Rapid Micromolding of Sub-100 µm Microfluidic Channels Using an 8K Stereolithographic Resin 3D Printer
Abstract
1. Introduction
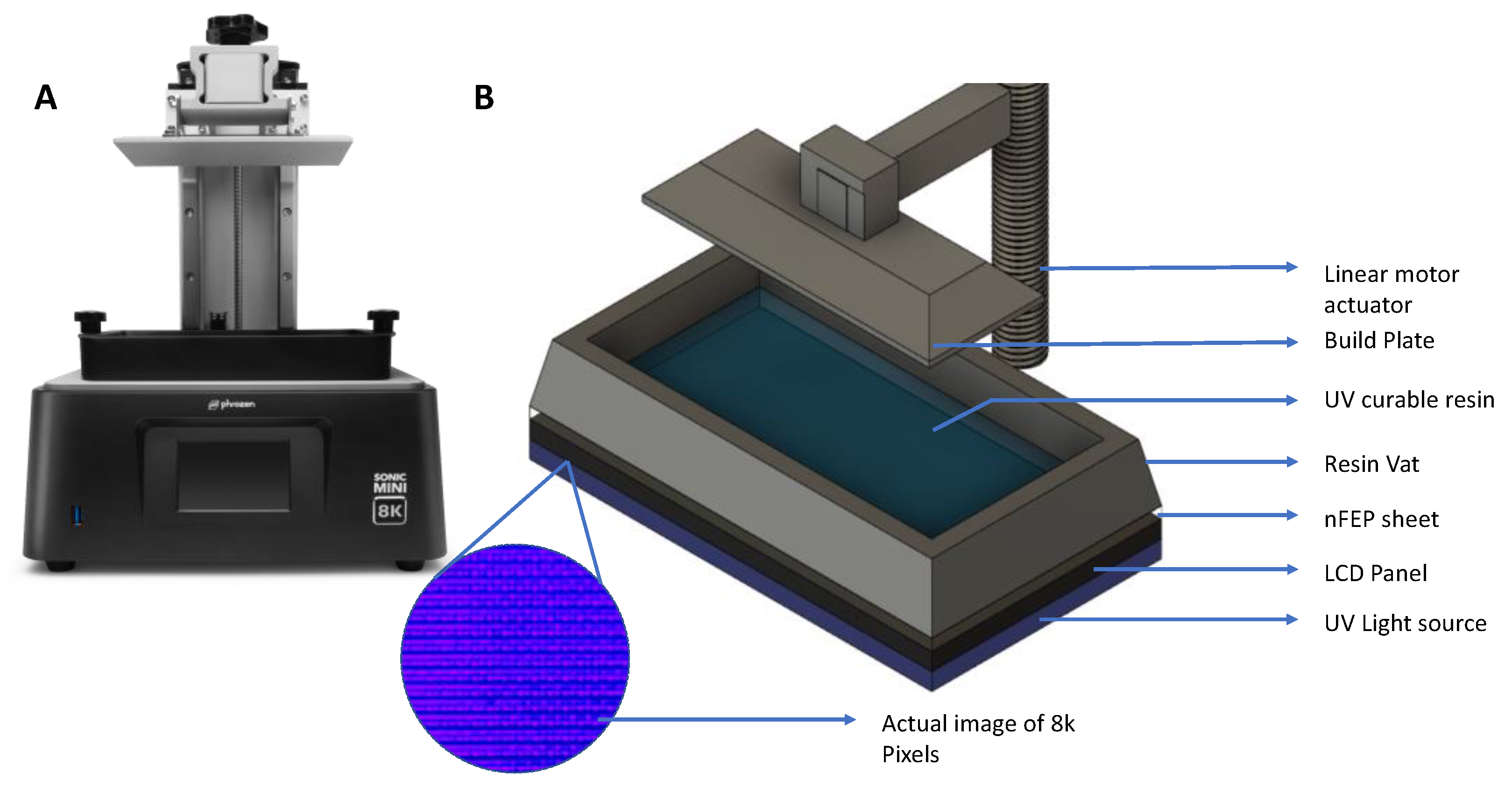
2. Methods and Experimental Setup
3. Results and Discussion
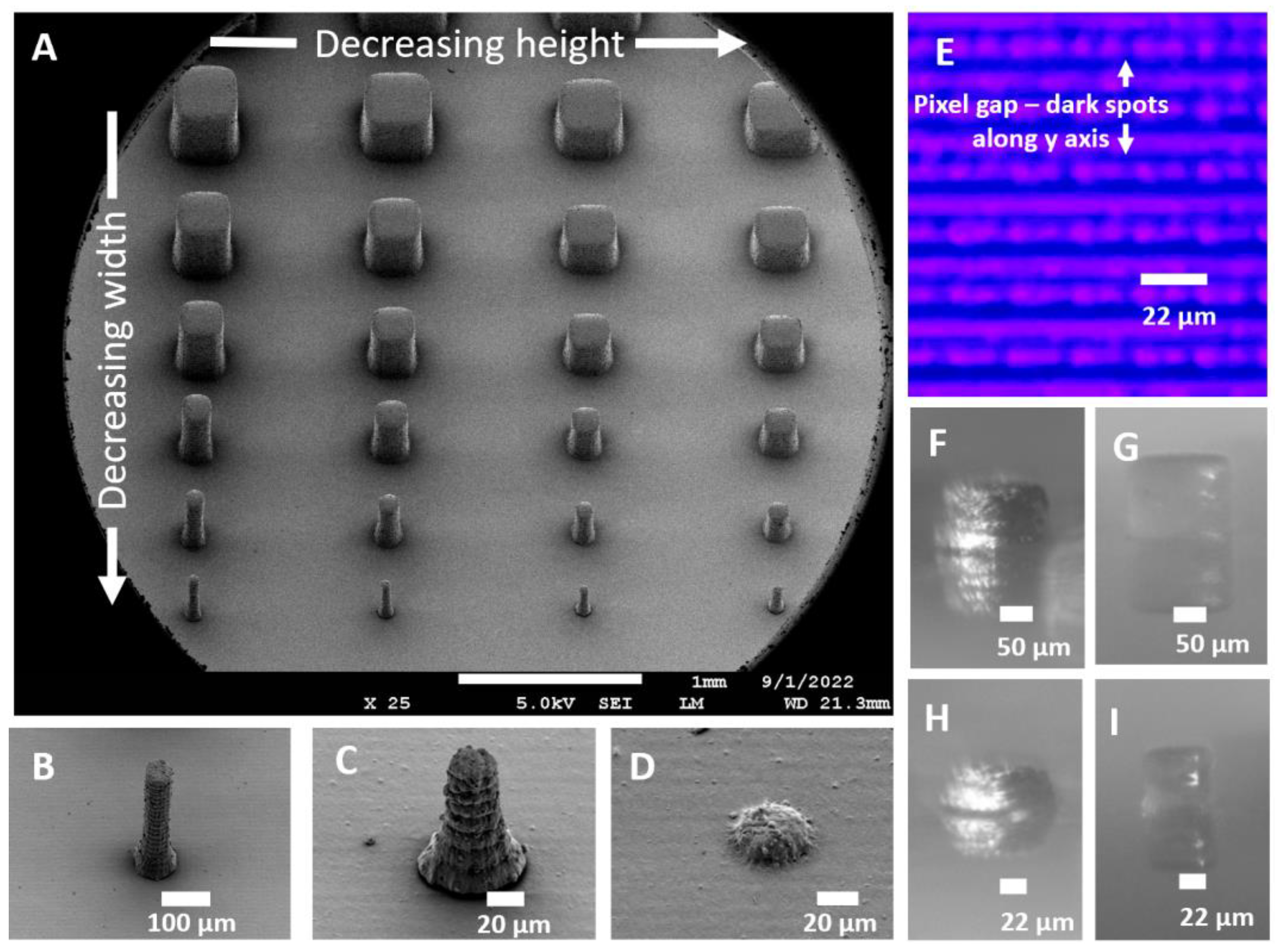
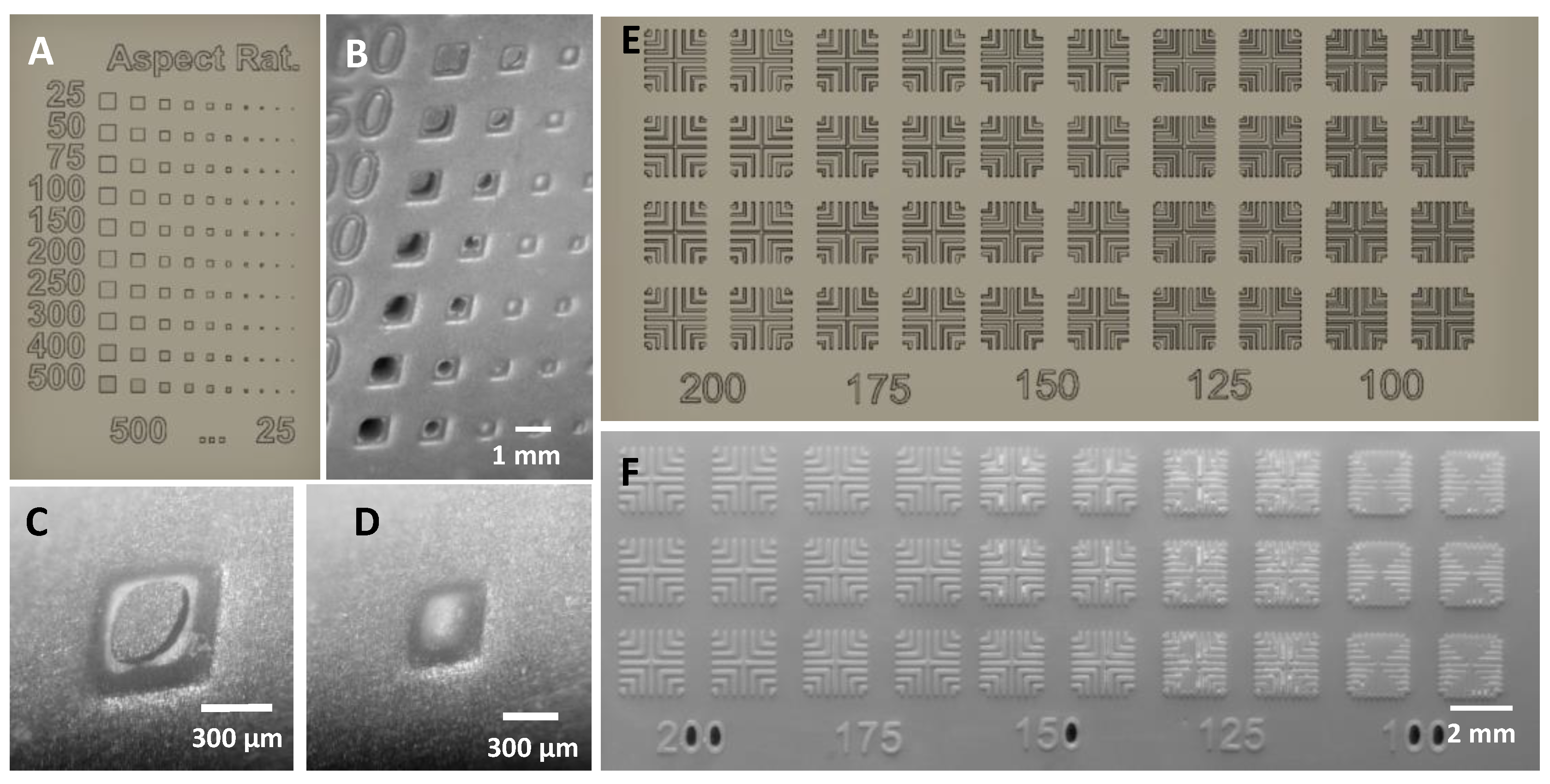
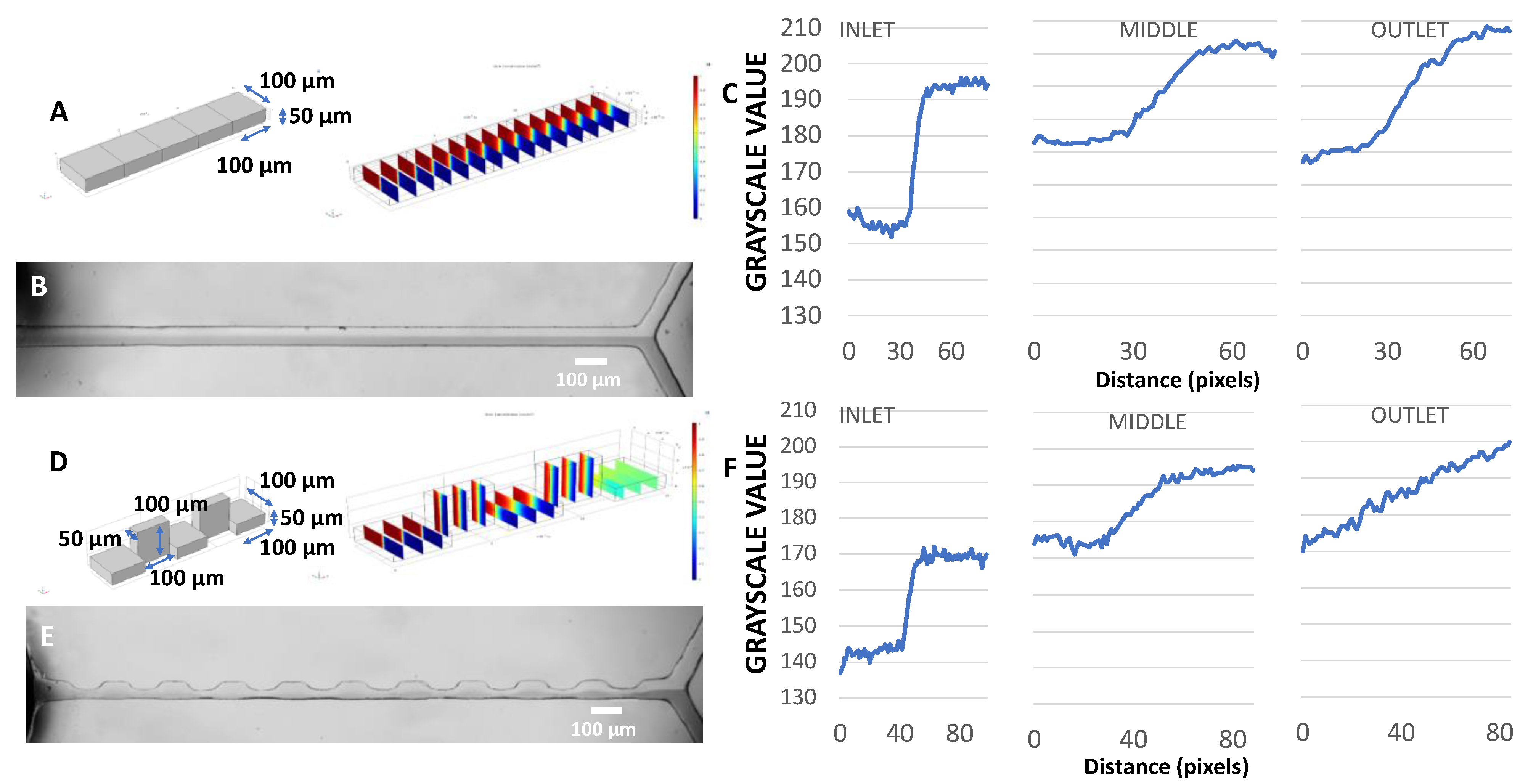
3.1. Extruded Mold Structures
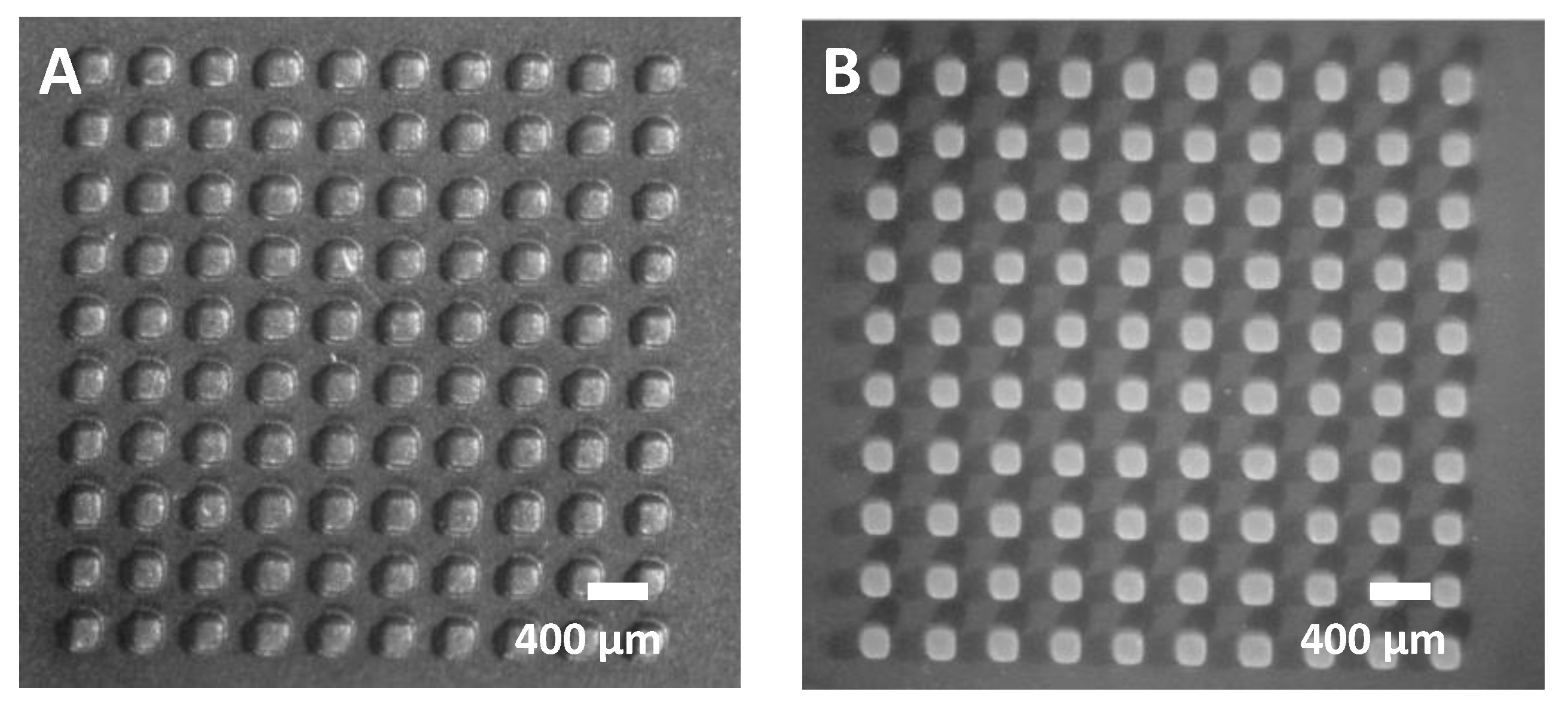
3.2. Cavity Mold Structures
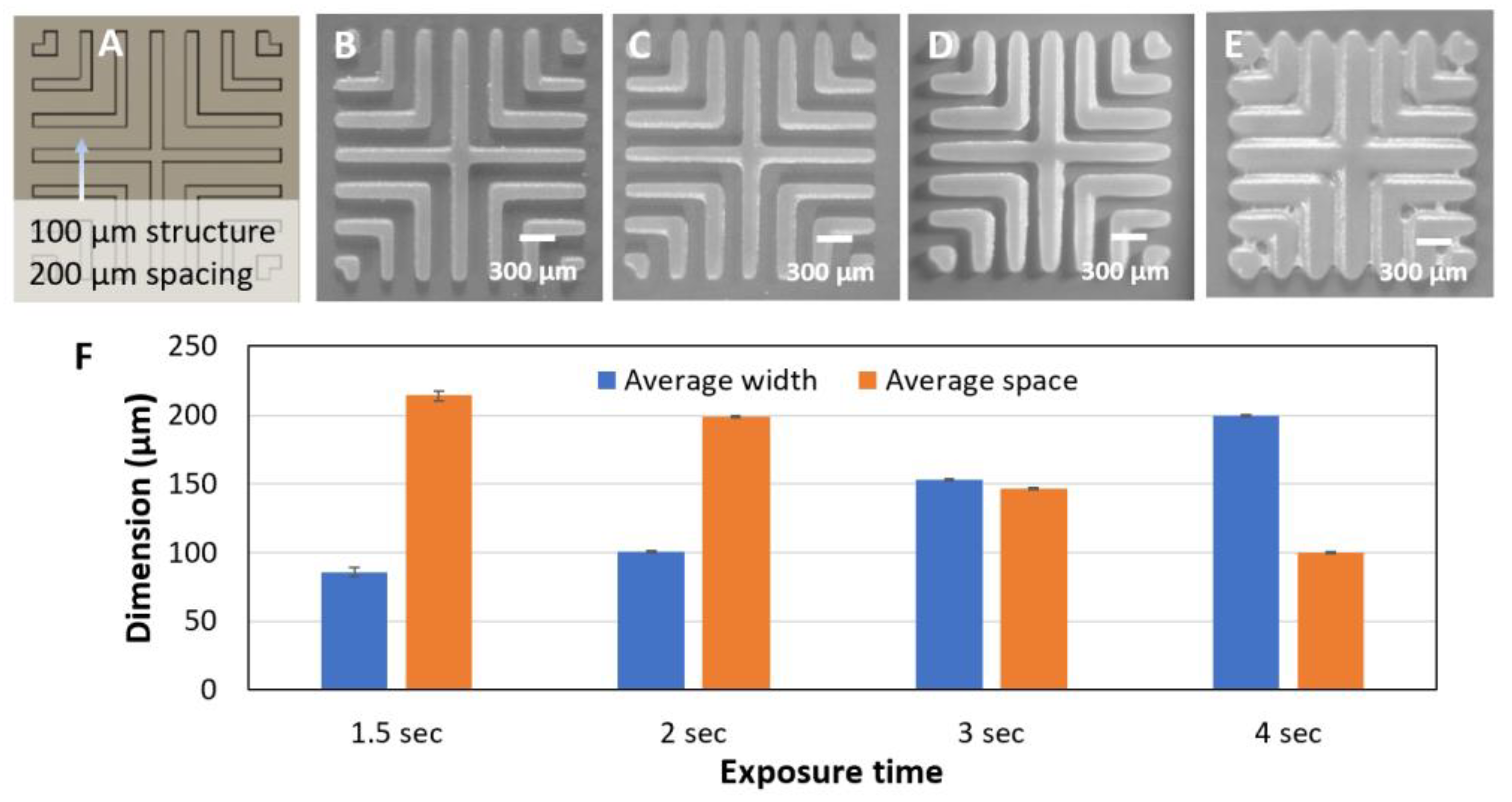
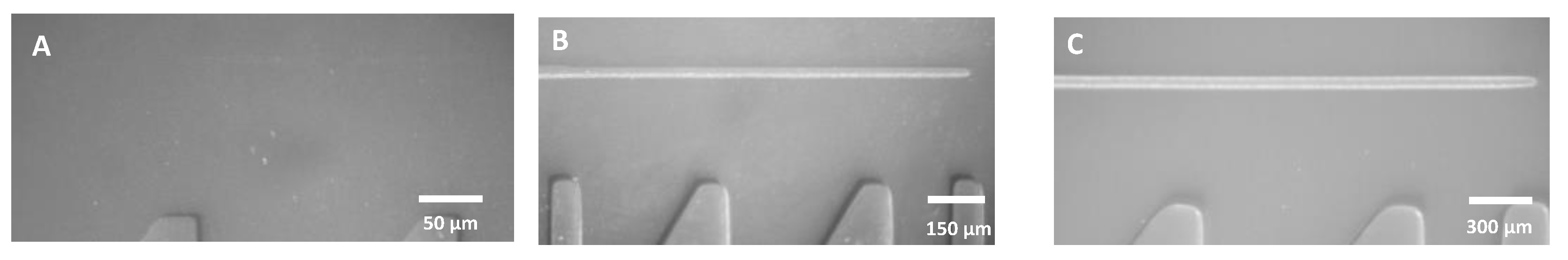
3.3. Line Structures
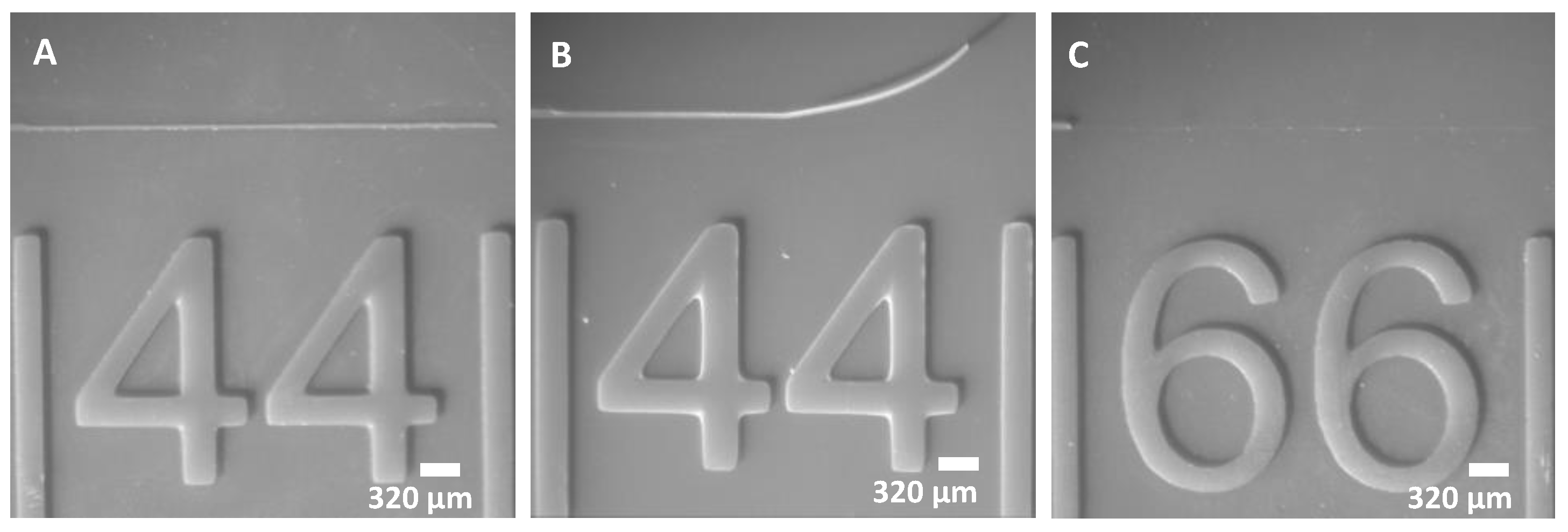
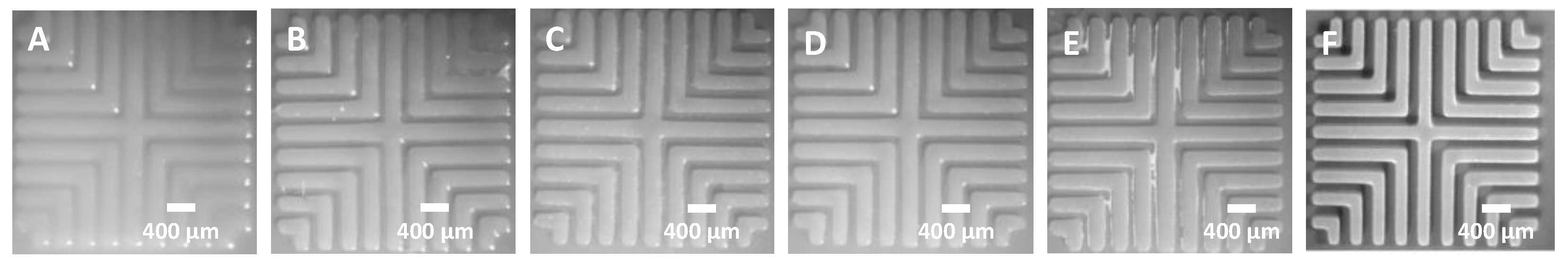
3.3.1. Effect of Exposure Time on Minimum Line Width and Spacing
3.3.2. Effect of Exposure Time on Adhesion
3.3.3. Effect of Retraction Velocity on Delamination
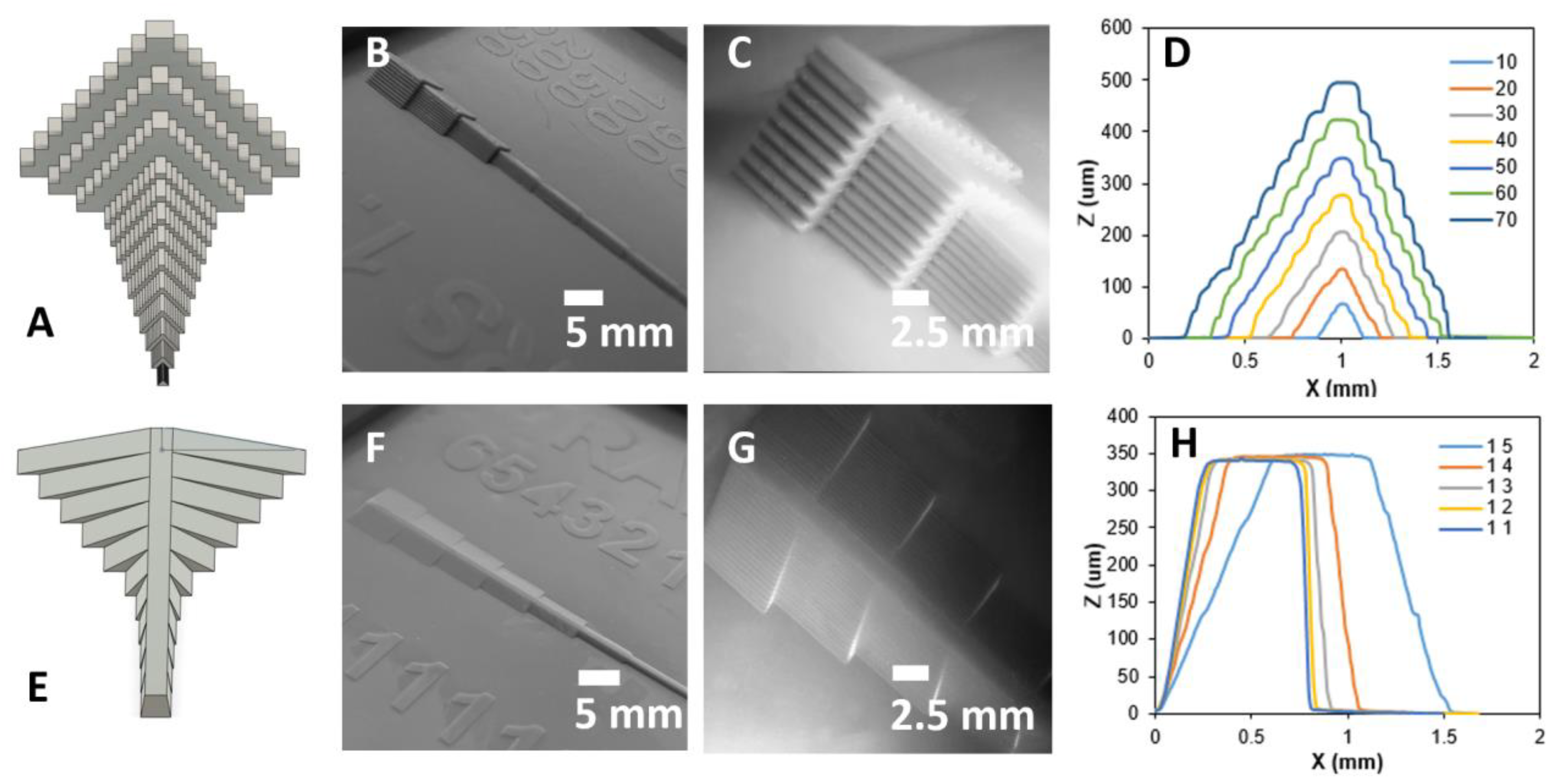
3.4. Mold Structures with Varying Heights
3.4.1. Stair and Ramp Test Structures
3.4.2. Optimization of Slice Layer Thickness and Exposure
3.5. IPA Sonication to Remove Uncured Resin
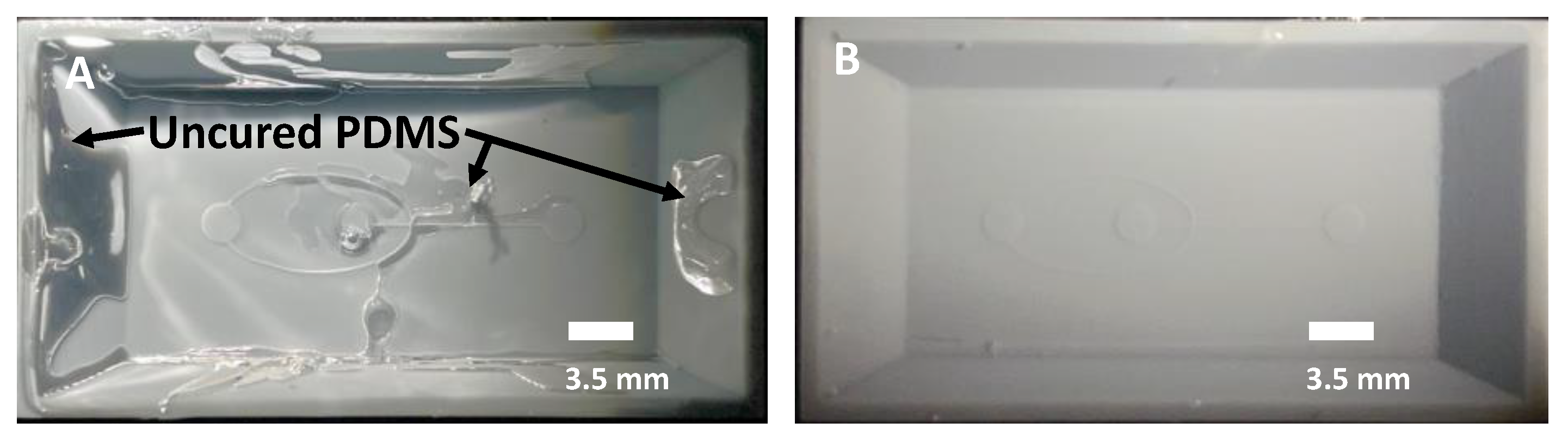
3.6. UV Curing to Eliminate Photoinitiators
3.7. Heat Treatment to Promote PDMS Curing
3.8. Anti-Stiction Coating to Facilitate PDMS Release
3.9. Sidewalls to Prevent Mold Warpage
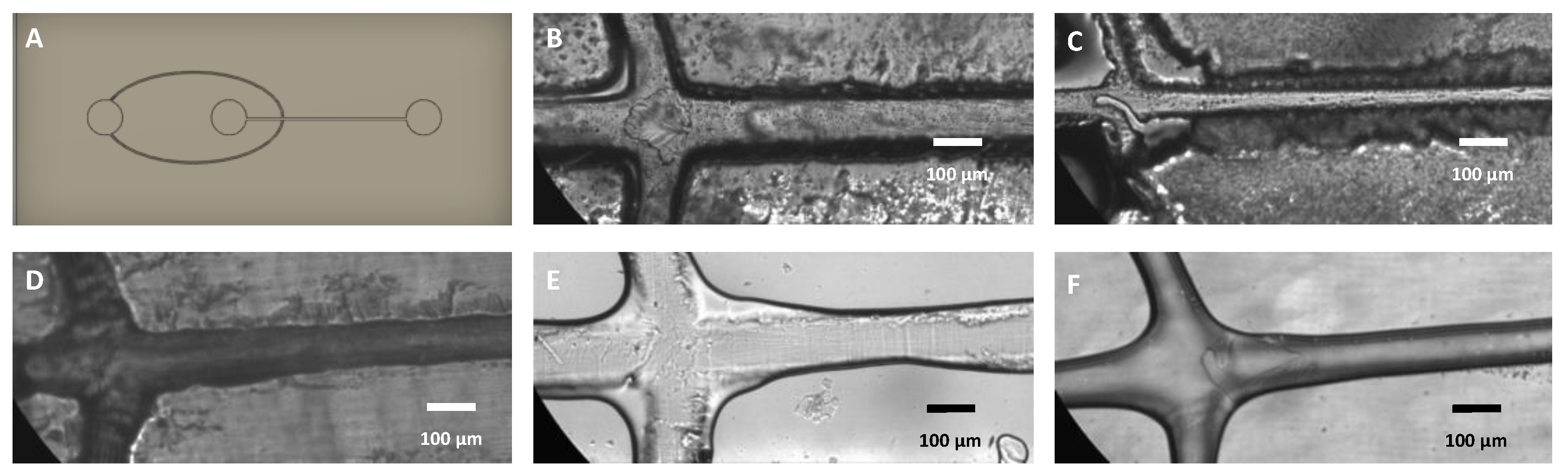
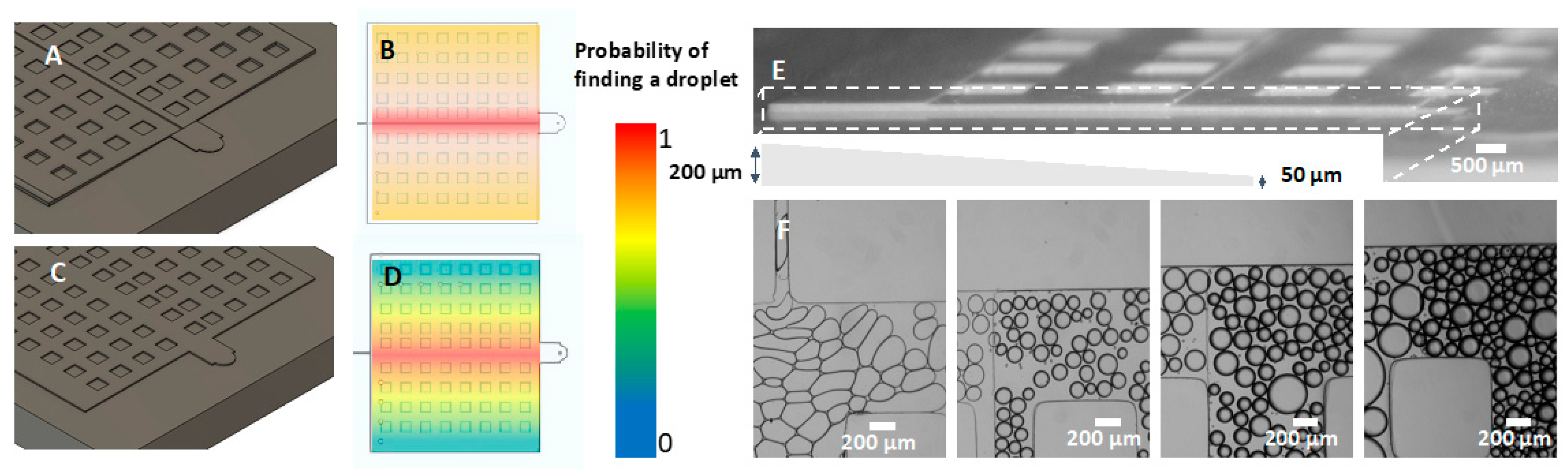
