Influence of Mn2+ and Eu3+ Concentration on Photoluminescence and Thermal Stability Properties in Eu3+-Activated ZnMoO4 Red Phosphor Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
3. Results and Discussion
3.1. Structure and Photoluminescence Performance
3.2. Electron Lifetime
3.3. CIE Chromaticity Coordinates
3.4. Thermal Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajeswari, R.; Jayasankar, C.K.; Ramachari, D.; Surendra Babu, S. Synthesis, structural and luminescence properties of near white light emitting Dy3+-doped Y2CaZnO5 nanophosphor for solid state lighting. Ceram. Int. 2013, 39, 7523–7529. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Yang, Z.; Guo, Q.; Dong, G. A white emitting phosphor BaMg2(PO4)2:Ce3+, Mn2+, Tb3+: Luminescence and energy transfer. Ceram. Int. 2014, 40, 15283–15292. [Google Scholar] [CrossRef]
- Seo, Y.W.; Noh, H.M.; Moon, B.K.; Jeong, J.H.; Yang, H.K.; Kim, J.H. Structural and luminescent properties of blue-emitting Sr2CeO4 phosphors by high-energy ball milling method. Ceram. Int. 2015, 41, 1249–1254. [Google Scholar] [CrossRef]
- Tang, J.; Chen, J.; Hao, L.; Xu, X.; Xie, W.; Li, Q. Green Eu2+-doped Ba3Si6O12N2 phosphor for white light-emitting diodes: Synthesis, characterization and theoretical simulation. J. Lumin. 2011, 131, 1101–1106. [Google Scholar] [CrossRef]
- Balci, M.H.; Chen, F.; Cunbul, A.B.; Svensen, Ø.; Akram, M.N.; Chen, X. Comparative study of blue laser diode driven cerium-doped single crystal phosphors in application of high-power lighting and display technologies. Opt. Rev. 2018, 25, 166–174. [Google Scholar] [CrossRef]
- Chen, F.; Akram, M.N.; Chen, X. Improved Photoluminescence Performance of Eu3+-Doped Y2(MoO4)3 Red-Emitting Phosphor via Orderly Arrangement of the Crystal Lattice. Molecules 2023, 28, 1014. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Akram, M.N.; Chen, X. Novel Red-Emitting Eu3+-Doped Y2(WxMo1-xO4)3 Phosphor with High Conversion Efficiency for Lighting and Display Applications. Molecules 2023, 28, 4624. [Google Scholar] [CrossRef]
- Zeshan, M.; Ali, M.; Alanazi, M.M.; Abdelmohsen, S.M.; Khosa, R.Y.; Al-Sehemi, A.G.; Ansari, M.Z.; Tayeb, R.A.; Tahir Farid, H.M.; Rahman, M.M. Study of SrEr0.04Fe1.96O4/PANI nano-composites for high-frequency applications. Ceram. Int. 2023, 49, 20536–20543. [Google Scholar] [CrossRef]
- Balci, M.H.; Chen, F.; Cunbul, A.B.; Svensen, O.; Akram, M.N.; Chen, X. Transparent Ce,Sm:TAG Luminescent Ceramics for High Power Blue Laser Driven Lightning and Display Technologies. In Electrochemical Society Meeting Abstracts; The Electrochemical Society, Inc.: Washington, DC, USA, 2017; Volume MA2017-02, p. 1764. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, K.; Lian, H.; Shang, M.; Lin, J. Crystal-site engineering control for the reduction of Eu3+ to Eu2+ in CaYAlO4: Structure refinement and tunable emission properties. ACS Appl. Mater. Interfaces 2015, 7, 2715–2725. [Google Scholar] [CrossRef]
