A Free-Standing Chitosan Membrane Prepared by the Vibration-Assisted Solvent Casting Method
Abstract
1. Introduction
2. Materials and Methods
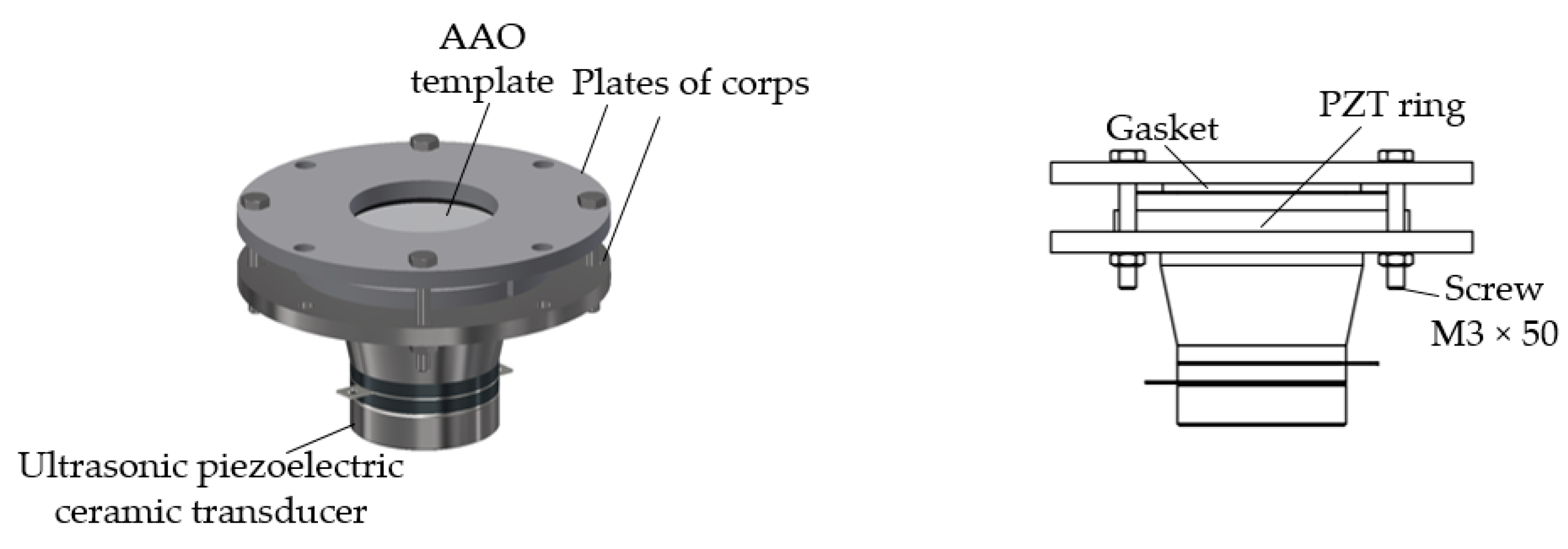
2.1. Fabrication of Nanoporous AAO Template
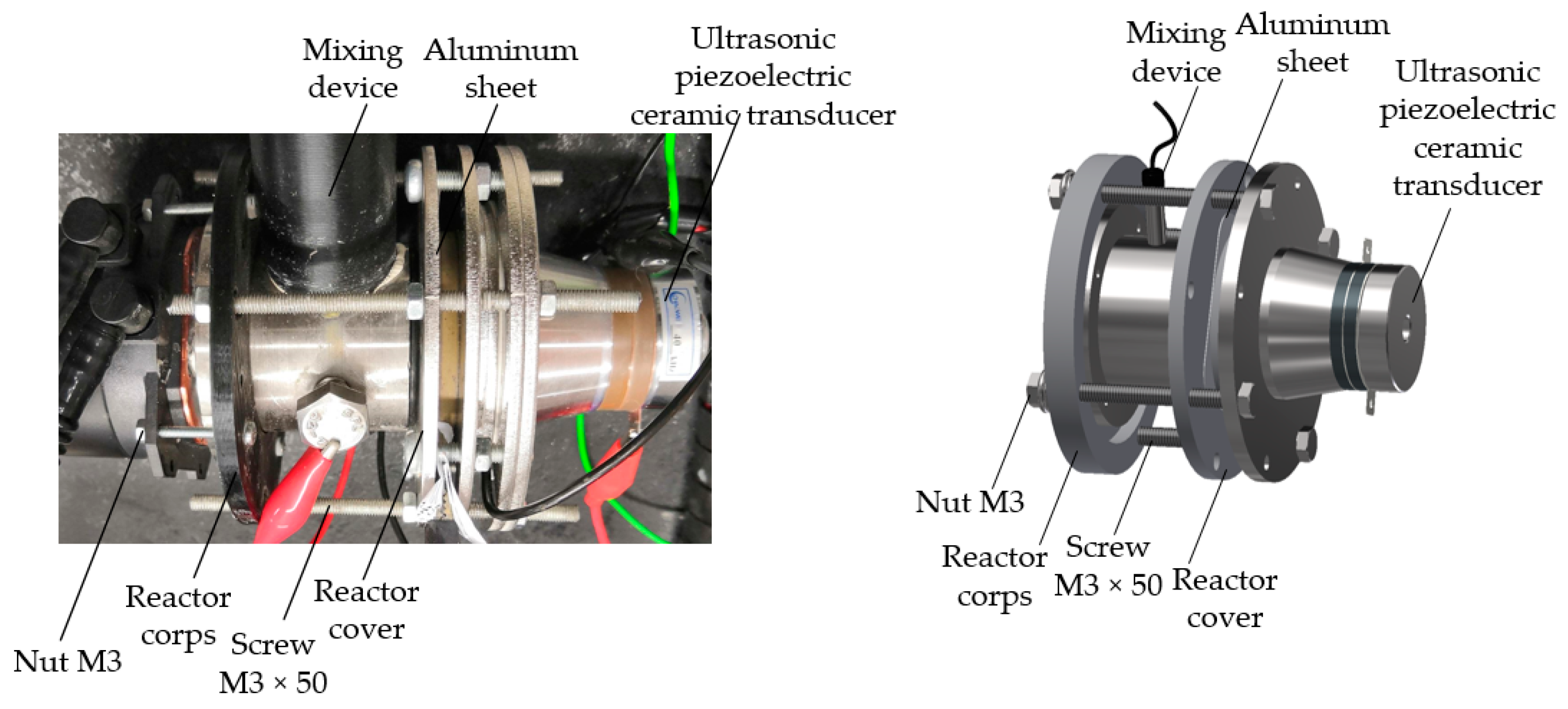
2.2. Fabrication of Nanopillared Chitosan Membrane
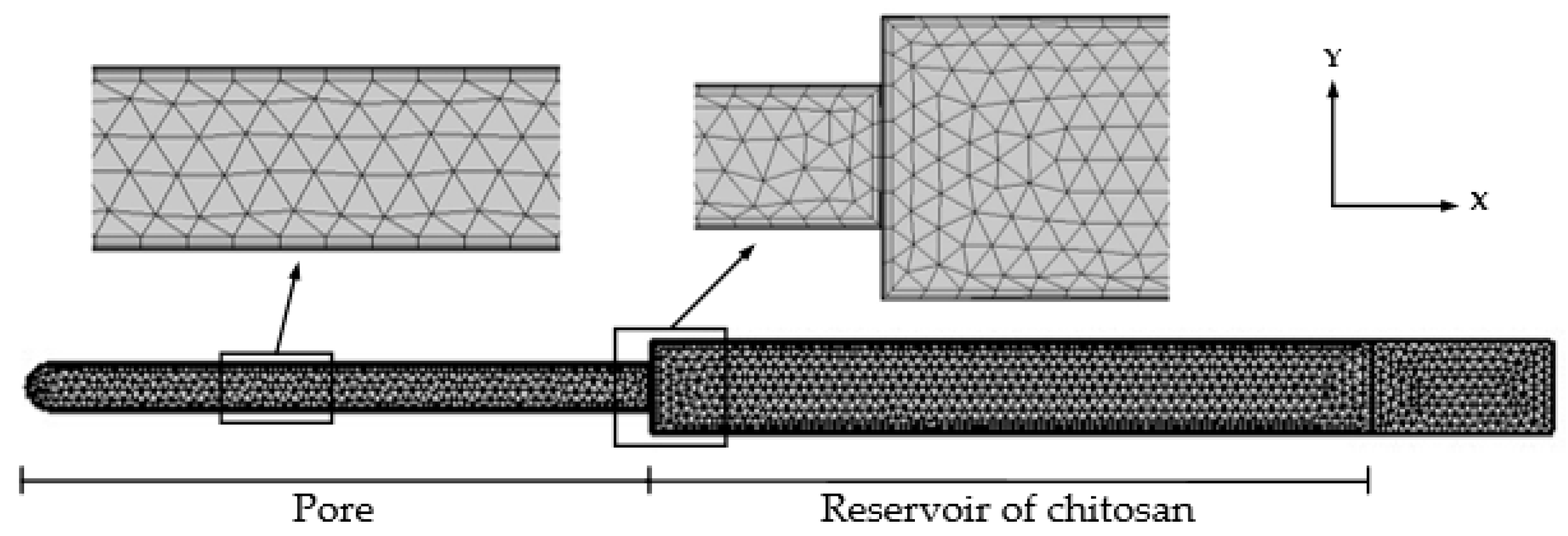
2.3. Simulation Method and Conditions of Vibration Process
3. Results and Discussion
3.1. Investigation of Porous AAO Template
3.2. Theoretical Results of the Flow of Different Concentrations of Chitosan Solution into the AAO Nanopores
3.3. Investigation of Nanopillared Chitosan Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, J.; Wang, B. Bio-thermo-viscoelastic behavior in multilayer skin tissue. J. Therm. Stress. 2022, 45, 559–575. [Google Scholar] [CrossRef]
- Priya, S.; Rath, S.N. Chapter 5—Artificial skin: Current advanced methods of fabrication and development. In Natural Polymers in Wound Healing and Repair; Sah, M.K., Kasoju, N., Mano, J.F., Eds.; Elsevier: Amsterdam, Netherlands, 2022; pp. 103–128. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef]
- Ray, P.; Chakraborty, R.; Banik, O.; Banoth, E.; Kumar, P. Surface Engineering of a Bioartificial Membrane for Its Application in Bioengineering Devices. ACS Omega 2023, 8, 3606–3629. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, P.; Fu, Z.; Meng, S.; Dai, L.; Yang, H. Applications of nanomaterials in tissue engineering. RSC Adv. 2021, 11, 19041. