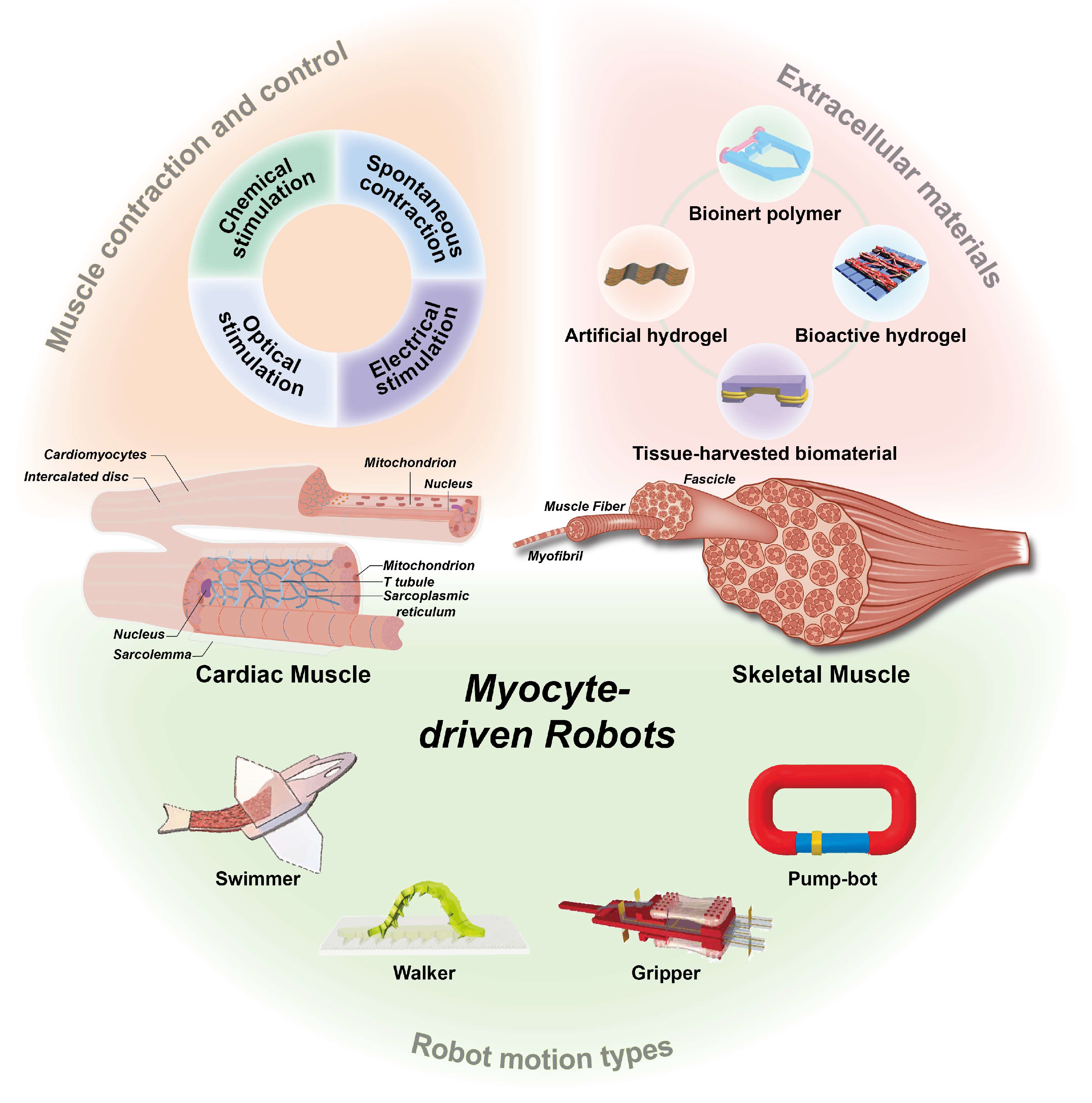
Biohybrid Soft Robots Powered by Myocyte: Current Progress and Future Perspectives
Abstract
1. Introduction

2. Myocytes
2.1. Cardiomyocytes
2.2. Skeletal Muscles
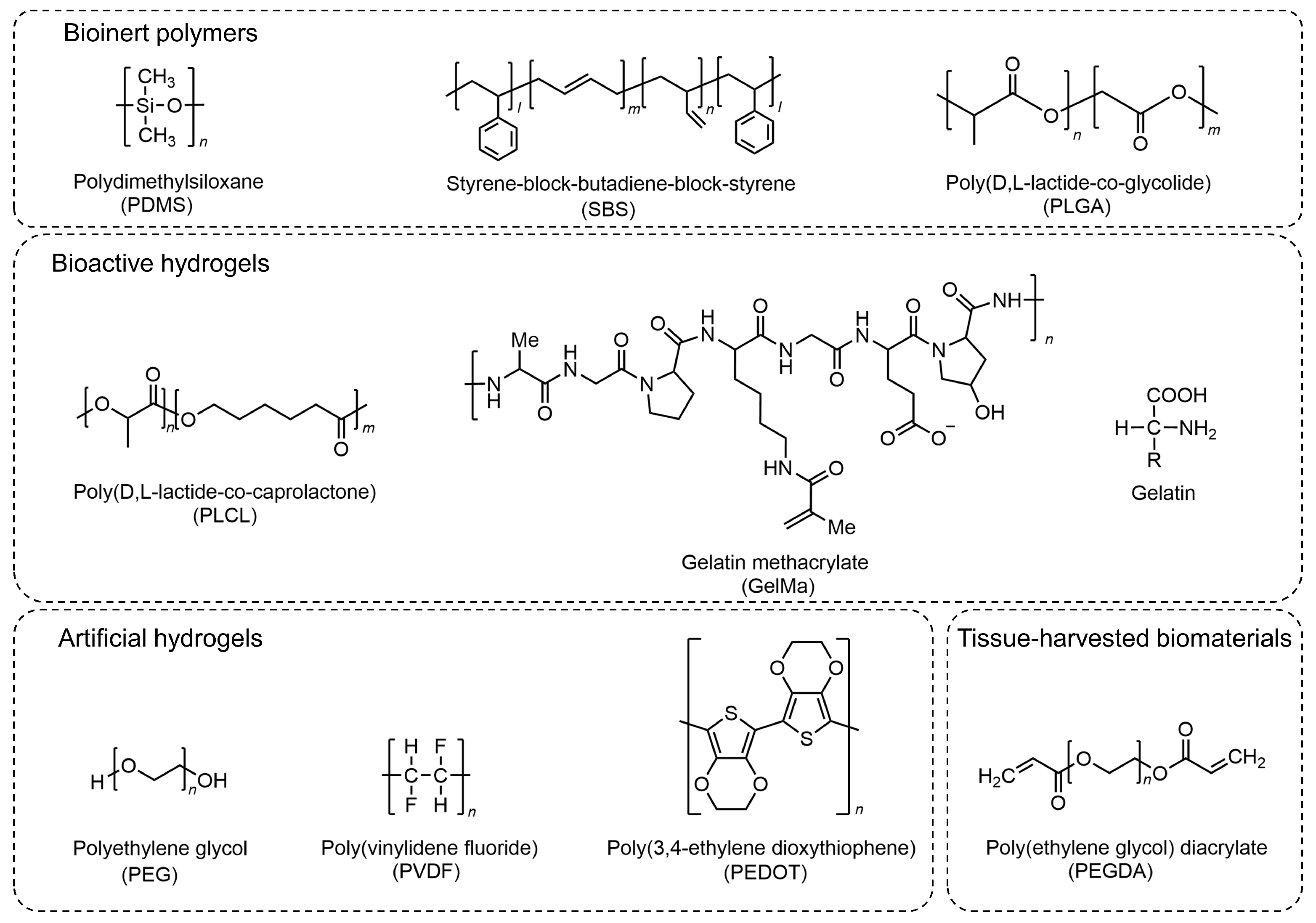
3. Extracellular Materials for Myocyte-Driven Robots
3.1. Bioinert Polymers
3.2. Hydrogel
3.2.1. Bioactive Hydrogels
3.2.2. Artificial Hydrogels
3.3. Tissue-Harvested Biomaterials
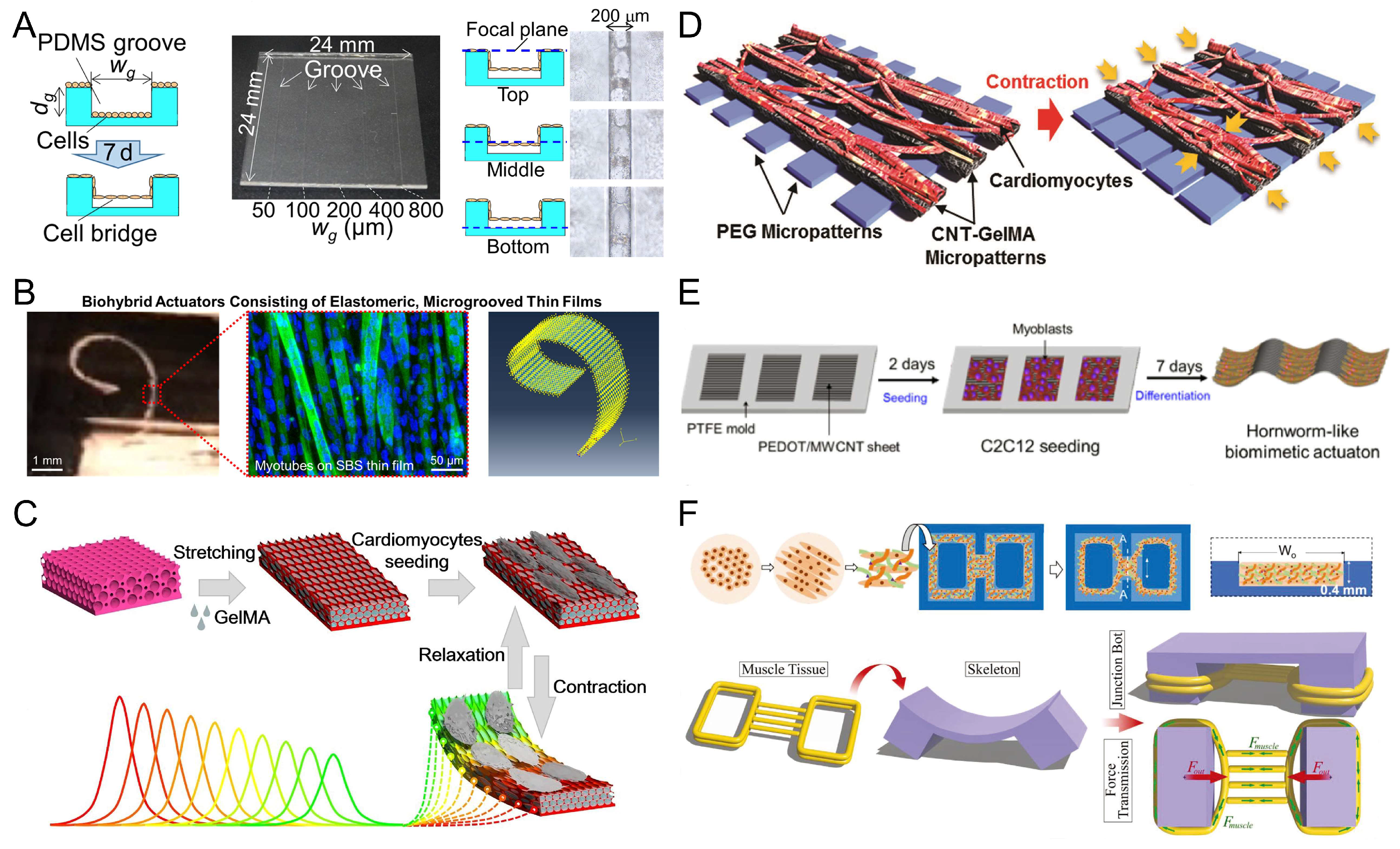
4. Contraction of Muscle Tissue and Control Methods
4.1. Spontaneous Contraction
4.2. Electrical Stimulation
4.3. Optical Stimulation
4.4. Chemical Stimulation
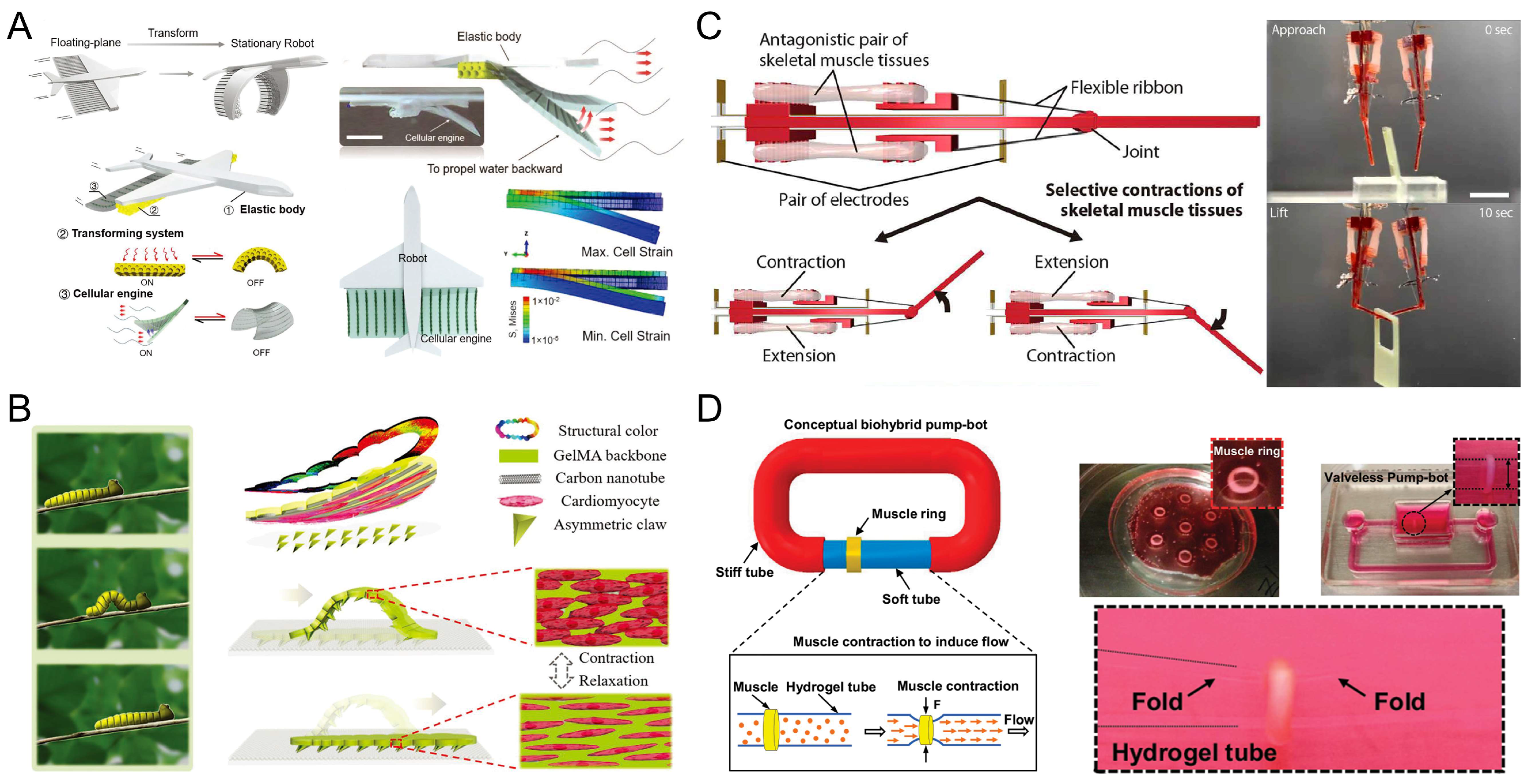
5. Various Applications of Myocyte-Driven Robots
5.1. Swimmers
5.2. Walkers
5.3. Grippers
5.4. Pump-Bots
6. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Z.; Polden, J.; Larkin, N.; Van Duin, S.; Norrish, J. Recent progress on programming methods for industrial robots. Robot. Comput.-Integr. Manuf. 2012, 28, 87–94. [Google Scholar] [CrossRef]
- Sparrow, R.; Howard, M. Robots in agriculture: Prospects, impacts, ethics, and policy. Precis. Agric. 2021, 22, 818–833. [Google Scholar] [CrossRef]
- Beasley, R.A. Medical Robots: Current Systems and Research Directions. J. Robot. 2012, 2012, 401613. [Google Scholar] [CrossRef]
- Gonzalez-Aguirre, J.A.; Osorio-Oliveros, R.; Rodríguez-Hernández, K.L.; Lizárraga-Iturralde, J.; Morales Menendez, R.; Ramírez-Mendoza, R.A.; Ramírez-Moreno, M.A.; Lozoya-Santos, J.D. Service Robots: Trends and Technology. Appl. Sci. 2021, 11, 10702. [Google Scholar] [CrossRef]
- Webster-Wood, V.A.; Akkus, O.; Gurkan, U.A.; Chiel, H.J.; Quinn, R.D. Organismal engineering: Toward a robotic taxonomic key for devices using organic materials. Sci. Robot. 2017, 2, eaap9281. [Google Scholar] [CrossRef] [PubMed]
- Sitti, M.; Ceylan, H.; Hu, W.; Giltinan, J.; Turan, M.; Yim, S.; Diller, E. Biomedical Applications of Untethered Mobile Milli/Microrobots. Proc. IEEE 2015, 103, 205–224. [Google Scholar] [CrossRef]
- Ma, K.Y.; Chirarattananon, P.; Fuller, S.B.; Wood, R.J. Controlled Flight of a Biologically Inspired, Insect-Scale Robot. Science 2013, 340, 603–607. [Google Scholar] [CrossRef]
- Hines, L.; Petersen, K.; Lum, G.Z.; Sitti, M. Soft Actuators for Small-Scale Robotics. Adv. Mater. 2017, 29, 1603483. [Google Scholar] [CrossRef]
- Go, G.; Jeong, S.-G.; Yoo, A.; Han, J.; Kang, B.; Kim, S.; Nguyen, K.T.; Jin, Z.; Kim, C.-S.; Seo, Y.R.; et al. Human adipose–derived mesenchymal stem cell–based medical microrobot system for knee cartilage regeneration in vivo. Sci. Robot. 2020, 5, eaay6626. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Jang, D.; Kim, D.; Lee, D.; Chung, S.K. Acoustic bubble-based drug manipulation: Carrying, releasing and penetrating for targeted drug delivery using an electromagnetically actuated microrobot. Sens. Actuators A Phys. 2020, 306, 111973. [Google Scholar] [CrossRef]
- Jafferis, N.T.; Helbling, E.F.; Karpelson, M.; Wood, R.J. Untethered flight of an insect-sized flapping-wing microscale aerial vehicle. Nature 2019, 570, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Helps, T.; Taghavi, M.; Wang, S.; Rossiter, J. Twisted Rubber Variable-Stiffness Artificial Muscles. Soft Robot. 2019, 7, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Wallin, T.J.; Pan, W.; Xu, P.; Wang, K.; Giannelis, E.P.; Mazzolai, B.; Shepherd, R.F. Autonomic perspiration in 3D-printed hydrogel actuators. Sci. Robot. 2020, 5, eaaz3918. [Google Scholar] [CrossRef]
- Shahsavan, H.; Aghakhani, A.; Zeng, H.; Guo, Y.; Davidson, Z.S.; Priimagi, A.; Sitti, M. Bioinspired underwater locomotion of light-driven liquid crystal gels. Proc. Natl. Acad. Sci. USA 2020, 117, 5125–5133. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Wang, W.; Xi, N.; Liu, L. A Manta Ray-Inspired Biosyncretic Robot with Stable Controllability by Dynamic Electric Stimulation. Cyborg Bionic Syst. 2022, 2022, 9891380. [Google Scholar] [CrossRef]
- Michas, C.; Karakan, M.Ç.; Nautiyal, P.; Seidman, J.G.; Seidman, C.E.; Agarwal, A.; Ekinci, K.; Eyckmans, J.; White, A.E.; Chen, C.S. Engineering a living cardiac pump on a chip using high-precision fabrication. Sci. Adv. 2022, 8, eabm3791. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Ansari, M.H.; Mahajan, M.; Srivastava, S.; Kashyap, S.; Dwivedi, P.; Pandit, V.; Katha, U. Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines 2020, 11, 448. [Google Scholar] [CrossRef]
- Xu, H.; Medina-Sánchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-Hybrid Micromotor for Targeted Drug Delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef]
- Morrow, J.; Shin, H.S.; Phillips-Grafflin, C.; Jang, S.H.; Torrey, J.; Larkins, R.; Dang, S.; Park, Y.L.; Berenson, D. Improving Soft Pneumatic Actuator fingers through integration of soft sensors, position and force control, and rigid fingernails. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 5024–5031. [Google Scholar]
- Stokes, A.A.; Shepherd, R.F.; Morin, S.A.; Ilievski, F.; Whitesides, G.M. A Hybrid Combining Hard and Soft Robots. Soft Robot. 2013, 1, 70–74. [Google Scholar] [CrossRef]
