A Review of Nitrogen-Doped Graphene Aerogel in Electromagnetic Wave Absorption
Abstract
:1. Introduction
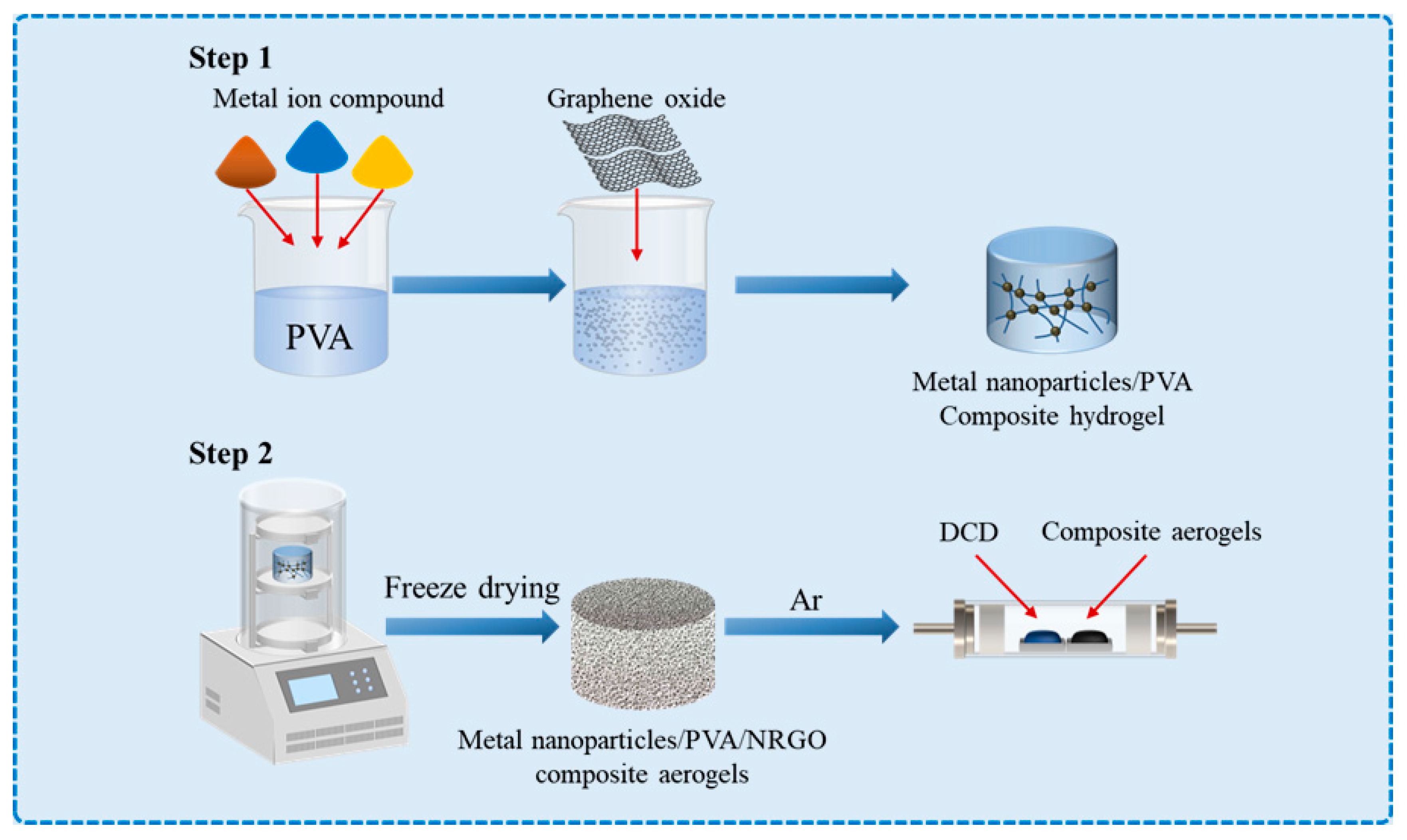
2. Synthesis of Nitrogen-Doped Graphene Aerogel
3. N-Doped Graphene Aerogel for Electromagnetic Wave Absorption
3.1. Pure N-Doped Graphene Aerogel for Electromagnetic Wave Absorption
- (1)
- Conduction loss: The three-dimensional conductive network formed by the GA provides a pathway for electron transport. The introduction of nitrogen atoms adds extra electrons to the system, thereby increasing the conduction loss in the graphene network. Additionally, under the influence of alternating EM fields, according to Cao’s electron jump model [62], electrons can absorb energy from the EMWs and move through the graphene network by migration and jumping, thus leading to conduction loss.