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Chapter 5—Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Elsevier: Amsterdam, Netherlands, 2018; pp. 121–139. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Dixon, D.T.; Gomillion, C.T. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. J. Funct. Biomater. 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.; Venault, A.; Chang, Y. Zwitterionized chitosan based soft membranes for diabetic wound healing. J. Membr. Sci. 2019, 591, 117319. [Google Scholar] [CrossRef]
- Han, W.; Ren, J.; Xuan, H.; Ge, L. Controllable degradation rates, antibacterial, free-standing and highly transparent films based on polylactic acid and chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2018, 541, 128–136. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Y.; Tu, Y.; Ay, B.; Sun, X.; Li, Y.; Wang, X.; Chen, X.; Zhang, L. Chitosan-based asymmetric topological membranes with cell-like features for healthcare applications. J. Mater. Chem. B 2019, 7, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Valiei, A.; Lin, N.; McKay, G.; Nguyen, D.; Moraes, C.; Hill, R.J.; Tufenkji, N. Surface Wettability Is a Key Feature in the Mechano-Bactericidal Activity of Nanopillars. ACS Appl. Mater. Interfaces 2022, 14, 27564–27574. [Google Scholar] [CrossRef] [PubMed]
- Gudur, A.; Ji, H.F. Bio-Applications of Nanopillars. Front. Nanosci. Nanotechnol. 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Velu, R.; Calais, T.; Jayakumar, A.; Raspall, F. A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique. Materials 2020, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Vakalopoulou, E.; Rath, T.; Warchomicka, F.G.; Carraro, F.; Falcaro, P.; Amenitsch, H.; Trimmel, G. Honeycomb-structured copper indium sulfide thin films obtained via a nanosphere colloidal lithography method. Mater. Adv. 2022, 3, 2884. [Google Scholar] [CrossRef]
- Yu, H.M.; Lee, J. Fabrication of nanomaterials using anodic aluminum oxide and their properties. Curr. Appl. Phys. 2011, 11, S339–S345. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef]
- Noormohammadi, M.; Arani, Z.S.; Ramazani, A.; Kashi, M.A.; Abbasimofrad, S. Super-fast fabrication of self-ordered nanoporous anodic alumina membranes by ultra-hard anodization. Electrochim. Acta 2020, 354, 136766. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M. Revisiting anodic alumina templates: From fabrication to applications. Nanoscale 2021, 13, 2227–2265. [Google Scholar] [CrossRef]
- Manzano, C.V.; Martín, J.; Martín-González, M.S. Ultra-narrow 12 nm pore diameter self-ordered anodic alumina templates. Microporous Mesoporous Mater. 2014, 184, 177–183. [Google Scholar] [CrossRef]
