Study on Low-Temperature Conductive Silver Pastes Containing Bi-Based Glass for MgTiO3 Electronic Power Devices
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
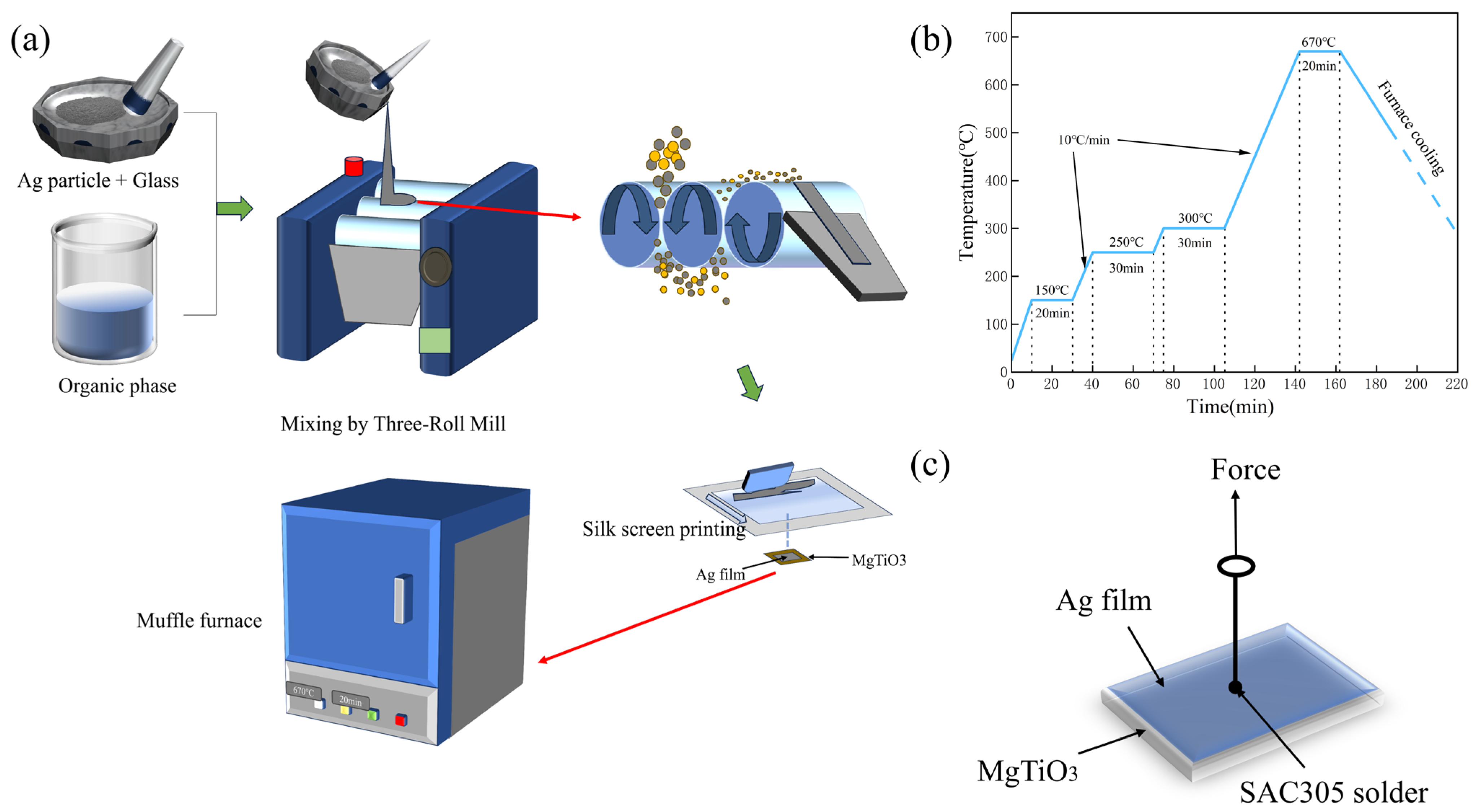
2.2. Synthesis of Glass Powder
2.3. Preparation of Silver Paste and Silver Film
2.4. Materials Characterization
3. Results and Discussion
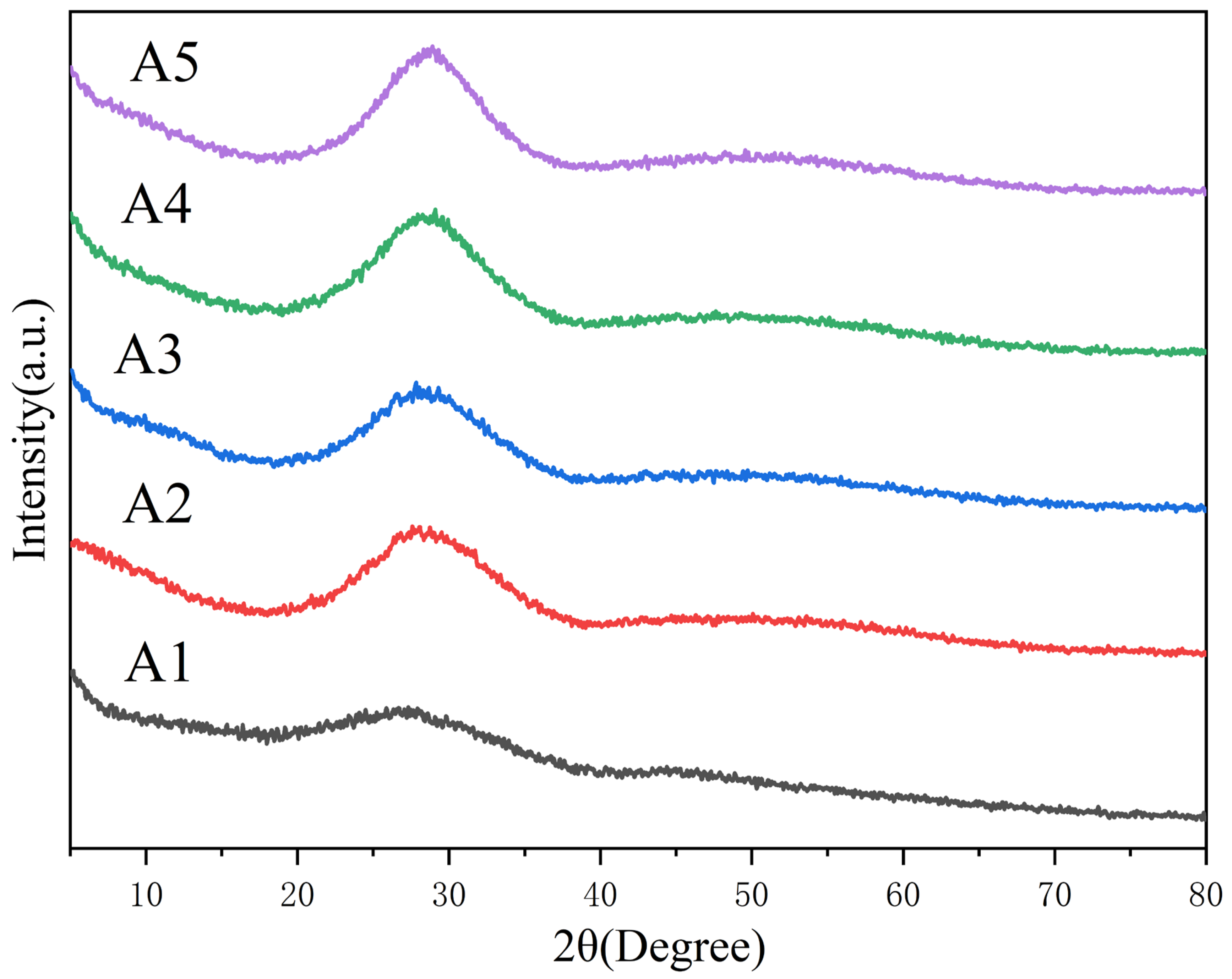
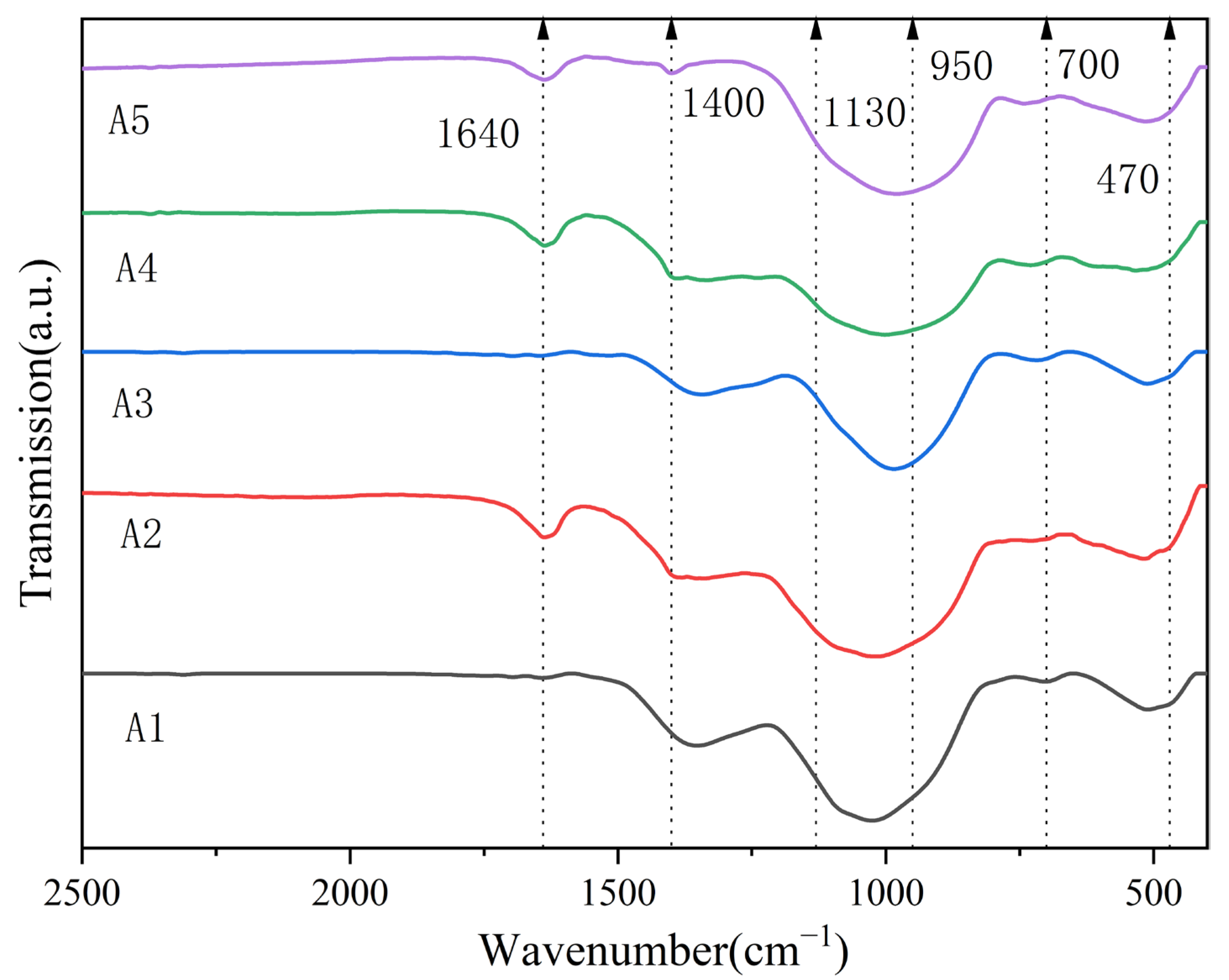
3.1. Characterization of the Glass Frits
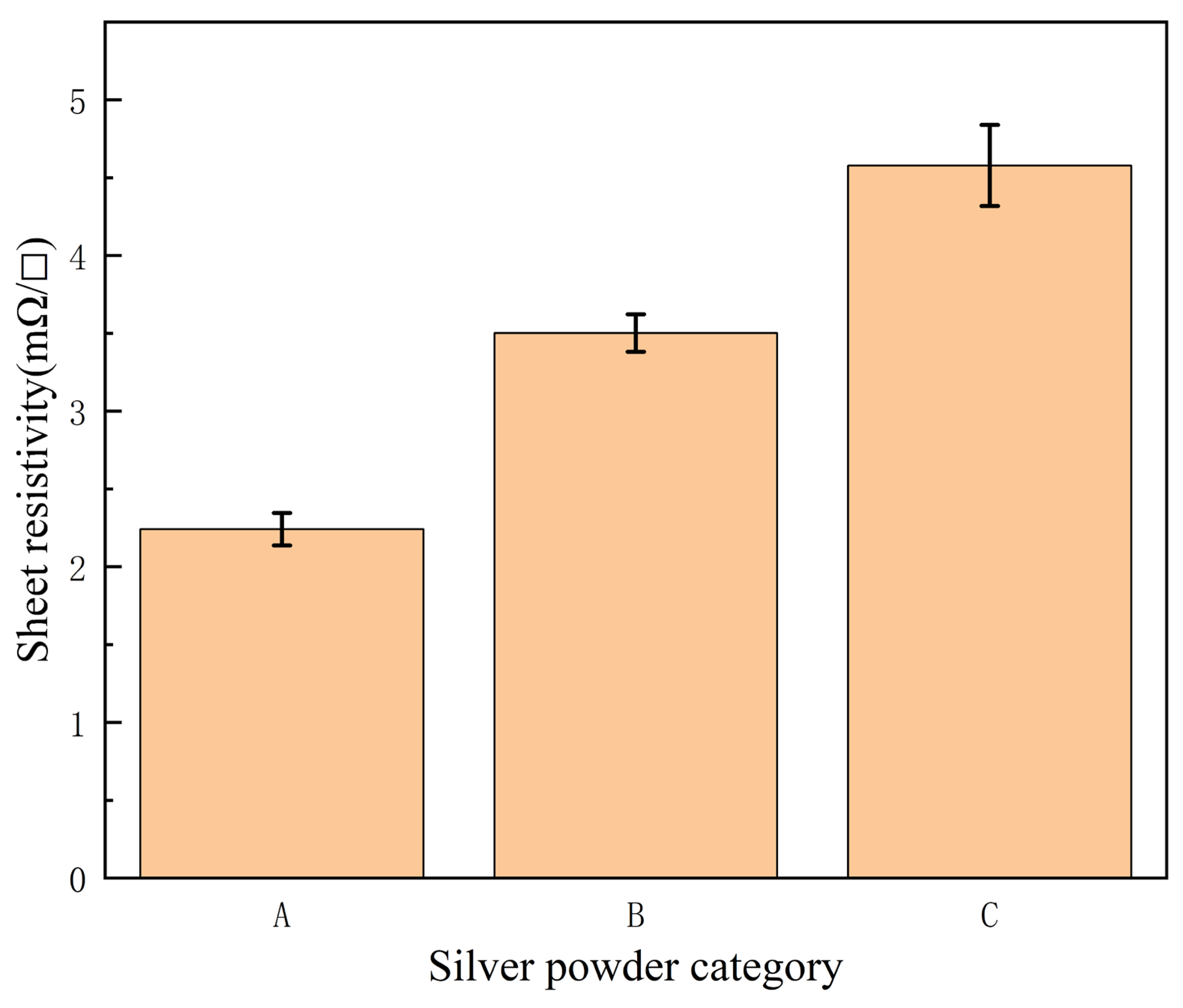
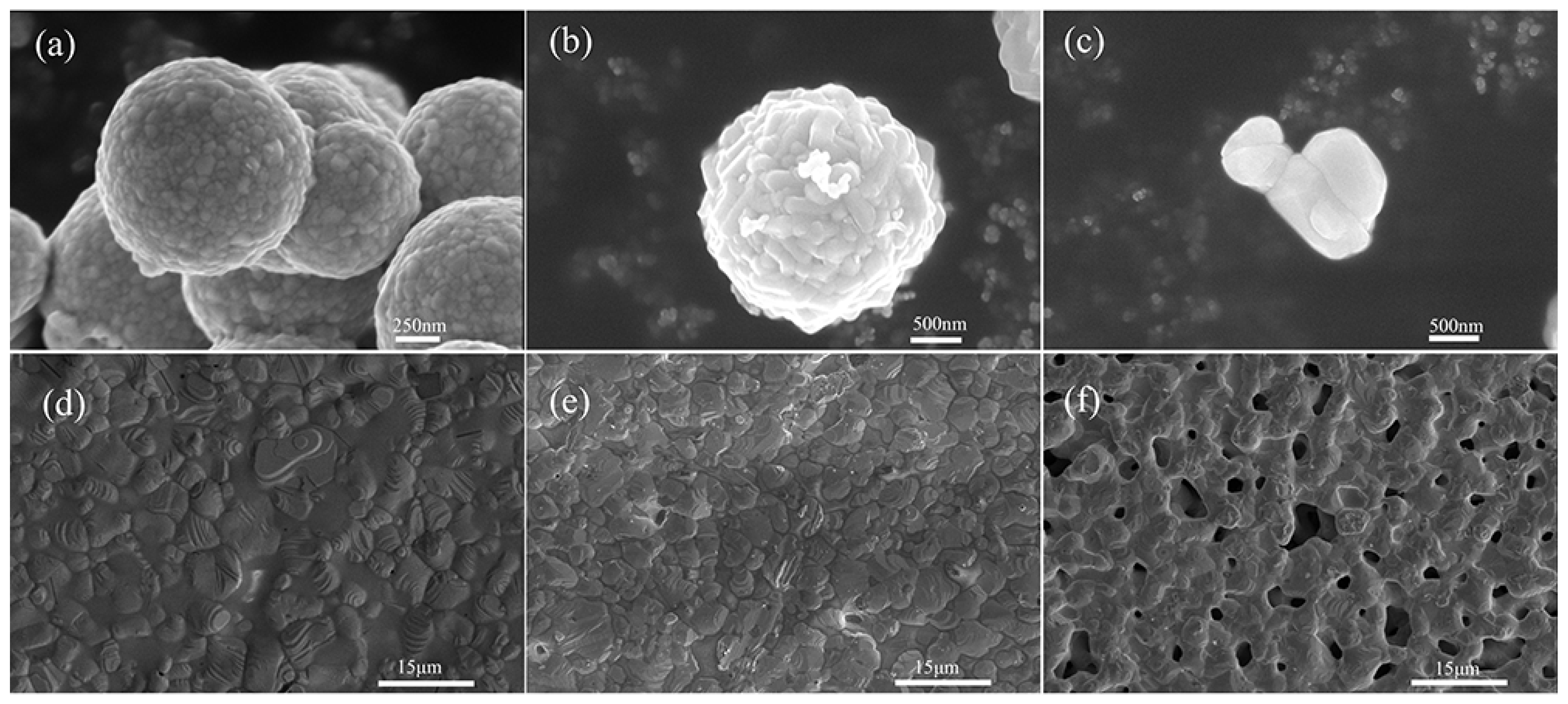
3.2. Effect of Silver Powder Category on Sintering Temperature
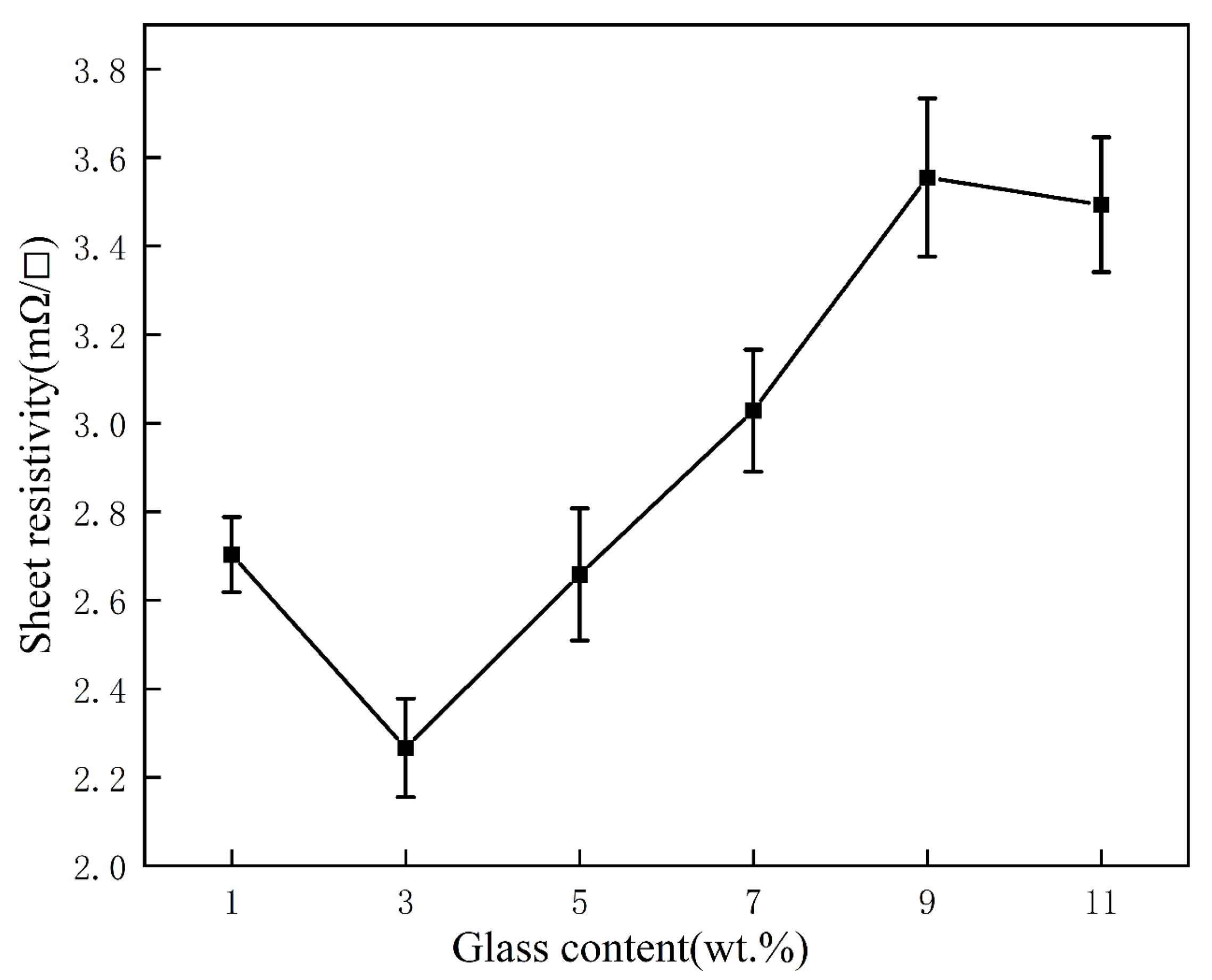
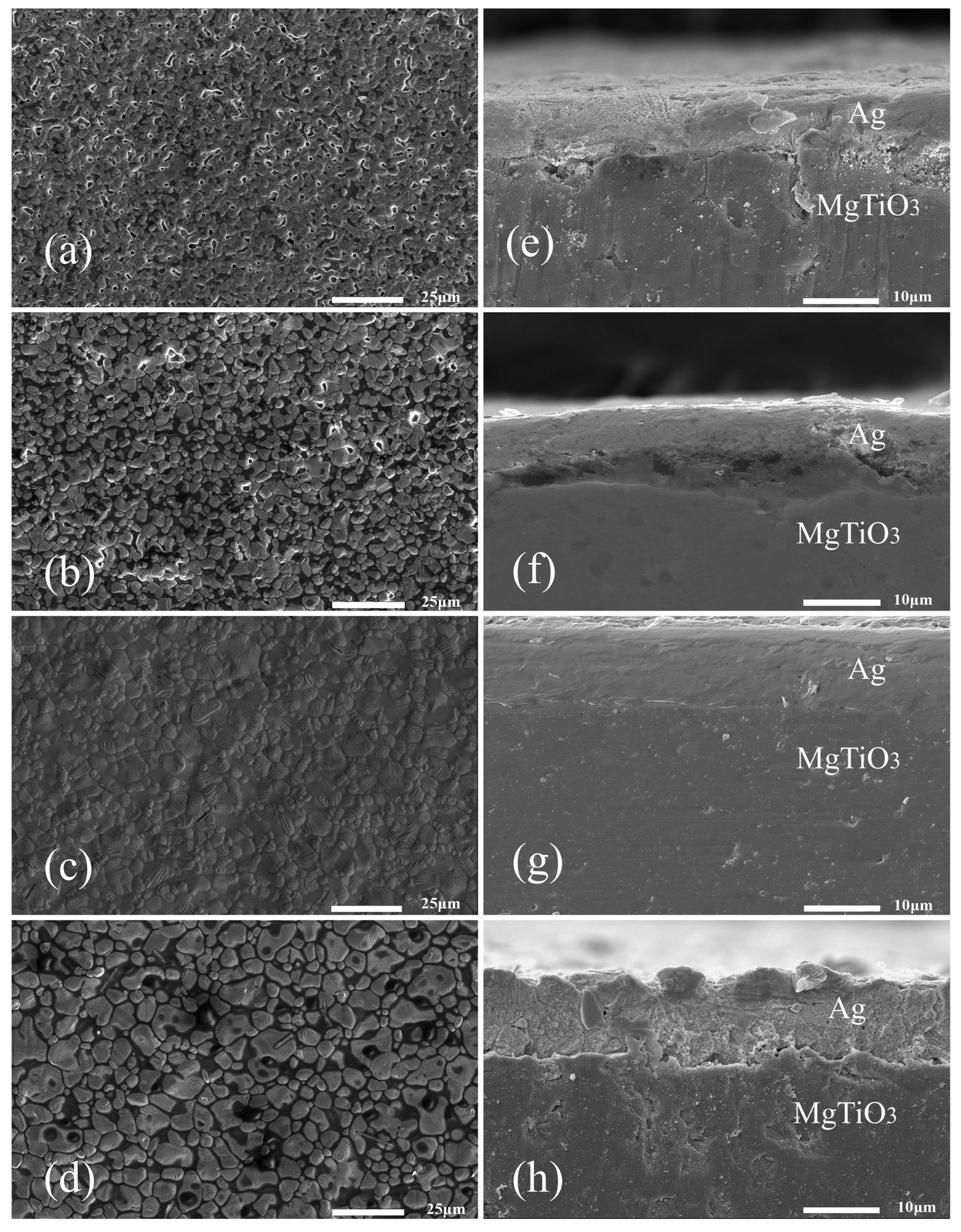
3.3. Effect of Glass Powder Content on the Properties of Silver Film
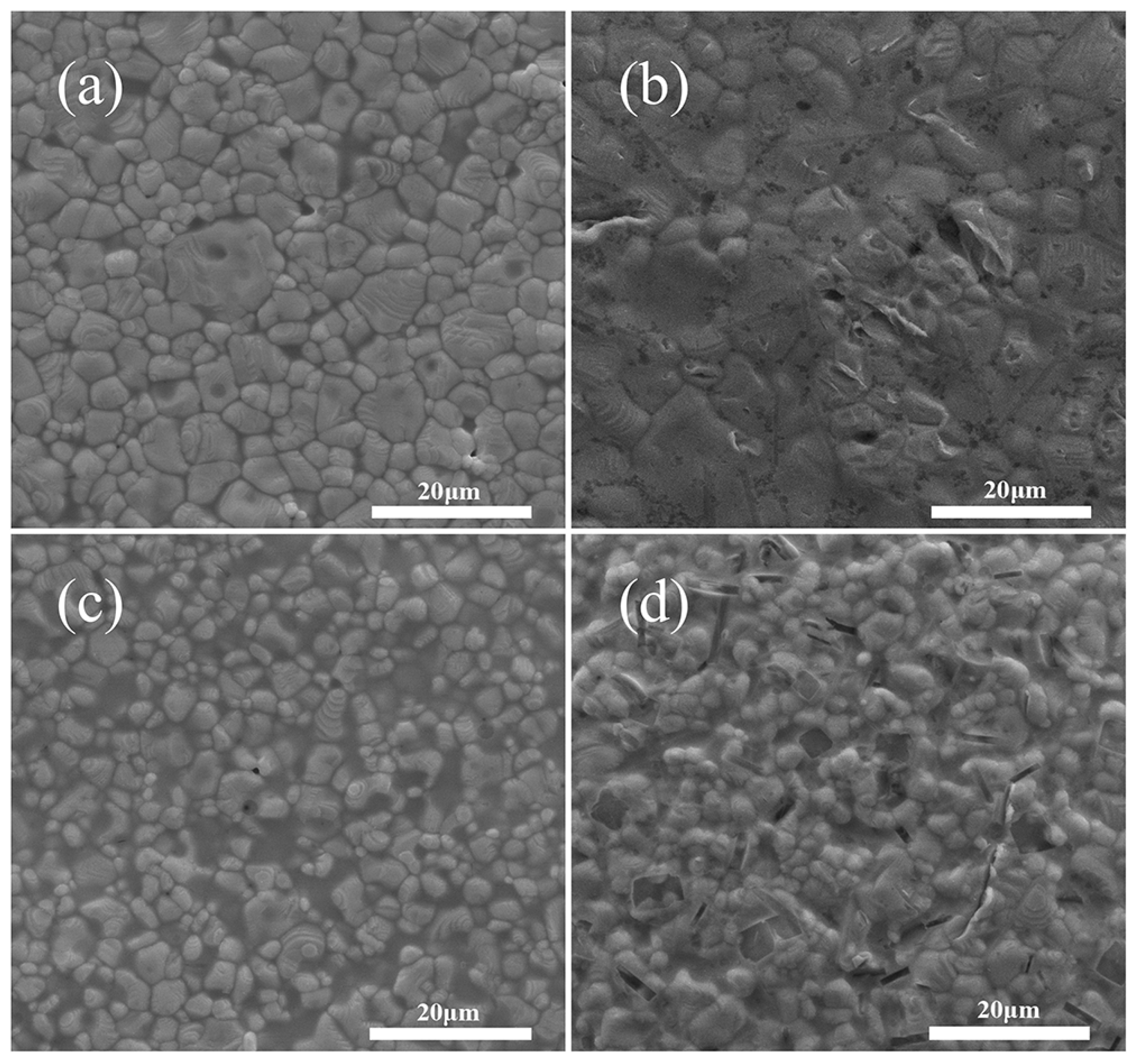
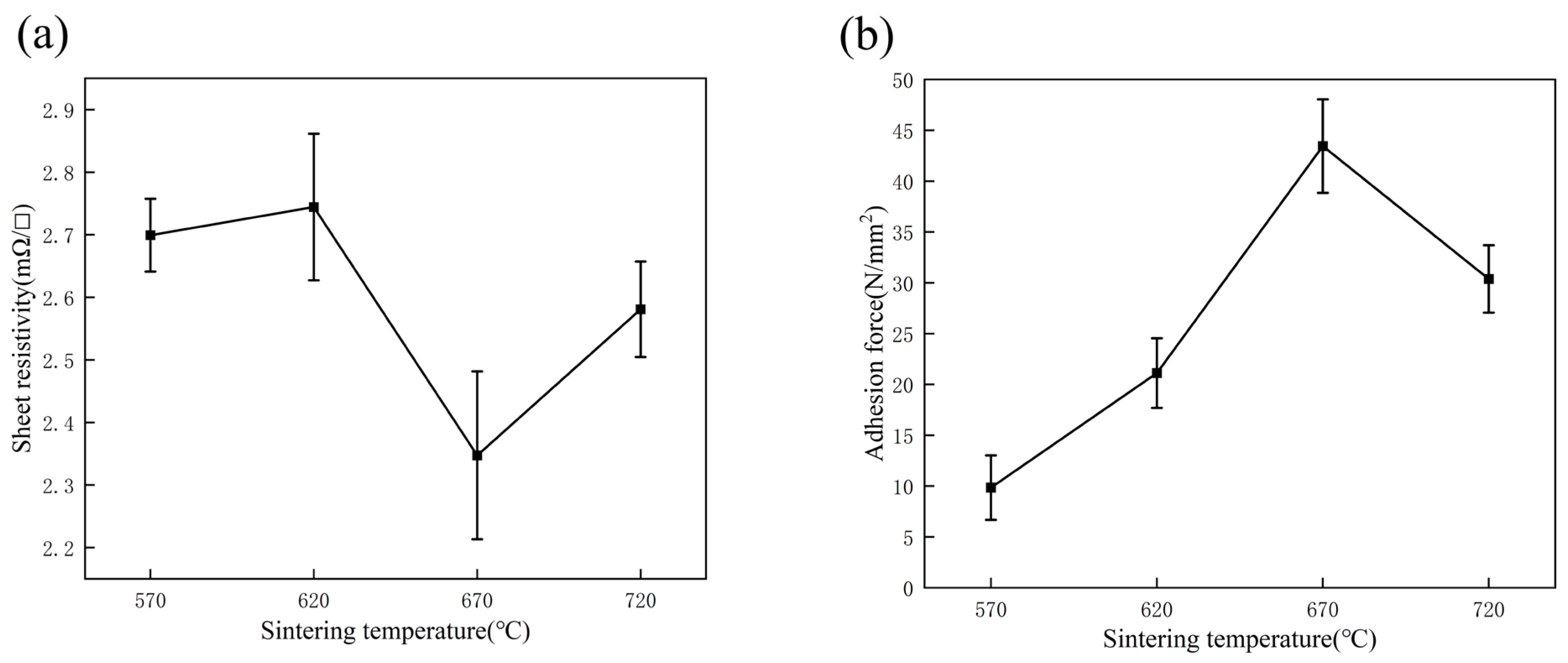
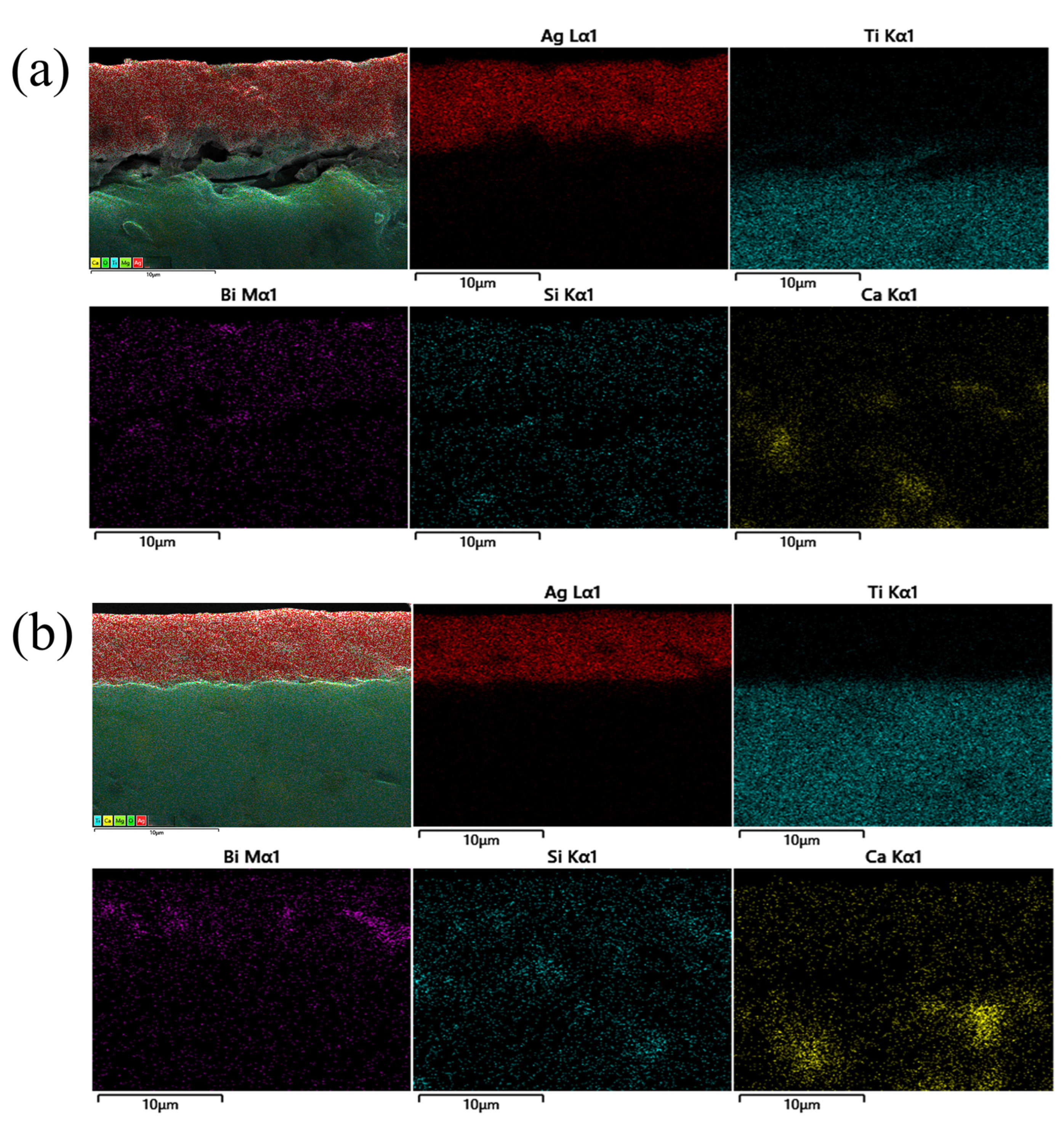
3.4. Effect of Sintering Temperature on the Properties of Silver Film
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, K.; Bahel, S. Effect of Co substitution on the structural, dielectric and reflection properties of MgTiO3 solid solutions. Mater. Res. Bull. 2023, 157, 112037. [Google Scholar] [CrossRef]
- Mase, A.; Kogi, Y.; Kuwahara, D.; Nagayama, Y.; Ito, N.; Maruyama, T.; Ikezi, H.; Wang, X.; Inutake, M.; Tokuzawa, T.; et al. Development and application of radar reflectometer using micro to infrared waves. Adv. Phys. X 2018, 3, 1472529. [Google Scholar] [CrossRef]