- Long, S.; Hou, J.; Zhang, G.; Huang, F.; Zeng, Y. High quantum efficiency red-emission tungstate based phosphor Sr(La1−xEux)2Mg2W2O12 for WLEDs application. Ceram. Int. 2013, 39, 6013–6017. [Google Scholar] [CrossRef]
- Lu, J.; Mu, Z.; Zhu, D.; Wang, Q.; Wu, F. Luminescence properties of Eu3+ doped La3Ga5GeO14 and effect of Bi3+ co-doping. J. Lumin. 2018, 196, 50–56. [Google Scholar] [CrossRef]
- Zhu, Y.; Tong, C.; Dai, R.; Xu, C.; Yang, L.; Li, Y. Luminescence properties of Na2Ca2Si3O9: Eu3+ phosphors via a sol-gel method. Mater. Lett. 2018, 213, 245–248. [Google Scholar] [CrossRef]
- Farid, H.M.T.; Gouadria, S.; Al-Moayid, S.M.; Algarni, H.; Ansari, M.Z.; Ali, H.E. Facile synthesis of CuCo2O4 spinel with rGO nanocomposite via hydrothermal approach for solid state supercapacitor application. J. Energy Storage 2023, 66, 107394. [Google Scholar] [CrossRef]
- Li, K.; Shang, M.; Lian, H.; Lin, J. Recent development in phosphors with different emitting colors via energy transfer. J. Mater. Chem. C 2016, 4, 5507–5530. [Google Scholar] [CrossRef]
- Spassky, D.A.; Vasil’ev, A.N.; Kamenskikh, I.A.; Mikhailin, V.V.; Savon, A.E.; Hizhnyi, Y.A.; Nedilko, S.G.; Lykov, P.A. Electronic structure and luminescence mechanisms in ZnMoO4 crystals. J. Phys. Condens. Matter 2011, 23, 365501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhang, Z.; Chu, Y.; Pan, Y.; You, M.; Zheng, T.; Jiayue, X. Phase transition and photoluminescence properties of Eu3+-doped ZnMoO4 red phosphors. Sci. China Technol. Sci. 2017, 60, 1473–1479. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Wei, J.-S.; Gong, F.-Z.; Huang, J.-L.; Yi, L.-H. A potential red phosphor ZnMoO4:Eu3+ for light-emitting diode application. J. Solid State Chem. 2008, 181, 1337–1341. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Zi, W.; Guo, N.; Zou, L.; Gan, S.; Ji, G. Self-assembled CaMoO4 and CaMoO4:Eu3+ hierarchical superstructures: Facile sonochemical route synthesis and tunable luminescent properties. J. Phys. Chem. Solids 2014, 75, 878–887. [Google Scholar] [CrossRef]
- Kim, M.-J.; Huh, Y.-D. Synthesis and optical properties of CaMoO4:Eu3+, Na+ nanophosphors and a transparent CaMoO4:Eu3+, Na+ suspension. Opt. Mater. 2012, 35, 263–267. [Google Scholar] [CrossRef]
- Krishna Bharat, L.; Lee, S.H.; Yu, J.S. Synthesis, structural and optical properties of BaMoO4:Eu3+ shuttle like phosphors. Mater. Res. Bull. 2014, 53, 49–53. [Google Scholar] [CrossRef]
- Sczancoski, J.C.; Cavalcante, L.S.; Marana, N.L.; da Silva, R.O.; Tranquilin, R.L.; Joya, M.R.; Pizani, P.S.; Varela, J.A.; Sambrano, J.R.; Siu Li, M.; et al. Electronic structure and optical properties of BaMoO4 powders. Curr. Appl. Phys. 2010, 10, 614–624. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, D. Luminescent properties and the morphology of SrMoO4:Eu3+ powders synthesized via combining sol-gel and solid-state route. Cent. Eur. J. Phys. 2010, 8, 766–770. [Google Scholar] [CrossRef]
