Investigation of Performance Parameters of Spherical Gold Nanoparticles in Localized Surface Plasmon Resonance Biosensing
Abstract
:1. Introduction
2. Materials and Methods
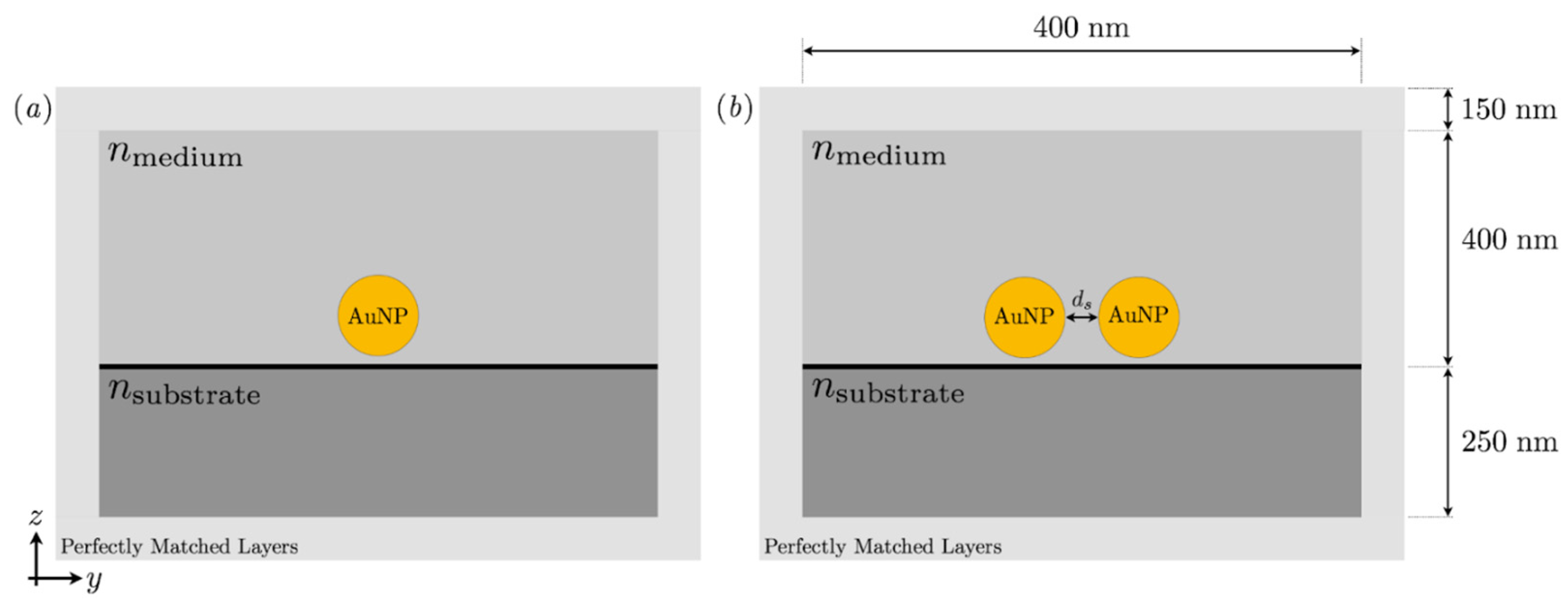
3. Numerical Modelling Analysis
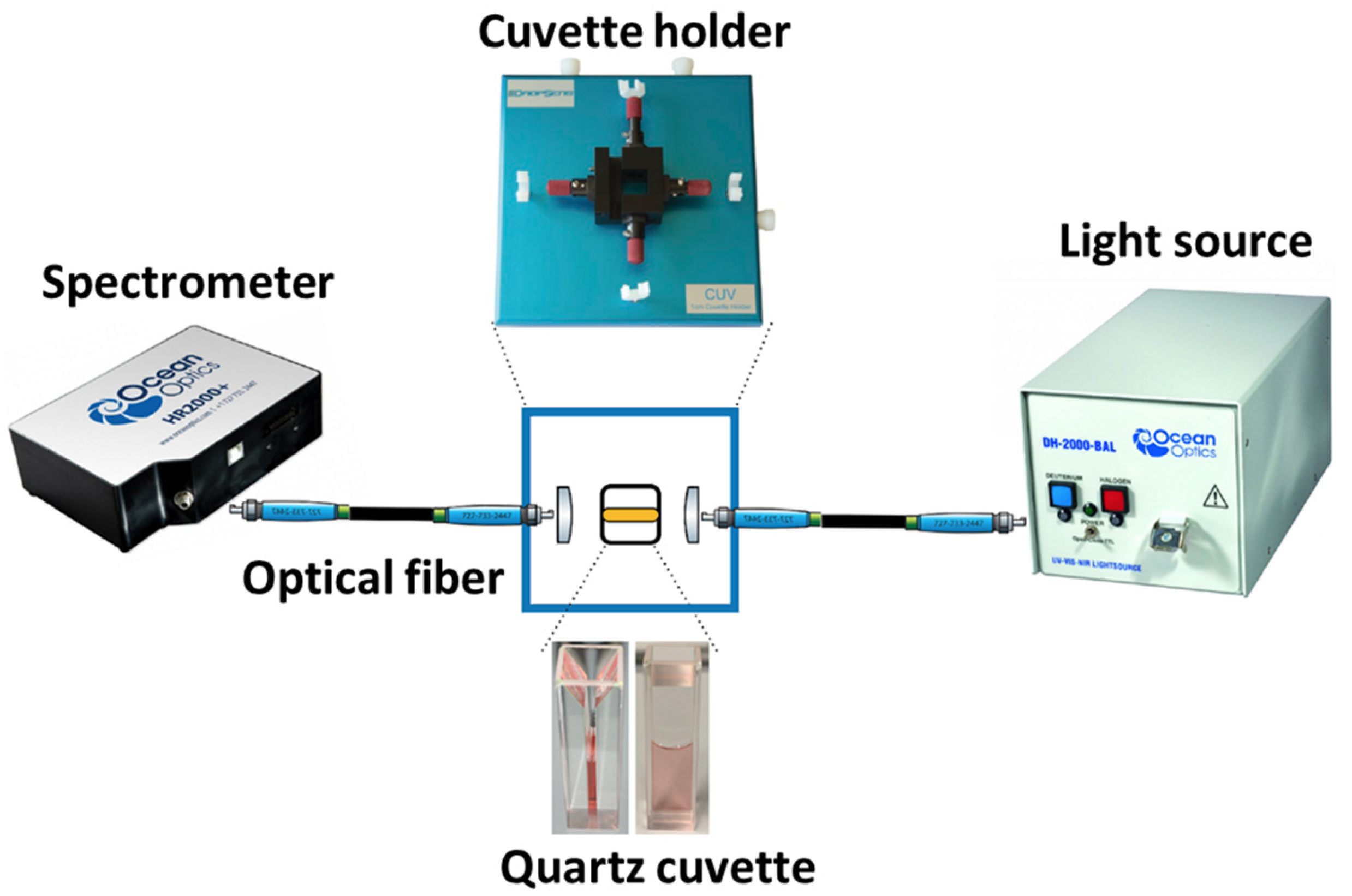
4. Experimental Setup
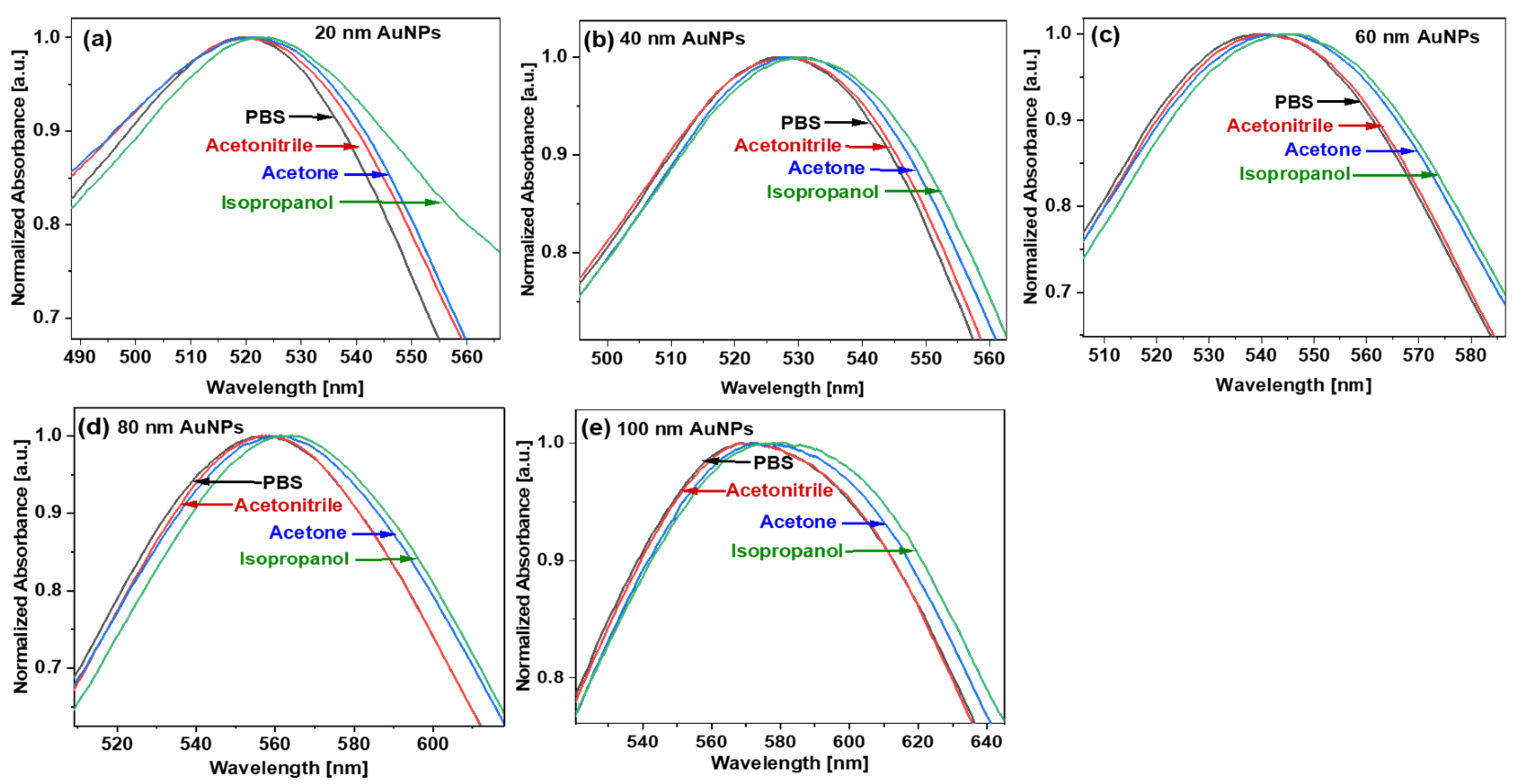
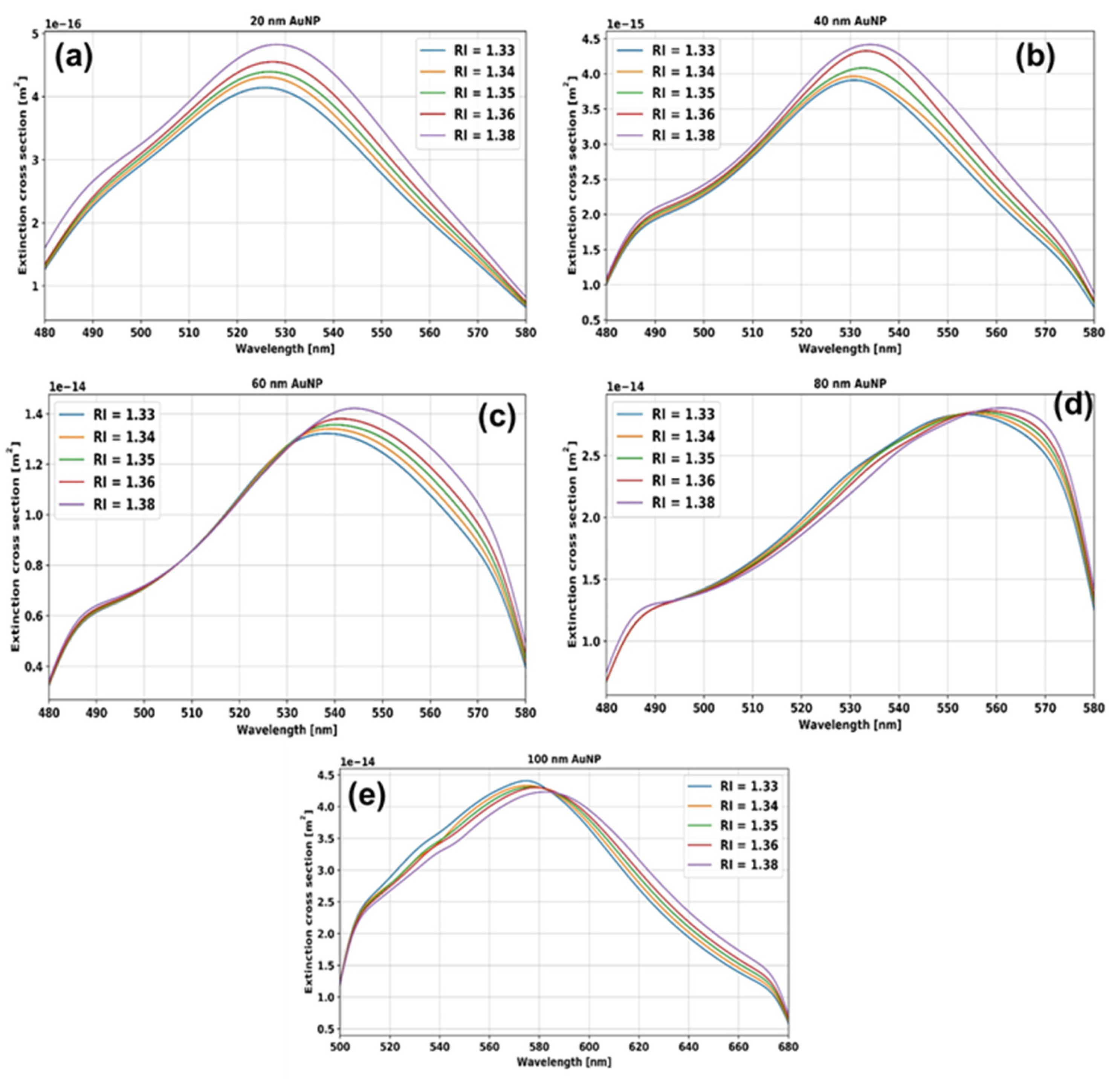
5. Results and Discussion