- Resende, P.M.; Martín-González, M. Sub-10 nm porous alumina templates to produce sub-10 nm nanowires. Microporous Mesoporous Mater. 2019, 284, 198–204. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Genesi, B.P.; Barbosa, R.M.; Severino, P.; Rodas, A.C.D.; Yoshida, C.M.P.; Mathor, M.B.; Lopes, P.S.; Viseras, C.; Souto, E.B.; Silva, C.F. Aloe vera and copaiba oleoresin-loaded chitosan films for wound dressings: Microbial permeation, cytotoxicity, and in vivo proof of concept. Int. J. Pharm. 2023, 634, 122648. [Google Scholar] [CrossRef]
- Ji, M.; Li, J.; Wang, Y.; Li, F.; Man, J.; Li, J.; Zhang, C.; Peng, S.; Wang, S. Advances in chitosan-based wound dressings: Modifications, fabrications, applications and prospects. Carbohydr. Polym. 2022, 297, 120058. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533. [Google Scholar] [CrossRef]
- Tricol Biomedical Inc. Available online: https://tricolbiomedical.com/product-category/trauma/ (accessed on 2 July 2023).
- Tang, W.; Wang, J.; Hou, H.; Li, Y.; Wang, J.; Fu, J.; Lu, L.; Gao, D.; Liu, Z.; Zhao, F.; et al. Review: Application of chitosan and its derivatives in medical materials. Int. J. Biol. Macromol. 2023, 240, 124398. [Google Scholar] [CrossRef] [PubMed]
- Herliana, H.; Yusuf, H.Y.; Laviana, A.; Wandawa, G.; Cahyanto, A. Characterization and Analysis of Chitosan-Gelatin Composite-Based Biomaterial Effectivity as Local Hemostatic Agent: A Systematic Review. Polymers 2023, 15, 575. [Google Scholar] [CrossRef]