- Hu, D.; Huan, W.; Wang, Y. Preparation and investigation of Eu3+-activated ZnMoO4 phosphors for white LED. J. Mater. Sci. Mater. Electron. 2015, 26, 7290–7294. [Google Scholar] [CrossRef]
- Ran, W.; Wang, L.; Yang, M.; Kong, X.; Qu, D.; Shi, J. Enhanced energy transfer from Bi3+ to Eu3+ ions relying on the criss-cross cluster structure in MgMoO4 phosphor. J. Lumin. 2017, 192, 141–147. [Google Scholar] [CrossRef]
- Han, C.; Luo, l.; He, J.; Wang, J.; Zhang, W. Synthesis and luminescence properties of ZnMoO4:Eu3+,M+ (M+ = Li+, Na+ and K+) phosphors. J. Mater. Sci. Mater. Electron. 2017, 28, 4409–4413. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kraus, H.; Wahl, D.; Ehrenberg, H.; Mykhaylyk, M.S. Optical and luminescence studies of ZnMoO4 using vacuum ultraviolet synchrotron radiation. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 562, 513–516. [Google Scholar] [CrossRef]
- Chen, F.; Akram, M.N.; Chen, X. Nanocomposite phosphor materials fabricated by solid-state reaction for optoelectronics application. In Proceedings of the 2020 IEEE 8th Electronics System-Integration Technology Conference (ESTC), Tønsberg, Norway, 15–18 September 2020; pp. 1–4. [Google Scholar]
- Xue, R.; Hong, W.; Pan, Z.; Jin, W.; Zhao, H.; Song, Y.; Zhou, J.; Liu, Y. Enhanced electrochemical performance of ZnMoO4/reduced graphene oxide composites as anode materials for lithium-ion batteries. Electrochim. Acta 2016, 222, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Balcı, M.; Xia, H.; Akram, M.; Chen, X. Photoluminescence Properties and Quantum Efficiency of Eu3+/Mn2+- Doped ZnMoO4 Red Phosphor. Key Eng. Mater. 2020, 843, 70–78. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, T.; Zou, L.; Gan, S. Ultrasound-assisted precipitation synthesis of PbMoO4 and PbMoO4:Eu3+ nanocrystals and photoluminescence properties. J. Photochem. Photobiol. A Chem. 2016, 314, 35–41. [Google Scholar] [CrossRef]
- Chen, G.; Zhuang, W.; Hu, Y.; Liu, Y.; Liu, R.; He, H. Luminescence properties of Eu2+-doped Ba3Si6O12N2 green phosphor: Concentration quenching and thermal stability. J. Rare Earths 2013, 31, 113–118. [Google Scholar] [CrossRef]
- Poncé, S.; Jia, Y.; Giantomassi, M.; Mikami, M.; Gonze, X. Understanding Thermal Quenching of Photoluminescence in Oxynitride Phosphors from First Principles. J. Phys. Chem. C 2016, 120, 4040–4047. [Google Scholar] [CrossRef]
- Ueda, J.; Tanabe, S.; Nakanishi, T. Analysis of Ce3+ Luminescence Quenching in Solid Solutions Between Y3Al5O12 and Y3Ga5O12 by Temperature Dependence of Photoconductivity Measurement. J. Appl. Phys. 2011, 110, 53102–531026. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Mao, Z.; Wang, D.; Bie, L. Investigation of thermal quenching and abnormal thermal quenching in mixed valence Eu co-doped LaAlO3 phosphor. J. Lumin. 2017, 186, 72–76. [Google Scholar] [CrossRef]