- Chan, V.; Asada, H.H.; Bashir, R. Utilization and control of bioactuators across multiple length scales. Lab Chip 2014, 14, 653–670. [Google Scholar] [CrossRef]
- Vo, V.T.K.; Ang, M.H.; Koh, S.J.A. Maximal Performance of an Antagonistically Coupled Dielectric Elastomer Actuator System. Soft Robot. 2020, 8, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Liu, X.; Cacucciolo, V.; Imboden, M.; Civet, Y.; El Haitami, A.; Cantin, S.; Perriard, Y.; Shea, H. An autonomous untethered fast soft robotic insect driven by low-voltage dielectric elastomer actuators. Sci. Robot. 2019, 4, eaaz6451. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zou, Z.; Mao, G.; Yang, X.; Liang, Y.; Li, C.; Qu, S.; Suo, Z.; Yang, W. Agile and Resilient Insect-Scale Robot. Soft Robot. 2018, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, L.; Liu, Y.; Leng, J. Review of Dielectric Elastomer Actuators and Their Applications in Soft Robots. Adv. Intell. Syst. 2021, 3, 2000282. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.; Wang, J.; Jiang, C.; Zhu, X.; Ge, Q.; Gu, G. Design of 3D Printed Programmable Horseshoe Lattice Structures Based on a Phase-Evolution Model. ACS Appl. Mater. Interfaces 2020, 12, 22146–22156. [Google Scholar] [CrossRef]
- Behl, M.; Kratz, K.; Zotzmann, J.; Nöchel, U.; Lendlein, A. Reversible Bidirectional Shape-Memory Polymers. Adv. Mater. 2013, 25, 4466–4469. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Lee, Y.-J.; Yang, S. Responsive and Foldable Soft Materials. Trends Chem. 2020, 2, 107–122. [Google Scholar] [CrossRef]
- Guo, Y.; Shahsavan, H.; Sitti, M. Microscale Polarization Color Pixels from Liquid Crystal Elastomers. Adv. Opt. Mater. 2020, 8, 1902098. [Google Scholar] [CrossRef]
- Zmyślony, M.; Dradrach, K.; Haberko, J.; Nałęcz-Jawecki, P.; Rogóż, M.; Wasylczyk, P. Optical Pliers: Micrometer-Scale, Light-Driven Tools Grown on Optical Fibers. Adv. Mater. 2020, 32, 2002779. [Google Scholar] [CrossRef]
- Ahn, C.; Liang, X.; Cai, S. Bioinspired Design of Light-Powered Crawling, Squeezing, and Jumping Untethered Soft Robot. Adv. Mater. Technol. 2019, 4, 1900185. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Yang, S. Multi-functional liquid crystal elastomer composites. Appl. Phys. Rev. 2022, 9, 011301. [Google Scholar] [CrossRef]
- Wang, C.; Sim, K.; Chen, J.; Kim, H.; Rao, Z.; Li, Y.; Chen, W.; Song, J.; Verduzco, R.; Yu, C. Soft Ultrathin Electronics Innervated Adaptive Fully Soft Robots. Adv. Mater. 2018, 30, 1706695. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zhao, X. Magnetic Soft Materials and Robots. Chem. Rev. 2022, 122, 5317–5364. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Zhang, X.; Feng, Y.; Zeng, H.; Wang, L.; Feng, W. Light-driven bimorph soft actuators: Design, fabrication, and properties. Mater. Horiz. 2021, 8, 728–757. [Google Scholar] [CrossRef] [PubMed]
- Sitti, M. Miniature soft robots—Road to the clinic. Nat. Rev. Mater. 2018, 3, 74–75. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Pena-Francesch, A.; Jung, H.; Demirel, M.C.; Sitti, M. Biosynthetic self-healing materials for soft machines. Nat. Mater. 2020, 19, 1230–1235. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Bellingham, J.; Dupont, P.E.; Fischer, P.; Floridi, L.; Full, R.; Jacobstein, N.; Kumar, V.; McNutt, M.; Merrifield, R.; et al. The grand challenges of Science Robotics. Sci. Robot. 2018, 3, eaar7650. [Google Scholar] [CrossRef]
- Xi, J.; Schmidt, J.J.; Montemagno, C.D. Self-assembled microdevices driven by muscle. Nat. Mater. 2005, 4, 180–184. [Google Scholar] [CrossRef]
- Nawroth, J.C.; Lee, H.; Feinberg, A.W.; Ripplinger, C.M.; McCain, M.L.; Grosberg, A.; Dabiri, J.O.; Parker, K.K. A tissue-engineered jellyfish with biomimetic propulsion. Nat. Biotechnol. 2012, 30, 792–797. [Google Scholar] [CrossRef]
- Park, S.-J.; Gazzola, M.; Park, K.S.; Park, S.; Di Santo, V.; Blevins, E.L.; Lind, J.U.; Campbell, P.H.; Dauth, S.; Capulli, A.K.; et al. Phototactic guidance of a tissue-engineered soft-robotic ray. Science 2016, 353, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Onoe, H.; Takeuchi, S. Biohybrid robot powered by an antagonistic pair of skeletal muscle tissues. Sci. Robot. 2018, 3, eaat4440. [Google Scholar] [CrossRef] [PubMed]
- Aydin, O.; Zhang, X.; Nuethong, S.; Pagan-Diaz, G.J.; Bashir, R.; Gazzola, M.; Saif, M.T.A. Neuromuscular actuation of biohybrid motile bots. Proc. Natl. Acad. Sci. USA 2019, 116, 19841–19847. [Google Scholar] [CrossRef]
- Lee, K.Y.; Park, S.-J.; Matthews, D.G.; Kim, S.L.; Marquez, C.A.; Zimmerman, J.F.; Ardoña, H.A.M.; Kleber, A.G.; Lauder, G.V.; Parker, K.K. An autonomously swimming biohybrid fish designed with human cardiac biophysics. Science 2022, 375, 639–647. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.Y.; Lim, T.H.; Park, C.H. Silk Fibroin Bioinks for Digital Light Processing (DLP) 3D Bioprinting. In Bioinspired Biomaterials: Advances in Tissue Engineering and Regenerative Medicine; Chun, H.J., Reis, R.L., Motta, A., Khang, G., Eds.; Springer: Singapore, 2020; pp. 53–66. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, Y.B.; Hinton, T.J.; Feinberg, A.W.; Alsberg, E. Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues. Mater. Today Chem. 2019, 12, 61–70. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Kort-Mascort, J.; Kinsella, J.M. Extrusion bioprinting of soft materials: An emerging technique for biological model fabrication. Appl. Phys. Rev. 2019, 6, 011310. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Tandon, N.; Godier, A.; Maidhof, R.; Marsano, A.; Martens, T.P.; Radisic, M. Challenges in Cardiac Tissue Engineering. Tissue Eng. Part B Rev. 2009, 16, 169–187. [Google Scholar] [CrossRef]
