ZnO QDs/GO/g-C3N4 Preparation and Photocatalytic Properties of Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Preparation Method of ZnO-GO-g-C3N4
2.3. Preparation of Water-Soluble ZnO QDs-GO-g-C3N4
2.4. Degradation of Rhodamine B Catalyzed by Visible Light
3. Results and Discussion
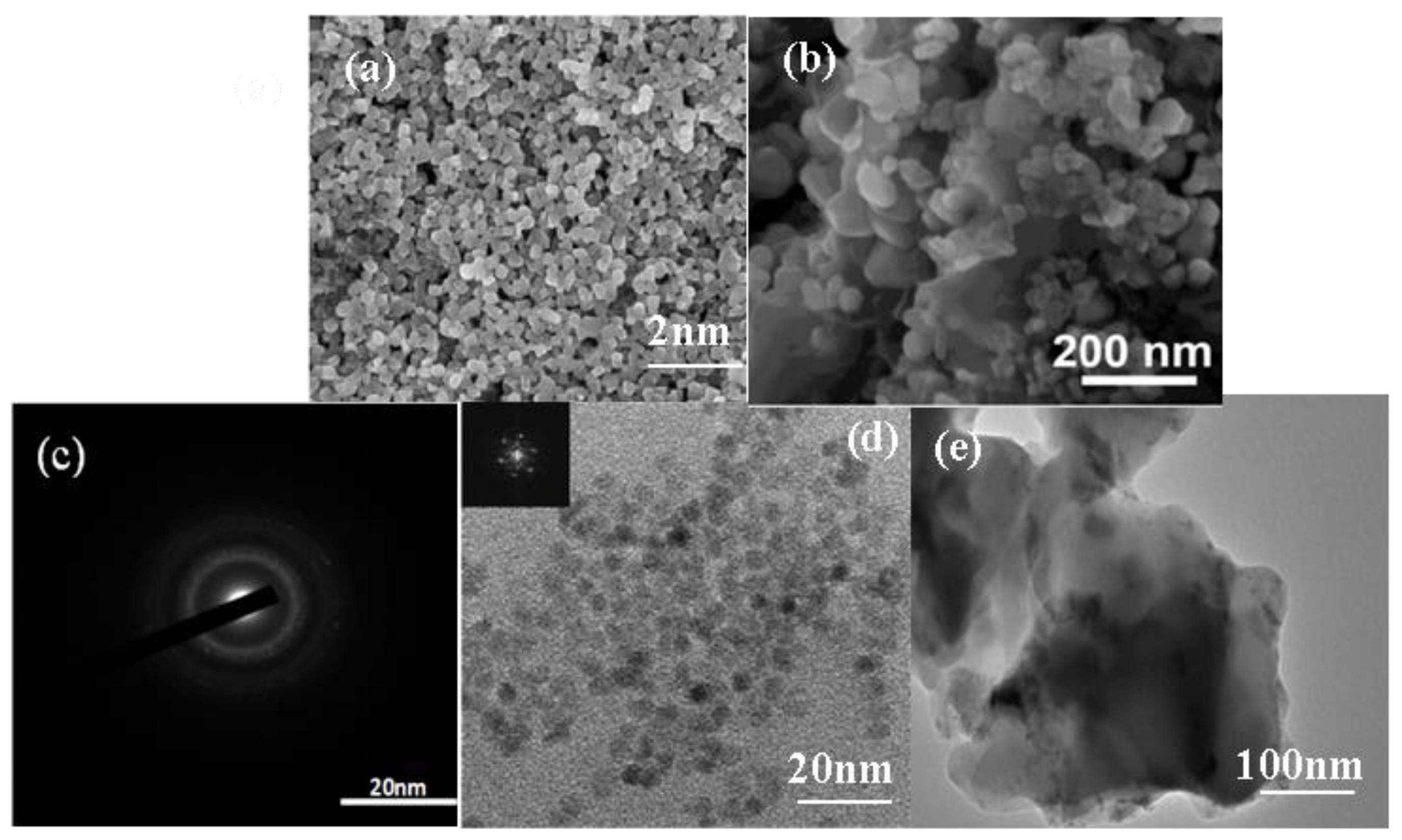
3.1. TEM and SEM
3.2. XRD
3.3. XPS
3.4. FT-IR
3.5. DRS Analysis
3.6. BET Analysis
3.7. Photocatalytic Performance
3.8. Kinetic Study
3.9. Cycling Stability
3.10. Free Radical Capture
3.11. Photocatalytic Mechanism
3.12. Comparative Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajapaksha, P.; Orrell-Trigg, R.; Shah, D.; Cheeseman, K.B.; Vu, S.T.; Ngo, B.J.; Murdoch, N.R.; Choudhury, H.; Yin, D.; Cozzolino, Y.B.; et al. Chapman Broad spectrum antibacterial zinc oxide-reduced graphene oxide nanocomposite for water depollution. Mater. Today Chem. 2023, 27, 101242. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; Kalpana, V.; Rajalakshmi, G.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A. Enhanced photocatalytic degradation of water pollutants using bio-green synthesis of zinc oxide nanoparticles (ZnO NPs). J. Environ. Chem. Eng. 2021, 9, 105772. [Google Scholar] [CrossRef]
- Ferdosi, E.; Bahiraei, H.; Ghanbari, D. Investigation the photocatalytic activity of CoFe2O4/ZnO and CoFe2O4/ZnO/Ag nanocomposites for purification of dye pollutants. Sep. Purif. Technol. 2019, 211, 35–39. [Google Scholar] [CrossRef]
- Yousefi, H.R.; Hashemi, B. Photocatalytic properties of Ag@ Ag-doped ZnO core-shell nanocomposite. J. Photochem. Photobiol. A Chem. 2019, 375, 71–76. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- Mohamed, W.A.A.; Abd El-Gawad, H.H.; Mekkey, S.D.; Galal, H.R.; Labib, A.A. Zinc oxide quantum dots: Confinement size, photophysical and tunning optical properties effect on photodecontamination of industrial organic pollutants. Opt. Mater. 2021, 118, 111242. [Google Scholar] [CrossRef]
- Banerjee, A.; Chattopadhyay, S.; Kundu, A.; Sharma, R.K.; Maiti, P.; Das, S. Vertically aligned zinc oxide nanosheet for high-performance photocatalysis of water pollutants. Ceram. Int. 2019, 45, 16821–16828. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Guo, Y.; Guo, W.; Wang, M.; Guo, Y.; Huo, M. Preparation and enhanced visible-light photocatalytic activity of graphitic carbon nitride/bismuth niobate heterojunctions. J. Hazard. Mater. 2013, 261, 235–245. [Google Scholar] [CrossRef]
- Ren, S.; Wang, B.; Zhang, H.; Ding, P.; Wang, Q. Sandwiched ZnO@Au@Cu2O nanorod films as efficient visible-light-driven plasmonic photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 4066–4074. [Google Scholar] [CrossRef]
- Ye, Y. Photoluminescence property adjustment of ZnO quantum dots synthesized via sol-gel method. J. Mater. Sci. Mater. Electron. 2018, 29, 4967–4974. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kobylański, M.P.; Gołąbiewska, A.; Nadolna, J.; Zaleska-Medynska, A.; Malankowska, A. Quantum dot-decorated semiconductor micro-and nanoparticles: A review of their synthesis, characterization and application in photocatalysis. Adv. Colloid Interface Sci. 2018, 256, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.K.; Voznyy, O.; Zhitomirsky, D.; Sargent, E.H. 25th anniversary article: Colloidal quantum dot materials and devices: A quarter-century of advances. Adv. Mater. 2013, 25, 4986–5010. [Google Scholar]
