A Comprehensive Review of In-Body Biomedical Antennas: Design, Challenges and Applications
Abstract
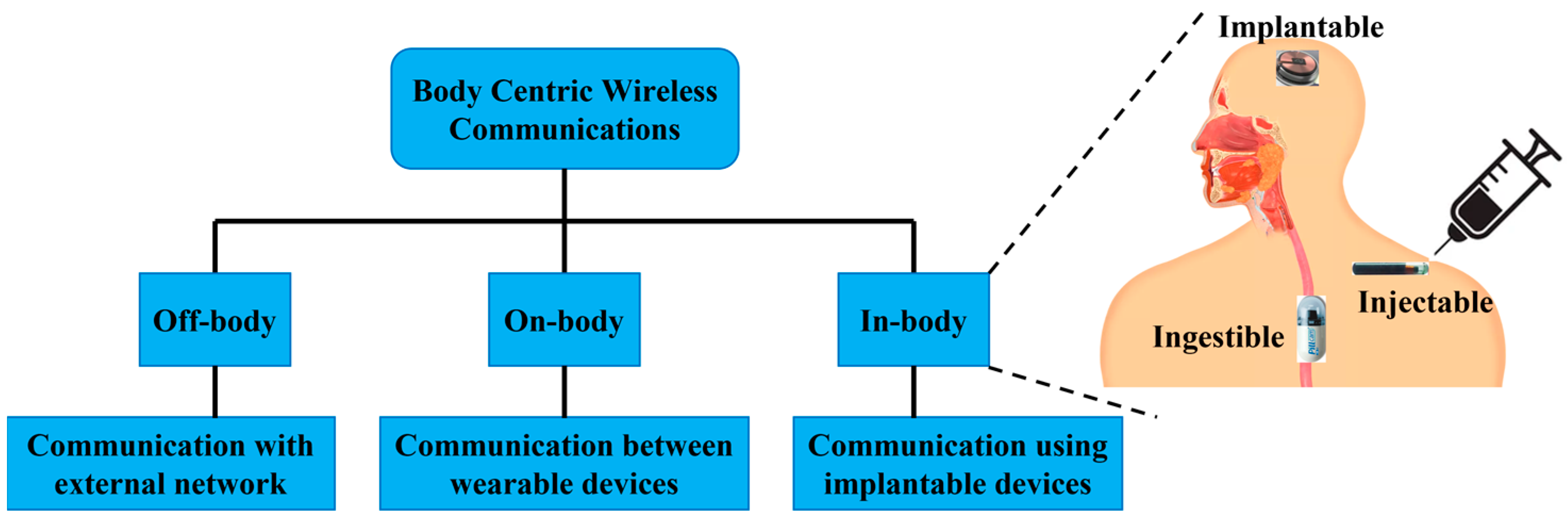
:1. Introduction
2. Design Specifications
2.1. Operation Frequency Bands
2.2. Miniaturization
2.2.1. High Permittivity Dielectric Substrate or Superstrate
2.2.2. Path Lengthening of Current Flow
2.2.3. Impedance Matching with Loading
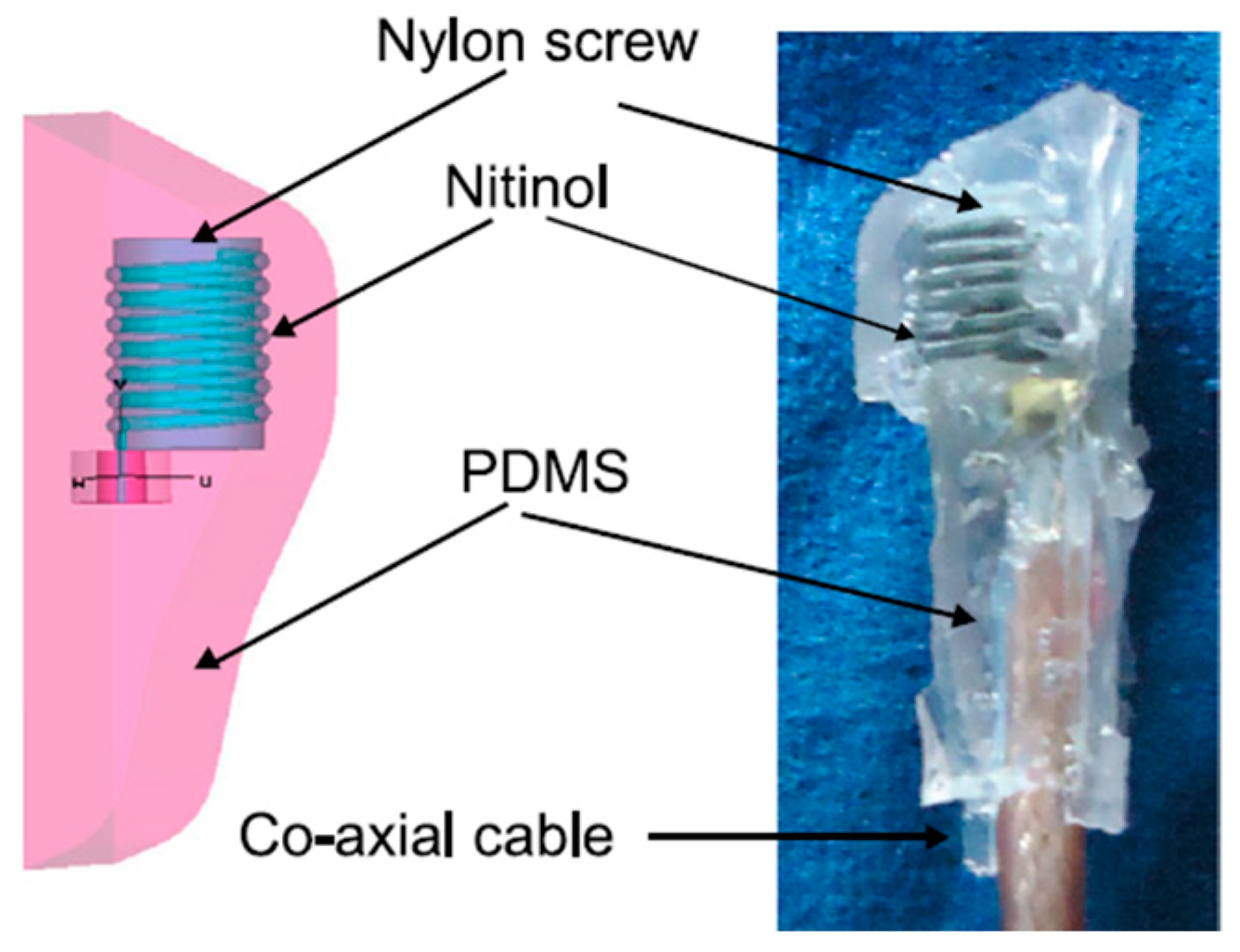
2.2.4. Pin Shorting
2.2.5. High Frequency Band
2.2.6. Modification of Ground Plane
2.2.7. Use of Metamaterial
2.3. Wireless Link Consideration
2.4. Powering
2.5. Biocompatibility
2.6. Safety Consideration
3. Antenna Design, Manufacture and Testing
3.1. Testing of In Vitro Antenna
3.2. Testing of In Vivo Antenna
4. Challenges That Influence the Design of In-Body Antennas
4.1. Effect of Tissue Diversification
4.2. Impact of Effective Wavelength on In-Body Antenna
4.3. Effect of Efficiency
4.4. Biocompatible Encapsulation
4.5. Effect on Antenna Bandwidth
4.6. Effect on Antenna Radiation Pattern
4.7. SAR Requirement
4.8. Effective Isotropic Radiated Power (EIRP)
4.9. Powering
5. In-Body Antenna Applications
5.1. Pacemaker
5.2. Blood Pressure Monitoring Implant
5.3. Brain Implant
5.4. Intracranial Pressure
5.5. Glucose Monitoring and Sensing
5.6. Orthopedic Implant Infection Monitoring
5.7. Cochlear Implant
5.8. Retinal Implant
5.9. Capsule Endoscopy
5.10. Cell Rover
5.11. Pharmacology and Optogenetics
6. Some Future Research Challenges
- Generally, the coupling of in-body antennas with lossy tissue causes the absorption of the EM wave in the near reactive and far field, which is commonly not considered in the design phase. This results in a significant reduction in the radiation efficiency and peak gain, causing inefficient antenna operation. This is inevitable in the far field. However, it is possible to reduce the absorption of the EM wave by covering the in-body antenna with biocompatible material in the near field. Therefore, designing in-body antennas with biocompatibility covering the near field will be a conceivable future research challenge.