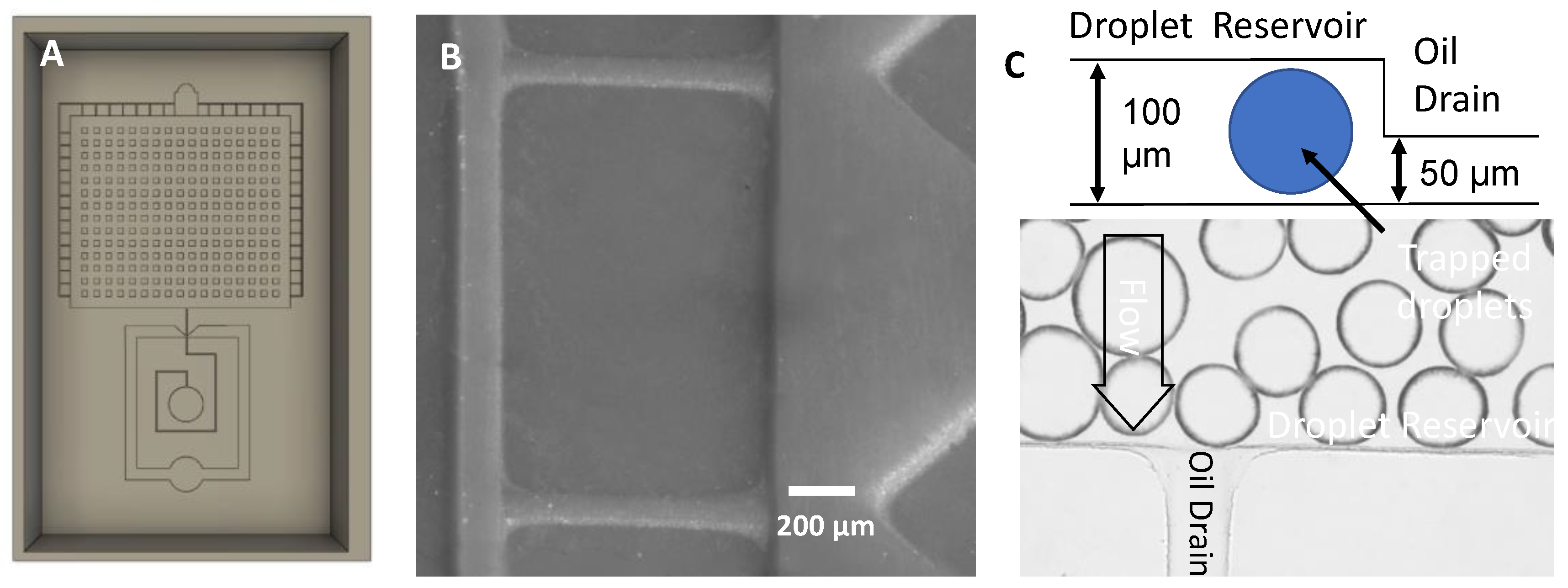
3.10. Applications: Microfluidic Devices Fabricated from 3D Printed Molds
3.11. Solvent Recycling
4. Summary and Conclusions
- The recommended channel width for highly reproducible printing is ~100 µm. Printing at 50 µm is possible, but there may be higher batch-to-batch variability.
- The minimum gap between square or circular cavity structures is 400 µm. To avoid the rounding of cavity corners, circular cavities are preferred.
- The minimum distance between adjacent microchannels can be as small as 200 µm.
- The minimum Z height for reproducible printing is 25 µm, using a 25 µm slice layer height. For taller structures, we recommend using the default 50 µm slices and exposure time to avoid extra exposures due to smaller slices.
- The optimal exposure time for the Phrozen Aqua Grey 8K resin is 3 s for a 50 µm slice layer height. Over and underexposure may result in larger structures (albeit for different reasons).
- The mold base under the channels should be at least 3 mm thick to avoid warping or breakage of the base layer.
- The side walls should be at least 2 mm tall to avoid the warping of the mold. Larger chips (for example, 35 × 85 mm) may require 5 mm sidewalls.