- Yang, W.; Yang, H.; Ding, W.; Zhang, B.; Zhang, L.; Wang, L.; Yu, M.; Zhang, Q. High quantum yield ZnO quantum dots synthesizing via an ultrasonication microreactor method. Ultrason. Sonochem. 2016, 33, 106–117. [Google Scholar] [CrossRef]
- Laurenti, M.; Canavese, G.; Sacco, A.; Fontana, M.; Bejtka, K.; Castellino, M.; Pirri, C.F.; Cauda, V. Nanobranched ZnO Structure: p-Type Doping Induces Piezoelectric Voltage Generation and Ferroelectric-Photovoltaic Effect. Adv. Mater. 2015, 27, 4218–4223. [Google Scholar] [CrossRef]
- Tu, Z.; Yang, G.; Song, H.; Wang, C. Amorphous ZnO quantum dot/mesoporous carbon bubble composites for a high-performance lithium-ion battery anode. ACS Appl. Mater. Interfaces 2017, 9, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, J.; Zhang, T.; Wang, Y.; Liu, D.; Chen, H.; Ji, L.; Liu, C.; Ahmad, W.; Chen, Z.D.; et al. Perovskite Solar Cells with ZnO Electron-Transporting Materials. Adv. Mater. 2018, 30, 1703737. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Edvinsson, T. Optical Quantum Confinement in Ultrasmall ZnO and the Effect of Size on Their Photocatalytic Activity. J. Phys. Chem. C 2020, 124, 6395–6404. [Google Scholar] [CrossRef]
- Sahoo, D.; Mandal, A.; Mitra, T.; Chakraborty, K.; Bardhan, M.; Dasgupta, A.K. Nanosensing of Pesticides by Zinc Oxide Quantum Dot: An Optical and Electrochemical Approach for the Detection of Pesticides in Water. J. Agric. Food Chem. 2018, 66, 414–423. [Google Scholar] [CrossRef]
- Lee, G.; Seo, Y.D.; Jang, J. ZnO quantum dot-decorated carbon nanofibers derived from electrospun ZIF-8/PVA nanofibers for high-performance energy storage electrodes. Chem. Commun. 2017, 53, 11441–11444. [Google Scholar] [CrossRef]
- Li, L.; Gu, L.; Lou, Z.; Fan, Z.; Shen, G. ZnO quantum dot decorated Zn2SnO4 nanowire heterojunction photodetectors with drastic performance enhancement and flexible ultraviolet image sensors. ACS Nano 2017, 11, 4067–4076. [Google Scholar] [CrossRef]
- Gong, M.; Liu, Q.; Cook, B.; Kattel, B.; Wang, T.; Chan, W.L.; Ewing, D.; Casper, M.; Stramel, A.; Wu, J.Z. All-printable ZnO quantum dots/graphenevan der Waals heterostructures for ultrasensitive detection of ultraviolet light. ACS Nano 2017, 4, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-sensitive ZnO quantum dots-doxorubicin nanoparticles for lung cancer targeted drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442. [Google Scholar] [CrossRef] [PubMed]
- Daumann, S.; Andrzejewskii, D.; Marcantonio, M.D.; Hagemann, U.; Wepfer, S.; Vollkommer, F.; Bacher, G.; Epple, E.; Nannen, E. Water-free synthesis of ZnO quantum dots for application as an electron injection layer in light-emitting electrochemical cells. J. Mater. Chem. C 2017, 5, 2344–2351. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Shen, J.; Liu, H.; Li, J.; Wang, A.; Hui, A.; Munir, H.A. Superoxide anion: Critical source of high performance antibacterial activity in Co-Doped ZnO QDs. Ceram. Int. 2020, 46, 15822–15830. [Google Scholar] [CrossRef]
