Highly Efficient Solar-Light-Active Ag-Decorated g-C3N4 Composite Photocatalysts for the Degradation of Methyl Orange Dye
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Synthesis of GCN and x% AgGCN Composite Catalysts
2.3. Characterizations of Materials
2.4. Photocatalytic Activity Measurement
3. Result and Discussion
3.1. XRD Analysis
3.2. FT-IR Analysis
3.3. UV-DRS Analysis
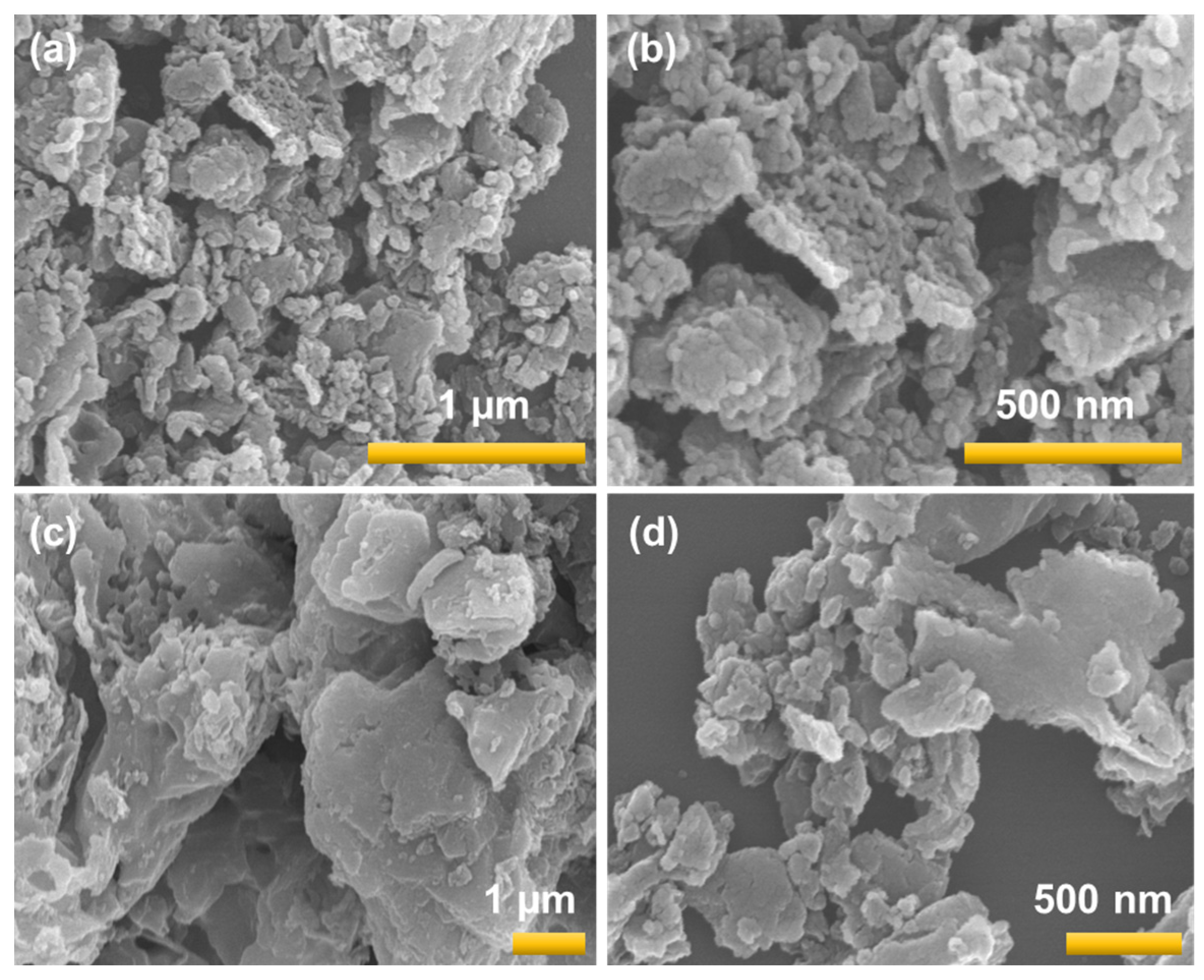
3.4. SEM/EDS Analysis
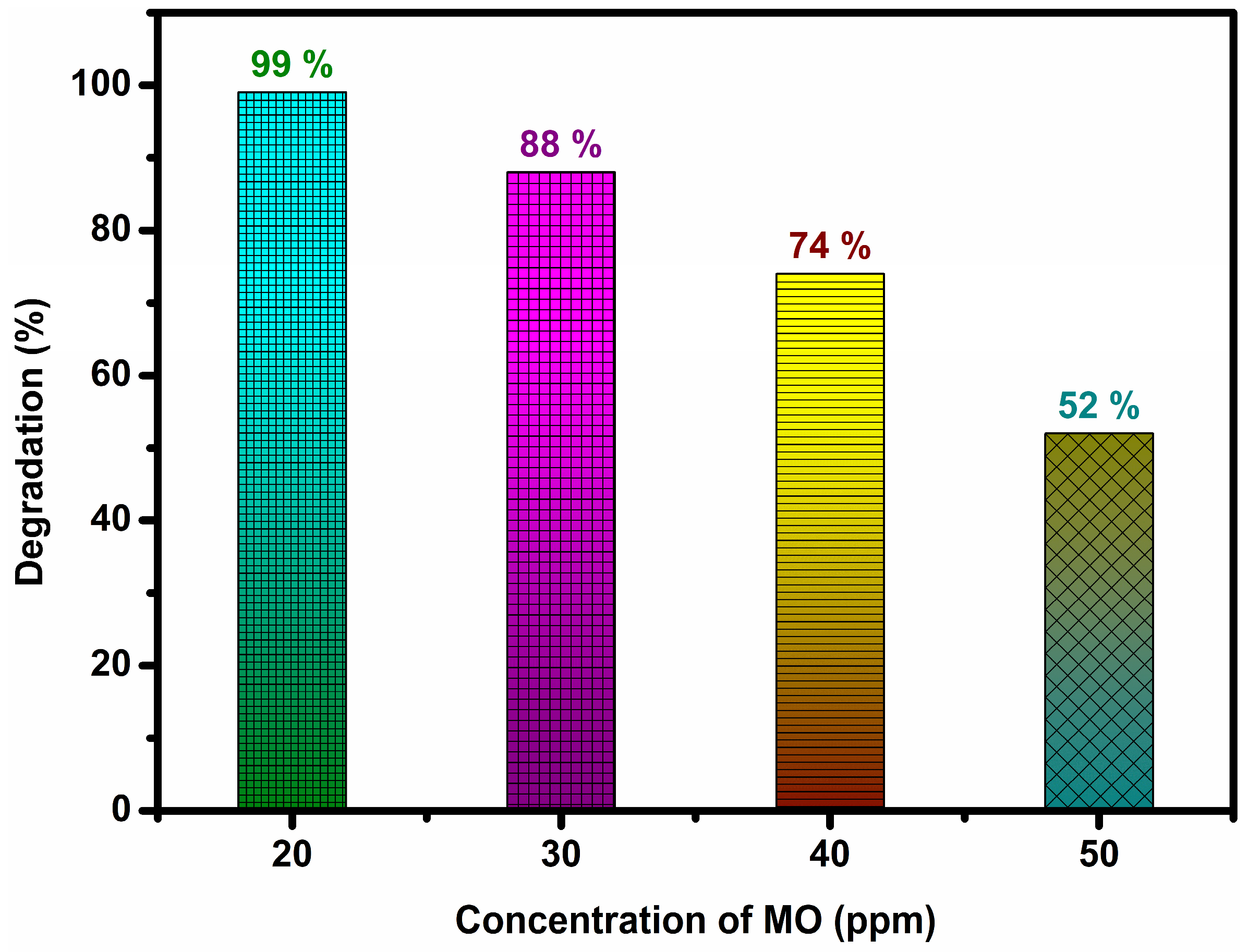
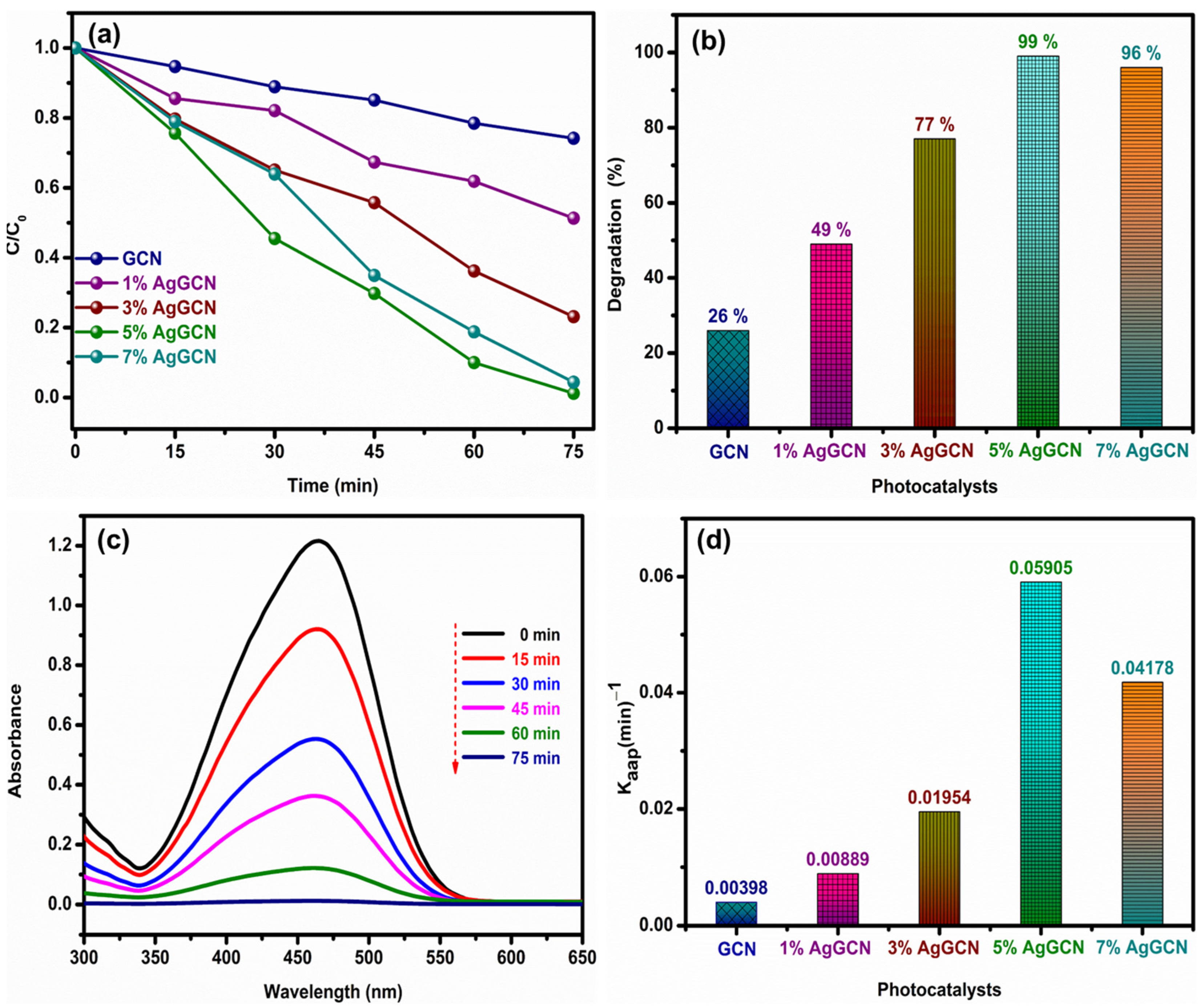
3.5. Photocatalytic Activity of Bare GCN and x% AgGCN Composite Photocatalysts
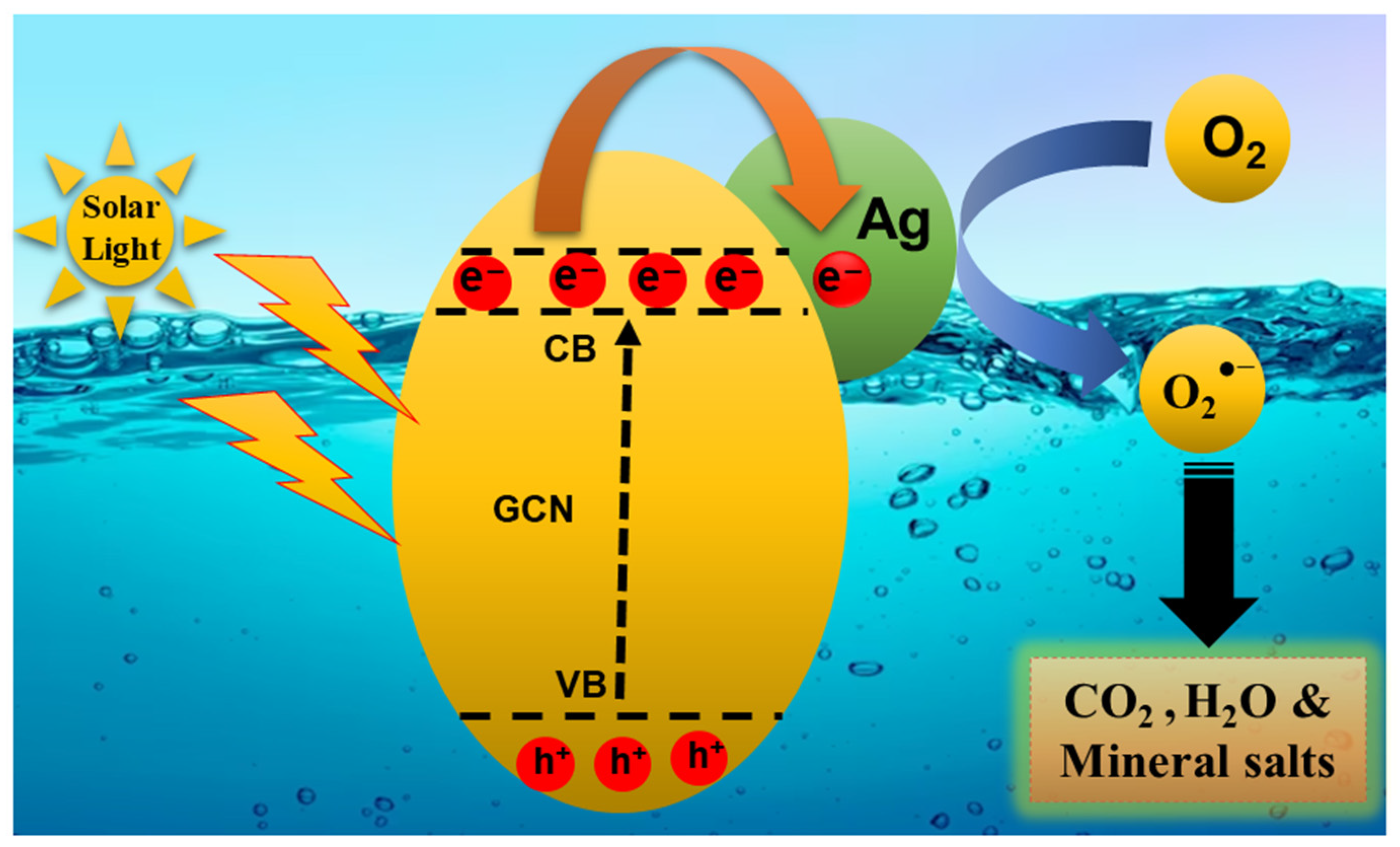
3.6. Catalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumaravel, S.; Thiripuranthagan, S.; Vembuli, T.; Erusappan, E.; Durai, M.; Sureshkumar, T.; Durai, M. Fabrication of mesoporous WO3-SBA-15 catalysts and enhanced photocatalytic degradation of harmful dyes. Optik 2021, 235, 166599. [Google Scholar] [CrossRef]
- Mohan, D.; Shukla, S.P. Hazardous consequences of textile mill effluents on soil and their remediation approaches. Clean. Eng. Technol. 2022, 7, 100434. [Google Scholar]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile dyeing industry: Environmental impacts and remediation. Environ. Sci. Pollut. Res. 2020, 27, 3803–3818. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Ferreira, L.F.R.; Hussain, C.M.; Mulla, S.I.; Bharagava, R.N. Degradation mechanism and toxicity reduction of methyl orange dye by a newly isolated bacterium Pseudomonas aeruginosa MZ520730. J. Water Process Eng. 2021, 43, 102300. [Google Scholar] [CrossRef]