- Post printing, the chips should be pre-rinsed with IPA, using a squeeze bottle to reduce contamination of the IPA bath. They can then be submerged in an IPA bath and sonicated for 15 min at room temperature. Too little time or ultrasonic power may result in uncured resin left in the mold, while too much time may delaminate fine structures below 50 µm.
- After 15 min, the IPA bath should be replaced with fresh IPA, followed by another 15 min of sonication. If any visible residue is observed, we suggest another round of sonication with fresh IPA for 15 min.
- The chips must be exposed to UV light for at least 30 min to cure any excess photo initiators that may interfere with PDMS curing.
- After exposure, the chips must be baked in an oven for 1 h at 60 °C to remove any additional volatile impurities that may interfere with PDMS curing.
- If uncured PDMS is observed after the molding process, consider increasing the duration of the UV exposure and heat treatment.
- Vapor deposition of trichloro (1H,1H,2H,2H—perfluorooctyl) silane should be performed on the chips for 30 min to provide an anti-stiction coating that facilitates the removal of PDMS from the mold. After coating, the chips must be placed in an IPA bath and sonicated for 30 min to remove any excess silane before PDMS casting.
- After the mold has been fabricated, soft lithography can be performed using typical procedures. A detailed standard operating procedure (SOP) is provided in the Appendix A.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Standard Operating Procedure (SOP)
- Phrozen Sonic Mini 8K 3D printer
- Phrozen 8K aqua grey resin
- N-FEP sheet compatible with Phrozen Sonic Mini 8K
- Computer with Chitubox slicing software
- Deiner electronic Zepto vacuum plasma chamber
- Vacuum desiccator
- Branson ultrasonic cleaner
- Elegoo wash and cure station, or similar system
- Temperature controlled oven
- Sylgard 184 PDMS elastomer kit
- IPA
- Trichloro(1H, 1H,2H, 2H-perfluorooctyl)silane
- 500 mL glass beaker
- Glass dish (Corning petri dish)
- 1.5 mm biopsy punch
- 60 mL syringe
- Shake the bottle of Phrozen 8K vigorously to homogenize the resin.
- Pour the resin into A 500 mL beaker and place it in the desiccator for 1–2 h to remove air bubbles. Check to make sure no air bubbles are formed on top.
- Pour the resin carefully into a corner of the vat, taking care not to incorporate any air bubbles into the resin. Air in resin would cause air bubbles and will result in improper prints.
- Export the CAD design to STL format.
- Open Chitubox and click the settings button.
- Add new printer—if Phrozen 8K sonic mini is not found, use the following settings:
- ○
- X/Y resolution 7500 × 3240 px;
- ○