- The human body is formed with inhomogeneous biological tissues and organs. Furthermore, the characteristics and dimensions of biological tissues vary every so often, including by gender. Therefore, the detuning effect of the in-body antenna inside the human body is considered as one of the primary research and design IBBD applications. To date, in-body antenna design and experiments are mostly restricted only to a single tissue environment, which will be a noteworthy shortcoming for diverse biological tissue environments. Therefore, the upcoming in-body antenna research focus must be the investigation of diverse biological environments for efficient in-body antenna operation.
- The implantation of a device operating at radio frequency inside the biological tissue may lead to a severe long-term health problem due to radiative power absorption. Therefore, an effective and optimized in-body antenna design with a SAR value limit as standard and an appropriate selection of biocompatible materials will be the key future research investigation.
- Traditional antenna miniaturization techniques tend toward narrow operational bandwidths. Such narrowband operation can cause a detuning effect of the in-body antenna inside the biological environment. Biocompatible encapsulation of the in-body antenna can be utilized to increase the radiation efficiency and gain. However, this inflates the overall IBBD thickness. Therefore, an antenna design technique with acceptable operational bandwidth, radiation efficiency and gain is still a challenging matter in IBBDs. Increasing the operation frequency band can increase the miniaturization scale. However, this increases the loss and tissue absorption, which introduces additional design challenge.
