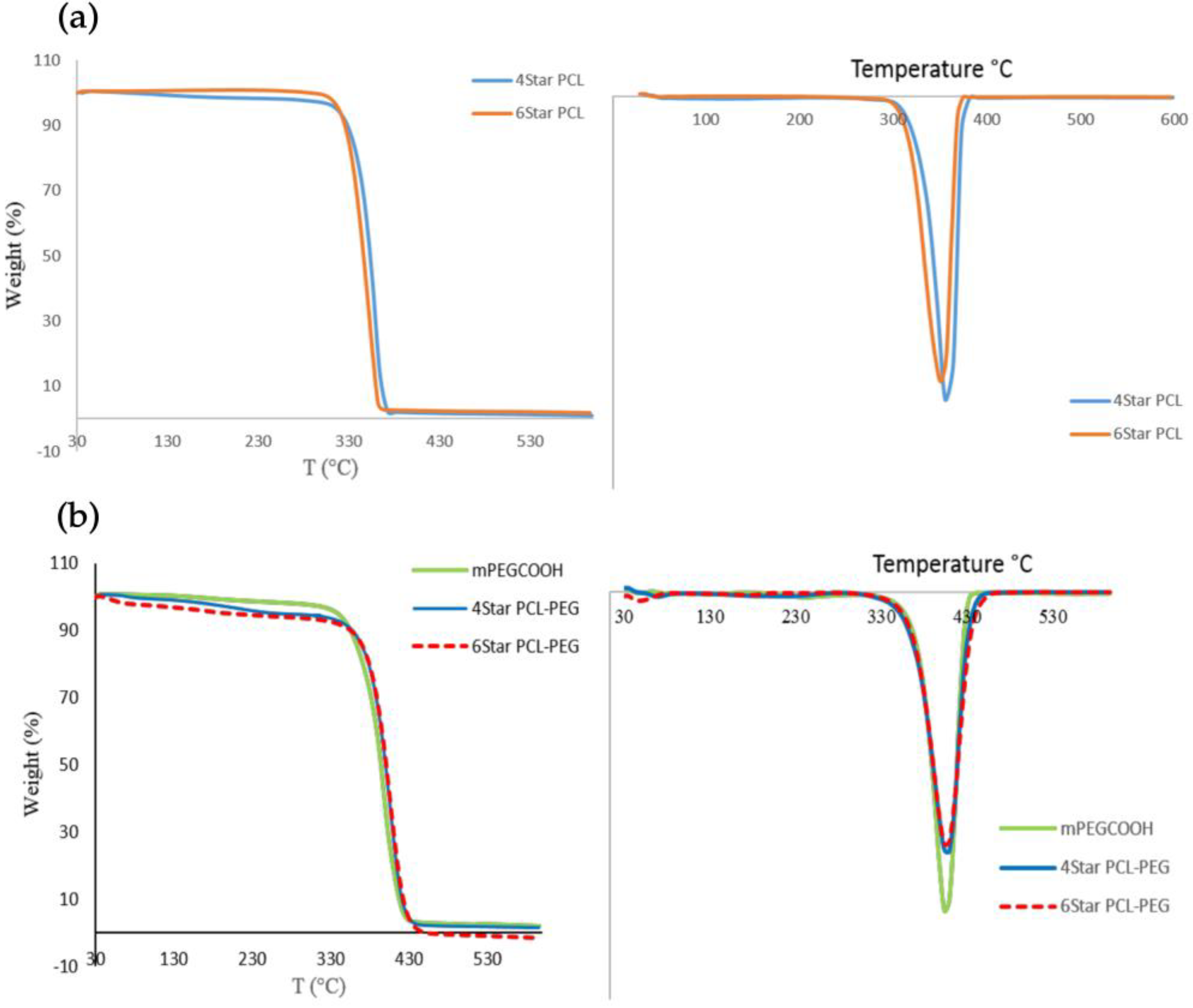
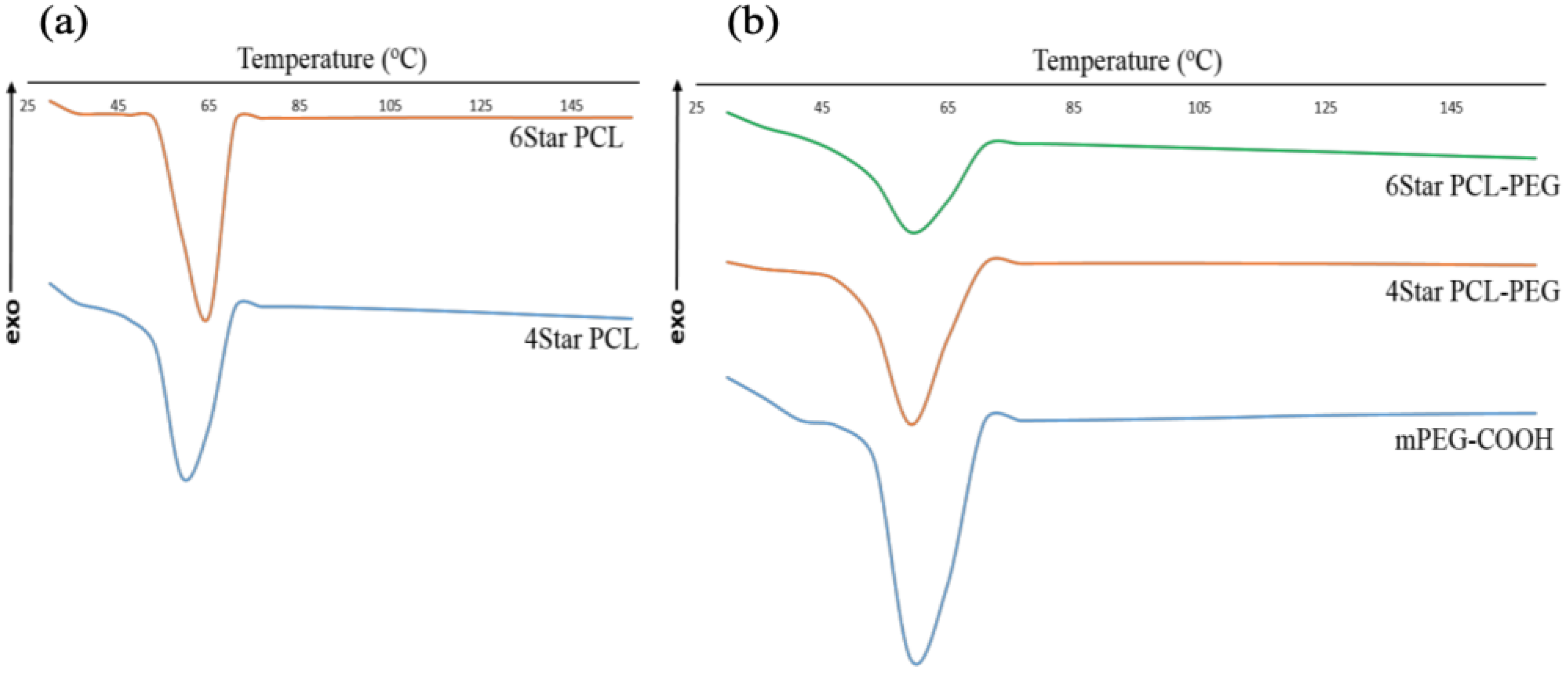
| Sample | Tm a (°C) | Td-max b (°C) |
|---|---|---|
| 4Star PCL | 60 | 356.6 |
| 6Star PCL | 56 | 327.5 |
| 4Star PCL-PEG | 59 | 409.2 |
| 4Star PCL | 60 | 409.1 |
| Formulations | % Drug Entrapment |
|---|---|
| 4SF | 99.20 ± 0.01 |
| 6SF | 99.25 ± 0.04 |
| Formulations | Coefficient, R2 | |||
|---|---|---|---|---|
| Zero Order | First Order | Higuchi | Korsmeyer–Peppas | |
| 4SF | 0.429 | 0.621 | 0.429 | 0.651 |
| 6SF | 0.394 | 0.269 | 0.394 | 0.680 |
| Formulations | Coefficient, R2 | n |
|---|---|---|
| 4SF | 0.651 | 0.005 |
| 6SF | 0.680 | 0.0159 |
| Microorganisms | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | Pseudomonas aeruginosa | Enterococcus faecalis | Streptococcus pyogenes | |||||||||||||
| Inhibition Zone (mm) | Inhibition Zone (mm) | Inhibition Zone (mm) | Inhibition Zone (mm) | |||||||||||||
| Time (Hour) | 3 | 6 | 12 | 24 | 3 | 6 | 12 | 24 | 3 | 6 | 12 | 24 | 3 | 6 | 12 | 24 |
| 4SF | X | 16.3 | 36.3 | 44.3 | X | 12.3 | 28.3 | 30.3 | X | 14.3 | 29.0 | 31.0 | X | 16.3 | 33.3 | 37.3 |
| 6SF | X | 16.7 | 35.3 | 43.3 | X | 12.7 | 28.7 | 31.0 | X | 13.7 | 28.0 | 31.0 | X | 15.7 | 34.7 | 38.3 |
| STN4 | X | 11.3 | 14.6 | 14.6 | X | X | X | X | X | X | X | X | X | 9.7 | 12.0 | 12.0 |