- Zhang, A.; Fan, H.; Yang, F.; Wang, W.; Sun, Y. Defects evolution and temperature stability of low-loss magnesium titanate stannate—Calcium strontium titanate microwave dielectric ceramics. J. Alloys Compd. 2022, 925, 166633. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Y. Eco-friendly, cost-effective electroless Ag plating based on a novel Ni–P activation process on magnesium titanate ceramic. Ceram. Int. 2022, 48, 27334–27342. [Google Scholar] [CrossRef]
- Jiang, C.; Lin, P.; Chen, Q.; Wang, C.; Lin, T.; He, P.; Zhao, X.; Xu, J.; Liu, Y.; Liu, H.; et al. Microstructure and properties of functional magnesium titanate ceramic joint brazed by bismuth-borate glass. J. Eur. Ceram. Soc. 2019, 39, 4901–4910. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, P.; Qu, S.-X.; Cheng, L.; Zhang, H. Microwave dielectric properties of Mg2TiO4 ceramics synthesized via high energy ball milling method. J. Alloys Compd. 2015, 623, 238–242. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.-G.; Yu, X.; Fu, D. Mechanical behavior of ceramic-metal joint under quasi-static and dynamic four point bending: Microstructures, damage and mechanisms. Ceram. Int. 2017, 43, 6684–6692. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, H.; Liu, D.; Song, X.; Tan, C.; Cao, J. Direct bonding of silicon nitride to copper via laser surface modification. Appl. Surf. Sci. 2022, 602, 154354. [Google Scholar] [CrossRef]
- Reboun, J.; Hromadka, K.; Hermansky, V.; Johan, J. Properties of power electronic substrates based on thick printed copper technology. Microelectron. Eng. 2017, 167, 58–62. [Google Scholar] [CrossRef]