- (2)
- Polarization loss: Defects and oxygen-containing functional groups present at the edges of graphene layers, such as hydroxyl and carboxylic groups, act as polarization centers, generating defect polarization and dipole polarization. Furthermore, the incorporation of nitrogen atoms disrupts the sp2 hybridization of the graphene lattice, leading to the formation of sp3 defects and disordered sites [14]. This results in the generation of dipole polarization with C–N electric dipoles as centers, as well as defect polarization with nitrogen atoms as centers. Additionally, the presence of heterogeneous interfaces between the graphene and paraffin matrices can result in interface polarization [63].
- (3)
- Multiple scattering: The three-dimensional network structure of the aerogel and layered structure of graphene allows for multiple reflections and scatterings of EMWs within the material. This phenomenon results in the attenuation of EMW energy.
3.2. N-Doped Graphene Aerogel/Magnetic Metal for Electromagnetic Wave Absorption
3.3. N-Doped Graphene Aerogel/Magnetic Oxide for Electromagnetic Wave Absorption
- (1)
- Pure ferrite
- (2)
- Cobalt and nickel ferrite
- (3)
- Composite ferrite
- (4)
- Other oxides
- (5)
- Combination with sulfides
3.4. N-Doped Graphene Aerogel/Carbon Nanotubes for Electromagnetic Wave Absorption
3.5. N-Doped Graphene Aerogel/Polymer for Electromagnetic Wave Absorption
4. Prospects
- (1)
- Low frequency
- (2)
- Multifunctional
- (3)
- Combination with advanced preparation methods
- (4)
- Innovation in structure
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhi, D.; Li, T.; Li, J.; Ren, H.; Meng, F. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part B Eng. 2021, 211, 108642. [Google Scholar] [CrossRef]
- Lv, H.; Yang, Z.; Pan, H.; Wu, R. Electromagnetic absorption materials: Current progress and new frontiers. Prog. Mater. Sci. 2022, 127, 100946. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, X.; Yu, R.; Stucky, G.D. Electromagnetic microwave absorption theory and recent achievements in microwave absorbers. Carbon 2020, 168, 606–623. [Google Scholar] [CrossRef]
- Green, M.; Chen, X. Recent progress of nanomaterials for microwave absorption. J. Materiomics 2019, 5, 503–541. [Google Scholar] [CrossRef]
- Song, Q.; Ye, F.; Kong, L.; Shen, Q.; Han, L.; Feng, L.; Yu, G.; Pan, Y.; Li, H. Graphene and MXene Nanomaterials: Toward High-Performance Electromagnetic Wave Absorption in Gigahertz Band Range. Adv. Funct. Mater. 2020, 30, 2000475. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Q.; Fu, Y.; Liu, T. A review on carbon/magnetic metal composites for microwave absorption. J. Mater. Sci. Technol. 2021, 86, 91–109. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Gu, J.; Liu, H.; Liu, C.; Luo, C.; Kong, J.; Shao, Q.; Wang, N.; Guo, Z.; et al. Ultralight, highly compressible and fire-retardant graphene aerogel with self-adjustable electromagnetic wave absorption. Carbon 2018, 139, 1126–1135. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Liu, Y.-J.; Sui, G.-X. Lightweight spongy bone-like graphene@SiC aerogel composites for high-performance microwave absorption. Chem. Eng. J. 2018, 337, 522–531. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Fu, Y.; Wu, X.; Wang, Q.; Zhang, W.; Luo, C. Enhanced microwave absorption performances of polyaniline/graphene aerogel by covalent bonding. Compos. Part B Eng. 2019, 169, 221–228. [Google Scholar] [CrossRef]