- Lee, J.H.; Baek, S.E.; Lee, H.S.; Khang, D.Y.; Lee, W. Soft-lithographically line-patterned In-doped ZnO quantum dots with hydrothermally grown ZnO nanocolumns for acetone detection. Sens. Actuators B Chem. 2021, 329, 129131. [Google Scholar] [CrossRef]
- Montes-de-Oca, L.M.; Hernandez-Prudencio, A.; Borjas-García, S.E.; Espinosa, G.; Gomez-Ortiz, N.M.; Martinez-Torres, P. Controlled Synthesis and Surface Functionalization of ZnO Quantum Dots. Microsc. Microanal. 2018, 24, 1782–1783. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, K.; Xu, B.; Yang, C.; Wang, H.; Zhan, P.; Xie, S. Synthesis of a novel Z-scheme Ag/WO3/g-C3N4 nanophotocatalyst for degradation of oxytetracycline hydrochloride under visible light. Mater. Sci. Semicond. Process. 2022, 137, 106168. [Google Scholar] [CrossRef]
- Zhong, S.; Wang, Y.; Li, S.; Wang, S.; Que, X.; Sheng, L.; Peng, J.; Zhao, L.; Yuan, L.; Zhai, M. Enhanced photo-reduction of chromium (VI) from aqueous solution by nanosheet hybrids of covalent organic framework and graphene-phase carbon nitride. Sep. Purif. Technol. 2022, 294, 121204. [Google Scholar] [CrossRef]
- Wang, W.; Lv, B.; Tao, F. NiO/g-C3N4 composite for enhanced photocatalytic properties in the wastewater treatment. Environ. Sci. Pollut. Res. 2022, 30, 25620–25634. [Google Scholar] [CrossRef]
- Li, X.; Huang, G.; Chen, X.; Huang, J.; Li, M.; Yin, J.; Liang, Y.; Yao, Y.; Li, Y. A review on graphitic carbon nitride (g-C3N4) based hybrid membranes for water and wastewater treatment. Sci. Total Environ. 2021, 792, 148462. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Shan, J.; Liu, J.; Huang, X. Preparation, characterization of graphitic carbon nitride photo-catalytic nanocomposites and their application in wastewater remediation: A review. Crystals 2021, 11, 723. [Google Scholar]
- Huang, Q.; Wang, C.; Hao, D.; Wei, W.; Wang, L.; Ni, B.-J. Ultralight biodegradable 3D-g-C3N4 aerogel for advanced oxidation water treatment driven by oxygen delivery channels and triphase interfaces. J. Clean. Prod. 2021, 288, 125091. [Google Scholar] [CrossRef]
- Leelavathi, H.; Abirami, N.; Muralidharan, R.; Kavitha, H.P.; Tamizharasan, S.; Arulmozhi, R. Sunlight-assisted degradation of textile pollutants and phytotoxicity evaluation using mesoporous ZnO/gC3N4 catalyst. RSC Adv. 2021, 11, 26800–26812. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ai, L.; Wang, L.; Ju, Y.; Liu, S.; Wang, J.; Fan, H. The stable and elastic graphitic carbon nitride/polyvinyl alcohol photocatalytic composite sponge: Simple synthesis and application for wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 107814. [Google Scholar] [CrossRef]
- Jirickova, A.; Jankovsky, O.; Sofer, Z.; Sedmidubsky, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Tiwari, S.; Purabgola, A.; Kandasubramanian, B. Functionalised graphene as flexible electrodes for polymer photovoltaics. J. Alloy. Compd. 2020, 825, 153954. [Google Scholar] [CrossRef]