- Yao, S.; Xing, J.; Zhang, J.; Xiong, S.; Yang, Y.; Yuan, X.; Li, H.; Tong, H. Microscopic investigation on sintering mechanism of electronic silver paste and its effect on electrical conductivity of sintered electrodes. J. Mater. Sci. Mater. Electron. 2018, 29, 18540–18546. [Google Scholar] [CrossRef]
- Wang, C.; Li, G.; Xu, L.; Li, J.; Zhang, D.; Zhao, T.; Sun, R.; Zhu, P. Low Temperature Sintered Silver Nanoflake Paste for Power Device Packaging and Its Anisotropic Sintering Mechanism. ACS Appl. Electron. Mater. 2021, 3, 5365–5373. [Google Scholar] [CrossRef]
- Tsai, J.-T.; Lin, L.-K.; Lin, S.-T.; Stanciu, L.; Jun, M.B.-G. The influence of Bi2O3 glass powder in the silver paste and the impact on silicon solar cell substrates. Mater. Des. 2021, 200, 109453. [Google Scholar] [CrossRef]
- Kim, B.-S.; Lim, E.-S.; Lee, J.-H.; Kim, J.-J. Effect of Bi2O3 content on sintering and crystallization behavior of low-temperature firing Bi2O3–B2O3–SiO2 glasses. J. Eur. Ceram. Soc. 2007, 27, 819–824. [Google Scholar] [CrossRef]
- Shim, H.; Cho, S.; Yie, H.; Kim, H. Crystallization behavior of bismuth oxide nano-glass studied via in situ transmission electron microscopy. Ceram. Int. 2015, 41, 2196–2201. [Google Scholar]
- Haily, E.; Bih, L.; El Bouari, A.; Lahmar, A.; Elmarssi, M.; Manoun, B. Effect of BaO–Bi2O3–P2O5 glass additive on structural, dielectric and energy storage properties of BaTiO3 ceramics. Mater. Chem. Phys. 2020, 241, 122434. [Google Scholar] [CrossRef]
- Guo, W.; Fu, L.; He, P.; Lin, T.; Wang, C. Crystallization and wetting behavior of bismuth–borate–zinc glass and its application in low temperature joining alumina ceramics. J. Manuf. Process. 2019, 39, 128–137. [Google Scholar] [CrossRef]