- Wu, L.; Liu, X.; Lv, G.; Zhu, R.; Tian, L.; Liu, M.; Li, Y.; Rao, W.; Liu, T.; Liao, L. Study on the adsorption properties of methyl orange by natural one-dimensional nano-mineral materials with different structures. Sci. Rep. 2021, 11, 10640. [Google Scholar] [CrossRef]
- Mani, D.; Tahawy, R.; Doustkhah, E.; Shanmugam, M.; Arivanandhan, M.; Jayavel, R.; Ide, Y. A rutile TiO2 nanobundle as a precursor of an efficient visible-light photocatalyst embedded with Fe2O3. Inorg. Chem. Front. 2021, 8, 4423–4430. [Google Scholar] [CrossRef]
- Kumaravel, S.; Thiripuranthagan, S.; Vembuli, T.; Kumaravel, S.; Erusappan, E.; Chicardi, E.; Chinnasamy, S. Detoxification of harmful pollutants using highly efficient visible light active Ru/TiO2/PVDF photocatalytic membranes. Mater. Res. Bull. 2023, 167, 112421. [Google Scholar] [CrossRef]
- Baral, A.; Das, D.P.; Minakshi, M.; Ghosh, M.K.; Padhi, D.K. Probing Environmental Remediation of RhB Organic Dye Using α-MnO2 under Visible-Light Irradiation: Structural, Photocatalytic and Mineralization Studies. ChemistrySelect 2016, 1, 4277–4285. [Google Scholar] [CrossRef]
- Ravikumar, S.; Mani, D.; Khan, M.R.; Ahmad, N.; Gajalakshmi, P.; Surya, C.; Durairaj, S.; Pandiyan, V.; Ahn, Y.H. Effect of silver incorporation on the photocatalytic degradation of Reactive Red 120 using ZnS nanoparticles under UV and solar light irradiation. Environ. Res. 2022, 209, 112819. [Google Scholar] [CrossRef]
- Baral, A.B.; Dash, B.; Ghosh, M.K.; Subbaiah, T.; Minakshi, M. Pathway of sucrose oxidation in manganese (pyrolusite) nodule. Ind. Eng. Chem. Res. 2015, 54, 12233–12241. [Google Scholar] [CrossRef]