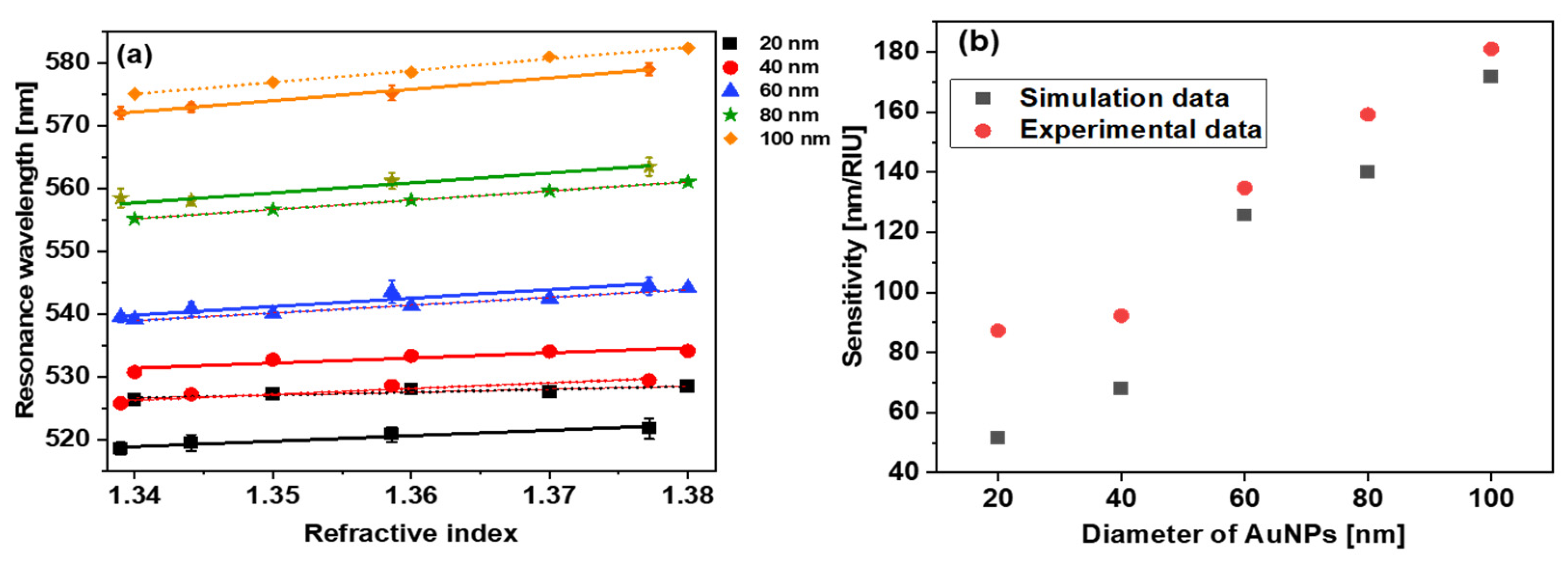
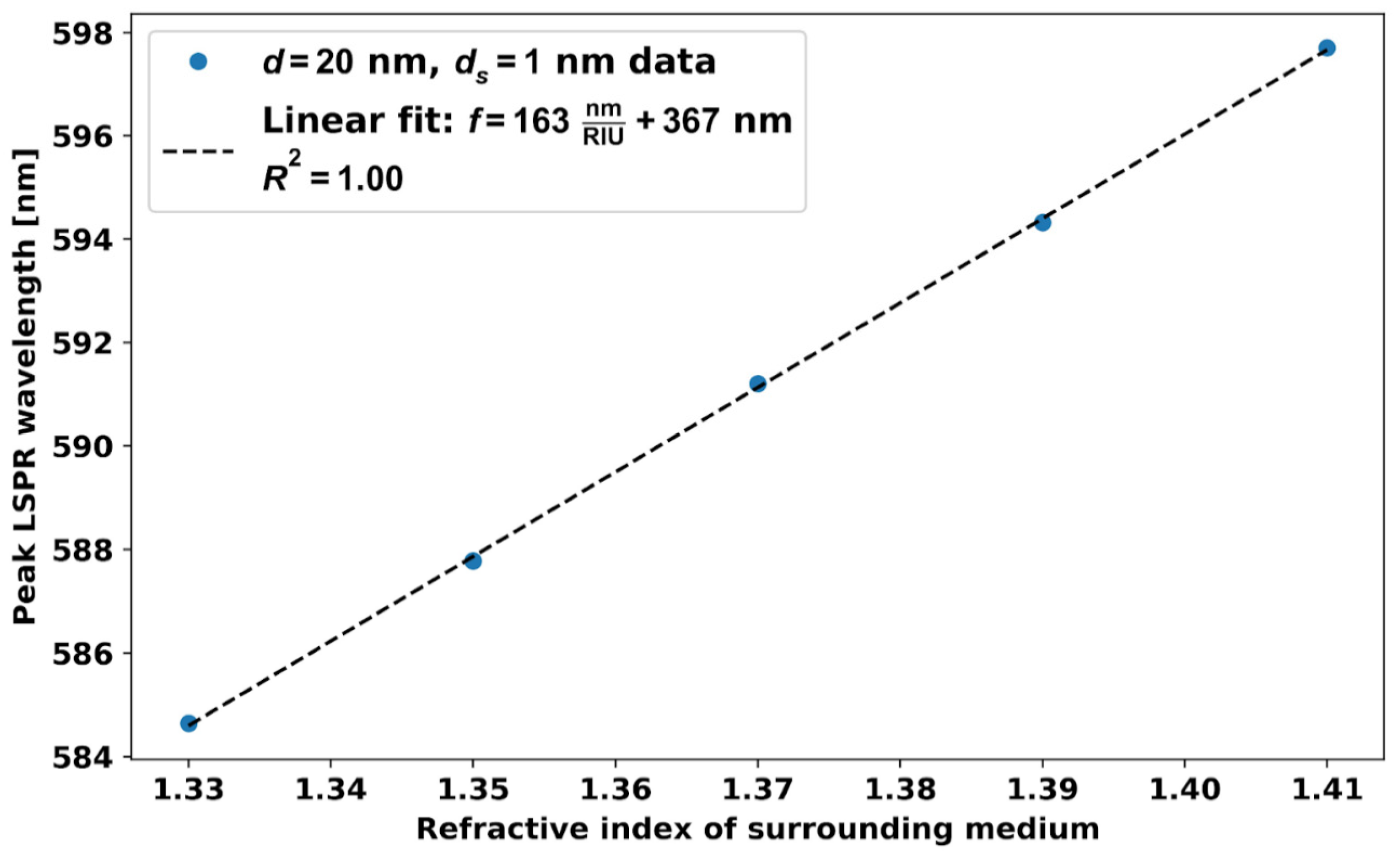
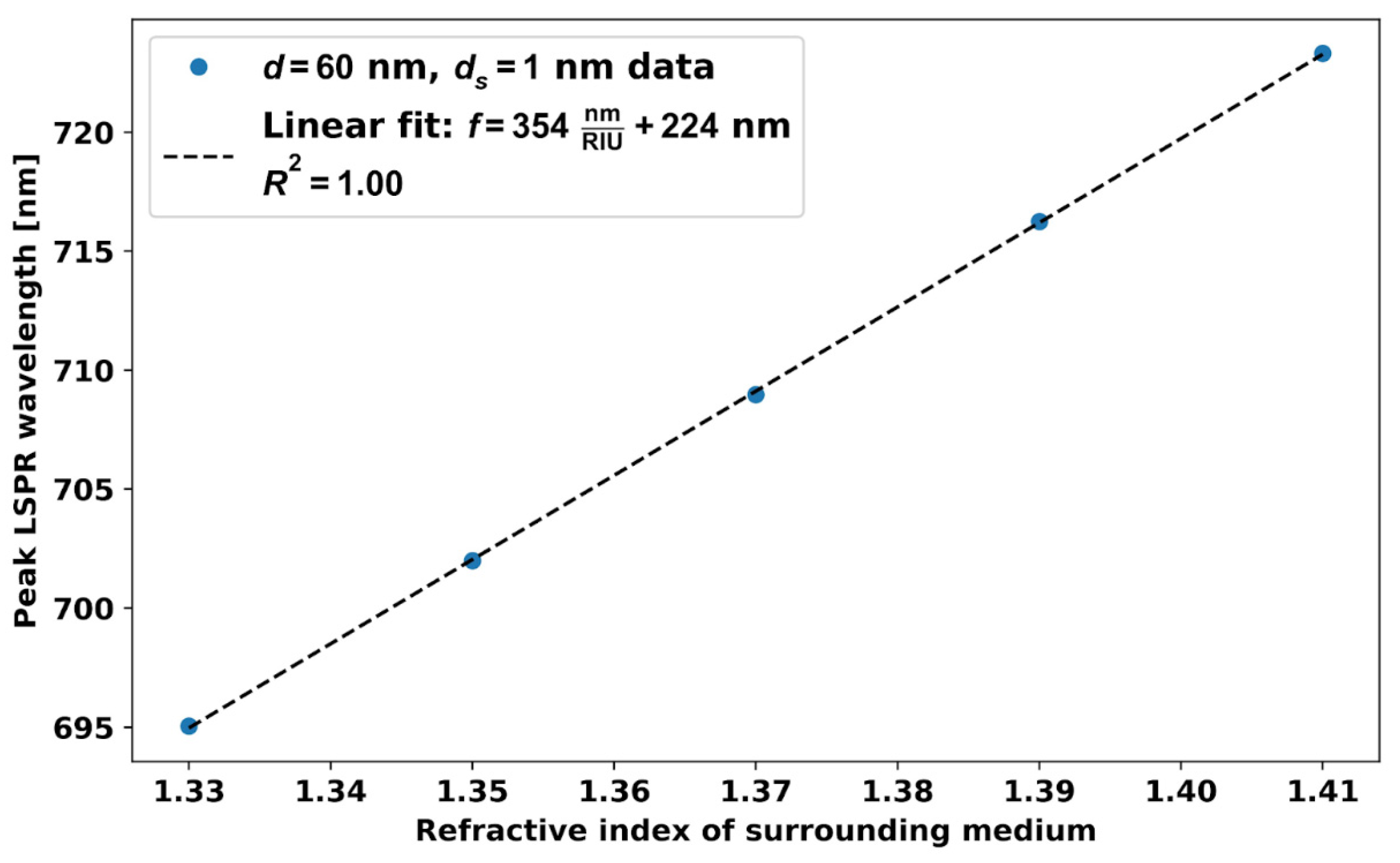
5.1. RI Sensitivity, Figures of Merit of Different Size AuNPs
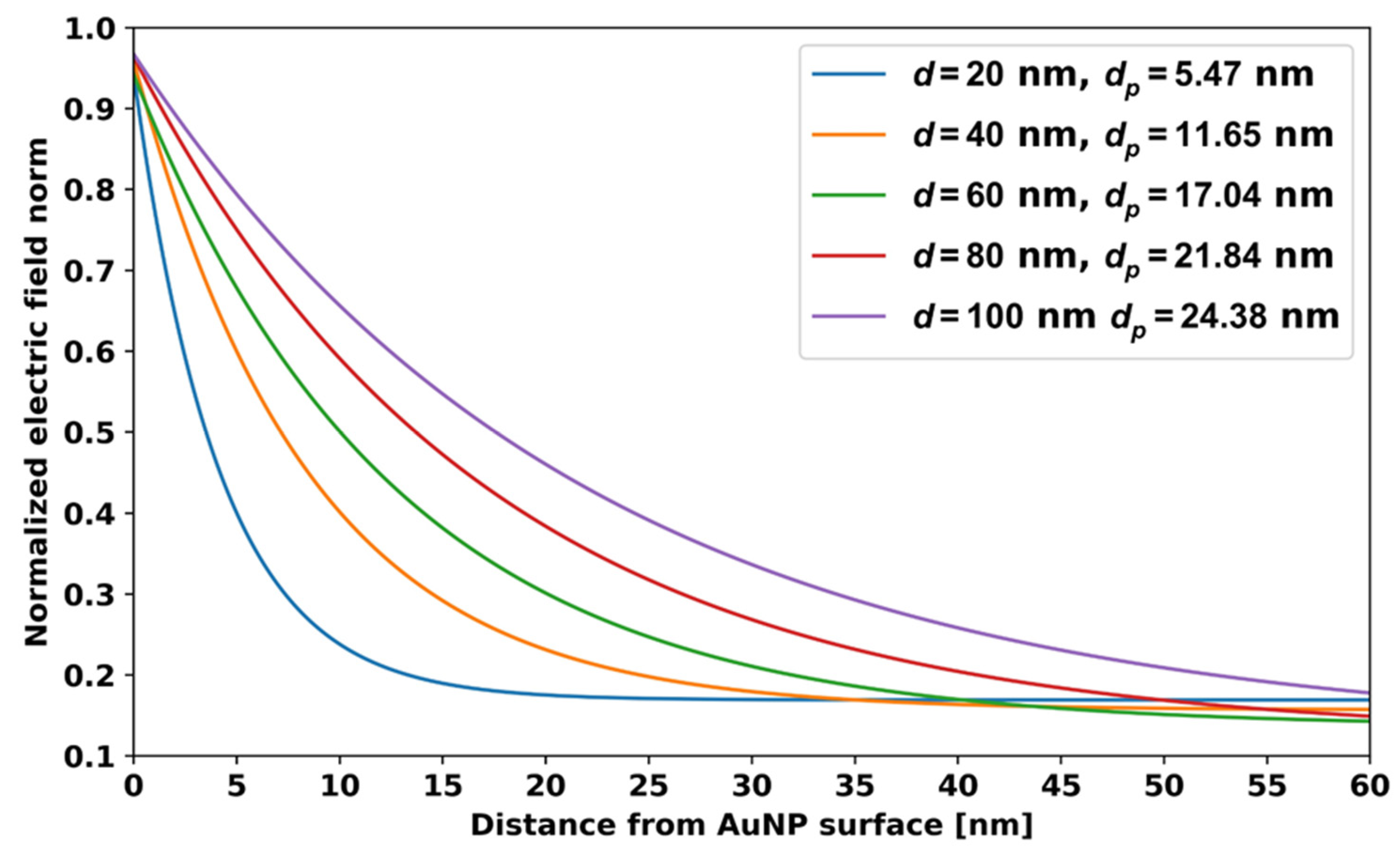
5.2. Localized Plasmon Penetration Depth for Spherical AuNPs
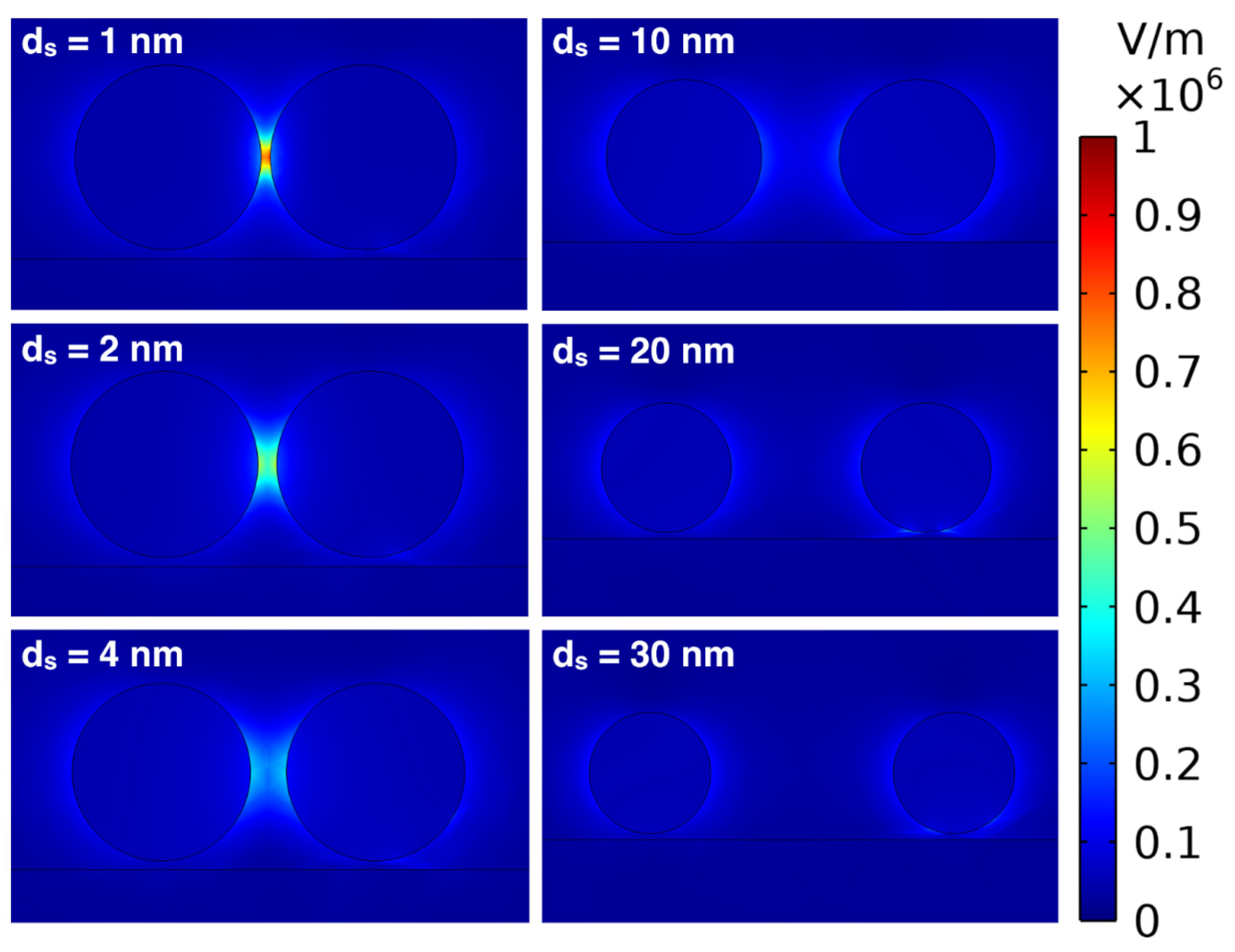
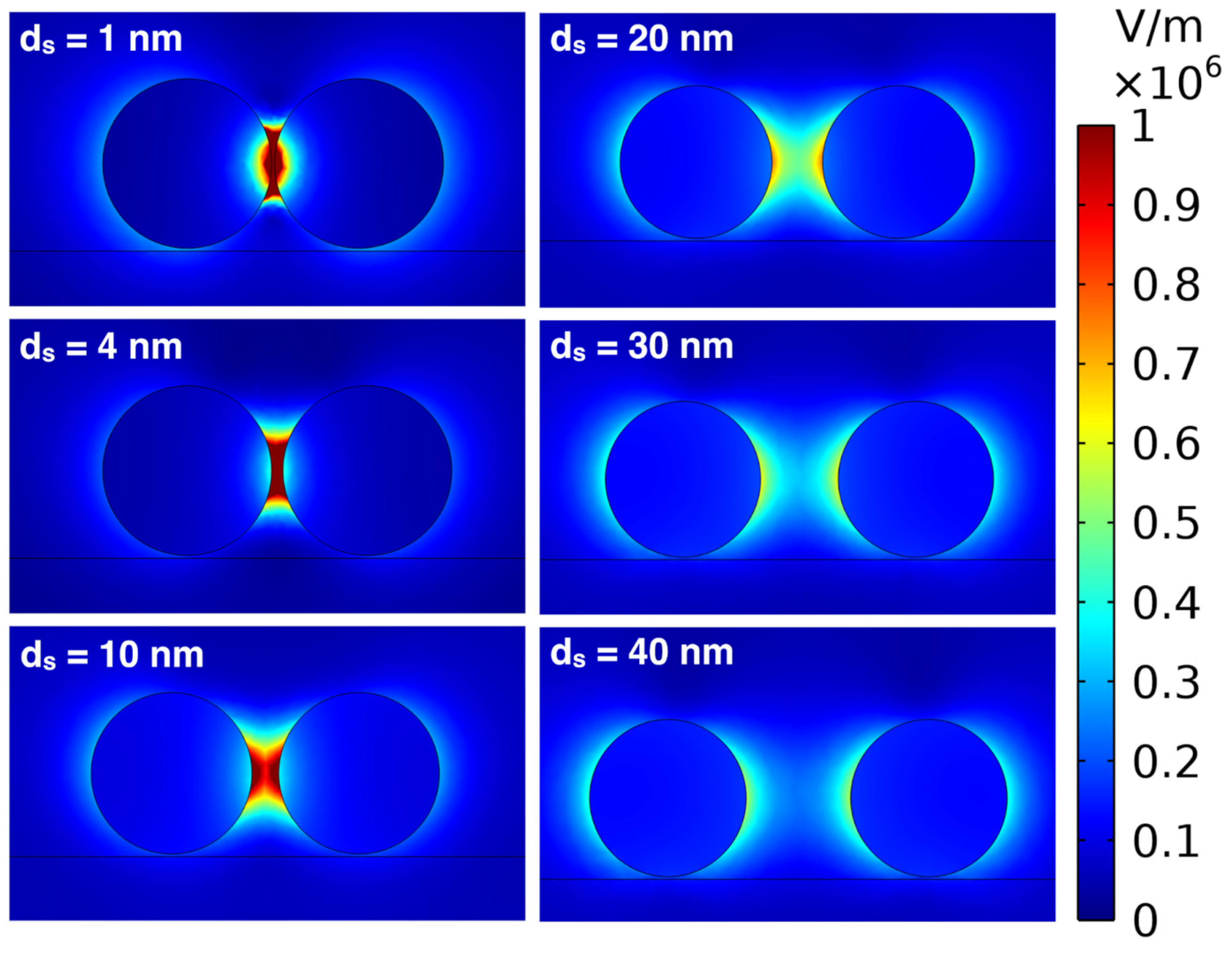
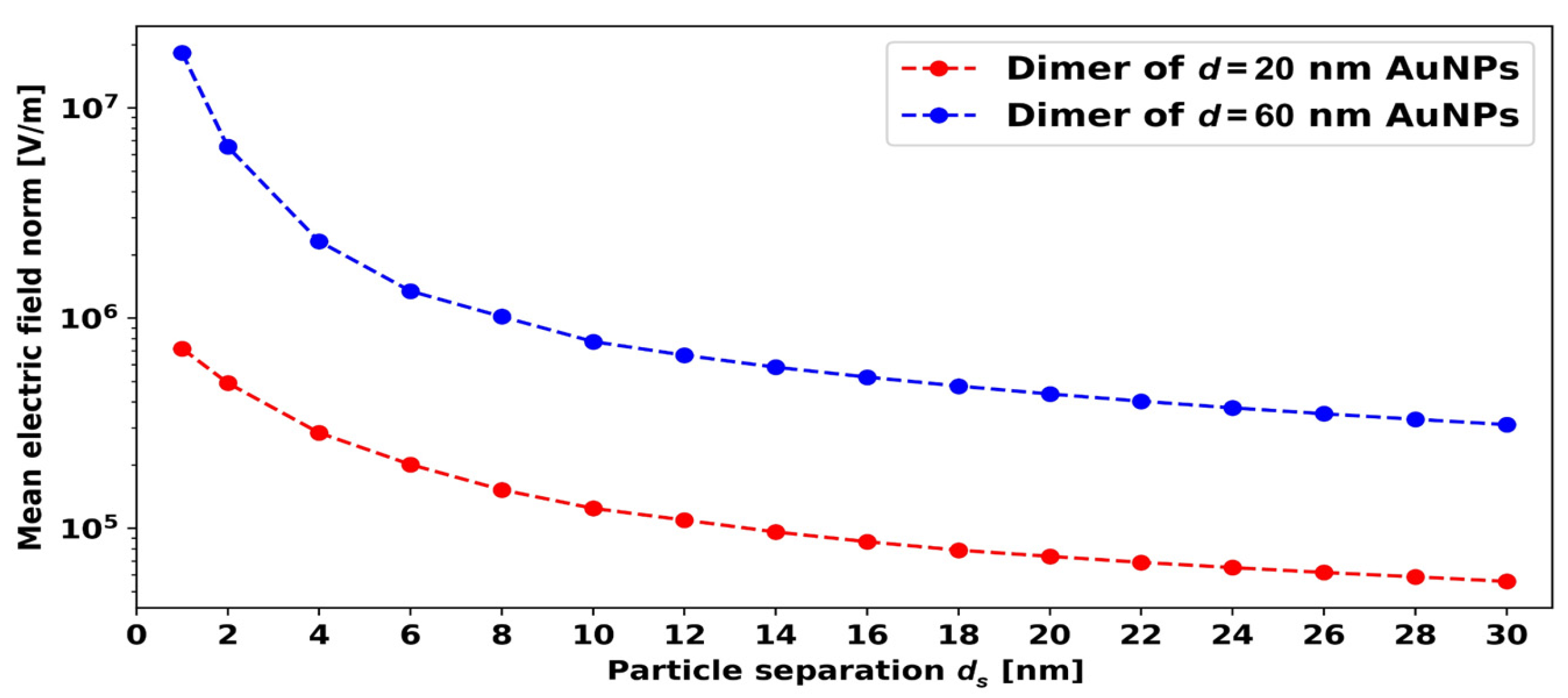
5.3. Effect of Interparticle Distance