- An, B.; Wang, Y.; Huang, Y.; Wang, X.; Liu, Y.; Xun, D.; Church, G.M.; Dai, Z.; Yi, X.; Tang, T.-C.; et al. Engineered Living Materials For Sustainability. Chem. Rev. 2023, 123, 2349–2419. [Google Scholar] [CrossRef]
- Martel, S.; Mohammadi, M.; Felfoul, O.; Zhao, L.; Pouponneau, P. Flagellated Magnetotactic Bacteria as Controlled MRI-trackable Propulsion and Steering Systems for Medical Nanorobots Operating in the Human Microvasculature. Int. J. Robot. Res. 2009, 28, 571–582. [Google Scholar] [CrossRef]
- Morimoto, Y.; Onoe, H.; Takeuchi, S. Biohybrid robot with skeletal muscle tissue covered with a collagen structure for moving in air. APL Bioeng. 2020, 4, 026101. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Loskill, P.; Deveshwar, N.; Spencer, C.I.; Judge, L.M.; Mandegar, M.A.; Fox, C.B.; Mohamed, T.M.A.; Ma, Z.; Mathur, A.; et al. Miniaturized iPS-Cell-Derived Cardiac Muscles for Physiologically Relevant Drug Response Analyses. Sci. Rep. 2016, 6, 24726. [Google Scholar] [CrossRef]
- Akiyama, Y.; Iwabuchi, K.; Furukawa, Y.; Morishima, K. Electrical stimulation of cultured lepidopteran dorsal vessel tissue: An experiment for development of bioactuators. Vitr. Cell. Dev. Biol.—Anim. 2010, 46, 411–415. [Google Scholar] [CrossRef]
- Yalikun, Y.; Uesugi, K.; Hiroki, M.; Shen, Y.; Tanaka, Y.; Akiyama, Y.; Morishima, K. Insect Muscular Tissue-Powered Swimming Robot. Actuators 2019, 8, 30. [Google Scholar] [CrossRef]
- Magdanz, V.; Medina-Sánchez, M.; Schwarz, L.; Xu, H.; Elgeti, J.; Schmidt, O.G. Spermatozoa as Functional Components of Robotic Microswimmers. Adv. Mater. 2017, 29, 1606301. [Google Scholar] [CrossRef]
- Asghar, Z.; Ali, N.; Waqas, M.; Nazeer, M.; Khan, W.A. Locomotion of an efficient biomechanical sperm through viscoelastic medium. Biomech. Model. Mechanobiol. 2020, 19, 2271–2284. [Google Scholar] [CrossRef] [PubMed]
- Magdanz, V.; Sanchez, S.; Schmidt, O.G. Development of a Sperm-Flagella Driven Micro-Bio-Robot. Adv. Mater. 2013, 25, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Seo, Y.; Aydin, O.; Elhebeary, M.; Kamm, R.D.; Kong, H.; Saif, M.T.A. Biohybrid valveless pump-bot powered by engineered skeletal muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 1543–1548. [Google Scholar] [CrossRef]
- Guix, M.; Mestre, R.; Patiño, T.; De Corato, M.; Fuentes, J.; Zarpellon, G.; Sánchez, S. Biohybrid soft robots with self-stimulating skeletons. Sci. Robot. 2021, 6, eabe7577. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Han, X.; Hu, Y.; Luo, Y.; Chen, C.H.; Chen, Z.; Shi, P. A Remotely Controlled Transformable Soft Robot Based on Engineered Cardiac Tissue Construct. Small 2019, 15, e1900006. [Google Scholar] [CrossRef]
- Wang, W.; Duan, W.; Ahmed, S.; Mallouk, T.E.; Sen, A. Small power: Autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 2013, 8, 531–554. [Google Scholar] [CrossRef]
- Williams, B.J.; Anand, S.V.; Rajagopalan, J.; Saif, M.T.A. A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun. 2014, 5, 3081. [Google Scholar] [CrossRef]
- Kim, Y.; Pagan-Diaz, G.; Gapinske, L.; Kim, Y.; Suh, J.; Solomon, E.; Harris, J.F.; Nam, S.; Bashir, R. Integration of Graphene Electrodes with 3D Skeletal Muscle Tissue Models. Adv. Healthc. Mater. 2020, 9, e1901137. [Google Scholar] [CrossRef]
- Pagan-Diaz, G.J.; Zhang, X.; Grant, L.; Kim, Y.; Aydin, O.; Cvetkovic, C.; Ko, E.; Solomon, E.; Hollis, J.; Kong, H.; et al. Simulation and Fabrication of Stronger, Larger, and Faster Walking Biohybrid Machines. Adv. Funct. Mater. 2018, 28, 1801145. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Z.; Bian, F.; Zhao, Y. Bioinspired Soft Robotic Caterpillar with Cardiomyocyte Drivers. Adv. Funct. Mater. 2020, 30, 1907820. [Google Scholar] [CrossRef]
- Kabumoto, K.; Hoshino, T.; Morishima, K. Bio-robotics using interaction between neuron and muscle for development of living prosthesis. In Proceedings of the 2010 3rd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, Tokyo, Japan, 26–29 September 2010; pp. 419–424. [Google Scholar]
- Kabumoto, K.; Hoshino, T.; Akiyama, Y.; Morishima, K. Voluntary movement controlled by the surface EMG signal for tissue-engineered skeletal muscle on a gripping tool. Tissue Eng. Part A 2013, 19, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Balance, W.C.; Joy, M.S.H.; Patel, S.; Hwang, J.; Kong, H.; Saif, M.T.A. Adaptive biohybrid pumping machine with flow loop feedback. Biofabrication 2022, 14, 025009. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Saif, M.T.A. Mechanics of Biohybrid Valveless Pump-Bot. J. Appl. Mech. 2021, 88, 111004. [Google Scholar] [CrossRef]
- Shutko, A.V.; Gorbunov, V.S.; Guria, K.G.; Agladze, K.I. Biocontractile microfluidic channels for peristaltic pumping. Biomedical Microdevices 2017, 19, 72. [Google Scholar] [CrossRef]