- Teichmann, C.; Töpfer, J. Sintering and electrical properties of Cu-substituted Zn-Co-Ni-Mn spinel ceramics for NTC thermistors thick films. J. Eur. Ceram. Soc. 2022, 42, 2261–2267. [Google Scholar] [CrossRef]
- Luo, T.; Tong, Y.; Lu, J.; Mou, Y.; Wang, Q.; Liu, J.; Peng, Y.; Chen, M. Enhanced Optical–Thermal Performances of High-Power LED by Plated Copper on Thick Film Ceramic Substrate. IEEE Trans. Electron Devices 2023, 70, 3774–3779. [Google Scholar] [CrossRef]
- Dai, X.; Xu, W.; Zhang, T.; Wang, T. Self-Reducible Cu Nanoparticles for Conductive Inks. Ind. Eng. Chem. Res. 2018, 57, 2508–2516. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Bae, S.; Lee, B.-S.; Jung, S.-B. Bonding of power device to ceramic substrate using Sn-coated Cu micro paste for high-temperature applications. Appl. Surf. Sci. 2020, 515, 146060. [Google Scholar] [CrossRef]
- Guo, H.-H.; Fu, M.-S.; Zhou, D.; Du, C.; Wang, P.-J.; Pang, L.-X.; Liu, W.-F.; Sombra, A.S.B.; Su, J.-Z. Design of a High-Efficiency and -Gain Antenna Using Novel Low-Loss, Temperature-Stable Li2Ti1–x(Cu1/3Nb2/3)xO3 Microwave Dielectric Ceramics. ACS Appl. Mater. Interfaces 2021, 13, 912–923. [Google Scholar] [PubMed]
- Liu, C.; Cai, C.; Xie, J.; Guo, W.; Qin, H.; Gao, P.; Xiao, H. Effect of surface brittle-to-ductile transition on high-temperature thermal shock resistance of Al2O3 ceramics. Ceram. Int. 2022, 48, 20627–20638. [Google Scholar]
- Gao, Y.; Ma, J.-J.; Chen, Y.; Wang, M.-H. Effect of Bi2O3 on the structure and thermal properties of Bi2O3-SiO2-B2O3 glasses prepared by sol-gel method. J. Sol-Gel Sci. Technol. 2022, 103, 713–721. [Google Scholar] [CrossRef]