- Sangeetha, M.; Madhan, D. Ultra sensitive molybdenum disulfide (MoS2)/graphene based hybrid sensor for the detection of NO2 and formaldehyde gases by fiber optic clad modified method. Opt. Laser Technol. 2020, 127, 106193. [Google Scholar] [CrossRef]
- Zheng, J.; Li, J.; Zhang, L.; Chen, X.; Huang, H. Post-graphene 2D materials-based antimicrobial agents: Focus on fabrication strategies and biosafety assessments. J. Mater. Sci. 2020, 55, 7226–7246. [Google Scholar] [CrossRef]
- Ming, H.D.; Feng, Y.D.; Fei, H.; Liu, J.L. Electron drift velocity and mobility in graphene. Front. Phys. 2018, 13, 137203. [Google Scholar]
- Wang, J.; Tan, H.; Xiao, D.; Navik, R.; Goto, M.; Zhao, Y. Preparation of waterborne graphene paste with high electrical conductivity. Chem. Phys. Lett. 2020, 741, 137098. [Google Scholar] [CrossRef]
- Ying, L.; Hui, L.; Shiwei, W.; Liu, W. Tuning the optical nonlinearity of graphene. J. Chem. Phys. 2020, 153, 080903. [Google Scholar]
- Li, Y.; Li, H.; Wu, S.; Liu, W. Chemical Stability of (3,1)-Chiral Graphene Nanoribbons. ACS Nano 2021, 15, 5610–5617. [Google Scholar]
- Liang, H.; Hua, P.; Zhou, Y.; Fu, J.; Tang, J.; Niu, J. Fabrication of Cu/RGO/MoS2 nanohybrid with energetic visible-light response for degradation of rhodamine B. Chin. Chem. Lett. 2019, 30, 2245–2248. [Google Scholar] [CrossRef]
- Lei, D.; Zhang, Q.; Liu, N.; Su, T.; Wang, L.; Ren, Z.; Zhang, Z.; Su, J.; Gao, Y. Self-Powered Graphene Oxide Humidity Sensor Based on Potentiometric Humidity Transduction Mechanism. Adv. Funct. Mater. 2021, 32, 2107330. [Google Scholar] [CrossRef]
- Zhou, Y.; Xue, C.; Gan, L.; Owens, G.; Chen, Z. Antibacterial activity of reduced graphene oxide prepared by microbe. Mater. Today Sustain. 2023, 22, 100341. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Z.; Jin, H.; Zhao, X.; Feng, H.; Li, P.; He, D. Compressed Graphene Assembled Film with Tunable Electrical Conductivity. Materials 2023, 16, 526. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, N.; Zhang, M.; Li, P.; Lin, Z. Effect of preparation methods on dispersion stability and electrochemical performance of graphene sheets. J. Solid State Chem. 2017, 249, 9–14. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Hao, Y.; Meng, J.; Zhao, Y.; Wang, S.; Serge, Z.; Xu, H. H+/g-C3N4/GO-COOH Composited by Acid-Treated g-C3N4 and Functionalized Graphene Oxide for Efficient Photocatalytic H2 Production. Energy Fuels 2022, 36, 6005–6012. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.T.; Shin, E.W. Effect of g-C3N4 precursors on the morphological structures of g-C3N4/ZnO composite photocatalysts. J. Alloy. Compd. 2019, 788, 1084–1092. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Jin, R.; Meng, Q.; Chen, Y.; Long, X.; Wang, L.; Guo, H.; Zhang, S. Ultrasonic-microwave assisted synthesis of GO/g-C3N4 composites for efficient photocatalytic H2 evolution. Solid State Sci. 2019, 97, 105990. [Google Scholar] [CrossRef]