- Kim, T.H.; Kwon, C.H.; Lee, C.; An, J.; Phuong, T.T.T.; Park, S.H.; Lima, M.D.; Baughman, R.H.; Kang, T.M.; Kim, S.J. Bio-inspired Hybrid Carbon Nanotube Muscles. Sci. Rep. 2016, 6, 26687. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Migliori, B.; Miccoli, B.; Li, Y.C.; Mostafalu, P.; Seo, J.; Mandla, S.; Enrico, A.; Antona, S.; Sabarish, R.; et al. Electrically Driven Microengineered Bioinspired Soft Robots. Adv. Mater. 2018, 30, 1704189. [Google Scholar] [CrossRef]
- de Tombe, P.P. Cardiac myofilaments: Mechanics and regulation. J. Biomech. 2003, 36, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Takemura, R.; Akiyama, Y.; Hoshino, T.; Morishima, K. Chemical switching of jellyfish-shaped micro robot consisting only of cardiomyocyte gel. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 2442–2445. [Google Scholar]
- Holley, M.T.; Nagarajan, N.; Danielson, C.; Zorlutuna, P.; Park, K. Development and characterization of muscle-based actuators for self-stabilizing swimming biorobots. Lab Chip 2016, 16, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.; Yang, S.; Baek, J.; Kim, B.; Lee, S.H.; Yoon, E.S.; Chun, K.; Park, S. Establishment of a fabrication method for a long-term actuated hybrid cell robot. Lab Chip 2007, 7, 1504–1508. [Google Scholar] [CrossRef]
- Chan, V.; Park, K.; Collens, M.B.; Kong, H.; Saif, T.A.; Bashir, R. Development of miniaturized walking biological machines. Sci. Rep. 2012, 2, 857. [Google Scholar] [CrossRef]
- Cvetkovic, C.; Raman, R.; Chan, V.; Williams, B.J.; Tolish, M.; Bajaj, P.; Sakar, M.S.; Asada, H.H.; Saif, M.T.; Bashir, R. Three-dimensionally printed biological machines powered by skeletal muscle. Proc. Natl. Acad. Sci. USA 2014, 111, 10125–10130. [Google Scholar] [CrossRef]
- Raman, R.; Cvetkovic, C.; Uzel, S.G.; Platt, R.J.; Sengupta, P.; Kamm, R.D.; Bashir, R. Optogenetic skeletal muscle-powered adaptive biological machines. Proc. Natl. Acad. Sci. USA 2016, 113, 3497–3502. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Park, J.; Park, I.; Kilicarslan, E.; Kim, Y.; Dou, Z.; Bashir, R.; Gazzola, M. Computationally Assisted Design and Selection of Maneuverable Biological Walking Machines. Adv. Intell. Syst. 2021, 3, 2000237. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morishima, K.; Shimizu, T.; Kikuchi, A.; Yamato, M.; Okano, T.; Kitamori, T. An actuated pump on-chip powered by cultured cardiomyocytes. Lab Chip 2006, 6, 362–368. [Google Scholar] [CrossRef]
- Park, J.; Kim, I.C.; Baek, J.; Cha, M.; Kim, J.; Park, S.; Lee, J.; Kim, B. Micro pumping with cardiomyocyte-polymer hybrid. Lab Chip 2007, 7, 1367–1370. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, K.; Shimizu, T.; Yamato, M.; Okano, T.; Kitamori, T. A micro-spherical heart pump powered by cultured cardiomyocytes. Lab Chip 2007, 7, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Yamashita, T.; Yalikun, Y.; Amaya, S.; Sato, A.; Vogel, V.; Tanaka, Y. An ultra-small fluid oscillation unit for pumping driven by self-organized three-dimensional bridging of pulsatile cardiomyocytes on elastic micro-piers. Sens. Actuators B Chem. 2019, 293, 256–264. [Google Scholar] [CrossRef]
- Nerbonne, J.M.; Kass, R.S. Molecular Physiology of Cardiac Repolarization. Physiol. Rev. 2005, 85, 1205–1253. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Qin, C.; Gu, C.; He, C.; Yuan, Q.; Liu, M.; Zhuang, L.; Wan, H.; Wang, P. A novel bionic in vitro bioelectronic tongue based on cardiomyocytes and microelectrode array for bitter and umami detection. Biosens. Bioelectron. 2019, 145, 111673. [Google Scholar] [CrossRef] [PubMed]
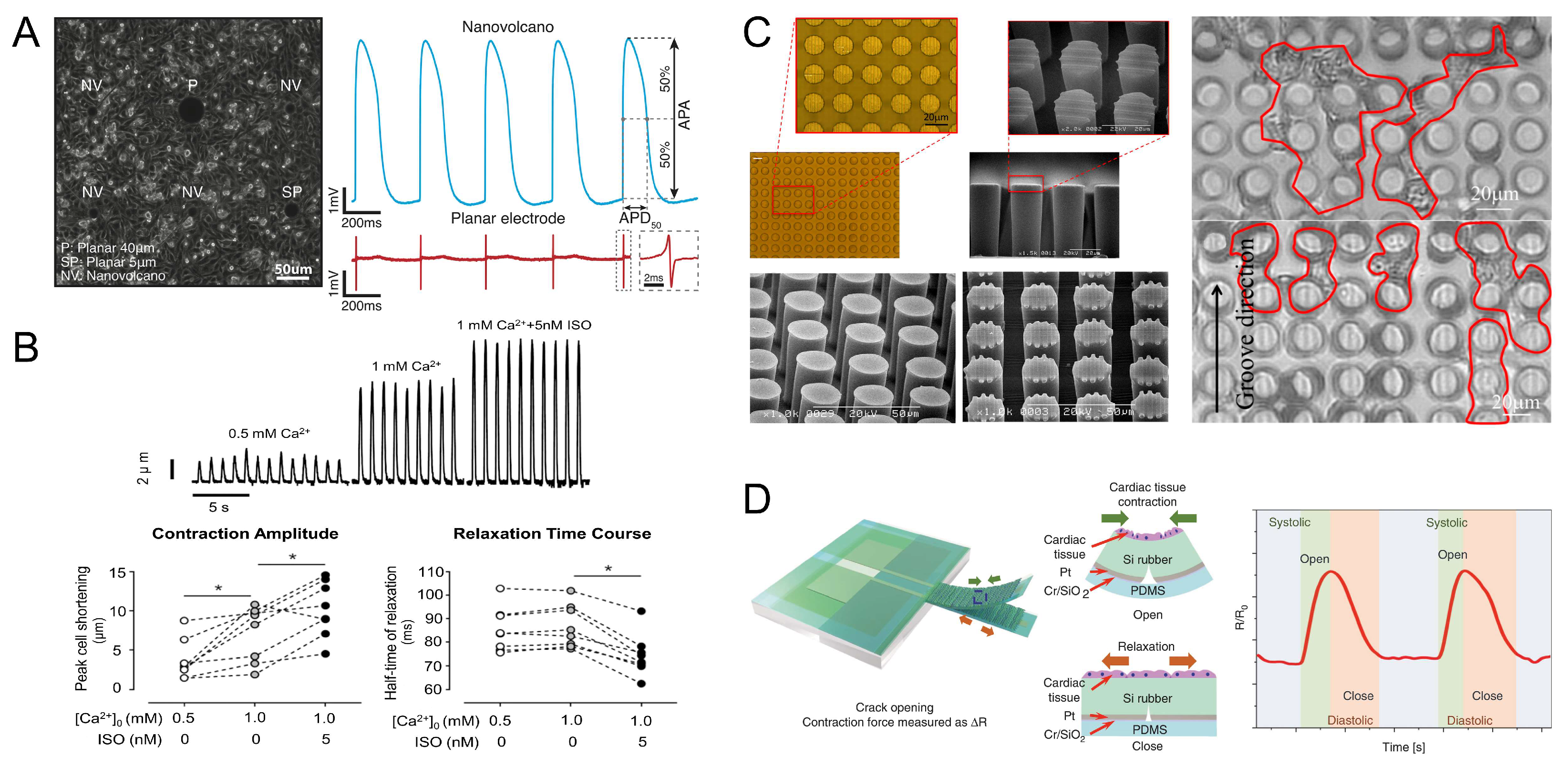
- Desbiolles, B.X.E.; de Coulon, E.; Bertsch, A.; Rohr, S.; Renaud, P. Intracellular Recording of Cardiomyocyte Action Potentials with Nanopatterned Volcano-Shaped Microelectrode Arrays. Nano Lett. 2019, 19, 6173–6181. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef]
- Göktepe, S.; Kuhl, E. Electromechanics of the heart: A unified approach to the strongly coupled excitation–contraction problem. Comput. Mech. 2009, 45, 227–243. [Google Scholar] [CrossRef]
- Bazan, C.; Barba, D.T.; Blomgren, P.; Paolini, P. Image Processing Techniques for Assessing Contractility in Isolated Adult Cardiac Myocytes. Int. J. Biomed. Imaging 2009, 2009, 352954. [Google Scholar] [CrossRef]
- Goulart, J.T.; Bassani, R.A.; Bassani, J.W. Application based on the Canny edge detection algorithm for recording contractions of isolated cardiac myocytes. Comput. Biol. Med. 2017, 81, 106–110. [Google Scholar] [CrossRef]
- Ahola, A.; Kiviaho, A.L.; Larsson, K.; Honkanen, M.; Aalto-Setälä, K.; Hyttinen, J. Video image-based analysis of single human induced pluripotent stem cell derived cardiomyocyte beating dynamics using digital image correlation. BioMed. Eng. OnLine 2014, 13, 39. [Google Scholar] [CrossRef]
- Oyunbaatar, N.E.; Lee, D.H.; Patil, S.J.; Kim, E.S.; Lee, D.W. Biomechanical Characterization of Cardiomyocyte Using PDMS Pillar with Microgrooves. Sensors 2016, 16, 1258. [Google Scholar] [CrossRef]