| STN6 | X | 12.0 | 16.3 | 16.3 | X | X | X | X | X | X | X | X | X | 10.9 | 12.3 | 12.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodir, W.K.W.A.; Ismail, M.W.; Hamid, S.A.; Daik, R.; Susanti, D.; Taher, M.; Guarino, V. Synthesis and Characterization of Ciprofloxacin Loaded Star-Shaped Polycaprolactone–Polyethylene Glycol Hydrogels for Oral Delivery. Micromachines 2023, 14, 1382. https://doi.org/10.3390/mi14071382
Khodir WKWA, Ismail MW, Hamid SA, Daik R, Susanti D, Taher M, Guarino V. Synthesis and Characterization of Ciprofloxacin Loaded Star-Shaped Polycaprolactone–Polyethylene Glycol Hydrogels for Oral Delivery. Micromachines. 2023; 14(7):1382. https://doi.org/10.3390/mi14071382
Chicago/Turabian StyleKhodir, Wan Khartini Wan Abdul, Mohamad Wafiuddin Ismail, Shafida Abd Hamid, Rusli Daik, Deny Susanti, Muhammad Taher, and Vincenzo Guarino. 2023. "Synthesis and Characterization of Ciprofloxacin Loaded Star-Shaped Polycaprolactone–Polyethylene Glycol Hydrogels for Oral Delivery" Micromachines 14, no. 7: 1382. https://doi.org/10.3390/mi14071382
APA StyleKhodir, W. K. W. A., Ismail, M. W., Hamid, S. A., Daik, R., Susanti, D., Taher, M., & Guarino, V. (2023). Synthesis and Characterization of Ciprofloxacin Loaded Star-Shaped Polycaprolactone–Polyethylene Glycol Hydrogels for Oral Delivery. Micromachines, 14(7), 1382. https://doi.org/10.3390/mi14071382