- Ravichandran, K.; Uma, R.; Sriram, S.; Balamurgan, D. Fabrication of ZnO: Ag/GO composite thin films for enhanced photocatalytic activity. Ceram. Int. 2017, 43, 10041–10051. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, C.; Zhang, Z.; Li, J.; Zhang, P.; Zhang, M.; Mu, J.; Guo, Z.; Liang, P.; Liu, Y. In situ Generation of Well-Dispersed ZnO Quantum Dots on Electrospun Silica Nanotubes with High Photocatalytic Activity. ACS Appl. Mater. Interfaces 2011, 4, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.; Perumal, S.; Karthikeyan, K.; Lee, Y. Facile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradation. J. Photochem. Photobiol. B Biol. 2016, 162, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, L.; Wang, C.; Lun, N.; Qi, Y. Sol-gel growth of hexagonal faceted ZnO prism quantum dots with polar surfaces for enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2010, 2, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-W.; Shi, H.-Q.; Li, W.-N.; Xiao, H.-M.; Fu, S.-Y.; Cao, X.-Z.; Li, Z.-X. Lanthanum-doped ZnO quantum dots with greatly enhanced fluorescent quantum yield. J. Mater. Chem. 2012, 22, 8221–8227. [Google Scholar] [CrossRef]
- Zhao, L.-H.; Zhang, R.; Zhang, J.; Sun, S.-Q. Synthesis and characterization of biocompatible ZnO nanoparticles. Cryst. Eng. Comm. 2012, 14, 945–950. [Google Scholar] [CrossRef]
- Vinogradov, A.V.; Vinogradov, V.V. Low-temperature sol–gel synthesis of crystalline materials. RSC Adv. 2014, 4, 45903–45919. [Google Scholar] [CrossRef]
- Sowik, J.; Miodyńska, M.; Bajorowicz, B.; Mikołajczyk, A.; Lisowski, W.; Klimczuk, T.; Kaczor, D.; Medynska, A.Z.; Malankowska, A. Optical and photocatalytic properties of rare earth metal-modified ZnO quantum dots. Appl. Surf. Sci. 2018, 464, 651–663. [Google Scholar] [CrossRef]
- Chennakesavulu, K.; Madhusudhana, M.; Reddy, G.; Rabel, A.M.; Brijitta, J.; Vinita, V.; Sasipraba, T.; Sreeramulu, J. Synthesis, characterization and photo catalytic studies of the composites by tantalum oxide and zinc oxide nanorods. J. Mol. Struct. 2015, 1091, 49–56. [Google Scholar] [CrossRef]
- Ghaderi, A.; Abbasi, S.; Farahbod, F. Synthesis of SnO2 and ZnO nanoparticles and SnO2-ZnO hybrid for the photocatalytic oxidation of methyl orange. Iran. J. Chem. Eng. (IJChE) 2015, 12, 96–105. [Google Scholar]
- Chen, L.; Li, J.; Yuan, L.; Huang, C.; Tran, T.T.; Cai, Q. Synthesis and photocatalytic application of Au/Ag nanoparticle-sensitized ZnO films. Appl. Surf. Sci. 2013, 273, 82–88. [Google Scholar] [CrossRef]
- Ali, M.M.; Chauveau, J.; Bretagnon, T. Evidence of exciton complexes in non polar ZnO/(Zn,Mg)O A-plane quantum well. Superlattice. Microst. 2018, 120, 410–418. [Google Scholar]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Yang, B.; Wu, M.; Xu, J.; Fu, Z.; Yan, L.; Guo, T.; Zhao, Y.; Zhu, C. Synthesis of Ag/ZnO nanorods array with enhanced photocatalytic performance. J. Hazard. Mater. 2010, 182, 123–129. [Google Scholar] [CrossRef]
- Niu, F.X.; Wang, Y.X.; Zhang, Y.T.; Xie, S.K.; Ma, L.R.; Mao, Y.P. A hierarchical architecting of PANI/APTES/SiC nano-composites with tunable dielectric for lightweight and strong microwave absorption. J. Mater. Sci. 2019, 54, 2181–2192. [Google Scholar] [CrossRef]