- Lastly, battery-powered IBBDs have a limited lifetime and bulky dimension, which can cause insufficiency in an in-body antenna power system. Furthermore, the replacement of the battery through a surgical procedure is complex and costly. Therefore, designing power-efficient IBBDs for in-body antennas is a crucial design challenge for the future.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, Y. Antennas and Propagation for Body Centric Wireless Communications; Artech House: New York, NY, USA, 2011; p. 3. [Google Scholar] [CrossRef]
- Yadav, S.; Chaudhary, K.P.; Gahlot, A.; Arya, Y.; Dahiya, A.; Garg, N. (Eds.) Recent Advances in Metrology; Springer: Singapore, 2022; ISBN 978-981-19-2467-5. [Google Scholar]
- Kiourti, A.; Nikita, K.S. A Review of Implantable Patch Antennas for Biomedical Telemetry: Challenges and Solutions [Wireless Corner]. IEEE Antennas Propag. Mag. 2012, 54, 210–228. [Google Scholar] [CrossRef]
- Kiourti, A.; Nikita, K.S. A Review of In-Body Biotelemetry Devices: Implantables, Ingestibles, and Injectables. IEEE Trans. Biomed. Eng. 2017, 64, 1422–1430. [Google Scholar] [CrossRef]
- Chow, E.Y.; Morris, M.M.; Irazoqui, P.P. Implantable RF Medical Devices: The Benefits of High-Speed Communication and Much Greater Communication Distances in Biomedical Applications. IEEE Microw. Mag. 2013, 14, 64–73. [Google Scholar] [CrossRef]
- Kiourti, A.; Lee, C.W.L.; Chae, J.; Volakis, J.L. A Wireless Fully Passive Neural Recording Device for Unobtrusive Neuropotential Monitoring. IEEE Trans. Biomed. Eng. 2015, 63, 131–137. [Google Scholar] [CrossRef]
- Yuce, M.R.; Dissanayake, T. Easy-to-Swallow Wireless Telemetry. IEEE Microw. Mag. 2012, 13, 90–101. [Google Scholar] [CrossRef]
- Singeap, A.-M.; Stanciu, C.; Trifan, A. Capsule endoscopy: The road ahead. World J. Gastroenterol. 2016, 22, 369–378. [Google Scholar] [CrossRef]
- Hafezi, H.; Robertson, T.L.; Moon, G.D.; Au-Yeung, K.-Y.; Zdeblick, M.J.; Savage, G.M. An Ingestible Sensor for Measuring Medication Adherence. IEEE Trans. Biomed. Eng. 2014, 62, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.; Lee, S.; Molnar, A.C.; Cash, S. Injectable wireless microdevices: Challenges and opportunities. Bioelectron. Med. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Mugisha, A.J.; Tsiamis, A.; Mitra, S. Commercial Off-the-Shelf Components (COTS) in Realizing Miniature Implantable Wireless Medical Devices: A Review. Sensors 2022, 22, 3635. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Saito, K.; Ito, K. Small Coil Antenna with Magnetic Sheet for Implantable Medical Device Communication in 40–60 MHz Band. IEEE J. Electromagn. RF Microw. Med. Biol. 2022, 6, 348–354. [Google Scholar] [CrossRef]
- Faerber, J.; Gregson, R.; Clutton, R.E.; Khan, S.R.; Cochran, S.; Desmulliez, M.P.Y.; Cummins, G.; Pavuluri, S.K.; Record, P.; Rodriguez, A.R.A.; et al. In Vivo Characterization of a Wireless Telemetry Module for a Capsule Endoscopy System Utilizing a Conformal Antenna. IEEE Trans. Biomed. Circuits Syst. 2017, 12, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.R.; Pavuluri, S.K.; Cummins, G.; Desmulliez, M.P.Y. Wireless Power Transfer Techniques for Implantable Medical Devices: A Review. Sensors 2020, 20, 3487. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xuan, X.-W.; Zhao, W.-Y.; Nie, H.-K. An Implantable Antenna Sensor for Medical Applications. IEEE Sens. J. 2021, 21, 14035–14042. [Google Scholar] [CrossRef]
- Wang, G.-B.; Xuan, X.-W.; Jiang, D.-L.; Li, K.; Wang, W. A miniaturized implantable antenna sensor for wireless capsule endoscopy system. AEU Int. J. Electron. Commun. 2021, 143, 154022. [Google Scholar] [CrossRef]
- Damaj, A.W.; El Misilmani, H.M.; Chahine, S.A. Implantable Antennas for Biomedical Applications: An Overview on Alternative Antenna Design Methods and Challenges. In Proceedings of the 2018 International Conference on High Performance Computing and Simulation, HPCS 2018, Orléans, France, 16–20 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 31–37. [Google Scholar]
- Cil, E.; Dumanli, S. The Design of a Reconfigurable Slot Antenna Printed on Glass for Wearable Applications. IEEE Access 2020, 8, 95417–95423. [Google Scholar] [CrossRef]
- Kim, J.; Rahmat-Samii, Y. Implanted Antennas Inside a Human Body: Simulations, Designs, and Characterizations. IEEE Trans. Microw. Theory Tech. 2004, 52, 1934–1943. [Google Scholar] [CrossRef]
- Ben Amar, A.; Kouki, A.B.; Cao, H. Power Approaches for Implantable Medical Devices. Sensors 2015, 15, 28889–28914. [Google Scholar] [CrossRef] [Green Version]
- Mahn, T.G.; Barritt, K.A. Wireless Medical Technologies: Navigating Government Regulation in the New Medical Age; Fish & Richardson: Boston, MA, USA, 2017. [Google Scholar]
- Symeonidis, S.; Whittow, W.G.; Zecca, M.; Panagamuwa, C. Bone fracture monitoring using implanted antennas in the radius, tibia and phalange heterogeneous bone phantoms. Biomed. Phys. Eng. Express 2018, 4, 45006. [Google Scholar] [CrossRef] [Green Version]