- Mani, D.; Elango, D.; Priyadharsan, A.; Al-Humaid, L.A.; Al-Dahmash, N.D.; Ragupathy, S.; Jayanthi, P.; Ahn, Y.H. Groundnut shell chemically treated with KOH to prepare inexpensive activated carbon: Methylene blue adsorption and equilibrium isotherm studies. Environ. Res. 2023, 231, 116026. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour.-Effic. Technol. 2016, 2, 175–184. [Google Scholar]
- Ahmed, M.; Mavukkandy, M.O.; Giwa, A.; Elektorowicz, M.; Katsou, E.; Khelifi, O.; Naddeo, V.; Hasan, S.W. Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy. npj Clean Water 2022, 5, 12. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nam, S.N.; Kim, J.; Oh, J. Photocatalytic degradation of dissolved organic matter under ZnO-catalyzed artificial sunlight irradiation system. Sci. Rep. 2020, 10, 13090. [Google Scholar] [CrossRef]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- Durmus, Z.; Maijenburg, A.W. A review on graphitic carbon nitride (g-C3N4)-metal organic framework (MOF) heterostructured photocatalyst materials for photo (electro) chemical hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 36784–36813. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Said, R.S.; Hrahsheh, F.; Haruna, K.; Mohammed, J. Tuning of Graphitic Carbon Nitride (g-C3N4) for Photocatalysis: A Critical Review. Arab. J. Chem. 2023, 16, 104542. [Google Scholar] [CrossRef]