- Wang, C.; Ye, S.; Zhang, Q. Unraveling the distinct luminescence thermal quenching behaviours of A/B-site Eu3+ ions in double perovskite Sr2CaMoO6:Eu3+. Opt. Mater. 2018, 75, 337–346. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Guo, Z.; Mo, F.; Wu, Z.-C. A zero-thermal-quenching and color-tunable phosphor LuVO4: Bi3+, Eu3+ for NUV LEDs. Dye. Pigment. 2018, 156, 67–73. [Google Scholar] [CrossRef]
- Shi, P.; Zhiguo, X.; Molokeev, M.; Atuchin, V. Crystal Chemistry and Luminescence Properties of red-Emitting CsGd1−xEux(MoO4)2 Solid-Solution Phosphors. Dalton Trans. 2014, 43, 9669–9676. [Google Scholar] [CrossRef]
- Cui, D.; Song, Z.; Zhiguo, X.; Liu, Q. Luminescence Tuning, Thermal Quenching, and Electronic Structure of Narrow-Band Red-Emitting Nitride Phosphors. Inorg. Chem. 2017, 56, 11837–11844. [Google Scholar] [CrossRef]
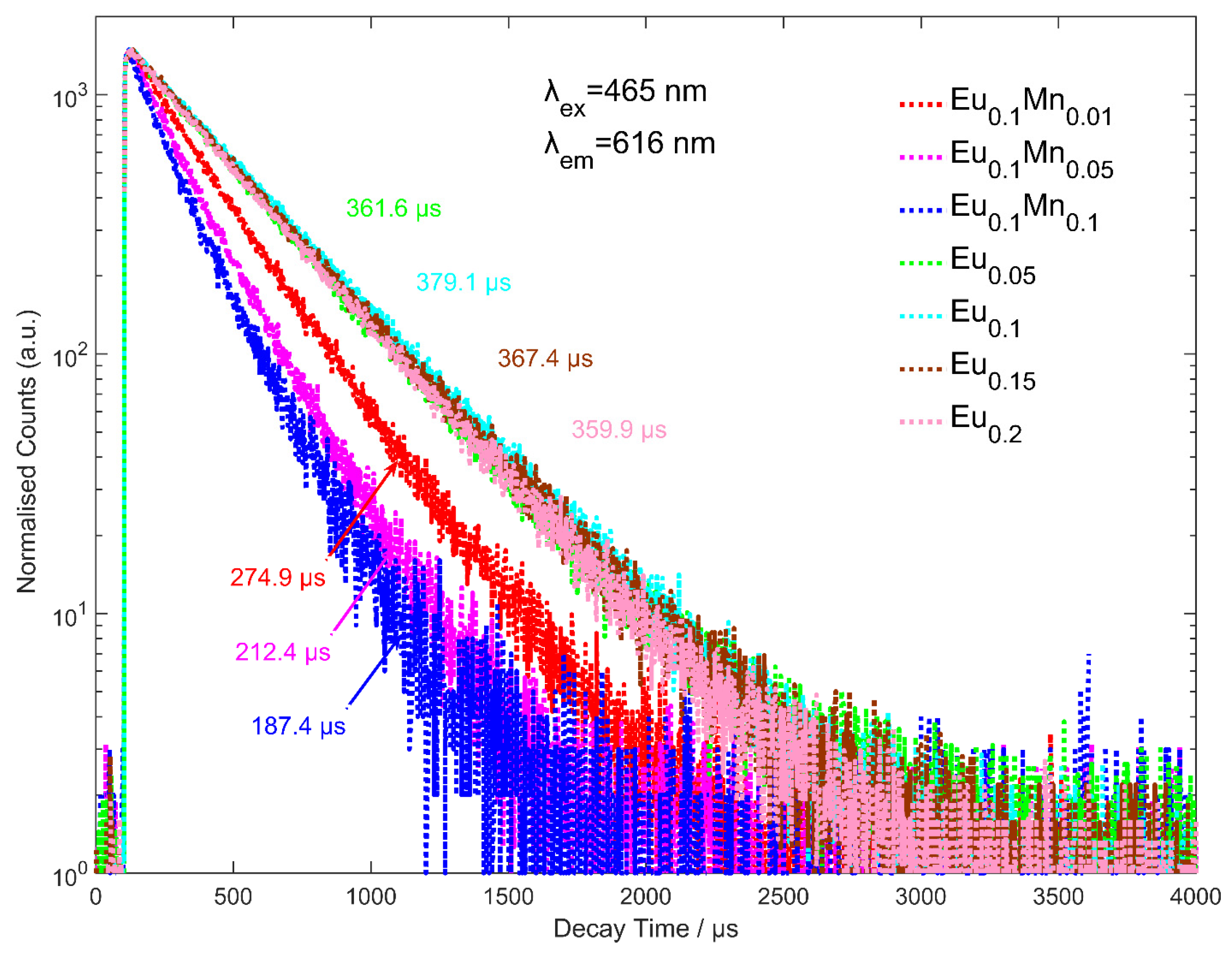



| Samples | A1 | τ1/µs | A1 | τ1/µs | τave |
|---|---|---|---|---|---|
| x = 0.05 | 5537.2 | 334.9 | 506.1 | 542.0 | 361.6 |
| x = 0.1 | 4879.9 | 355.4 | 1755.2 | 432.8 | 379.0 |
| x = 0.15 | 2773.5 | 329.1 | 1841.6 | 413.3 | 367.4 |
| x = 0.2 | 3963.1 | 324.0 | 1391.5 | 435.8 | 359.9 |
| y = 0.01 | 1744.4 | 216.1 | 1877.8 | 312.6 | 274.9 |
| y = 0.05 | 999.6 | 144.2 | 1342.4 | 242.6 | 212.4 |
| y = 0.1 | 482.6 | 109.3 | 782.0 | 212.2 | 187.4 |
| Samples | 395 nm | 465 nm | 535 nm | |||
|---|---|---|---|---|---|---|
| x | y | x | y | x | y | |
| x = 0.05 | 0.6722 | 0.3276 | 0.6711 | 0.3286 | 0.6634 | 0.3363 |
| x = 0.1 | 0.6725 | 0.3272 | 0.6719 | 0.3279 | 0.6682 | 0.3315 |
| x = 0.15 | 0.6723 | 0.3275 | 0.6714 | 0.3284 | 0.6678 | 0.3319 |
| x = 0.2 | 0.6717 | 0.3280 | 0.6709 | 0.3289 | 0.6667 | 0.3330 |
| y = 0.01 | 0.6674 | 0.3324 | 0.6689 | 0.3308 | 0.6595 | 0.3401 |
| y = 0.05 | 0.6523 | 0.3473 | 0.6643 | 0.3354 | 0.6472 | 0.3524 |
| y = 0.1 | 0.6360 | 0.3636 | 0.6577 | 0.3419 | 0.6264 | 0.3730 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Akram, M.N.; Chen, X. Influence of Mn2+ and Eu3+ Concentration on Photoluminescence and Thermal Stability Properties in Eu3+-Activated ZnMoO4 Red Phosphor Materials. Micromachines 2023, 14, 1605. https://doi.org/10.3390/mi14081605
Chen F, Akram MN, Chen X. Influence of Mn2+ and Eu3+ Concentration on Photoluminescence and Thermal Stability Properties in Eu3+-Activated ZnMoO4 Red Phosphor Materials. Micromachines. 2023; 14(8):1605. https://doi.org/10.3390/mi14081605
Chicago/Turabian StyleChen, Fan, Muhammad Nadeem Akram, and Xuyuan Chen. 2023. "Influence of Mn2+ and Eu3+ Concentration on Photoluminescence and Thermal Stability Properties in Eu3+-Activated ZnMoO4 Red Phosphor Materials" Micromachines 14, no. 8: 1605. https://doi.org/10.3390/mi14081605