- Liu, J.; Xu, X.; Zheng, T.; Guo, Y.; Lv, J. Effect of Bi2O3 content on the structure and properties of Bi2O3-B2O3-BaO-ZnO glass. J. Non-Cryst. Solids 2022, 575, 121211. [Google Scholar]
- Chen, L.-T.; Lai, Y.-S. Thermal, structural, and metallization properties of 0.95[50B2O3-30BaO-(20−x)ZnO-xBi2O3]+0.05V2O5 glasses. J. Non-Cryst. Solids 2021, 556, 120552. [Google Scholar]
- Yang, Y.; Wu, L.; Teng, Y.; Jiang, F.; Lei, J.; Chen, H.; Cheng, J. Effect of ZnO content on microstructure, crystallization behavior, and thermal properties of xZnO-30B2O3-(65−x)Bi2O3-5BaO glass. J. Non-Cryst. Solids 2019, 511, 29–35. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Miao, W.; Lei, Q.; Li, M. Dependence of glass transition on the structure in Bi B Zn oxide glass. J. Alloys Compd. 2018, 742, 151–158. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Jiang, Q.; Li, A.; Yu, J.; Zeng, H. Effect of properties and crystallization behavior of BaO–ZnO–B2O3–SiO2 glass on the electrical conductivity of Cu paste. J. Am. Ceram. Soc. 2022, 105, 5687–5697. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Lai, S.-S.; Li, Y.-J.; Lin, H.-J.; Chiang, T.-H. Investigation of SiO2-B2O3-ZnO-Bi2O3 glass frits on the interface reaction of silver front contacts. J. Alloys Compd. 2021, 858, 157646. [Google Scholar] [CrossRef]
- Zhu, X.; Mai, C.; Li, M. Effects of B2O3 content variation on the Bi ions in Bi2O3–B2O3–SiO2 glass structure. J. Non-Cryst. Solids 2014, 388, 55–61. [Google Scholar]