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anker, J.N.; Hall, W.P.; Lyandres, O.L.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with Plasmonic Nanosensors. Nanosci. Technol. A Collect. Rev. Nat. J. 2009, 7, 308–319. [Google Scholar]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Semwal, V.; Gupta, B.D. LSPR and SPR-Based Fiber-Optic Cholesterol Sensor Using Immobilization of Cholesterol Oxidase Over Silver Nanoparticles Coated Graphene Oxide Nanosheets. IEEE Sens. J. 2018, 18, 1039–1046. [Google Scholar] [CrossRef]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-based Nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Ali, M.H.; Elsherbiny, M.E.; Emara, M. Updates on Aptamer Research. Int. J. Mol. Sci. 2019, 20, 2511. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based Biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Guo, L.; Xu, Y.; Ferhan, A.R.; Chen, G.; Kim, D.H. Oriented Gold Nanoparticle Aggregation for Colorimetric Sensors with Surprisingly High Analytical Figures of Merit. J. Am. Chem. Soc. 2013, 135, 12338–12345. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold Nanoparticle-Based Colorimetric Biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, J.; Prodan, E.; Nordlander, P. Quantum Description of the Plasmon Resonances of a Nanoparticle Dimer. Nano Lett. 2009, 9, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Tuersun, P.; Yusufu, T.; Yimiti, A.; Sidike, A. Refractive Index Sensitivity Analysis of Gold Nanoparticles. Optik 2017, 149, 384–390. [Google Scholar] [CrossRef]
- Martinsson, E.; Sepulveda, B.; Chen, P.; Elfwing, A.; Liedberg, B.; Aili, D. Optimizing the Refractive Index Sensitivity of Plasmonically Coupled Gold Nanoparticles. Plasmonics 2014, 9, 773–780. [Google Scholar] [CrossRef]
- Wang, H. Plasmonic Refractive Index Sensing Using Strongly Coupled Metal Nanoantennas: Nonlocal Limitations. Sci. Rep. 2018, 8, 9589. [Google Scholar] [CrossRef] [PubMed]
- COMSOL Multiphysics. COMSOL Multiphysics Programming Reference Manual 5.6. 2020. Available online: https://doc.comsol.com/5.6/doc/com.comsol.help.comsol/COMSOL_ProgrammingReferenceManual.pdf (accessed on 1 November 2022).
