Marcus Theory and Tunneling Method for the Electron Transfer Rate Analysis in Quantum Dot Sensitized Solar Cells in the Presence of Blocking Layer
Abstract
:1. Introduction
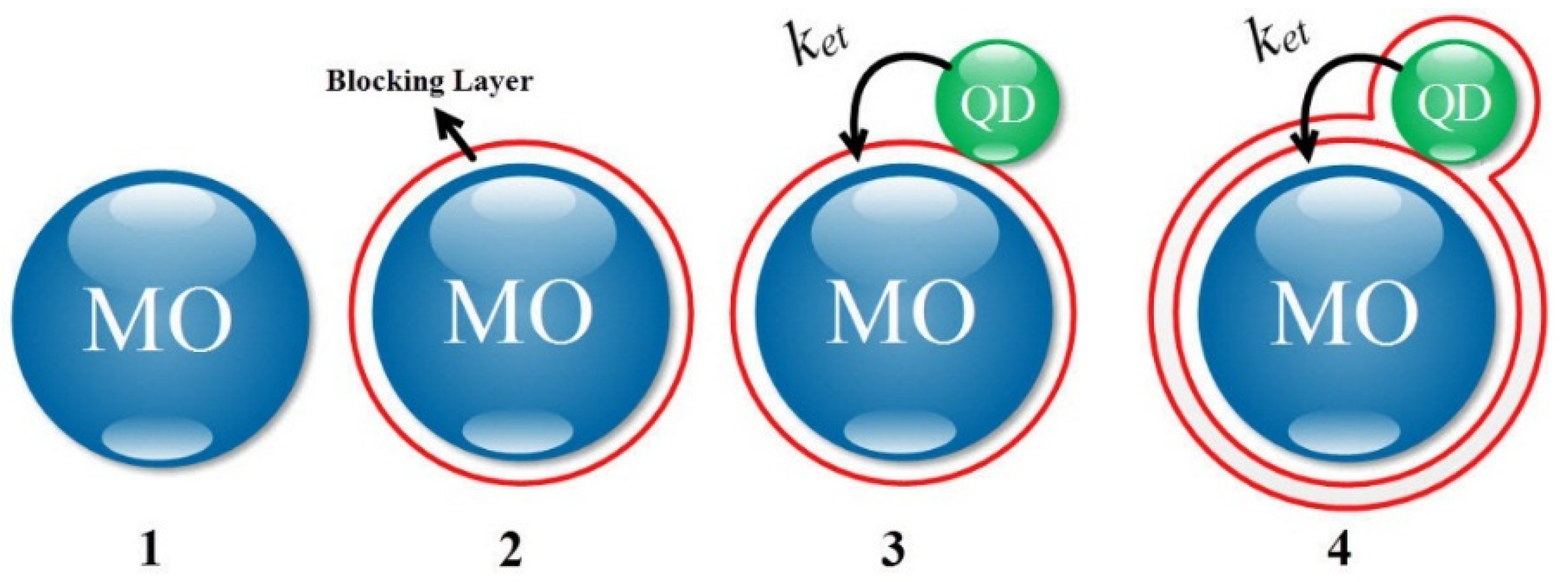
2. The Structure, Theory, and Modeling
2.1. The Marcus Model
2.2. Tunneling between Two Spheres
3. Simulation Results and Discussion
3.1. The Effect of the Blocking Layer Thickness
3.2. The Effect of the Type of Blocking Layer
3.3. The Effect of Temperature
3.4. The Effect of the QD Size
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, X.; Wang, W.; Mattoussi, H. Controlling the spectroscopic properties of quantum dots via energy transfer and charge transfer interactions: Concepts and applications. Nano Today 2016, 11, 98–121. [Google Scholar] [CrossRef]
- Sharma, D.; Jha, R.; Kumar, S. Quantum dot sensitized solar cell: Recent advances and future perspectives in photoanode. Sol. Energy Mater. Sol. Cells 2016, 155, 294–322. [Google Scholar] [CrossRef]
- Kershaw, S.V.; Susha, A.S.; Rogach, A.L. Narrow bandgap colloidal metal chalcogenide quantum dots: Synthetic methods, heterostructures, assemblies, electronic and infrared optical properties. Chem. Soc. Rev. 2013, 42, 3033–3087. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Wu, K.; Lian, T. Charge Transfer Dynamics from Photoexcited Semiconductor Quantum Dots. Annu. Rev. Phys. Chem. 2016, 67, 259–281. [Google Scholar] [CrossRef]
- Toyoda, T.; Yindeesuk, W.; Kamiyama, K.; Katayama, K.; Kobayashi, H.; Hayase, S.; Shen, Q. The Electronic Structure and Photoinduced Electron Transfer Rate of CdSe Quantum Dots on Single Crystal Rutile TiO2: Dependence on the Crystal Orientation of the Substrate. J. Phys. Chem. C 2016, 120, 2047–2057. [Google Scholar] [CrossRef]
- Vafapoor, B.; Fathi, D.; Eskandari, M. ZnS/Al2S3 Layer as a Blocking Layer in Quantum Dot Sensitized Solar Cells. J. Electron. Mater. 2018, 47, 1932–1936. [Google Scholar] [CrossRef]
- Askari, F.; Fathi, D.; Eskandari, M. Copper sulfide/cuprous selenide as a new counter electrode for quantum-dot-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2020, 31, 1789–1796. [Google Scholar] [CrossRef]
- Fahimi, M.J.; Eskandari, M.; Fathi, D. Performance enhancement of quantum dot sensitized solar cells by treatment of ZnO nanorods by magnesium acetate. J. Mater. Sci. Mater. Electron. 2018, 29, 4569–4574. [Google Scholar] [CrossRef]
- Kamat, P.V. Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. J. Phys. Chem. C 2008, 112, 18737–18753. [Google Scholar] [CrossRef]
- Jun, H.K.; Careem, M.A.; Arof, A.K. Quantum dot-sensitized solar cells—Perspective and recent developments: A review of Cd chalcogenide quantum dots as sensitizers. Renew. Sustain. Energy Rev. 2013, 22, 148–167. [Google Scholar] [CrossRef]
- Sun, Z.; Sitbon, G.; Pons, T.; Bakulin, A.A.; Chen, Z. Reduced Carrier Recombination in PbS-CuInS2 Quantum Dot Solar Cells. Sci. Rep. 2015, 5, 10626. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Masala, S.; Sargent, E.H. Charge-extraction strategies for colloidal quantum dot photovoltaics. Nat. Mater. 2014, 13, 233–240. [Google Scholar] [CrossRef]