- Chen, C.; Suganuma, K. Microstructure and mechanical properties of sintered Ag particles with flake and spherical shape from nano to micro size. Mater. Des. 2019, 162, 311–321. [Google Scholar] [CrossRef]
- Chiodi, M.; Cancellieri, C.; Moszner, F.; Andrzejczuk, M.; Janczak-Rusch, J.; Jeurgens, L.P.H. Massive Ag migration through metal/ceramic nano-multilayers: An interplay between temperature, stress-relaxation and oxygen-enhanced mass transport. J. Mater. Chem. C 2016, 4, 4927–4938. [Google Scholar] [CrossRef]
- Wang, W.; Zou, G.; Jia, Q.; Zhang, H.; Feng, B.; Deng, Z.; Liu, L. Mechanical properties and microstructure of low temperature sintered joints using organic-free silver nanostructured film for die attachment of SiC power electronics. Mater. Sci. Eng. A 2020, 793, 139894. [Google Scholar]
- Huang, B.R.; Yeh, C.Y.; Hsu, Y.H.; Tsai, C.P.; Lee, Y.F.; Wang, J.Y.; Liu, C.Y. Cu Sintering Process Modified by Adding a Low Temperature Liquid Sintering Step. JOM 2022, 74, 1730–1738. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Yuan, X.; Zhao, H.; Yang, Y.; Li, H.; Tong, H. Relation of silver electrode solderability to intermetallic compound growth rate. J. Mater. Sci. Mater. Electron. 2019, 30, 7209–7215. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, Y.; Yi, F.; Chen, C.; Song, X. Surface metallization of alumina ceramics: Effects of sintering time and substrate etching. Ceram. Int. 2014, 40, 12709–12715. [Google Scholar]
- Zou, Y.; Fu, R.; Liu, X.; Liu, H.; Wang, H. Enhanced adhesion strength of silver paste on AlN ceramic substrate via sintered nano-CuO. Ceram. Int. 2021, 47, 9471–9476. [Google Scholar]