- Murphy, O.H.; Bahmanyar, M.R.; Borghi, A.; McLeod, C.N.; Navaratnarajah, M.; Yacoub, M.H.; Toumazou, C. Continuous in vivo blood pressure measurements using a fully implantable wireless SAW sensor. Biomed. Microdev. 2013, 15, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Yuce, M.R. Review of Medical Implant Communication System (MICS) band and network. ICT Express 2016, 2, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Li, Z.; Qi, L.; Shen, W.; Li, G. A compact and miniaturized implantable antenna for ISM band in wireless cardiac pacemaker system. Sci. Rep. 2022, 12, 238. [Google Scholar] [CrossRef]
- Denes, E.; Barrière, G.; Poli, E.; Lévêque, G. Alumina Biocompatibility. J. Autom. Inf. Sci. 2018, 28, 9–13. [Google Scholar] [CrossRef]
- Kiourti, A.; Christopoulou, M.; Nikita, K.S. Performance of a Novel Miniature Antenna Implanted in the Human Head for Wireless Biotelemetry. In Proceedings of the 2011 IEEE International Symposium on Antennas and Propagation (APSURSI), Spokane, Washington, DC, USA, 3–8 July 2011; pp. 392–395. [Google Scholar]
- Karacolak, T.; Hood, A.Z.; Topsakal, E. Design of a Dual-Band Implantable Antenna and Development of Skin Mimicking Gels for Continuous Glucose Monitoring. IEEE Trans. Microw. Theory Tech. 2008, 56, 1001–1008. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.-X.; Xiao, S. Capacitively Loaded Circularly Polarized Implantable Patch Antenna for ISM Band Biomedical Applications. IEEE Trans. Antennas Propag. 2014, 62, 2407–2417. [Google Scholar] [CrossRef]
- Soontornpipit, P.; Furse, C.; Chung, Y.C. Miniaturized biocompatible microstrip antenna using genetic algorithm. IEEE Trans. Antennas Propag. 2005, 53, 1939–1945. [Google Scholar] [CrossRef]
- Chien, T.-F.; Cheng, C.-M.; Yang, H.-C.; Jiang, J.-W.; Luo, C.-H. Development of Nonsuperstrate Implantable Low-Profile CPW-Fed Ceramic Antennas. IEEE Antennas Wirel. Propag. Lett. 2010, 9, 599–602. [Google Scholar] [CrossRef]
- Khan, M.U.; Sharawi, M.S.; Mittra, R. Microstrip patch antenna miniaturisation techniques: A review. IET Microw. Antennas Propag. 2015, 9, 913–922. [Google Scholar] [CrossRef]
- Kiourti, A.; Nikita, K.S. Meandered versus Spiral Novel Miniature PIFAs Implanted in the Human Head: Tuning and Performance. Lect. Notes Inst. Comput. Sci. Soc. Inform. Telecommun. Eng. 2012, 83, 80–87. [Google Scholar] [CrossRef]
- Soontornpipit, P.; Furse, C.M.; Chung, Y.C. Design of Implantable Microstrip Antenna for Communication with Medical Implants. IEEE Trans. Microw. Theory Tech. 2004, 52, 1944–1951. [Google Scholar] [CrossRef]
- Hashemi, S.; Rashed-Mohassel, J. Miniaturization of dual band implantable antennas. Microw. Opt. Technol. Lett. 2016, 59, 36–40. [Google Scholar] [CrossRef]
- Qing, X.; Chen, Z.N.; See, T.S.P.; Goh, C.K.; Chiam, T.M. Characterization of RF Transmission in Human Body. In Proceedings of the 2010 IEEE International Symposium on Antennas and Propagation and CNC-USNC/URSI Radio Science Meeting—Leading the Wave, AP-S/URSI 2010, Toronto, ON, Canada, 11–17 July 2010. [Google Scholar] [CrossRef]
- Huang, J. The finite ground plane effect on the microstrip antenna radiation patterns. IEEE Trans. Antennas Propag. 1983, 31, 649–653. [Google Scholar] [CrossRef]
- Lier, E.; Jakobsen, K. Rectangular microstrip patch antennas with infinite and finite ground plane dimensions. IEEE Trans. Antennas Propag. 1983, 31, 978–984. [Google Scholar] [CrossRef]
- Bhattacharyya, A. Effects of finite ground plane on the radiation characteristics of a circular patch antenna. IEEE Trans. Antennas Propag. 1990, 38, 152–159. [Google Scholar] [CrossRef]
- Xia, W.; Saito, K.; Takahashi, M.; Ito, K. Performances of an Implanted Cavity Slot Antenna Embedded in the Human Arm. IEEE Trans. Antennas Propag. 2009, 57, 894–899. [Google Scholar] [CrossRef]
- Sihvola, A. Metamaterials in electromagnetics. Metamaterials 2007, 1, 2–11. [Google Scholar] [CrossRef]
- Hussain, M.; Awan, W.A.; Alzaidi, M.S.; Hussain, N.; Ali, E.M.; Falcone, F. Metamaterials and Their Application in the Performance Enhancement of Reconfigurable Antennas: A Review. Micromachines 2023, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Karia, D.C. A metamaterial-inspired circularly polarized antenna for implantable applications. Eng. Rep. 2020, 2, e12251. [Google Scholar] [CrossRef]
- Khan, S.R.; Pavuluri, S.K.; Desmulliez, M.P.Y. Accurate Modeling of Coil Inductance for Near-Field Wireless Power Transfer. IEEE Trans. Microw. Theory Tech. 2018, 66, 4158–4169. [Google Scholar] [CrossRef]
- Li, P.; Principe, J.C.; Bashirullah, R. A Wireless Power Interface for Rechargeable Battery Operated Neural Recording Implants. IEEE Trans. Circuits Syst. II Express Briefs 2006, 2006, 6253–6256. [Google Scholar] [CrossRef]
- Karami, M.A.; Inman, D.J. Powering pacemakers from heartbeat vibrations using linear and nonlinear energy harvesters. Appl. Phys. Lett. 2012, 100, 42901. [Google Scholar] [CrossRef]
- Cadei, A.; Dionisi, A.; Sardini, E.; Serpelloni, M. Kinetic and thermal energy harvesters for implantable medical devices and biomedical autonomous sensors. Meas. Sci. Technol. 2013, 25, 12003. [Google Scholar] [CrossRef]