- Altuntas, S.; Dhaliwal, H.K.; Bassous, N.J.; Radwan, A.E.; Alpaslan, P.; Webster, T.; Buyukserin, F.; Amiji, M. Nanopillared Chitosan/Gelatin Films: A Biomimetic Approach for Improved Osteogenesis. ACS Biomater. Sci. Eng. 2019, 5, 4311–4322. [Google Scholar] [CrossRef]
- De Masi, A.; Tonazzini, I.; Masciullo, C.; Mezzena, R.; Chiellini, F.; Puppi, D.; Cecchini, M. Chitosan films for regenerative medicine: Fabrication methods and mechanical characterization of nanostructured chitosan films. Biophys. Rev. 2019, 11, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.Y.; Yang, C.Y.; Chen, W.S.; Wang, Y.K.; Yeh, J.A.; Cheng, C.M. Probing neural cell behaviors through micro-/nano-patterned chitosan substrates. Biofabrication 2015, 7, 045007. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, S.; Dhaliwal, H.K.; Radwan, A.E.; Amiji, M.; Buyukserin, F. Local epidermal growth factor delivery using nanopillared chitosan–gelatin films for melanogenesis and wound healing. Biomater. Sci. 2023, 11, 181–194. [Google Scholar] [CrossRef]
- Cigane, U.; Palevicius, A.; Janusas, G. Vibration-Assisted Synthesis of Nanoporous Anodic Aluminum Oxide (AAO) Membranes. Micromachines 2022, 13, 2236. [Google Scholar] [CrossRef]