- Yang, W.; Sun, Q.; Lei, Q.; Zhu, W.; Li, Y.; Wei, J.; Li, M. Formation of a highly conductive thick film by low-temperature sintering of silver paste containing a Bi2O3-B2O3-ZnO glass frit. J. Mater. Process. Technol. 2019, 267, 61–67. [Google Scholar]
- Li, Y.; Jing, H.; Han, Y.; Xu, L.; Lu, G. Microstructure and joint properties of nano-silver paste by ultrasonic-assisted pressureless sintering. J. Electron. Mater. 2016, 45, 3003–3012. [Google Scholar]
- Chen, C.; Choe, C.; Kim, D.; Zhang, Z.; Long, X.; Zhou, Z.; Wu, F.; Suganuma, K. Effect of oxygen on microstructural coarsening behaviors and mechanical properties of ag sinter paste during high-temperature storage from macro to micro. J. Alloys Compd. 2020, 834, 155173. [Google Scholar]

| Sample | Bi2O3 | B2O3 | SiO2 | ZnO | Al2O3 | CaO |
|---|---|---|---|---|---|---|
| A1 | 30 | 40 | 15 | 9 | 3 | 3 |
| A2 | 40 | 30 | 15 | 9 | 3 | 3 |
| A3 | 50 | 20 | 15 | 9 | 3 | 3 |
| A4 | 60 | 10 | 15 | 9 | 3 | 3 |
| A5 | 70 | 0 | 15 | 9 | 3 | 3 |
| Component | Ethyl Cellulose | Terpineol | Dibutyl Phthalate | Hydrogenated Castor Oil | Siban 85 |
|---|---|---|---|---|---|
| Content | 12 | 56 | 30 | 0.5 | 1.5 |
| Sample | Ag | Glass Frit | Organic Vehicle |
|---|---|---|---|
| #1 | 82 | 1 | 17 |
| #2 | 80 | 3 | 17 |
| #3 | 78 | 5 | 17 |
| #4 | 76 | 7 | 17 |
| #5 | 74 | 9 | 17 |
| #6 | 72 | 11 | 17 |
| Sample | Tg (°C) | Tf (°C) | Tc (°C) | ΔT = Tc − Tg (°C) |
|---|---|---|---|---|
| A1 | 477 | 587 | 614 | 137 |
| A2 | 452 | 583 | 627 | 175 |
| A3 | 408 | 565 | 603 | 195 |
| A4 | 465 | 547 | 609 | 144 |