- Gao, R.H.; Ge, Q.; Jiang, N.; Cong, H.; Liu, M.; Zhang, Y.Q. Graphitic carbon nitride (g-C3N4)-based photocatalytic materials for hydrogen evolution. Front. Chem. 2022, 10, 1048504. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent advances of graphitic carbon nitride-based structures and applications in catalyst, sensing, imaging, and LEDs. Nano Micro Lett. 2017, 9, 4. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, L.; Wang, S.; Li, J.; Chi, B. Recent progress of g-C3N4 applied in solar cells. J. Mater. 2021, 7, 728–741. [Google Scholar] [CrossRef]
- Marschall, R.; Wang, L. Non-metal doping of transition metal oxides for visible-light photocatalysis. Catal. Today 2014, 225, 111–135. [Google Scholar] [CrossRef]
- Bora, T.; Zoepfl, D.; Dutta, J. Importance of plasmonic heating on visible light driven photocatalysis of gold nanoparticle decorated zinc oxide nanorods. Sci. Rep. 2016, 6, 26913. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yang, Z.; Yang, Y.; Mei, Y.; Zhou, H.; Yang, S. One-step introduction of metallic Bi and non-metallic C in Bi2WO6 with enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2019, 30, 1310–1321. [Google Scholar] [CrossRef]
- Wang, J.; Shao, B.L.X.; Ji, X.; Tian, G.; Ge, H. CdS and Ag synergistically improved the performance of g-C3N4 on visible-light photocatalytic degradationof pollution. Environ. Sci. Pollut. Res. 2022, 29, 48348–48357. [Google Scholar] [CrossRef]
- Sureshkumar, T.; Thiripuranthagan, S.; Paskalis, S.M.K.; Kumaravel, S.; Kannan, K.; Devarajan, A. Synthesis, characterization and photodegradation activity of graphitic C3N4-SrTiO3 nanocomposites. J. Photochem. Photobiol. A 2018, 356, 425–439. [Google Scholar] [CrossRef]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A.; Ranganathan, S.; Vembuli, T.; Kumaravel, S.; Erusappan, E. Fabrication of novel Bi2MoO6/N-rGO catalyst for the efficient photocatalytic degradation of harmful dyes. Mater. Res. Bull. 2020, 125, 110782. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Qi, K.; Li, Y.; Xie, Y.; Liu, S.Y.; Zheng, K.; Chen, Z.; Wang, R. Ag loading enhanced photocatalytic activity of g-C3N4 porous nanosheets for decomposition of organic pollutants. Front. Chem. 2019, 7, 91. [Google Scholar] [CrossRef]
- Hao, X.; Zhou, J.; Cui, Z.; Wang, Y.; Wang, Y.; Zou, Z. Zn-vacancy mediated electron-hole separation in ZnS/g-C3N4 heterojunction for efficient visible-light photocatalytic hydrogen production. Appl. Catal. B 2018, 229, 41–51. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J.; Li, Y. Enhanced visible light photocatalytic activity of novel polymeric g-C3N4 loaded with Ag nanoparticles. Appl. Catal. A 2011, 409, 215–222. [Google Scholar] [CrossRef]
- Mohanta, D.; Mahanta, A.; Mishra, S.R.; Jasimuddin, S.; Ahmaruzzaman, M. Novel SnO2@ ZIF-8/gC3N4 nanohybrids for excellent electrochemical performance towards sensing of p-nitrophenol. Environ. Res. 2021, 197, 111077. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, D.; Makkar, P.; Korde, R.; Das, M.R.; Ghosh, N.N. Exfoliated gC3N4 supported CdS nanorods as a S-scheme heterojunction photocatalyst for the degradation of various textile dyes. Adv. Powder Technol. 2022, 33, 103801. [Google Scholar] [CrossRef]
- Alhaddad, M.; Navarro, R.M.; Hussein, M.A.; Mohamed, R.M. Visible light production of hydrogen from glycerol over Cu2O-gC3N4 nanocomposites with enhanced photocatalytic efficiency. J. Mater. Res. Technol. 2020, 9, 15335–15345. [Google Scholar] [CrossRef]