- Lei, J.; Cheng, F.; Li, K. Numerical Simulation of Boundary-Driven Acoustic Streaming in Microfluidic Channels with Circular Cross-Sections. Micromachines 2020, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhou, M.; Yuan, S.; Fan, C.; Jiang, B. Numerical investigation of particle deflection in tilted-angle standing surface acoustic wave microfluidic devices. Appl. Math. Model. 2022, 101, 517–532. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, X.; Gong, M.; Wang, H.; Liu, X. Acoustic radiation force and motion of a free cylinder in a viscous fluid with a boundary defined by a plane wave incident at an arbitrary angle. J. Appl. Phys. 2020, 128, 044902. [Google Scholar] [CrossRef]
- Wang, Q.; Riaud, A.; Zhou, J.; Gong, Z.; Baudoin, M. Acoustic Radiation Force on Small Spheres Due to Transient Acoustic Fields. Phys. Rev. Appl. 2021, 15, 044034. [Google Scholar] [CrossRef]
- Basch, T.; Pavlic, A.; Dual, J. Acoustic radiation force acting on a heavy particle in a standing wave can be dominated by the acoustic microstreaming. Phys. Rev. E 2019, 100, 061102. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Q.; Jin, T. Numerical simulation of nonlinear phenomena in a standing-wave thermoacoustic engine with gas-liquid coupling oscillation. Appl. Therm. Eng. 2022, 207, 118131. [Google Scholar] [CrossRef]
- Das, P.K.; Snider, A.D.; Bhethanabotla, V.R. Acoustic streaming in second-order fluids. Phys. Fluids 2020, 32, 123103. [Google Scholar] [CrossRef]
- Rubio, L.D.; Collins, M.; Sen, A.; Aranson, I.S. Ultrasound Manipulation and Extrusion of Active Nanorods. Small 2023, 2300028. [Google Scholar] [CrossRef] [PubMed]