| A5 | 421 | 544 | 615 | 194 |
| Band Position (cm−1) | Assignment |
|---|---|
| 430–520 | Bi-O-Bi in the [BiO6] octahedral unit [23] |
| 690–730 | Bending vibrations of oxygen bridges between two [BO3] units (BIII-O-BIII) [25] |
| 830–900 | Symmetric stretching vibrations of Bi-O bonds in [BiO3] pyramidal units [30] |
| 990–1080 | Stretching vibrations of B-O bonds in [BO4] units from tri-, tetra-, and penta-borate groups [25,26] |
| 1130 | B-O-B stretching vibration in [BO4] [27] |
| 1300–1400 | Asymmetric stretching of B-O bond in [BO3] units [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Yu, X.; Liu, L.; Tang, X.; Li, J.; Gan, G. Study on Low-Temperature Conductive Silver Pastes Containing Bi-Based Glass for MgTiO3 Electronic Power Devices. Micromachines 2023, 14, 1663. https://doi.org/10.3390/mi14091663
Fu Y, Yu X, Liu L, Tang X, Li J, Gan G. Study on Low-Temperature Conductive Silver Pastes Containing Bi-Based Glass for MgTiO3 Electronic Power Devices. Micromachines. 2023; 14(9):1663. https://doi.org/10.3390/mi14091663
Chicago/Turabian StyleFu, Yunsheng, Xianglei Yu, Li Liu, Xianjie Tang, Junpeng Li, and Guoyou Gan. 2023. "Study on Low-Temperature Conductive Silver Pastes Containing Bi-Based Glass for MgTiO3 Electronic Power Devices" Micromachines 14, no. 9: 1663. https://doi.org/10.3390/mi14091663
APA StyleFu, Y., Yu, X., Liu, L., Tang, X., Li, J., & Gan, G. (2023). Study on Low-Temperature Conductive Silver Pastes Containing Bi-Based Glass for MgTiO3 Electronic Power Devices. Micromachines, 14(9), 1663. https://doi.org/10.3390/mi14091663