- Kim, D.-S.; Choi, Y.W.; Shanmugasundaram, A.; Jeong, Y.-J.; Park, J.; Oyunbaatar, N.-E.; Kim, E.-S.; Choi, M.; Lee, D.-W. Highly durable crack sensor integrated with silicone rubber cantilever for measuring cardiac contractility. Nat. Commun. 2020, 11, 535. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, K.; Shimizu, T.; Yamato, M.; Okano, T.; Kitamori, T. Biological cells on microchips: New technologies and applications. Biosens Bioelectron 2007, 23, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, X.; Zhan, C.; Wu, J.; Xu, J.; Cheung, J. Measuring Single Cardiac Myocyte Contractile Force via Moving a Magnetic Bead. Biophys. J. 2005, 88, 1489–1495. [Google Scholar] [CrossRef]
- McCain, M.L.; Agarwal, A.; Nesmith, H.W.; Nesmith, A.P.; Parker, K.K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials 2014, 35, 5462–5471. [Google Scholar] [CrossRef]
- Nakajima, T.; Sankai, Y.; Takata, S.; Kobayashi, Y.; Ando, Y.; Nakagawa, M.; Saito, T.; Saito, K.; Ishida, C.; Tamaoka, A.; et al. Cybernic treatment with wearable cyborg Hybrid Assistive Limb (HAL) improves ambulatory function in patients with slowly progressive rare neuromuscular diseases: A multicentre, randomised, controlled crossover trial for efficacy and safety (NCY-3001). Orphanet J. Rare Dis. 2021, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Costa, J.M.; Fernández-Garibay, X.; Velasco-Mallorquí, F.; Ramón-Azcón, J. Bioengineered in vitro skeletal muscles as new tools for muscular dystrophies preclinical studies. J. Tissue Eng. 2021, 12, 2041731420981339. [Google Scholar] [CrossRef] [PubMed]
- Santoso, J.W.; Li, X.; Gupta, D.; Suh, G.C.; Hendricks, E.; Lin, S.; Perry, S.; Ichida, J.K.; Dickman, D.; McCain, M.L. Engineering skeletal muscle tissues with advanced maturity improves synapse formation with human induced pluripotent stem cell-derived motor neurons. APL Bioeng. 2021, 5, 036101. [Google Scholar] [CrossRef]
- Fujita, H.; Van Dau, T.; Shimizu, K.; Hatsuda, R.; Sugiyama, S.; Nagamori, E. Designing of a Si-MEMS device with an integrated skeletal muscle cell-based bio-actuator. Biomed Microdevices 2011, 13, 123–129. [Google Scholar] [CrossRef]
- Vannozzi, L.; Mazzocchi, T.; Hasebe, A.; Takeoka, S.; Fujie, T.; Ricotti, L. A Coupled FEM-SPH Modeling Technique to Investigate the Contractility of Biohybrid Thin Films. Adv. Biosyst. 2020, 4, 1900306. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Nakayama, A.; Nakano, S.; Amiya, R.; Hirose, J. An Electrical Stimulation Culture System for Daily Maintenance-Free Muscle Tissue Production. Cyborg Bionic Syst. 2021, 2021, 9820505. [Google Scholar] [CrossRef]
- Raman, R.; Bashir, R. Biomimicry, Biofabrication, and Biohybrid Systems: The Emergence and Evolution of Biological Design. Adv. Healthc. Mater. 2017, 6, 1700496. [Google Scholar] [CrossRef]
- Donnelly, K.; Khodabukus, A.; Philp, A.; Deldicque, L.; Dennis, R.G.; Baar, K. A Novel Bioreactor for Stimulating Skeletal Muscle In Vitro. Tissue Eng. Part C Methods 2009, 16, 711–718. [Google Scholar] [CrossRef]
- Martin, N.R.W.; Passey, S.L.; Player, D.J.; Mudera, V.; Baar, K.; Greensmith, L.; Lewis, M.P. Neuromuscular Junction Formation in Tissue-Engineered Skeletal Muscle Augments Contractile Function and Improves Cytoskeletal Organization. Tissue Eng. Part A 2015, 21, 2595–2604. [Google Scholar] [CrossRef]
- Gao, H.; Cao, X.; Dong, H.; Fu, X.; Wang, Y. Influence of 3D Microgrooves on C2C12 Cell Proliferation, Migration, Alignment, F-actin Protein Expression and Gene Expression. J. Mater. Sci. Technol. 2016, 32, 901–908. [Google Scholar] [CrossRef]
- Gao, H.; Xiao, J.; Wei, Y.; Wang, H.; Wan, H.; Liu, S. Regulation of Myogenic Differentiation by Topologically Microgrooved Surfaces for Skeletal Muscle Tissue Engineering. ACS Omega 2021, 6, 20931–20940. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, V.; Ahadian, S.; Ostrovidov, S.; Camci-Unal, G.; Chen, S.; Kaji, H.; Ramalingam, M.; Khademhosseini, A. Engineered Contractile Skeletal Muscle Tissue on a Microgrooved Methacrylated Gelatin Substrate. Tissue Eng. Part A 2012, 18, 2453–2465. [Google Scholar] [CrossRef]
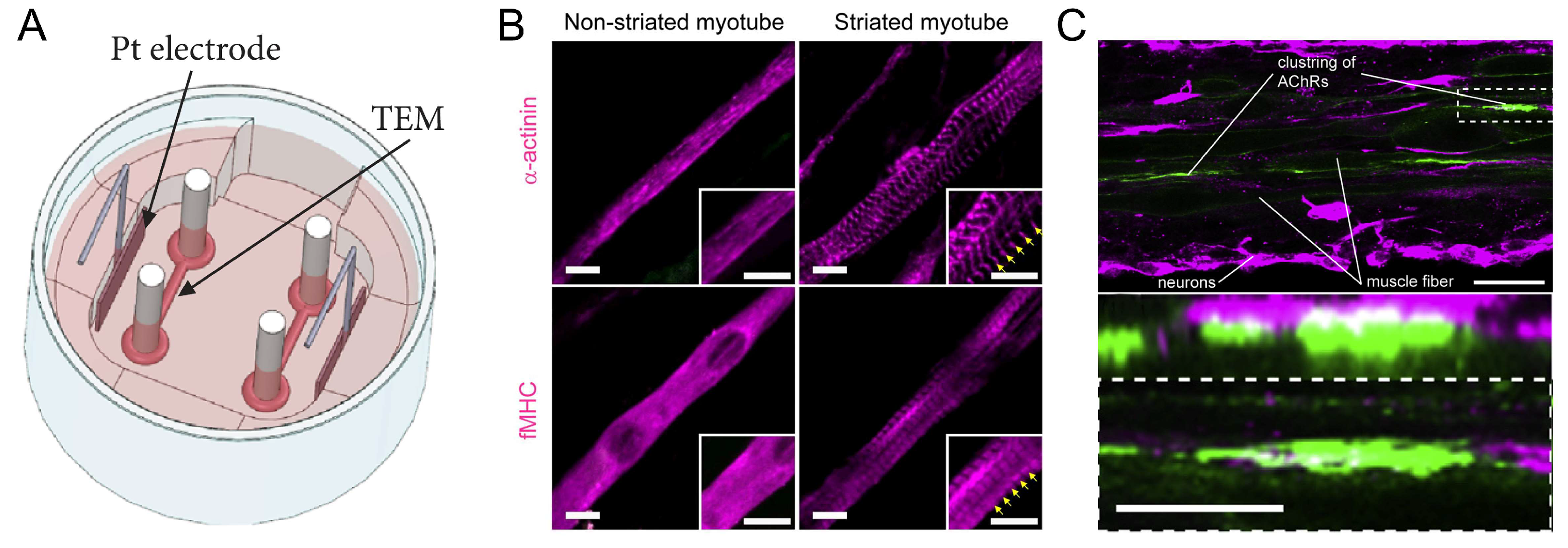
- Ahadian, S.; Ramón-Azcón, J.; Ostrovidov, S.; Camci-Unal, G.; Hosseini, V.; Kaji, H.; Ino, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Interdigitated array of Pt electrodes for electrical stimulation and engineering of aligned muscle tissue. Lab Chip 2012, 12, 3491–3503. [Google Scholar] [CrossRef] [PubMed]
- Khodabukus, A.; Baar, K. Defined Electrical Stimulation Emphasizing Excitability for the Development and Testing of Engineered Skeletal Muscle. Tissue Eng. Part C Methods 2011, 18, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Ishizuka, T.; Morishima, K.; Yawo, H. Optogenetic induction of contractile ability in immature C2C12 myotubes. Sci. Rep. 2015, 5, 8317. [Google Scholar] [CrossRef]
- Asano, T.; Ishizua, T.; Yawo, H. Optically controlled contraction of photosensitive skeletal muscle cells. Biotechnol. Bioeng. 2012, 109, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Sakar, M.S.; Neal, D.; Boudou, T.; Borochin, M.A.; Li, Y.; Weiss, R.; Kamm, R.D.; Chen, C.S.; Asada, H.H. Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab Chip 2012, 12, 4976–4985. [Google Scholar] [CrossRef]