- COMSOL AB. Acoustic Streaming in a Microchannel Cross Section, COMSOL Multiphysics® v. 6.1; COMSOL AB: Stockholm, Sweden, 2023; pp. 2–24. [Google Scholar]
- COMSOL AB. Rising Bubble, COMSOL Multiphysics® v. 6.1; COMSOL AB: Stockholm, Sweden, 2023; pp. 2–14. [Google Scholar]


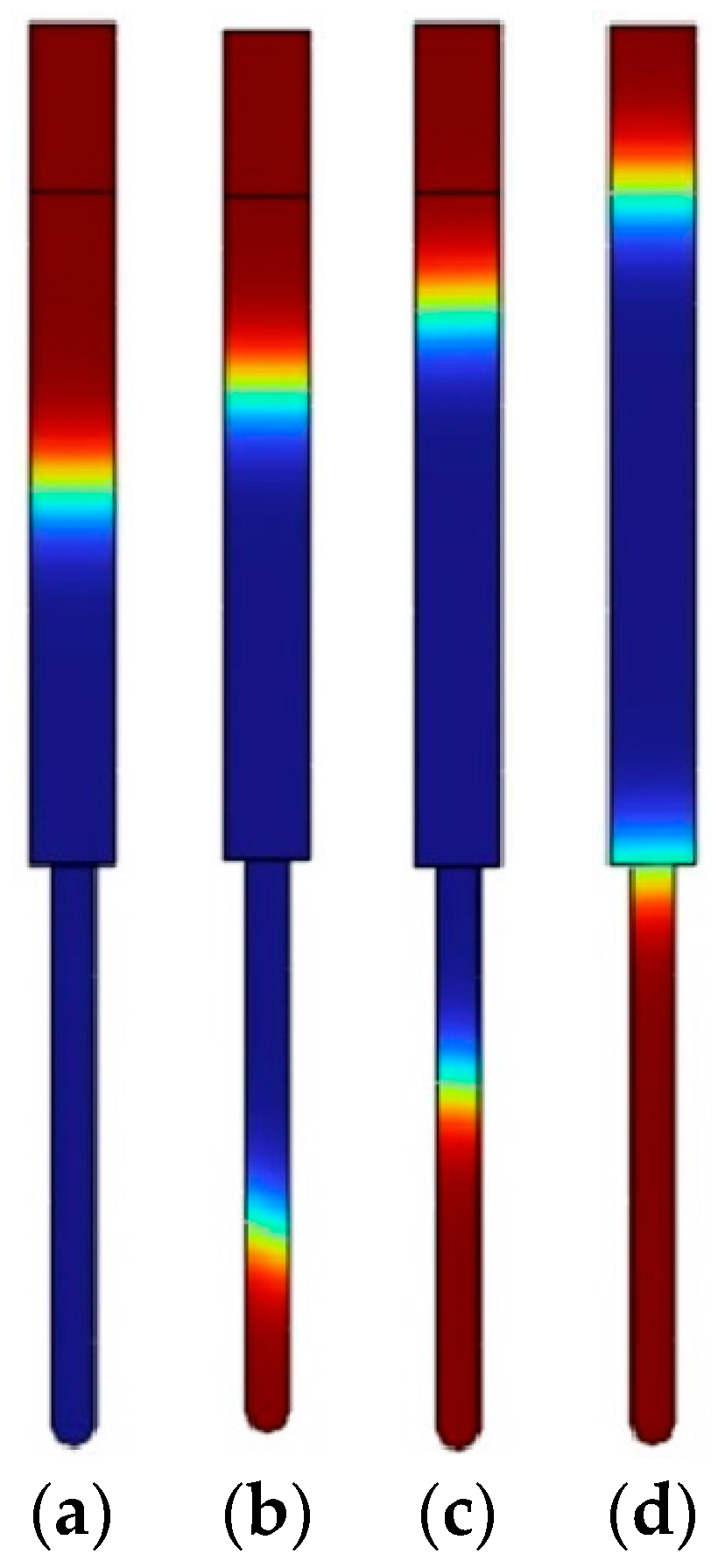

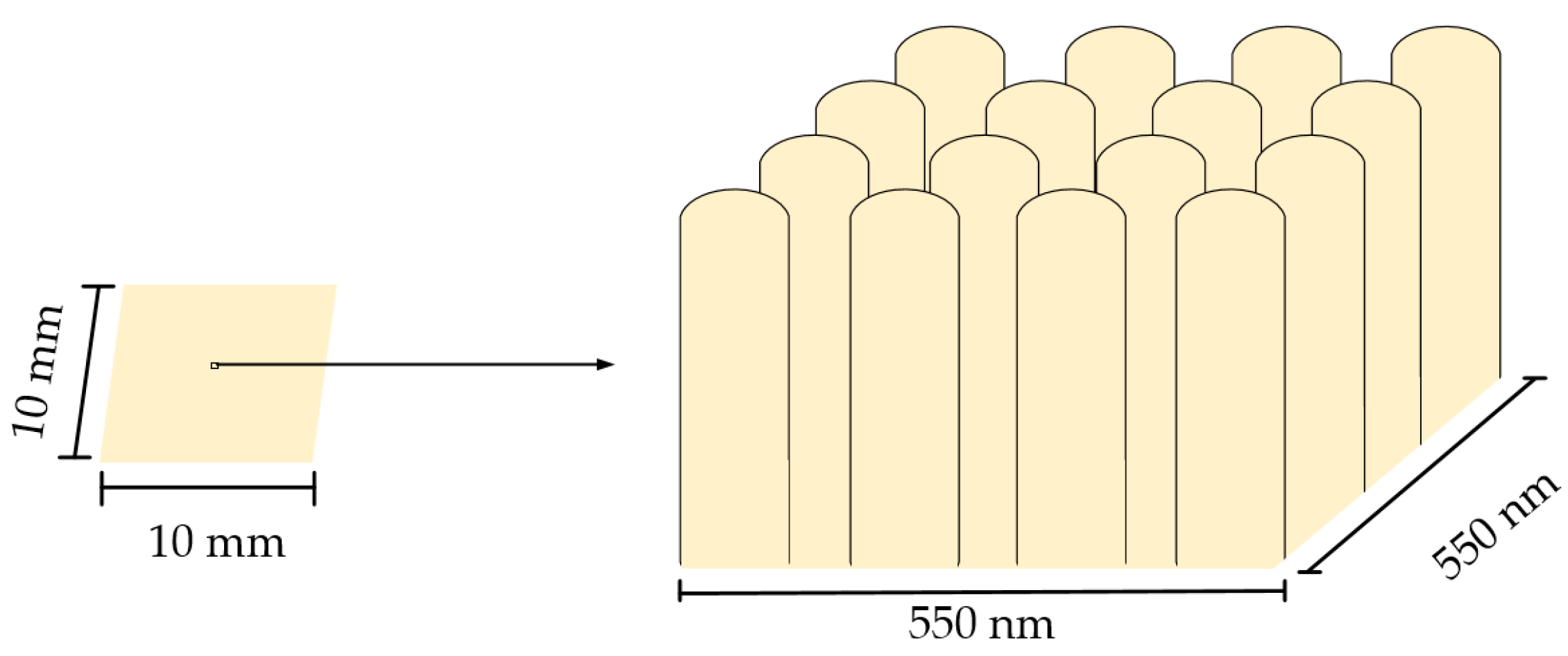
| Parameter | Symbol | Inscription | Value |
|---|---|---|---|
| Frequency | f0 | 40 [kHz] | 40.000 Hz |
| Ambient temperature | T0 | 20 [°C] | 293.15 K |
| Ambient pressure | p0 | 1 [atm] | 1.0133 × 105 Pa |
| Study angular frequency | omega0 | 2 × pi × f0 | 2.5132 × 105 Hz |
| Channel cross-section width | W | 100 [nm] | 1 × 10−7 m |
| Channel cross-section height | H | 1000 [nm] | 1 × 10−6 m |
| Wall displacement | d0 | 100 [nm] | 1× 10−7 m |
| Time | t | 4 [s] | 4 s |
| Parameter | Value | Image |
|---|---|---|
| Pore diameter, nm | 100 ± 10 |  |
| Interpore distance, nm | 150 ± 10 | |
| Thickness, µm | 45 ± 1 | 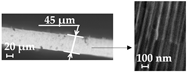 |
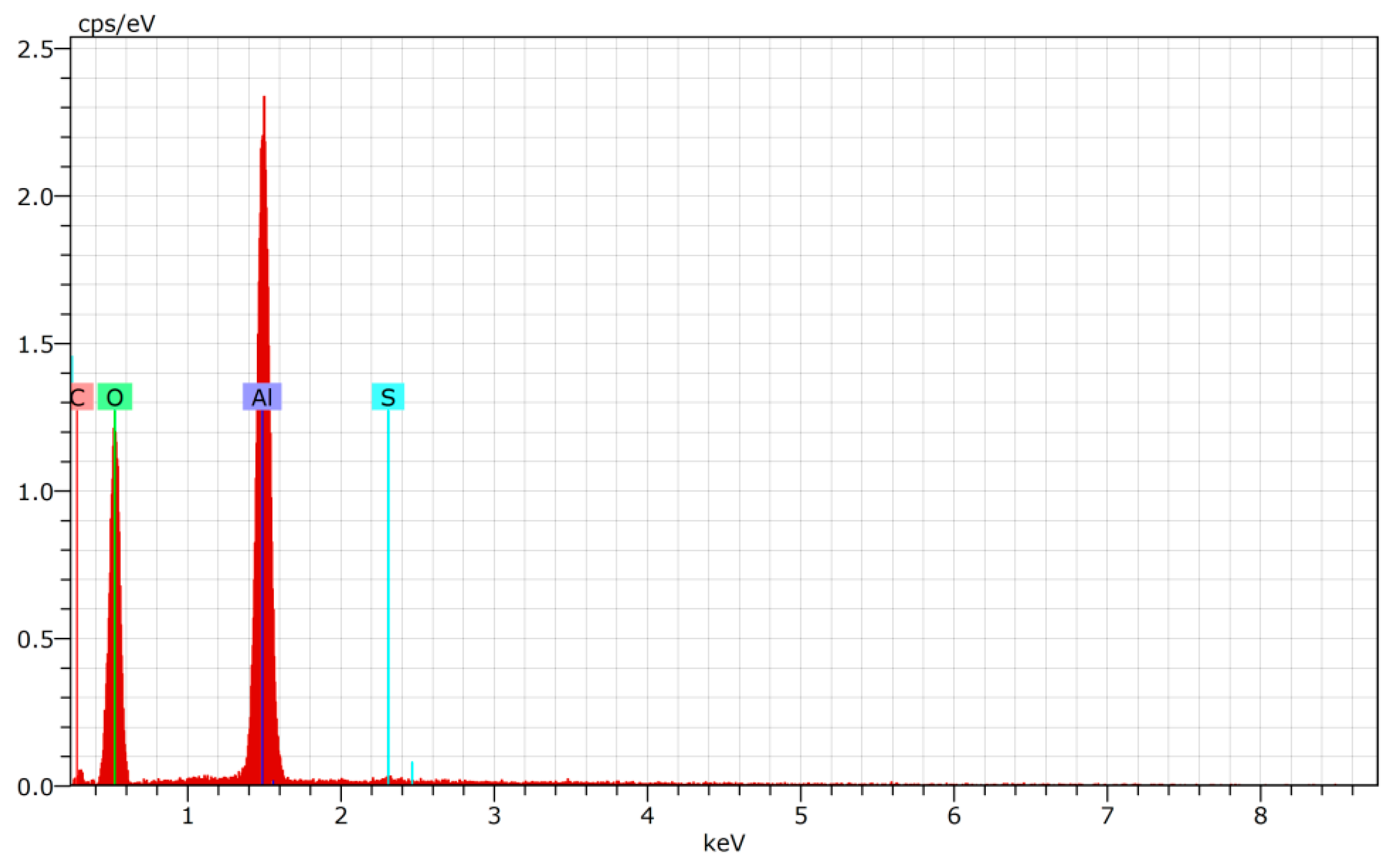
| Element | ||||
|---|---|---|---|---|
| Aluminum | Oxygen | Carbon | Sulfur | |
| Normalised concentration, wt% | 44.90 | 53.38 | 1.24 | 0.48 |
| Atomic concentration, at% | 32.51 | 65.18 | 2.02 | 0.29 |
| Error, % | 2.4 | 7.8 | 0.5 | 0.1 |
| Parameter | 1 wt% | 2 wt% | 3 wt% |
|---|---|---|---|