- Lock Ratio: on
- ○
- Size X/Y/Z: 165 mm/71.28 mm/180 mm
- ○
- Build Area Offset: off
- ○
- Mirror: LCD mirror
- If using a configuration file downloaded from the internet, click the import profile button from the top right. Select the config file for the resin being used. Select resin and close the window.
- Drag the STL file into the Chitubox window. This action pastes the design on the print area. Repeat this step to arrange and print multiple objects at once.
- Click slice and save the .ctb file. This file is copied to a USB drive and used for printing.
- Calibration settings may be changed in the Chitubox print Table.
- The calibration settings for several resins may be found at https://phrozen3d.com/pages/resin-mini-8K (accessed on 1 June 2023).
- If the calibration file for a given resin is not found, use the sample calibration file provided in the Supplementary Information ‘3D printing test structures_4mm base v1.stl’.
- While printing the calibration file, check for manufacturer settings and print the design at the nominal exposure time. Reprint the design at +/− 1 s exposure.
- Observe the printed structures under a microscope to determine the optimal exposure time.
- ○
- If the structures and the gaps are the same width, use that exposure time.
- ○
- If the structure is larger than the gap, this is overexposed. Use a shorter exposure time.
- ○
- If the structure is smaller than the gap, it is underexposed. Use a longer exposure time.
- Remove the 4 corner locating screws.
- Remove the tensioning screws of the screen holder in an alternating manner, one half turn at a time. This step is to ensure even tension is released and thus minimizes the potential for warping.
- Once the screws are removed, flip the cover over and remove the screws that hold the sheet to the frame.
- Place all screws in the IPA to clean any residue.
- Place the new nFEP sheet (cut to size) on the frame. Once sandwiched, use a hot soldering iron or sharp tip to puncture screw holes. The frame will act as a guide for the screw holes.
- Tighten all the screws in the same order as above. Note that it is normal for the nFEP sheet to be loose in the frame. Once it is tightened to the vat walls, tension is applied by the wall design.
- Connect the USB with the .ctb file to the 3D printer. The .ctb file must be in the root directory.
- Press print on the touch screen to begin.
- Remove the build plate from the printer. Hang it over the vat at about a 90-degree angle and drain excess resin back into the vat for 30 min.
- Detach the print from the build plate. Use a plastic spatula to avoid scratching the build plate.