- Romanazzo, S.; Forte, G.; Morishima, K.; Taniguchi, A. IL-12 involvement in myogenic differentiation of C2C12 in vitro. Biomater. Sci. 2015, 3, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Kato-Negishi, M.; Onoe, H.; Takeuchi, S. Three-dimensional neuron–muscle constructs with neuromuscular junctions. Biomaterials 2013, 34, 9413–9419. [Google Scholar] [CrossRef]
- Distler, T.; Solisito, A.A.; Schneidereit, D.; Friedrich, O.; Detsch, R.; Boccaccini, A.R. 3D printed oxidized alginate-gelatin bioink provides guidance for C2C12 muscle precursor cell orientation and differentiation via shear stress during bioprinting. Biofabrication 2020, 12, 045005. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.V.; Yakut Ali, M.; Saif, M.T.A. Cell culture on microfabricated one-dimensional polymeric structures for bio-actuator and bio-bot applications. Lab Chip 2015, 15, 1879–1888. [Google Scholar] [CrossRef]
- Feinberg, A.W.; Feigel, A.; Shevkoplyas, S.S.; Sheehy, S.; Whitesides, G.M.; Parker, K.K. Muscular Thin Films for Building Actuators and Powering Devices. Science 2007, 317, 1366–1370. [Google Scholar] [CrossRef]
- Nagamine, K.; Kawashima, T.; Sekine, S.; Ido, Y.; Kanzaki, M.; Nishizawa, M. Spatiotemporally controlled contraction of micropatterned skeletal muscle cells on a hydrogel sheet. Lab Chip 2011, 11, 513–517. [Google Scholar] [CrossRef]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.-J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H.; et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef]
- Vannozzi, L.; Ricotti, L.; Cianchetti, M.; Bearzi, C.; Gargioli, C.; Rizzi, R.; Dario, P.; Menciassi, A. Self-assembly of polydimethylsiloxane structures from 2D to 3D for bio-hybrid actuation. Bioinspiration Biomim. 2015, 10, 056001. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Ramón-Azcón, J.; Ahadian, S.; Estili, M.; Liang, X.; Ostrovidov, S.; Kaji, H.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; et al. Dielectrophoretically Aligned Carbon Nanotubes to Control Electrical and Mechanical Properties of Hydrogels to Fabricate Contractile Muscle Myofibers. Adv. Mater. 2013, 25, 4028–4034. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Selimović, Š.; Acevedo Cox, J.P.; Ribas, J.; Afshar Bakooshli, M.; Heintze, D.; Weiss, A.S.; Cropek, D.; Khademhosseini, A. Hydrogel-coated microfluidic channels for cardiomyocyte culture. Lab Chip 2013, 13, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Roshanbinfar, K.; Mohammadi, Z.; Sheikh-Mahdi Mesgar, A.; Dehghan, M.M.; Oommen, O.P.; Hilborn, J.; Engel, F.B. Carbon nanotube doped pericardial matrix derived electroconductive biohybrid hydrogel for cardiac tissue engineering. Biomater. Sci. 2019, 7, 3906–3917. [Google Scholar] [CrossRef]
- Hahn, M.S.; Taite, L.J.; Moon, J.J.; Rowland, M.C.; Ruffino, K.A.; West, J.L. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials 2006, 27, 2519–2524. [Google Scholar] [CrossRef]
- Raman, R.; Grant, L.; Seo, Y.; Cvetkovic, C.; Gapinske, M.; Palasz, A.; Dabbous, H.; Kong, H.; Pinera, P.P.; Bashir, R. Damage, Healing, and Remodeling in Optogenetic Skeletal Muscle Bioactuators. Adv. Healthc. Mater. 2017, 6, 1700030. [Google Scholar] [CrossRef]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas sorption, diffusion, and permeation in poly(dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Kuddannaya, S.; Bao, J.; Zhang, Y. Enhanced In Vitro Biocompatibility of Chemically Modified Poly(dimethylsiloxane) Surfaces for Stable Adhesion and Long-term Investigation of Brain Cerebral Cortex Cells. ACS Appl. Mater. Interfaces 2015, 7, 25529–25538. [Google Scholar] [CrossRef]
- Chen, W.; Lam, R.H.W.; Fu, J. Photolithographic surface micromachining of polydimethylsiloxane (PDMS). Lab Chip 2012, 12, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, A.; Suematsu, Y.; Takeoka, S.; Mazzocchi, T.; Vannozzi, L.; Ricotti, L.; Fujie, T. Biohybrid Actuators Based on Skeletal Muscle-Powered Microgrooved Ultrathin Films Consisting of Poly(styrene-block-butadiene-block-styrene). ACS Biomater. Sci. Eng. 2019, 5, 5734–5743. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Chen, Z.; Fu, F.; Sun, L.; Shao, C.; Jin, W.; Liu, H.; Zhao, Y. Cardiomyocyte-Driven Structural Color Actuation in Anisotropic Inverse Opals. ACS Nano 2019, 13, 796–802. [Google Scholar] [CrossRef]
- Yoon, J.; Eyster, T.W.; Misra, A.C.; Lahann, J. Cardiomyocyte-Driven Actuation in Biohybrid Microcylinders. Adv. Mater. 2015, 27, 4509–4515. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, H.; Lu, Y.; Li, S.; Lin, L.; Du, Y.; Wang, X. In vitro cardiomyocyte-driven biogenerator based on aligned piezoelectric nanofibers. Nanoscale 2016, 8, 7278–7286. [Google Scholar] [CrossRef]
- Webster, V.A.; Hawley, E.L.; Akkus, O.; Chiel, H.J.; Quinn, R.D. Effect of actuating cell source on locomotion of organic living machines with electrocompacted collagen skeleton. Bioinspiration Biomim. 2016, 11, 036012. [Google Scholar] [CrossRef]