- Kumaravel, S.; Thiripuranthagan, S.; Erusappan, E.; Durai, M. Mesoporous Ru/Sn-SBA-15 catalysts: Synthesis, characterization and catalytic activity towards hydrogenation of levulinic acid. J. Porous Mater. 2022, 29, 1083–1095. [Google Scholar] [CrossRef]
- Kumaravel, S.; Thiripuranthagan, S.; Erusappan, E.; Durai, M.; Vembuli, T.; Durai, M. Catalytic conversion of levulinic acid under noncorrosive conditions using Ru/Zr/Al-SBA-15 catalysts. Microporous Mesoporous Mater. 2020, 305, 110298. [Google Scholar] [CrossRef]
- Kumaravel, S.; Thiripuranthagan, S.; Durai, M.; Erusappan, E.; Vembuli, T. Catalytic transfer hydrogenation of biomass-derived levulinic acid to γ-valerolactone over Sn/Al-SBA-15 catalysts. New J. Chem. 2020, 44, 8209–8222. [Google Scholar] [CrossRef]
- Sundaram, M.M.; Appadoo, D. Traditional salt-in-water electrolyte vs. water-in-salt electrolyte with binary metal oxide for symmetric supercapacitors: Capacitive vs. faradaic. Dalton Trans. 2020, 49, 11743–11755. [Google Scholar] [CrossRef]
- Ramamoorthy, M.; Mani, D.; Karunanithi, M.; Mini, J.J.; Babu, A.; Mathivanan, D.; Ragupathy, S.; Ahn, Y.H. Influence of metal doping and non-metal loading on photodegradation of methylene blue using SnO2 nanoparticles. J. Mol. Struct. 2023, 1286, 135564. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Deng, H. Ag modified g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Mol. Catal. A Chem. 2016, 423, 270–276. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, X.; Ma, C.; Song, M.; Gao, N.; Wang, Y.; Huo, P.; Lu, Z.; Yan, Y. Fabrication of conductive and high-dispersed Ppy@Ag/g-C3N4 composite photocatalysts for removing various pollutants in water. Appl. Surf. Sci. 2016, 387, 366–374. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, H.Y.; Ma, C.C.; Mao, F.; Xue, B. Ag/g-C3N4 photocatalysts: Microwave-assisted synthesis and enhanced visible-light photocatalytic activity. Catal. Commun. 2016, 79, 45–48. [Google Scholar] [CrossRef]
- Palanivel, B.; Mani, A. Conversion of a type-II to a Z-scheme heterojunction by intercalation of a 0D electron mediator between the integrative NiFe2O4/g-C3N4 composite nanoparticles: Boosting the radical production for photo-fenton degradation. ACS Omega 2020, 5, 19747–19759. [Google Scholar] [CrossRef] [PubMed]
- Thorat, N.; Yadav, A.; Yadav, M.; Gupta, S.; Varma, R.; Pillai, S.; Fernandes, R.; Patel, M.; Patel, N. Ag loaded B-doped-g C3N4 nanosheet with efficient properties for photocatalysis. J. Environ. Manag. 2019, 247, 57–66. [Google Scholar] [CrossRef]
- Liu, R.; Yang, W.; He, G.; Zheng, W.; Li, M.; Tao, W.; Tian, M. Ag-modified g-C3N4 prepared by a one-step calcination method for enhanced catalytic efficiency and stability. ACS Omega 2020, 5, 19615–19624. [Google Scholar] [CrossRef]







| S. No. | Composites | Pollutants and Concentration | Light Source and Irradiation Time | Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| 1 | PPy@Ag/g-C3N4 | MO, 10 ppm | Visible, 60 min | 89% | [41] |
| 2 | 1.0 wt%Ag/g-C3N4 | MO, 10 ppm | Visible, 180 min | 100% | [31] |
| 3 | Ag/g-C3N4-2 | RhB, 10 ppm | Visible, 120 min | 98% | [42] |