- Hijazi, M.; Stambouli, V.; Rieu, M.; Barnier, V.; Tournier, G.; Demes, T.; Viricelle, J.P.; Pijolat, C. Synthesis and characterization of tin dioxide thick film modiled by APTES in vapor and liquid phases. J. Mater. Sci. 2018, 53, 727–738. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M. Facile sonochemical-assisted synthesis of Cu/ZnO/Al2O3 nanocomposites under vacuum: Optical and photocatalytic studies. Ultrason. Sonochem. 2019, 58, 104636. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Han, S. The synthesis of Ag–ZnO nanohybrid with plasmonic photocatalytic activity under visible light irradiation: The relationship between tunable optical absorption, defect chemistry and photocatalytic activity. Cryst. Eng. Commun. 2016, 18, 1933–1943. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S. Porous C3N4 Nanolayers@N-graphene flms as catalyst electrodes for highly efficient hydrogen evolution. ACS Nano 2015, 9, 931–940. [Google Scholar] [CrossRef]
- Vinayagam, R.; Selvaraj, R.; Arivalagan, P.; Varadavenkatesan, T. Synthesis, characterization and photocatalytic dye degradation capability of Calliandra haematocephala -mediated zinc oxide nanoflowers. J. Photochem. Photobiol. B Biol. 2020, 203, 111760. [Google Scholar] [CrossRef]
- Abbas, A.; Zuhra, M.; Aijaz, B. Efficient photocatalytic degradation of industrial wastewater dye by Grewia asiatica mediated zinc oxide nanoparticles. Optik 2023, 272, 170352. [Google Scholar]
- Nomita, K.D.; Anju, S.D.; Joychandra, W.S.; Jugeshwar, K.S. Nickel doped zinc oxide with improved photocatalytic activity for Malachite Green Dye degradation and parameters affecting the degradation. J. Mater. Sci. Mater. Electron. 2021, 32, 8733–8745. [Google Scholar]
- Abebe, B.; Prakash, O.Y.; Tania, D. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. Int. 2016, 23, 25485–25493. [Google Scholar]
- Yusuff, S.A.; Bello, A.K.; Azeez, M.T. Photocatalytic degradation of an anionic dye in aqueous solution by visible light responsive zinc oxide-termite hill composite. React. Kinet. Mech. Catal. 2020, 131, 537–554. [Google Scholar] [CrossRef]
- Srishti, K.; Kiran, M.; Sudhish, K.; Rakshit, A.; Chetna, A. Kinetics of sonophotocatalytic degradation of an anionic dye nigrosine with doped and undoped zinc oxide. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2019, 80, 1466–1475. [Google Scholar]
- Kumar, R.V.; Vinoth, S.; Baskar, V.; Arun, M.; Gurusaravanan, P. Synthesis of zinc oxide nanoparticles mediated by Dictyota dichotoma endophytic fungi and its photocatalytic degradation of fast green dye and antibacterial applications. S. Afr. J. Bot. 2022, 151, 337–344. [Google Scholar] [CrossRef]
- Inderyas, A.; Bhatti, I.A.; Ashar, A.; Ashraf, M.; Ghani, A.; Yousaf, M.; Mohsin, M.; Ahmad, M.; Rafique, S.; Masood, N.; et al. Synthesis of immobilized ZnO over polyurethane and photocatalytic activity evaluation for the degradation of azo dye under UV and solar light irardiation. Mater. Res. Express 2020, 7, 025033. [Google Scholar] [CrossRef]