- Bowers, B.J.; Arnold, D.P. Spherical, rolling magnet generators for passive energy harvesting from human motion. J. Micromech. Microeng. 2009, 19, 94008. [Google Scholar] [CrossRef]
- Cosnier, S.; Le Goff, A.; Holzinger, M. Towards glucose biofuel cells implanted in human body for powering artificial organs: Review. Electrochem. Commun. 2013, 38, 19–23. [Google Scholar] [CrossRef]
- Warty, R.; Tofighi, M.-R.; Kawoos, U.; Rosen, A. Characterization of Implantable Antennas for Intracranial Pressure Monitoring: Reflection by and Transmission Through a Scalp Phantom. IEEE Trans. Microw. Theory Tech. 2008, 56, 2366–2376. [Google Scholar] [CrossRef]
- Karacolak, T.; Cooper, R.; Butler, J.; Fisher, S.; Topsakal, E. In Vivo Verification of Implantable Antennas Using Rats as Model Animals. IEEE Antennas Wirel. Propag. Lett. 2010, 9, 334–337. [Google Scholar] [CrossRef]
- Abadia, J.; Merli, F.; Zürcher, J.-F.; Mosig, J.R.; Skrivervik, A.K. 3D-Spiral Small Antenna Design and Realization for Biomedical Telemetry in the MICS Band. Radioengineering 2009, 18, 359–367. [Google Scholar]
- Skrivervik, A.K.; Merli, F. Design strategies for implantable antennas. In Proceedings of the LAPC 2011—2011 Loughborough Antennas and Propagation Conference, Loughborough, UK, 14–15 November 2011. [Google Scholar] [CrossRef]
- Merli, F.; Fuchs, B.; Mosig, J.R.; Skrivervik, A.K. The Effect of Insulating Layers on the Performance of Implanted Antennas. IEEE Trans. Antennas Propag. 2010, 59, 21–31. [Google Scholar] [CrossRef]
- Duan, Z.; Guo, Y.X.; Je, M.; Kwong, D.-L. Design and in Vitro Test of a Differentially Fed Dual-Band Implantable Antenna Operating at MICS and ISM Bands. IEEE Trans. Antennas Propag. 2014, 62, 2430–2439. [Google Scholar] [CrossRef]
- IEEE Standards Coordinating Committee 28, on N.-I.R.Hazards. IEEE-SA Standards Board; IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3kHz to 300 GHz; IEEE: Piscataway, NJ, USA, 1999; p. 73. [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for Limiting Exposure to Electromagnetic Fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- IEEE International Committee on Electromagnetic Safety. IEEE-SA Standards Board. IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz; IEEE: Piscataway, NJ, USA, 2006; p. 238. [Google Scholar]
- Khan, S.R.; Choi, G. Optimization of planar strongly coupled wireless power transfer system for biomedical applications. Microw. Opt. Technol. Lett. 2016, 58, 1861–1866. [Google Scholar] [CrossRef]
- Khan, S.R.; Choi, G. Analysis and Optimization of Four-Coil Planar Magnetically Coupled Printed Spiral Resonators. Sensors 2016, 16, 1219. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.R.; Desmulliez, M.P. Towards a Miniaturized 3D Receiver WPT System for Capsule Endoscopy. Micromachines 2019, 10, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.R.; Pavuluri, S.K.; Cummins, G.; Desmulliez, M.P.Y. Miniaturized 3-D Cross-Type Receiver for Wirelessly Powered Capsule Endoscopy. IEEE Trans. Microw. Theory Tech. 2019, 67, 1985–1993. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Guo, L.; Xiong, J. Efficient and Wideband Implantable Antenna Based on Magnetic Structures. IEEE Trans. Antennas Propag. 2019, 67, 7242–7251. [Google Scholar] [CrossRef]
- Djellid, A.; Pichon, L.; Koulouridis, S.; Bouttout, F. Miniaturization of a PIFA Antenna for Biomedical Applications Using Artificial Neural Networks. Prog. Electromagn. Res. M 2018, 70, 1–10. [Google Scholar] [CrossRef]
- Kiourti, A.; Nikita, K.S. Implantable Antennas: A Tutorial on Design, Fabrication, and In Vitro\/In Vivo Testing. IEEE Microw. Mag. 2014, 15, 77–91. [Google Scholar] [CrossRef]
- Basir, A.; Yoo, H. Efficient Wireless Power Transfer System with a Miniaturized Quad-Band Implantable Antenna for Deep-Body Multitasking Implants. IEEE Trans. Microw. Theory Tech. 2020, 68, 1943–1953. [Google Scholar] [CrossRef]
- Zaki, A.Z.A.; Hamad, E.K.I.; Abouelnaga, T.G.; Elsadek, H.A.; Khaleel, S.A.; Al-Gburi, A.J.A.; Zakaria, Z. Design and Modeling of Ultra-Compact Wideband Implantable Antenna for Wireless ISM Band. Bioengineering 2023, 10, 216. [Google Scholar] [CrossRef]
- Shah, I.A.; Zada, M.; Yoo, H. Design and Analysis of a Compact-Sized Multiband Spiral-Shaped Implantable Antenna for Scalp Implantable and Leadless Pacemaker Systems. IEEE Trans. Antennas Propag. 2019, 67, 4230–4234. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.-G.; Nam, Y.-H.; Lee, J.-H. Investigation on Wireless Link for Medical Telemetry Including Impedance Matching of Implanted Antennas. Sensors 2021, 21, 1431. [Google Scholar] [CrossRef]
- Khan, S.R.; Mitra, S.; Desmulliez, M.P.Y. Use of a 3-D Wireless Power Transfer Technique as a Method for Capsule Localization. IEEE Access 2021, 9, 131685–131695. [Google Scholar] [CrossRef]
- Tissue Frequency Chart » IT’IS Foundation. Available online: https://itis.swiss/virtual-population/tissue-properties/database/tissue-frequency-chart/ (accessed on 27 February 2019).