- Place the prints in a glass dish with channels facing down so that any sediment falls from the chip. Cover the print with IPA.
- Place the dish in the bottom of the ultrasonic bath and run for 15 min.
- Replace with clean IPA, and run the ultrasonic cleaner for another 15 min.
- Remove the print from the IPA and air dry.
- Place print on top of an inverted glass beaker inside the Elegoo wash and cure station or similar system. The beaker will expose the bottom side of the print to UV. Perform UV exposure for 30 min to cure any remaining uncured resin.
- Heat the mold in the oven at 60 °C for 1 h to remove any volatile components that may inhibit PDMS curing.
- Place the mold in a vacuum desiccator. In a small beaker, add 500–750 µL (depending on the number/size of molds) of trichloro(1H, 1H,2H, 2H-perfluorooctyl)silane. Turn on the vacuum for 20–30 min.
- Perform an ultrasonic wash for 30 min with the mold upside down to remove any excess silane.
- Remove the plunger from a 60 mL syringe and close the mouth with a stopper.
- Pour PDMS and curing agent in a 10:1 ratio into the syringe.
- Mix thoroughly for 10 min using the helical mixer attachment.
- Place the syringe in a desiccator under vacuum for 1–2 h to remove all bubbles.
- Carefully put the plunger back while removing the stopper from the mouth.
- Once the molds are ready, pour PDMS carefully into the molds without introducing air bubbles. If air bubbles are introduced, desiccate for 20–30 min in a vacuum.
- Cure PDMS for 60 min at 65 °C in a laboratory oven. Cover with glass to avoid bubbling.
- Remove the PDMS from the mold carefully by running a razor blade through the edges and gently lifting. Work slowly to avoid ripping the PDMS, and use a sharp razor blade to avoid fraying the edges. Excessive fraying may indicate a poor silanization process.
- Place a piece of tape on the top and bottom of the PDMS to prevent dust buildup before bonding.
- If the mold does not already include reservoirs or holes for fluidic connections, use a biopsy punch to bore the appropriately-sized hole for tubing. For example, use a 1.5 mm biopsy punch for 1.6 outer diameter (OD) tubing.
- Turn on the plasma treater, ensuring that the exhaust of the plasma system is vented outdoors or in a fume hood. Vent the plasma chamber to atmospheric pressure. Remove the chamber lid and place the PDMS with the channel side to be bonded facing upward. In the chamber, add a glass slide or flat piece of PDMS to which the molded PDMS channel will be bonded. It is helpful to place a small piece of tape to mark which side is exposed to the plasma. The side facing up will have optimal plasma treatment.
- Replace the plasma chamber lid and activate the vacuum to pump down the chamber. Hold lid in place until vacuum is established (about 10 s). After 10 min, open the oxygen flow valve for 30 s. Set flow rate at 1.0 nl/h.
- Turn on the plasma RF generator for 10–15 s. Ideally, the process gas in the plasma chamber will have deep blue/purple color, indicating the formation of ozone. A mildly purple glow indicates the presence of some N2 but will still perform sufficient surface functionalization for bonding. However, a pale blue color suggests that O3 is not being formed, and the chip may not bond. Excessively long treatments may damage the PDMS.
- Once the exposure is complete, turn off the generator, turn off the vacuum, and vent the chamber. Place the PDMS on the glass slide and gently apply pressure without collapsing channels.
- To strengthen the bond, heat the chip in an oven at 35 °C for 10 min.
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Prabhakar, P.; Sen, R.K.; Dwivedi, N.; Khan, R.; Solanki, P.R.; Srivastava, A.K.; Dhand, C. 3D-Printed Microfluidics and Potential Biomedical Applications. Front. Nanotechnol. 2021, 3, 609355. [Google Scholar] [CrossRef]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel Fabrication on Glass Materials for Microfluidic Devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Raj M, K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Gorissen, B.; Van Hoof, C.; Reynaerts, D.; De Volder, M. SU8 etch mask for patterning PDMS and its application to flexible fluidic microactuators. Microsyst. Nanoeng. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Asri, M.A.M.; Nordin, A.N.; Ramli, N. Low-cost and cleanroom-free prototyping of microfluidic and electrochemical biosensors: Techniques in fabrication and bioconjugation. Biomicrofluidics 2021, 15, 061502. [Google Scholar] [CrossRef]
- Bell, N.A.W.; Molloy, J.E. Microfluidic flow-cell with passive flow control for microscopy applications. PLoS ONE 2020, 15, e0244103. [Google Scholar] [CrossRef]
- Epilog Laser Specifications and List of Materials That Can Be Cut. Available online: https://www.epiloglaser.com/laser-machines/fusion-edge-laser-cutter-engraver/ (accessed on 15 March 2023).
- Klank, H.; Geschke, O.; Kutter, J.P. CO2-laser micromachining and back-end processing for rapid production of PMMA-based microfluidic systems. Lab Chip 2002, 2, 242–246. [Google Scholar] [CrossRef]
- Dixon, C.; Lamanna, J.; Wheeler, A.R. Printed Microfluidics. Adv. Funct. Mater. 2017, 27, 1604824. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef]
- Moore, J.L.; McCuiston, A.; Mittendorf, I.; Ottway, R.; Johnson, R.D. Behavior of capillary valves in centrifugal microfluidic devices prepared by three-dimensional printing. Microfluid. Nanofluid. 2010, 10, 877–888. [Google Scholar] [CrossRef]
- Nelson, M.D.; Ramkumar, N.; Gale, B.K. Flexible, transparent, sub-100 µm microfluidic channels with fused deposition modeling 3D-printed thermoplastic polyurethane. J. Micromech. Microeng. 2019, 29, 095010. [Google Scholar] [CrossRef]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Kadri, N.A.; ABU Osman, N.A. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. Mater. 2015, 16, 033502. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Chabinyc, M.L.; Metallo, S.J.; Anderson, J.R.; Stroock, A.D.; Whitesides, G.M. Prototyping of Microfluidic Devices in Poly(dimethylsiloxane) Using Solid-Object Printing. Anal. Chem. 2002, 74, 1537–1545. [Google Scholar] [CrossRef]
- Hwang, Y.; Paydar, O.H.; Candler, R.N. Pneumatic microfinger with balloon fins for linear motion using 3D printed molds. Sens. Actuators A Phys. 2015, 234, 65–71. [Google Scholar] [CrossRef]
- Haske, W.; Chen, V.W.; Hales, J.M.; Dong, W.; Barlow, S.; Marder, S.R.; Perry, J.W. 65 nm feature sizes using visible wavelength 3-D multiphoton lithography. Opt. Express 2007, 15, 3426–3436. [Google Scholar] [CrossRef] [PubMed]
- Kumi, G.; Yanez, C.O.; Belfield, K.D.; Fourkas, J.T. High-speed multiphoton absorption polymerization: Fabrication of microfluidic channels with arbitrary cross-sections and high aspect ratios. Lab Chip 2010, 10, 1057–1060. [Google Scholar] [CrossRef]
- Zhou, W.; Kuebler, S.M.; Braun, K.L.; Yu, T.; Cammack, J.K.; Ober, C.K.; Perry, J.W.; Marder, S.R. An Efficient Two-Photon-Generated Photoacid Applied to Positive-Tone 3D Microfabrication. Science 2002, 296, 1106–1109. [Google Scholar] [CrossRef]
- Keating, S. Beyond D Printing: The New Dimensions of Additive Fabrication. In Designing for Emerging Technologies: UX for Genomics, Robotics, and the Internet of Things; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2014. [Google Scholar]
- Sugioka, K.; Cheng, Y. Femtosecond laser three-dimensional micro- and nanofabrication. Appl. Phys. Rev. 2014, 1, 041303. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 638905, 19 December 1989. Available online: https://cir.nii.ac.jp/crid/1571135649588411008 (accessed on 23 July 2023).