- Choi, H.; Nicolaescu, R.; Paek, S.; Ko, J.; Kamat, P.V. Supersensitization of CdS quantum dots with a near-infrared organic dye: Toward the design of panchromatic hybrid-sensitized solar cells. ACS Nano 2011, 5, 9238–9245. [Google Scholar] [CrossRef]
- Zhao, K.; Pan, Z.; Mora-Seró, I.; Cánovas, E.; Wang, H.; Song, Y.; Gong, X.; Wang, J.; Bonn, M.; Bisquert, J.; et al. Boosting Power Conversion Efficiencies of Quantum-Dot-Sensitized Solar Cells Beyond 8% by Recombination Control. J. Am. Chem. Soc. 2015, 137, 5602–5609. [Google Scholar] [CrossRef]
- McDaniel, H.; Fuke, N.; Makarov, N.S.; Pietryga, J.M.; Klimov, V.I. An integrated approach to realizing high-performance liquid-junction quantum dot sensitized solar cells. Nat. Commun. 2013, 4, 2887. [Google Scholar] [CrossRef]
- Seo, M.H.; Hwang, W.P.; Kim, Y.K.; Lee, J.K.; Kim, M.R. Improvement of quantum dot-sensitized solar cells based on Cds and CdSe quantum dots. In Proceedings of the 2011 37th IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 19–24 June 2011; pp. 2652–2655. [Google Scholar]
- Abbas, M.A.; Basit, M.A.; Park, T.J.; Bang, J.H. Enhanced performance of PbS-sensitized solar cells via controlled successive ionic-layer adsorption and reaction. Phys. Chem. Chem. Phys. 2015, 17, 9752–9760. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Nahm, C.; Moon, J.; Kim, C.; Nam, S.; Jung, D.R.; Park, B. The effect of a blocking layer on the photovoltaic performance in CdS quantum-dot-sensitized solar cells. J. Power Sources 2011, 196, 10526–10531. [Google Scholar] [CrossRef]
- Meng, K.; Surolia, P.K.; Byrne, O.; Thampi, K.R. Efficient CdS quantum dot sensitized solar cells made using novel Cu2S counter electrode. J. Power Sources 2014, 248, 218–223. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, H.; Bao, H.; Zhong, X. Optimization of TiO2 photoanode films for highly efficient quantum dot-sensitized solar cells. J. Mater. Chem. A 2014, 2, 13033–13040. [Google Scholar] [CrossRef]
- Shen, Q.; Kobayashi, J.; Diguna, L.J.; Toyoda, T. Effect of ZnS coating on the photovoltaic properties of CdSe quantum dot-sensitized solar cells. J. Appl. Phys. 2008, 103, 084304. [Google Scholar] [CrossRef]
- Kang, J.S.; Park, M.-A.; Kim, J.-Y.; Park, S.H.; Chung, D.Y.; Yu, S.-H.; Kim, J.; Park, J.; Choi, J.-W.; Lee, K.J.; et al. Reactively sputtered nickel nitride as electrocatalytic counter electrode for dye-and quantum dot-sensitized solar cells. Sci. Rep. 2015, 5, 10450. [Google Scholar] [CrossRef]
- Mora-Sero, I.; Gimenez, S.; Fabregat-Santiago, F.; Gomez, R.; Shen, Q.; Toyoda, T.; Bisquert, J. Recombination in quantum dot sensitized solar cells. Acc. Chem. Res. 2009, 42, 1848–1857. [Google Scholar] [CrossRef]