- Sunkara, V.; Cho, Y.K. Aminosilane Layers on the Plasma Activated Thermoplastics: Influence of Solvent on its Structure and Morphology. J. Colloid Interface Sci. 2013, 411, 122–128. [Google Scholar] [CrossRef]
- Markov, A.; Wolf, N.; Yuan, X.; Mayer, D.; Maybeck, V.; Offenhäusser, A.; Wördenweber, R. Controlled Engineering of Oxide Surfaces for Bioelectronics Applications using Organic Mixed Monolayers. ACS Appl. Mater. Interfaces 2017, 9, 29265–29272. [Google Scholar] [CrossRef]
- Attaran, A.; Emami, S.D.; Soltanian, M.R.K.; Penny, R.; Behbahani, F.; Harun, S.W.; Ahmad, H.; Abdul-Rashid, H.A.; Moghavvemi, M. Circuit Model of Fano Resonance on Tetramers, Pentamers, and Broken Symmetry Pentamers. Plasmonics 2014, 9, 1303–1313. [Google Scholar] [CrossRef]
- Mandelkern, M.; Elias, J.G.; Eden, D.; Crothers, D.M. The Dimensions of DNA in Solution. J. Mol. Biol. 1981, 152, 153–161. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Fang, X.; Li, Z.; Pu, H.; Chang, J.; Chen, J.; Mao, S. Ultratrace Antibiotic Sensing Using Aptamer/Graphene-Based Field-Effect Transistors. Biosens. Bioelectron. 2018, 126, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Thacker, V.; Herrmann, L.; Sigle, D.; Zhang, T.; Liedl, T.; Baumberg, J.J.; Keyser, U.F. DNA Origami based Assembly of Gold Nanoparticle Dimers for Surface-Enhanced Raman Scattering. Nat. Commun. 2014, 5, 3448. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Ma, Z.; Park, S.; Kawai, K.; Hirai, Y.; Tsuchiya, T.; Tabata, O. Formation of Gold Nanoparticle Dimers on Silicon by Sacrificial DNA Origami Technique. Micro. Nano Lett. 2017, 12, 854–859. [Google Scholar] [CrossRef]
- Sung, K.M.; Mosley, D.W.; Peelle, B.R.; Zhang, S.; Jacobson, J.M. Synthesis of Monofunctionalized Gold Nanoparticles by Fmoc Solid-Phase Reactions. J. Am. Chem. Soc. 2004, 126, 5064–5065. [Google Scholar] [CrossRef] [PubMed]
- Sardar, R.; Heap, T.B.; Shumaker-Parry, J.S. Versatile Solid Phase Synthesis of Gold Nanoparticle Dimers Using an Asymmetric Functionalization Approach. J. Am. Chem. Soc. 2007, 129, 5356–5357. [Google Scholar] [CrossRef] [PubMed]
- Navas, M.P.; Soni, R.K. Laser-Generated Bimetallic Ag-Au and Ag-Cu Core-Shell Nanoparticles for Refractive Index Sensing. Plasmonics 2015, 10, 681–690. [Google Scholar] [CrossRef]
- Sugawa, K.; Tahara, H.; Yamashita, A.; Otsuki, J.; Sagara, T.; Harumoto, T.; Yanagida, S. Refractive Index Susceptibility of the Plasmonic Palladium Nanoparticle: Potential as the Third Plasmonic Sensing Material. ACS Nano 2015, 9, 1895–1904. [Google Scholar] [CrossRef]
- Lelièvre, H.Y.; Lussier, F.; Masson, J.F. Influence of the Particle Shape and Density of Self-Assembled Gold Nanoparticle Sensors on LSPR and SERS. J. Phys. Chem. C 2015, 119, 28577–28585. [Google Scholar] [CrossRef]



| Diameter of AuNPs [nm] | Sensitivity [nm/RIU] | Figure of Merit (FoM) [RIU−1] | Penetration Depth [nm] | Ref. |
|---|---|---|---|---|
| 15 | 44 | 0.6 | Not mentioned | [13] |
| 25 | 60 | Not mentioned | Not mentioned | [15] |
| 50 | 80 | |||
| 100 | 180 | |||
| 10–50 | 128–233 | 1.5–3.5 | Not mentioned | [27] |
| 60 | 126 | Not mentioned | Not mentioned | [28] |
| 60 | 97 | 1.62 | 18 | [29] |
| 20 | 51 | 1.4 | 5.47 | Present work |
| 40 | 68 | 1.8 | 11.65 | |
| 60 | 125 | 2.9 | 17.04 | |
| 80 | 140 | 2.6 | 21.84 | |
| 100 | 170 | 2.5 | 24.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semwal, V.; Jensen, O.R.; Bang, O.; Janting, J. Investigation of Performance Parameters of Spherical Gold Nanoparticles in Localized Surface Plasmon Resonance Biosensing. Micromachines 2023, 14, 1717. https://doi.org/10.3390/mi14091717
Semwal V, Jensen OR, Bang O, Janting J. Investigation of Performance Parameters of Spherical Gold Nanoparticles in Localized Surface Plasmon Resonance Biosensing. Micromachines. 2023; 14(9):1717. https://doi.org/10.3390/mi14091717
Chicago/Turabian StyleSemwal, Vivek, Oliver Rishøj Jensen, Ole Bang, and Jakob Janting. 2023. "Investigation of Performance Parameters of Spherical Gold Nanoparticles in Localized Surface Plasmon Resonance Biosensing" Micromachines 14, no. 9: 1717. https://doi.org/10.3390/mi14091717
APA StyleSemwal, V., Jensen, O. R., Bang, O., & Janting, J. (2023). Investigation of Performance Parameters of Spherical Gold Nanoparticles in Localized Surface Plasmon Resonance Biosensing. Micromachines, 14(9), 1717. https://doi.org/10.3390/mi14091717