- Sun, Y.; Duffy, R.; Lee, A.; Feinberg, A.W. Optimizing the structure and contractility of engineered skeletal muscle thin films. Acta Biomater. 2013, 9, 7885–7894. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Langelaan, M.L.P.; Boonen, K.J.M.; Rosaria-Chak, K.Y.; van der Schaft, D.W.J.; Post, M.J.; Baaijens, F.P.T. Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells. J. Tissue Eng. Regen. Med. 2011, 5, 529–539. [Google Scholar] [CrossRef]
- Juhas, M.; Engelmayr, G.C.; Fontanella, A.N.; Palmer, G.M.; Bursac, N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 5508–5513. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G.R. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Wang, W.; Xi, N.; Wang, Y. Regulation of C2C12 Differentiation and Control of the Beating Dynamics of Contractile Cells for a Muscle-Driven Biosyncretic Crawler by Electrical Stimulation. Soft Robot. 2018, 5, 748–760. [Google Scholar] [CrossRef]
- Vurro, V.; Venturino, I.; Lanzani, G. A perspective on the use of light as a driving element for bio-hybrid actuation. Appl. Phys. Lett. 2022, 120, 080502. [Google Scholar] [CrossRef]
- Pastrana, E. Optogenetics: Controlling cell function with light. Nat. Methods 2011, 8, 24–25. [Google Scholar] [CrossRef]
- Tan, P.; He, L.; Huang, Y.; Zhou, Y. Optophysiology: Illuminating cell physiology with optogenetics. Physiol. Rev. 2022, 102, 1263–1325. [Google Scholar] [CrossRef]
- Toettcher, J.E.; Voigt, C.A.; Weiner, O.D.; Lim, W.A. The promise of optogenetics in cell biology: Interrogating molecular circuits in space and time. Nat. Methods 2011, 8, 35–38. [Google Scholar] [CrossRef]
- Chan, V.; Neal, D.M.; Uzel, S.G.M.; Kim, H.; Bashir, R.; Asada, H.H. Fabrication and characterization of optogenetic, multi-strip cardiac muscles. Lab Chip 2015, 15, 2258–2268. [Google Scholar] [CrossRef]
- Bruegmann, T.; van Bremen, T.; Vogt, C.C.; Send, T.; Fleischmann, B.K.; Sasse, P. Optogenetic control of contractile function in skeletal muscle. Nat. Commun. 2015, 6, 7153. [Google Scholar] [CrossRef]
- Frigault, M.M.; Lacoste, J.; Swift, J.L.; Brown, C.M. Live-cell microscopy—Tips and tools. J. Cell Sci. 2009, 122, 753–767. [Google Scholar] [CrossRef]
- Cheng, K.P.; Kiernan, E.A.; Eliceiri, K.W.; Williams, J.C.; Watters, J.J. Blue Light Modulates Murine Microglial Gene Expression in the Absence of Optogenetic Protein Expression. Sci. Rep. 2016, 6, 21172. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Häder, D.-P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Imagawa, K.; Horiguchi, H.; Ikeda, K.; Akiyama, Y.; Hoshino, T.; Maruo, S.; Morishima, K. Autonomous beating and fluid pumping gel by cardiomycytes drug stimulation. In Proceedings of the TRANSDUCERS 2009—2009 International Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009; pp. 769–772. [Google Scholar]
- Horiguchi, H.; Imagawa, K.; Hoshino, T.; Akiyama, Y.; Morishima, K. Fabrication and Evaluation of Reconstructed Cardiac Tissue and Its Application to Bio-actuated Microdevices. IEEE Trans. NanoBiosci. 2009, 8, 349–355. [Google Scholar] [CrossRef]
- Pignier, C.; Potreau, D. Characterization of nifedipine-resistant calcium current in neonatal rat ventricular cardiomyocytes. Am. J. Physiol.-Heart Circ. Physiol. 2000, 279, H2259–H2268. [Google Scholar] [CrossRef]
- Yoshioka, K.; Ito, A.; Kawabe, Y.; Kamihira, M. Novel neuromuscular junction model in 2D and 3D myotubes co-cultured with induced pluripotent stem cell-derived motor neurons. J. Biosci. Bioeng. 2020, 129, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Uzel, S.G.M.; Kamm, R.D. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv. 2018, 4, eaat5847. [Google Scholar] [CrossRef] [PubMed]
- Hofemeier, A.D.; Limon, T.; Muenker, T.M.; Wallmeyer, B.; Jurado, A.; Afshar, M.E.; Ebrahimi, M.; Tsukanov, R.; Oleksiievets, N.; Enderlein, J.; et al. Global and local tension measurements in biomimetic skeletal muscle tissues reveals early mechanical homeostasis. eLife 2021, 10, e60145. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, F.; Yu, Y.; Wang, H.; Shang, Y.; Zhao, Y. Cardiomyocytes-Actuated Morpho Butterfly Wings. Adv. Mater. 2019, 31, 1805431. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Weiss, D.E.; Liu, Q.; Tian, B. Biomimetic approaches toward smart bio-hybrid systems. Nano Res. 2018, 11, 3009–3030. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Xi, N.; Wang, Y.; Liu, L. Development and Future Challenges of Bio-Syncretic Robots. Engineering 2018, 4, 452–463. [Google Scholar] [CrossRef]
- Hoshino, T.; Morishima, K. Muscle-powered Cantilever for Microtweezers with an Artificial Micro Skeleton and Rat Primary Myotubes. J. Biomech. Sci. Eng. 2010, 5, 245–251. [Google Scholar] [CrossRef][Green Version]
- Ren, D.; Xia, Y.; Wang, J.; You, Z. Micropatterning of single cell arrays using the PEG-Silane and Biotin–(Strept)Avidin System with photolithography and chemical vapor deposition. Sens. Actuators B Chem. 2013, 188, 340–346. [Google Scholar] [CrossRef]
- Heinrich, M.K.; von Mammen, S.; Hofstadler, D.N.; Wahby, M.; Zahadat, P.; Skrzypczak, T.; Soorati, M.D.; Krela, R.; Kwiatkowski, W.; Schmickl, T.; et al. Constructing living buildings: A review of relevant technologies for a novel application of biohybrid robotics. J. R. Soc. Interface 2019, 16, 20190238. [Google Scholar] [CrossRef]