- Choi, H.; Kim, J.; Nahm, C.; Kim, C.; Nam, S.; Kang, J.; Lee, B.; Hwang, T.; Kang, S.; Choi, D.J.; et al. The role of ZnO-coating-layer thickness on the recombination in CdS quantum-dot-sensitized solar cells. Nano Energy 2013, 2, 1218–1224. [Google Scholar] [CrossRef]
- Yang, L.; McCue, C.; Zhang, Q.; Uchaker, E.; Mai, Y.; Cao, G. Highly efficient quantum dot-sensitized TiO2 solar cells based on multilayered semiconductors (ZnSe/CdS/CdSe). Nanoscale 2015, 7, 3173–3180. [Google Scholar] [CrossRef]
- Zheng, K.; Zidek, K.; Abdellah, M.; Zhang, W.; Chábera, P.; Lenngren, N.; Yartsev, A.; Pullerits, T. Ultrafast charge transfer from CdSe quantum dots to p-type NiO: Hole injection vs hole trapping. J. Phys. Chem. C 2014, 118, 18462–18471. [Google Scholar] [CrossRef]
- Jiao, S.; Shen, Q.; Mora-Seró, I.; Wang, J.; Pan, Z.; Zhao, K.; Kuga, Y.; Zhong, X.; Bisquert, J. Band Engineering in Core/Shell ZnTe/CdSe for Photovoltage and Efficiency Enhancement in Exciplex Quantum Dot Sensitized Solar Cells. ACS Nano 2015, 9, 908–915. [Google Scholar] [CrossRef]
- Bora, T.; Kyaw, H.H.; Dutta, J. Zinc oxide–zinc stannate core–shell nanorod arrays for CdS quantum dot sensitized solar cells. Electrochim. Acta 2012, 68, 141–145. [Google Scholar] [CrossRef]
- González-Pedro, V.; Xu, X.; Mora-Sero, I.; Bisquert, J. Modeling high-efficiency quantum dot sensitized solar cells. ACS Nano 2010, 4, 5783–5790. [Google Scholar] [CrossRef]
- Chang, J.Y.; Su, L.F.; Li, C.H.; Chang, C.C.; Lin, J.M. Efficient “green” quantum dot-sensitized solar cells based on Cu2S–CuInS2–ZnSe architecture. Chem. Commun. 2012, 48, 4848–4850. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Y.; Ali, G.; Yoo, S.H.; Cho, S.O. Improved conversion efficiency of CdS quantum dots-sensitized TiO2 nanotube array using ZnO energy barrier layer. Nanotechnology 2011, 22, 015202. [Google Scholar] [CrossRef]
- Fahimi, M.J.; Fathi, D.; Bastami, H. Blocking layer modeling for temperature analysis of electron transfer rate in quantum dot sensitized solar cells. J. Fundam. Appl. Sci. 2016, 8, 54–70. [Google Scholar] [CrossRef]
- Fahimi, M.J.; Fathi, D. The impact of blocking layer permittivity on the electron transfer rate in quantum dot sensitized solar cells. In Proceedings of the 24th Iranian Conference on Electrical Engineering (ICEE), Shiraz, Iran, 10–12 May 2016; pp. 1712–1717. [Google Scholar]
- Sung, S.D.; Lim, I.; Kim, M.S.; Lee, W.I. Effect of Ultrathin Al2O3 Layer on TiO2 Surface in CdS/CdSe Co-Sensitized Quantum Dot Solar Cells. Bull. Korean Chem. Soc. 2013, 34, 411–414. [Google Scholar] [CrossRef]
- Rao, S.S.; Durga, I.K.; Gopi, C.V.; Tulasivarma, C.V.; Kim, S.K.; Kim, H.J. The effect of TiO2 nanoflowers as a compact layer for CdS quantum-dot sensitized solar cells with improved performance. Dalton Trans. 2015, 44, 12852–12862. [Google Scholar] [CrossRef]
- Tachibana, Y.; Umekita, K.; Otsuka, Y.; Kuwabata, S. Performance improvement of CdS quantum dots sensitized TiO2 solar cells by introducing a dense TiO2 blocking layer. J. Phys. D Appl. Phys. 2008, 41, 102002. [Google Scholar] [CrossRef]