| 4 | 3% Ag/g-C3N4 | RhB, 10 ppm | Simulated sunlight, 120 min | 100% | [29] |
| 5 | Ag-B-NS-gC3N4 | RhB, 10 ppm | Visible, 60 min | 99% | [44] |
| 6 | Ag/CN-8 | MO, 10 ppm | Visible, 120 min | 98.7% | [45] |
| 7 | 5% AgGCN | MO, 20 ppm | Solar, 75 min | 99% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumaravel, S.; Chandrasatheesh, C.; Palanisamy, G.; Lee, J.; Hasan, I.; Kumaravel, S.; Avula, B.; Pongiya, U.D.; Balu, K. Highly Efficient Solar-Light-Active Ag-Decorated g-C3N4 Composite Photocatalysts for the Degradation of Methyl Orange Dye. Micromachines 2023, 14, 1454. https://doi.org/10.3390/mi14071454
Kumaravel S, Chandrasatheesh C, Palanisamy G, Lee J, Hasan I, Kumaravel S, Avula B, Pongiya UD, Balu K. Highly Efficient Solar-Light-Active Ag-Decorated g-C3N4 Composite Photocatalysts for the Degradation of Methyl Orange Dye. Micromachines. 2023; 14(7):1454. https://doi.org/10.3390/mi14071454
Chicago/Turabian StyleKumaravel, Sakthivel, Chandramoorthy Chandrasatheesh, Govindasamy Palanisamy, Jintae Lee, Imran Hasan, Saranraj Kumaravel, Balakrishna Avula, Uma Devi Pongiya, and Krishnakumar Balu. 2023. "Highly Efficient Solar-Light-Active Ag-Decorated g-C3N4 Composite Photocatalysts for the Degradation of Methyl Orange Dye" Micromachines 14, no. 7: 1454. https://doi.org/10.3390/mi14071454
APA StyleKumaravel, S., Chandrasatheesh, C., Palanisamy, G., Lee, J., Hasan, I., Kumaravel, S., Avula, B., Pongiya, U. D., & Balu, K. (2023). Highly Efficient Solar-Light-Active Ag-Decorated g-C3N4 Composite Photocatalysts for the Degradation of Methyl Orange Dye. Micromachines, 14(7), 1454. https://doi.org/10.3390/mi14071454