| Velocity, nm/s | 250 | 169 | 94 |
| Height of nanopillars, nm | 1000 | 675 | 375 |
| Surface area of a single nanopillar, µm2 | 0.314 | 0.212 | 0.118 |
| Parameter | 1 wt% | 2 wt% | 3 wt% |
|---|---|---|---|
| Diameter of nanopillars, nm | 99 ± 10 | 101 ± 10 | 100 ± 10 |
| Height of nanopillars, nm | 1007 ± 10 | 665 ± 10 | 377 ± 10 |
| Average surface area of a single nanopillar, µm2 | 0.313 | 0.211 | 0.118 |
| Calculated experimental velocity, nm/s | 201 | 133 | 75 |
| Calculated surface area, cm2 | 15.05 | 10.28 | 6.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cigane, U.; Palevicius, A.; Janusas, G. A Free-Standing Chitosan Membrane Prepared by the Vibration-Assisted Solvent Casting Method. Micromachines 2023, 14, 1419. https://doi.org/10.3390/mi14071419
Cigane U, Palevicius A, Janusas G. A Free-Standing Chitosan Membrane Prepared by the Vibration-Assisted Solvent Casting Method. Micromachines. 2023; 14(7):1419. https://doi.org/10.3390/mi14071419
Chicago/Turabian StyleCigane, Urte, Arvydas Palevicius, and Giedrius Janusas. 2023. "A Free-Standing Chitosan Membrane Prepared by the Vibration-Assisted Solvent Casting Method" Micromachines 14, no. 7: 1419. https://doi.org/10.3390/mi14071419
APA StyleCigane, U., Palevicius, A., & Janusas, G. (2023). A Free-Standing Chitosan Membrane Prepared by the Vibration-Assisted Solvent Casting Method. Micromachines, 14(7), 1419. https://doi.org/10.3390/mi14071419