- Yamamoto, T.; Sano, K.; Koshiji, K.; Chen, X.; Yang, S.; Abe, M.; Fukuda, A. Development of electromagnetic phantom at low-frequency band. IEEE Eng. Med. Biol. Soc. 2013, 2013, 1887–1890. [Google Scholar] [CrossRef]
- Ito, K.; Furuya, K.; Okano, Y.; Hamada, L. Development and characteristics of a biological tissue-equivalent phantom for microwaves. Electron. Commun. Jpn. (Part I Commun.) 2000, 84, 67–77. [Google Scholar] [CrossRef]
- Kiourti, A.; Christopoulou, M.; Koulouridis, S.; Nikita, K.S. Design of a Novel Miniaturized Implantable PIFA for Biomedical Telemetry. Lect. Notes Inst. Comput. Sci. Soc. Inform. Telecommun. Eng. 2011, 55, 127–134. [Google Scholar]
- Bocan, K.N.; Mickle, M.H.; Sejdic, E. Multi-Disciplinary Challenges in Tissue Modeling for Wireless Electromagnetic Powering: A Review. IEEE Sens. J. 2017, 17, 6498–6509. [Google Scholar] [CrossRef]
- Jilani, M.T.; Wen, W.P.; Cheong, L.Y.; Rehman, M.Z.U. A Microwave Ring-Resonator Sensor for Non-Invasive Assessment of Meat Aging. Sensors 2016, 16, 52. [Google Scholar] [CrossRef] [Green Version]
- Khadase, R.B.; Nandgaonkar, A.; Iyer, B.; Wagh, A.E. Multilayered Implantable Antenna Biosensor for Continuous Glucose Monitoring: Design and Analysis. Prog. Electromagn. Res. C 2021, 114, 173–184. [Google Scholar] [CrossRef]
- Skrivervik, A. Implantable Antennas: The Challenge of Efficiency. In Proceedings of the 7th European Conference on Antennas and Propagation (EuCAP), Göteborg, Sweden, 8–11 April 2013; IEEE: Gothenburg, Sweden, 2013. [Google Scholar]
- Pozar, D.M. Microwave Engineering, 4th ed.; John Wiley &Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–756. [Google Scholar]
- Griffiths, D.J. Introduction to Electrodynamics, 3rd ed.; Prentice-Hall: London, UK, 1999; ISBN 0-13-805326-X. [Google Scholar]
- Wu, T.; Rappaport, T.S.; Collins, C.M. The human body and millimeter-wave wireless communication systems: Interactions and implications. In Proceedings of the 2015 IEEE International Conference on Communications (ICC), London, UK, 8–12 June 2015; pp. 2423–2429. [Google Scholar] [CrossRef] [Green Version]
- Furse, C.; Christensen, D.A.; Durney, C.H. Basic Introduction to Bioelectromagnetics, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9780429136740. [Google Scholar]
- Matthew, N.O. Sadiku Elements of Electromagnetics; Oxford University Press: Oxford, UK, 2018; ISBN 9780190698614. [Google Scholar]
- Rudge, A. Antenna Theory and Design. Electron. Power 1982, 28, 267. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Tsai, C.-L.; Yang, C.-L. Compact broadband implantable monopole antenna with gain enhancement. In Proceedings of the 2014 IEEE Antennas and Propagation Society International Symposium (APSURSI), Memphis, TN, USA, 6–11 July 2014; pp. 1590–1591. [Google Scholar] [CrossRef]
- Islam, S.M.; Esselle, K.P.; Bull, D.; Pilowsky, P.M. Bandwidth enhancement of an implantable RFID tag antenna at 900 MHz ISM band for RF telemetry. In Proceedings of the 2012 International Symposium on Communications and Information Technologies, Gold Coast, Australia, 2–5 October 2012; pp. 741–745. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Xiao, S. A Review of Implantable Antennas for Wireless Biomedical Devices. Forum Electromagn. Res. Methods Appl. Technol. 2016, 14. Available online: https://efermat.github.io/articles/liu-qrt-2016-vol14-marapr-003/ (accessed on 10 May 2023).
- Chang, G.; Maity, S.; Chatterjee, B.; Sen, S. Design Considerations of a Sub-50 Mu-W Receiver Front-End for Implantable Devices in MedRadio Band. In Proceedings of the IEEE International Conference on VLSI Design, Pune, India, 6–10 January 2018; IEEE: Piscataway, NJ, USA, 2018; Volume 2018, pp. 329–334. [Google Scholar]
- Liu, C.; Zhang, Y.; Liu, X. Circularly Polarized Implantable Antenna for 915 MHz ISM-Band Far-Field Wireless Power Transmission. IEEE Antennas Wirel. Propag. Lett. 2018, 17, 373–376. [Google Scholar] [CrossRef]
- Sulaiman, N.H.; Samsuri, N.A.; Rahim, M.K.A.; Seman, F.C.; Inam, M. Compact Meander Line Telemetry Antenna for Implantable Pacemaker Applications. Indones. J. Electr. Eng. Comput. Sci. 2018, 10, 883–889. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chen, S.-C.; Hsiao, H.-M. A Single-Connector Stent Antenna for Intravascular Monitoring Applications. Sensors 2019, 19, 4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hout, S.; Chung, J.-Y. Design and Characterization of a Miniaturized Implantable Antenna in a Seven-Layer Brain Phantom. IEEE Access 2019, 7, 162062–162069. [Google Scholar] [CrossRef]