- Meng, K.; Surolia, P.K.; Byrne, O.; Thampi, K.R. Quantum dot and quantum dot-dye co-sensitized solar cells containing organic thiolate–disulfide redox electrolyte. J. Power Sources 2015, 275, 681–687. [Google Scholar] [CrossRef]
- Santra, P.K.; Nair, P.V.; George Thomas, K.; Kamat, P.V. CuInS2-sensitized quantum dot solar cell. Electrophoretic deposition, excited-state dynamics, and photovoltaic performance. J. Phys. Chem. Lett. 2013, 4, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Chettiar, U.K.; Engheta, N. Internal homogenization: Effective permittivity of a coated sphere. Opt. Express 2012, 20, 22976–22986. [Google Scholar] [CrossRef]
- Chukwuocha, E.O.; Onyeaju, M.C.; Harry, T.S.T. Theoretical Studies on the Effect of Confinement on Quantum Dots Using the Brus Equation. World J. Condens. Matter Phys. 2012, 2, 96–100. [Google Scholar] [CrossRef]
- Tvrdy, K.; Frantsuzov, P.A.; Kamat, P.V. Photoinduced electron transfer from semiconductor quantum dots to metal oxide nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 29–34. [Google Scholar] [CrossRef]
- Tvrdy, K. Electron Transfer Reactions in Quantum Dot Sensitized SOLAR cells. Ph.D. Dissertation, University of Notre Dame, Notre Dame, Indiana, 2011. [Google Scholar]
- Shevarenkov, D.N.; Shchurov, A.F. Dielectric properties of polycrystalline ZnS. Semiconductors 2006, 40, 33–35. [Google Scholar] [CrossRef]
- Chin, A.; Liao, C.C.; Lu, C.H.; Chen, W.J.; Tsai, C. Device and reliability of high-k Al2O3 gate dielectric with good mobility and low Dit. In Proceedings of the 1999 Symposium on VLSI Technology, Digest of Technical Papers (IEEE Cat. No.99CH36325), Kyoto, Japan, 14–16 June 1999; pp. 135–136. [Google Scholar]
- Fahimi, M.J.; Fathi, D.; Ansari-Rad, M. Accurate analysis of electron transfer from quantum dots to metal oxides in quantum dot sensitized solar cells. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 73, 148–155. [Google Scholar] [CrossRef]















| Blocking Layer | Relative Permittivity |
|---|---|
| ZnS | 8.3 [43] |
| Al2O3 | 9.4 [44] |
| ZnO | 9.9 [41] |
| TiO2 | 10.5 [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahimi, M.J.; Fathi, D.; Eskandari, M.; Das, N. Marcus Theory and Tunneling Method for the Electron Transfer Rate Analysis in Quantum Dot Sensitized Solar Cells in the Presence of Blocking Layer. Micromachines 2023, 14, 1731. https://doi.org/10.3390/mi14091731
Fahimi MJ, Fathi D, Eskandari M, Das N. Marcus Theory and Tunneling Method for the Electron Transfer Rate Analysis in Quantum Dot Sensitized Solar Cells in the Presence of Blocking Layer. Micromachines. 2023; 14(9):1731. https://doi.org/10.3390/mi14091731
Chicago/Turabian StyleFahimi, Mohammad Javad, Davood Fathi, Mehdi Eskandari, and Narottam Das. 2023. "Marcus Theory and Tunneling Method for the Electron Transfer Rate Analysis in Quantum Dot Sensitized Solar Cells in the Presence of Blocking Layer" Micromachines 14, no. 9: 1731. https://doi.org/10.3390/mi14091731