- Zhang, D.; Shadrin, I.Y.; Lam, J.; Xian, H.-Q.; Snodgrass, H.R.; Bursac, N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 2013, 34, 5813–5820. [Google Scholar] [CrossRef] [PubMed]
- Goldfracht, I.; Efraim, Y.; Shinnawi, R.; Kovalev, E.; Huber, I.; Gepstein, A.; Arbel, G.; Shaheen, N.; Tiburcy, M.; Zimmermann, W.H.; et al. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater. 2019, 92, 145–159. [Google Scholar] [CrossRef] [PubMed]



| Robot Types | Advantage | Disadvantage | References |
|---|---|---|---|
| Traditional rigid robots | High output power; High speed; High accuracy; Easy manipulation | Complex structure; Less flexible; Poor reliability; Low energy conversion rate | [19,20,21] |
| Flexible material-driven robots | Light weight; High adaptability to target shapes; High flexibility | Low lifetime; Inefficient movement | [22,23,24,25,26,27] |
| Biomaterial-driven robots | Excellent biocompatibility; High sensitivity; High stability; High energy conversion rate; Self-assembly and self-healing capability | Low lifetime; Ethical Issues; Cell survival environment issues; Simple function | [36,37,38,39] |
| Motion Types | Year | Myocytes | Extracellular Materials | Performance Parameters | Control Methods | References |
|---|---|---|---|---|---|---|
| Swimmers | 2011 | Cardiomyocytes | Collagen gel | Speed: 6.9 μm/s. | Chemical control | [76] |
| 2012 | Cardiomyocytes | PDMS | Speed: 2.4 mm/s. | Electric control | [41] | |
| 2014 | Cardiomyocytes | PDMS | Speed: 81 μm/s. | No control | [63] | |
| 2016 | Cardiomyocytes | PDMS; Au | Speed: 1.5 mm/s. | Optical control | [42] | |
| 2016 | Cardiomyocytes | PDMS | Speed: 142 μm/s. | No control | [77] | |
| 2018 | Cardiomyocytes | PEG;CNT–GelMA Hydrogel; Au | Response time: 0.3 s. | Electric control | [73] | |
| 2019 | Cardiomyocytes | FN | Speed: 0.6 ± 0.2 mm/s. | Optical control | [61] | |
| 2019 | Skeletal muscles | PDMS | Speed: 0.7 μm/s. | Optical control | [44] | |
| 2021 | Skeletal muscles | PDMS | Speed: 800 μm/s. | No control | [60] | |
| 2022 | Cardiomyocytes | Gelatin | Speed:15 mm/s. | Optical control | [45] | |
| 2022 | Skeletal muscles | PDMS | Speed: 70 μm/s. | Electric control | [15] | |
| Walkers | 2007 | Cardiomyocytes | PDMS | Average step stroke: 77.6 mm; Speed: 100 μm/s. | No control | [78] |
| 2012 | Cardiomyocytes | PEGDA | Per stroke: 354 µm; Speed: 236 μm/s. | No control | [79] | |
| 2014 | Skeletal muscles | PEGDA | Speed: 156 μm/s. | Electric control | [80] | |
| 2016 | Skeletal muscles | PEGDA | Speed: 310 μm/s. | Optical control | [81] | |
| 2018 | Skeletal muscles | PEGDA | Speed: 0.5 mm/s. | Electric control | [65] | |
| 2019 | Cardiomyocytes | CNT–GelMA | Speed: 20 μm/s. | Chemical control | [66] | |
| 2021 | Skeletal muscles | PEGDA | Speed: 5.9 mm/min. | Electric control | [82] | |
| Grippers | 2010 | Skeletal muscles | PDMS | Manipulate objects sized: 200 µm; Displacement: ~8 µm. | Electric control | [67] |
| 2013 | Skeletal muscles | PDMS | Displacement: ~5 µm. | Electric control | [68] | |
| 2018 | Skeletal muscles | Photo-reactive acrylate resin | Rotation angle: 90°. | Electric control | [43] | |
| 2020 | Skeletal muscles | Photo-reactive acrylate resin | Rotation angle: 90°. | Electric control | [52] | |
| Pump-bots | 2006 | Cardiomyocytes | PDMS | Flow rate: 0.24 μL/min. | No control | [83] |
| 2007 | Cardiomyocytes | PDMS; Cr/Au | Flow rate: 0.226 μL/min. | No control | [84] | |
| 2007 | Cardiomyocytes | PDMS | Flow rate: 0.047 μL/min. | No control | [85] | |
| 2017 | Cardiomyocytes | PDMS/FN | Flow rate: 6–8 μm/min. | No control | [71] | |
| 2019 | Cardiomyocytes | PDMS | Flow rate: 1.0 nL/min. | No control | [86] | |
| 2019 | Skeletal muscles | PDMS | Flow rate: 22.5 μL/min. | Electric control | [59] | |
| 2022 | Cardiomyocytes | Photoresist IP-S; PDMS | Flow rate: 0.3 μL/s. | No control | [16] | |
| 2022 | Skeletal muscles | PDMS | Flow rate: 13.62 μL/min. | No control | [69] |
| Types of Extracellular Materials | Biocompatibility | Chemical Stability | Biotoxicity | Mechanical Property |
|---|---|---|---|---|
| Bioinert polymers | Excellent | Excellent | Low | Excellent |
| Bioactive hydrogels | Excellent | General | Low | General |
| Artificial hydrogels | General | Excellent | Low | Excellent |
| Tissue-harvested biomaterials | Excellent | General | Low | General |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Guo, Q.; Jin, D.; Zhang, P.; Yang, W. Biohybrid Soft Robots Powered by Myocyte: Current Progress and Future Perspectives. Micromachines 2023, 14, 1643. https://doi.org/10.3390/mi14081643
Yuan Z, Guo Q, Jin D, Zhang P, Yang W. Biohybrid Soft Robots Powered by Myocyte: Current Progress and Future Perspectives. Micromachines. 2023; 14(8):1643. https://doi.org/10.3390/mi14081643
Chicago/Turabian StyleYuan, Zheng, Qinghao Guo, Delu Jin, Peifan Zhang, and Wenguang Yang. 2023. "Biohybrid Soft Robots Powered by Myocyte: Current Progress and Future Perspectives" Micromachines 14, no. 8: 1643. https://doi.org/10.3390/mi14081643
APA StyleYuan, Z., Guo, Q., Jin, D., Zhang, P., & Yang, W. (2023). Biohybrid Soft Robots Powered by Myocyte: Current Progress and Future Perspectives. Micromachines, 14(8), 1643. https://doi.org/10.3390/mi14081643