- Lee, C.W.L.; Kiourti, A.; Volakis, J.L. Miniaturized Fully Passive Brain Implant for Wireless Neuropotential Acquisition. IEEE Antennas Wirel. Propag. Lett. 2016, 16, 645–648. [Google Scholar] [CrossRef]
- Elyassi, R.; Moradi, G. Flexible and moon-shaped slot UWB implantable antenna design for head implants. Int. J. Microw. Wirel. Technol. 2017, 9, 1559–1567. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Yoo, H. Scalp-Implantable Antenna Systems for Intracranial Pressure Monitoring. IEEE Trans. Antennas Propag. 2018, 66, 2170–2173. [Google Scholar] [CrossRef]
- Khan, M.W.A.; Moradi, E.; Sydänheimo, L.; Björninen, T.; Rahmat-Samii, Y.; Ukkonen, L. Miniature Coplanar Implantable Antenna on Thin and Flexible Platform for Fully Wireless Intracranial Pressure Monitoring System. Int. J. Antennas Propag. 2017, 2017, 9161083. [Google Scholar] [CrossRef] [Green Version]
- Kiourti, A.; Nikita, K.S. Miniature Scalp-Implantable Antennas for Telemetry in the MICS and ISM Bands: Design, Safety Considerations and Link Budget Analysis. IEEE Trans. Antennas Propag. 2012, 60, 3568–3575. [Google Scholar] [CrossRef]
- Khan, M.W.A.; Bjorninen, T.; Sydanheimo, L.; Ukkonen, L. Remotely Powered Piezoresistive Pressure Sensor: Toward Wireless Monitoring of Intracranial Pressure. IEEE Microw. Wirel. Compon. Lett. 2016, 26, 549–551. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wu, Z.T.; Fan, Y.; Tentzeris, E.M. A Miniaturized CSRR Loaded Wide-Beamwidth Circularly Polarized Implantable Antenna for Subcutaneous Real-Time Glucose Monitoring. IEEE Antennas Wirel. Propag. Lett. 2016, 16, 577–580. [Google Scholar] [CrossRef]
- Mujeeb-U-Rahman, M.; Nazari, M.H.; Sencan, M.; Van Antwerp, W. A Novel Needle-Injectable Millimeter scale Wireless Electrochemical Glucose Sensing Platform for Artificial Pancreas Applications. Sci. Rep. 2019, 9, 17421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avaltroni, P.; Nappi, S.; Marrocco, G. Antennifying Orthopedic Bone-Plate Fixtures for the Wireless Monitoring of Local Deep Infections. IEEE Sensors J. 2021, 21, 21012–21021. [Google Scholar] [CrossRef]
- Vorobyov, A.; Hennemann, C.; Vasylchenko, A.; Decotignie, J.-D.; Baumgartner, J. Folded loop antenna as a promissing solution for a cochlear implant. In Proceedings of the 8th European Conference on Antennas and Propagation, EuCAP 2014, The Hague, The Netherlands, 6–11 April 2014; pp. 1735–1738. [Google Scholar] [CrossRef]
- Liapatis, O.; Nikita, K.S. Development of a biocompatible patch antenna for retinal prosthesis: Comparison of biocompatible coatings. In Proceedings of the 20th International Conference on Bioinformatics and Bioengineering, BIBE 2020, Cincinnati, OH, USA, 26–28 October 2020; pp. 819–825. [Google Scholar] [CrossRef]
- Christoe, M.J.; Phaoseree, N.; Han, J.; Michael, A.; Atakaramians, S.; Kalantar-Zadeh, K. Meandering Pattern 433 MHz Antennas for Ingestible Capsules. IEEE Access 2021, 9, 91874–91882. [Google Scholar] [CrossRef]
- Zainud-Deen, S.H.; Malhat, H.A.E.-A.; Balabel, A.A. Octafilar Helical Antenna for Circular Polarization Wireless Capsule Endoscopy Applications. Wirel. Pers. Commun. 2019, 108, 569–579. [Google Scholar] [CrossRef]
- Alemaryeen, A. Compact wideband antenna for wireless capsule endoscopy system. Appl. Phys. A 2021, 127, 271. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Liu, X.; Cao, H.; Zhang, Y.; Yang, X.; Guo, H. A Conformal Differentially Fed Antenna for Ingestible Capsule System. IEEE Trans. Antennas Propag. 2018, 66, 1695–1703. [Google Scholar] [CrossRef]
- Biswas, B.; Karmakar, A.; Chandra, V. Miniaturised wideband ingestible antenna for wireless capsule endoscopy. IET Microw. Antennas Propag. 2020, 14, 293–301. [Google Scholar] [CrossRef]
- Alazemi, A.J.; Iqbal, A. A compact and wideband MIMO antenna for high-data-rate biomedical ingestible capsules. Sci. Rep. 2022, 12, 14290. [Google Scholar] [CrossRef]
- Joy, B.; Cai, Y.; Bono, D.C.; Sarkar, D. Cell Rover—A miniaturized magnetostrictive antenna for wireless operation inside living cells. Nat. Commun. 2022, 13, 5210. [Google Scholar] [CrossRef]
- Zhang, Y.; Castro, D.C.; Han, Y.; Wu, Y.; Guo, H.; Weng, Z.; Xue, Y.; Ausra, J.; Wang, X.; Li, R.; et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc. Natl. Acad. Sci. USA 2019, 116, 21427–21437. [Google Scholar] [CrossRef]


