- Nida, A.; Shehzadi, S.; Samreen, F.; Tasneem, F. Photocatalytic Degradation of Synthetic Dyes Using Cyanobacteria-Derived Zinc Oxide Nanoparticles. BioNanoScience 2023, 13, 365–375. [Google Scholar]
- Singh, J.; Kaur, S.; Kaur, G.; Basu, S.; Rawat, M. Biogenic ZnO nanoparticles: A study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight. Green Process. Synth. 2019, 8, 272–280. [Google Scholar] [CrossRef] [Green Version]












| Sample Name | Regression Equation | k | R2 |
|---|---|---|---|
| ZnO-GO-g-C3N4 | y = 0.4768x − 0.4780 | 0.00778 | 0.9822 |
| ZnO QDs-GO-g-C3N4 | y = 0.559x − 0.6134 | 0.00784 | 0.9826 |
| Number of Use | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| decolourization ratio (%) | 97.16 | 93.64 | 90.76 | 89.78 |
| Articles | Degradation Rate |
|---|---|
| Our Articles | The degradation of RhB solutions by ZnO-GO-g-C3N4 and ZnO QDs-GO-g-C3N4 could reach 95.25% and 97.16%. |
| [71] | The photocatalytic properties of the nanoparticles were established by degrading methylene blue (MB) dye in response to solar radiation. The degradation rate was up to 88% over a period of 270 min. |
| [72] | Methylene blue degrades approximately 92% in 70 min. |
| [73] | The photocatalytic activity of the synthesized samples was studied for the degradation of peacock green (MG) dye under UV light irradiation. Among the studied samples, Zn0.94Ni0.06O was the better photocatalyst, degrading 77% of the dye under 4 h of irradiation. |
| [74] | Optimal photocatalyst loading of ZnO prepared by precipitation and sol-gel was required to reach 250 mg/L to achieve up to 81% and 92.5% dye degradation by ZnO. |
| [75] | The anionic dye (acid blue 113, AB113) was degraded by zinc oxide-termite hills complex (ZnO-TH) in aqueous solution and the maximum decolorization efficiency was found to be 92.21% under optimal conditions. |
| [76] | The degradation rate can reach 92%. |
| [77] | Synthesized nanoparticles show potential dye degradation efficiency under light irradiation (~90%) |
| [78] | ZnO/PUF showed good efficiency in the degradation of AB1 dyes, up to 86% and 65% at neutral pH, 4% H2O2, 240 min/sunlight and 75 min/UV irradiation time under UV and sunlight. |
| [79] | Biological ZnO NPs (10 mg/L) exhibited 91% degradation of methylene blue and 86% degradation of methyl orange. |
| [80] | The photocatalytic degradation of reactive yellow 186 (RY 186) dye was carried out under direct sunlight, and its degradation efficiency was 93.38%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Ma, H.; Geng, J.; Liu, C.; Song, C.; Lv, Y. ZnO QDs/GO/g-C3N4 Preparation and Photocatalytic Properties of Composites. Micromachines 2023, 14, 1501. https://doi.org/10.3390/mi14081501
Ren Z, Ma H, Geng J, Liu C, Song C, Lv Y. ZnO QDs/GO/g-C3N4 Preparation and Photocatalytic Properties of Composites. Micromachines. 2023; 14(8):1501. https://doi.org/10.3390/mi14081501
Chicago/Turabian StyleRen, Zhixin, Huachao Ma, Jianxin Geng, Cuijuan Liu, Chaoyu Song, and Yuguang Lv. 2023. "ZnO QDs/GO/g-C3N4 Preparation and Photocatalytic Properties of Composites" Micromachines 14, no. 8: 1501. https://doi.org/10.3390/mi14081501