- Chan, H.N.; Chen, Y.; Shu, Y.; Chen, Y.; Tian, Q.; Wu, H. Direct, one-step molding of 3D-printed structures for convenient fabrication of truly 3D PDMS microfluidic chips. Microfluid. Nanofluid. 2015, 19, 9–18. [Google Scholar] [CrossRef]
- Shallan, A.I.; Smejkal, P.; Corban, M.; Guijt, R.M.; Breadmore, M.C. Cost-Effective Three-Dimensional Printing of Visibly Transparent Microchips within Minutes. Anal. Chem. 2014, 86, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Woolley, A.T.; Nordin, G.P. High density 3D printed microfluidic valves, pumps, and multiplexers. Lab Chip 2016, 16, 2450–2458. [Google Scholar] [CrossRef]
- Van der Linden, P.J.E.M.; Popov, A.M.; Pontoni, D. Accurate and rapid 3D printing of microfluidic devices using wavelength selection on a DLP printer. Lab Chip 2020, 20, 4128–4140. [Google Scholar] [CrossRef] [PubMed]
- Urrios, A.; Parra-Cabrera, C.; Bhattacharjee, N.; Gonzalez-Suarez, A.M.; Rigat-Brugarolas, L.G.; Nallapatti, U.; Samitier, J.; DeForest, C.A.; Posas, F.; Garcia-Cordero, J.L.; et al. 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab Chip 2016, 16, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Vedhanayagam, A.; (Wayne State Unviersity, Detroit, MI, USA); Basu, A.; (Wayne State Unviersity, Detroit, MI, USA); Phrozen, E.; (Support Team). Personal communication, 2022.
- Montgomery, S.M.; Demoly, F.; Zhou, K.; Qi, H.J. Pixel-Level Grayscale Manipulation to Improve Accuracy in Digital Light Processing 3D Printing. Adv. Funct. Mater. 2023, 33, 2213252. [Google Scholar] [CrossRef]
- Montgomery, S.M.; Hamel, C.M.; Skovran, J.; Qi, H.J. A reaction–diffusion model for grayscale digital light processing 3D printing. Extrem. Mech. Lett. 2022, 53, 101714. [Google Scholar] [CrossRef]
- Flowers, G.; Switzer, S. Background Material Properties of Selected Silicone Potting Compounds and Raw Materials for Their Substitutes. Mason and Hanger-Silas Mason Co., Inc.: Amarillo, TX, USA, 1978. [Google Scholar] [CrossRef]
- De Almeida Monteiro Melo Ferraz, M.; Henning, H.H.; Ferreira da Costa, P.; Malda, J.; Le Gac, S.; Bray, F.; Van Duursen, M.B.; Brouwers, J.F.; Van De Lest, C.H.; Bertijn, I.; et al. Potential Health and Environmental Risks of Three-Dimensional Engineered Polymers. Environ. Sci. Technol. Lett. 2018, 5, 80–85. [Google Scholar] [CrossRef]
- Oskui, S.M.; Diamante, G.; Liao, C.; Shi, W.; Gan, J.; Schlenk, D.; Grover, W.H. Assessing and Reducing the Toxicity of 3D-Printed Parts. Environ. Sci. Technol. Lett. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Carve, M.; Wlodkowic, D. 3D-Printed Chips: Compatibility of Additive Manufacturing Photopolymeric Substrata with Biological Applications. Micromachines 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Venzac, B.; Deng, S.; Mahmoud, Z.; Lenferink, A.; Costa, A.; Bray, F.; Otto, C.; Rolando, C.; Le Gac, S. PDMS Curing Inhibition on 3D-Printed Molds: Why? Also, How to Avoid It? Anal. Chem. 2021, 93, 7180–7187. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.S. Digital Assays Part I: Partitioning Statistics and Digital PCR. SLAS Technol. Transl. Life Sci. Innov. 2017, 22, 369–386. [Google Scholar] [CrossRef]
- Basu, A.S. Digital Assays Part II: Digital Protein and Cell Assays. SLAS Technol. Transl. Life Sci. Innov. 2017, 22, 387–405. [Google Scholar] [CrossRef]
- Melin, J.; Quake, S.R. Microfluidic Large-Scale Integration: The Evolution of Design Rules for Biological Automation. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, V.; Geng, J.; Huang, Y. Fabrication of Tapered 3D Microstructure Arrays Using Dual-Exposure Lithography (DEL). Micromachines 2020, 11, 903. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Z.; Zhang, Q.; Qi, H.J.; Fang, D. Mechanics of shape distortion of DLP 3D printed structures during UV post-curing. Soft Matter 2019, 15, 6151–6159. [Google Scholar] [CrossRef]
- Vedhanayagam, A.; Basu, A.S. Imaging Flow Cytometry at >13K events/s Using GPU-Accelerated Computer Vision. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Li, Z.; Leshansky, A.M.; Pismen, L.M.; Tabeling, P. Step-emulsification in a microfluidic device. Lab Chip 2014, 15, 1023–1031. [Google Scholar] [CrossRef]
- Dangla, R.; Kayi, S.C.; Baroud, C.N. Droplet microfluidics driven by gradients of confinement. Proc. Natl. Acad. Sci. USA 2013, 110, 853–858. [Google Scholar] [CrossRef]
- Umbanhowar, P.B.; Prasad, V.; Weitz, D.A. Monodisperse Emulsion Generation via Drop Break off in a Coflowing Stream. Langmuir 2000, 16, 347–351. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, P.; Xiao, G.; Lin, M.; Cao, X. Focusing-enhanced mixing in microfluidic channels. Biomicrofluidics 2008, 2, 014101. [Google Scholar] [CrossRef]
- Recycle Isopropyl Alcohol FAST! | Recovering IPA from 3D Printer Resin Wash. [Online Video]. 7 November 2021. Available online: https://www.youtube.com/watch?v=HxT8rEr-tFI (accessed on 19 June 2023).
- Jiang, J.-Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]






| Device < $USD 1000 | Z Axis Variation | Minimum Feature | Mold/Chip | Time to Fabricate | |
|---|---|---|---|---|---|
| Photolithography [7] | No | Hard | 50 nm | Mold | Slow |
| FDM [16] | Yes | Easy | 40 µm | Both | Moderate |
| 2PP [21] | No | Easy | 110 nm | Both | Slow |
| i3DP [17] | No | Easy | 250 µm | Mold | Moderate |
| DLP [28] | No | Easy | 100 µm | Mold | Moderate |
| Laser Cutter [12] | No | Hard | 250 µm | Chip | Fast |
| Cricut [11] | Yes | Hard | 200 µm | Chip | Fast |
| Inkjet printing [13] | Yes | Hard | 150 µm | Chip | Fast |
| 8K LCD resin printer | Yes | Easy | 44 µm | Mold | Moderate |
| Process Step | Minimum Time Required (Minutes) | Maximum Time Required (Minutes) |
|---|---|---|
| Fusion 360 design | 10 | 60+ |
| 3D printing | 25 | 60+ |
| IPA wash 1 | 30 | 60 |
| UV exposure | 30 | 60 |
| Heat treatment | 60 | 480 |
| Silane vapor deposition | 20 | 30 |
| IPA wash 2 | 30 | 30 |
| PDMS casting | 60 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedhanayagam, A.; Golfetto, M.; Ram, J.L.; Basu, A.S. Rapid Micromolding of Sub-100 µm Microfluidic Channels Using an 8K Stereolithographic Resin 3D Printer. Micromachines 2023, 14, 1519. https://doi.org/10.3390/mi14081519
Vedhanayagam A, Golfetto M, Ram JL, Basu AS. Rapid Micromolding of Sub-100 µm Microfluidic Channels Using an 8K Stereolithographic Resin 3D Printer. Micromachines. 2023; 14(8):1519. https://doi.org/10.3390/mi14081519
Chicago/Turabian StyleVedhanayagam, Arpith, Michael Golfetto, Jeffrey L. Ram, and Amar S. Basu. 2023. "Rapid Micromolding of Sub-100 µm Microfluidic Channels Using an 8K Stereolithographic Resin 3D Printer" Micromachines 14, no. 8: 1519. https://doi.org/10.3390/mi14081519
APA StyleVedhanayagam, A., Golfetto, M., Ram, J. L., & Basu, A. S. (2023). Rapid Micromolding of Sub-100 µm Microfluidic Channels Using an 8K Stereolithographic Resin 3D Printer. Micromachines, 14(8), 1519. https://doi.org/10.3390/mi14081519