| Antenna | Ref, Application | Frequency | Size (mm × mm × mm) | Substrate/Material | Gain (dBi) | Bandwidth | SAR10g mW/kg |
|---|---|---|---|---|---|---|---|
| Planar | [90], Pacemaker | 402.5 MHz | 30.5 × 21.02 × 6.4 | FR-4, εr = 4.7, tan δ = 0.025 | - | 33.5% | - |
| [25], Pacemaker | 2.4 to 2.48 GHz | 3 × 3 × 0.5 | Rogers 3010, εr = 10.2, tan δ = 0.0023 | −24.9 | 22% | 147.7 | |
| [92], Brain implant | 2.4 GHz | 10 × 10 × 1.5 | Taconic RF-35, εr = 3.5, tan δ = 0.0018 | −20.75 | 14.9% | 0.3 | |
| [93], Brain implant | 2.4/4.8 GHz | 10 × 8.7 × 0.76 | Rogers TMM13i, εr = 12.2, tan δ = 0.0019 | - | 69 | ||
| [95], Intracranial pressure | 915 MHz, 2.45 GHz | 8 × 6 × 0.5 | Rogers 6010, εr = 10.2, tan δ = 0.0023 | −28.5, −22.8 | 9.84%, 8.57% | 2000 | |
| [96], Intracranial pressure | 2.45 GHz | 6 × 5 × 1 | Polymide | −19.63 | - | 10 | |
| [101], Orthopedic | 860 to 960 MHz | 14 × 6 × 3 | FR4 | −22 | - | - | |
| PIFA | [97], Intracranial pressure | 402, 433, 868 and 915 MHz | Dia = 12 mm, Thick = 1.8 mm | Rogers RO 3210, εr = 10.2, tan δ = 0.003 | −36.90, −35.99, −35.14 and −32.94 dB | - | 2000 |
| [103], Retinal implant | 401–406 MHz | Dia = 12 mm, Thick = 1.8 mm | Rogers RO 3210, εr = 10.2, tan δ = 0.003 | −36.82 | 3.4% | 2000 | |
| Wire | [23], Blood pressure monitoring | 863 to 870 MHz | Dia = 3 mm Length = 9.44 mm | Nilton wire, 0.33 mm thick | Directivity = 2.65 dBi | - | - |
| [91], Intravascular monitoring | 2.07 GHz | Dia = 2 mm, Length = 18 mm | Co–Cr alloy | −1.38 | - | - | |
| [102], Cochlear implant | 2.45 GHz | 38 × 38 × 2.2 | Metal wire, 0.3 mm thick | – 0.1 | 8.57% | - | |
| Spiral | [68], Brain implant and pacemaker | 402 MHz, 1.6 GHz and 2.45 GHz | 7 × 6.5 × 0.377 | Rogers RT/Duroid 6010, εr = 10.2, tan δ = 0.0035 | −30.5, −22.6, −18.2 | 36.8%, 10.8% and 3.4% | - |
| [98], Intracranial pressure | 11 MHz | 12.88 × 13.46 × 0.05 | Flexible polyimide, εr = 3.3, tan δ = 0.002 | –2.17 dB | - | - | |
| Slot | [94], Brain implant | 2.45 GHz | 8 × 9 × 0.2 | RO4003C, εr = 3.48, tan δ = 0.0027 | −13 | - | <1 W/kg for 1 g of tissue |
| [99], Glucose monitoring | 2.40 to 2.48 GHz | 8.5 × 8.5 × 1.27 | Rogers RO 3210, εr = 10.2, tan δ = 0.003 | −17 | 12.2% | - |
| Antenna | Ref, Application | Frequency | Size (mm × mm × mm) | Substrate/Material | Gain (dBi) | Bandwidth | SAR10g mW/kg |
|---|---|---|---|---|---|---|---|
| Planar | [104], CE | 433 MHz | 28 × 12 × 0.035 | Polyimide, εr = 3.5, tan δ = 0.0027 | −39 dBi | - | - |
| Wire | [105], CE | 2.4 GHz | Dia = 6.6 mm, Length = 8.85 mm | PEC wire, 0.4 mm thick | −19.83 | 12% | |
| [61], CE | 1 MHz | Dia = 8.9 mm, Thick = 4.8 mm | Copper wire, 0.2 mm thick | 0.7% PTE | - | 66 | |
| Conformal | [106], CE | 402 MHz, 433 MHz, 915 MHz and 2.45 GHz | 12 × 6 × 0.17 | Kapton substrate,εr = 3.5, tan δ = 0.0027 | −32.5, −30.4, −17.9 and −19.0 | - | - |
| [107], CE | 915 MHz | 32 × 5.8 × 0.15 | Polyimide, εr = 3.5, tan δ = 0.008 | −21 | 8.9% | ||
| Slot | [108], CE | 915 MHz | 7 × 7 × 0.675 | Silicon substrate, εr = 11.9 | −35.5 dBi | 32.8% | 8 mW/kg for 1 g of tissue |
| MIMO | [109], CE | 2.45 GHz | 5 × 4.2 × 0.12 | Rogers RO 3010, εr = 10.2, tan δ = 0.0022 | −20.6 | 25% | 2000 |
| Antenna | Ref, Application | Frequency | Size (mm × mm × mm) | Substrate/Material | Gain (dBi) | Bandwidth | SAR10g mW/kg |
|---|---|---|---|---|---|---|---|
| Spiral | [100], Glucose sensing | 900 MHz | 3 × 0.6 | Silicon substrate (Photolithography process) | −30 dB | - | - |
| [111], Pharmacology and optogenetics | 13.56 MHz | Dia = 5 mm | Polyimide | - | - | - | |
| Wire | [110], Cell rover | 4.5 MHz | 2 × 1 | AWG 47 (0.0355 mm) | - | 0.63% | 0.0226 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliqab, K.; Nadeem, I.; Khan, S.R. A Comprehensive Review of In-Body Biomedical Antennas: Design, Challenges and Applications. Micromachines 2023, 14, 1472. https://doi.org/10.3390/mi14071472
Aliqab K, Nadeem I, Khan SR. A Comprehensive Review of In-Body Biomedical Antennas: Design, Challenges and Applications. Micromachines. 2023; 14(7):1472. https://doi.org/10.3390/mi14071472
Chicago/Turabian StyleAliqab, Khaled, Iram Nadeem, and Sadeque Reza Khan. 2023. "A Comprehensive Review of In-Body Biomedical Antennas: Design, Challenges and Applications" Micromachines 14, no. 7: 1472. https://doi.org/10.3390/mi14071472
APA StyleAliqab, K., Nadeem, I., & Khan, S. R. (2023). A Comprehensive Review of In-Body Biomedical Antennas: Design, Challenges and Applications. Micromachines, 14(7), 1472. https://doi.org/10.3390/mi14071472