- Guan, X.; Yang, Z.; Zhou, M.; Yang, L.; Peymanfar, R.; Aslibeiki, B.; Ji, G. 2D MXene Nanomaterials: Synthesis, Mechanism, and Multifunctional Applications in Microwave Absorption. Small Struct. 2022, 3, 2200102. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Lv, X.; Cui, G.; Gu, G. Facile synthesis 3D porous MXene Ti3C2Tx@RGO composite aerogel with excellent dielectric loss and electromagnetic wave absorption. J. Alloys Compd. 2020, 828, 154251. [Google Scholar] [CrossRef]
- Liang, L.; Li, Q.; Yan, X.; Feng, Y.; Wang, Y.; Zhang, H.-B.; Zhou, X.; Liu, C.; Shen, C.; Xie, X. Multifunctional Magnetic Ti3C2Tx MXene/Graphene Aerogel with Superior Electromagnetic Wave Absorption Performance. ACS Nano 2021, 15, 6622–6632. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhou, Y.; Tong, Y.; Song, Z.; Feng, S.; Bu, X.; He, M. Ultralight Hierarchically Structured RGO Composite Aerogels Embedded with MnO2/Ti3C2Tx for Efficient Microwave Absorption. Langmuir 2022, 38, 14733–14744. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Farbod, M.; Laal Dolatabad, Z.; Ahangarpour, A. Fabrication of graphene and N-doped graphene aerogels and comparing their electrical, mechanical, and adsorption capacity properties. Fuller. Nanotub. Carbon Nanostruct. 2022, 30, 906–912. [Google Scholar] [CrossRef]
- Xu, P.; Gao, Q.; Ma, L.; Li, Z.; Zhang, H.; Xiao, H.; Liang, X.; Zhang, T.; Tian, X.; Liu, C. A high surface area N-doped holey graphene aerogel with low charge transfer resistance as high performance electrode of non-flammable thermostable supercapacitors. Carbon 2019, 149, 452–461. [Google Scholar] [CrossRef]
- Ling, R.; Cao, B.; Qi, W.; Yang, C.; Shen, K.; Sang, Z.; Guo, J.; Xie, D.; Cai, S.; Sun, X. Three-dimensional Na3V2(PO4)3@carbon/N-doped graphene aerogel: A versatile cathode and anode host material with high-rate and ultralong-life for sodium storage. J. Alloys Compd. 2021, 869, 159307. [Google Scholar] [CrossRef]
- Ghazitabar, A.; Naderi, M.; Fatmehsari Haghshenas, D. A facile chemical route for synthesis of nitrogen-doped graphene aerogel decorated by Co3O4 nanoparticles. Ceram. Int. 2018, 44, 23162–23171. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W.; et al. High-Performance Energy Storage and Conversion Materials Derived from a Single Metal–Organic Framework/Graphene Aerogel Composite. Nano Lett. 2017, 17, 2788–2795. [Google Scholar] [CrossRef]
- Chen, H.; Lu, X.; Zhang, L.; Sui, D.; Wang, C.; Meng, F.; Qi, W. Enhanced electrochemical performance of MnO2 nanoparticles: Graphene aerogels as conductive substrates and capacitance contributors. Dalton Trans. 2021, 50, 8776–8784. [Google Scholar] [CrossRef]
- Mahamad Yusoff, N.F.; Idris, N.H.; Md Din, M.F.; Majid, S.R.; Harun, N.A. Enhanced Electrochemical Performances of Mn3O4/Heteroatom-Doped Reduced Graphene Oxide Aerogels as an Anode for Sodium-Ion Batteries. Nanomaterials 2022, 12, 3569. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Li, P.; Xing, B.; Tian, Q.; Zhang, X.; Xu, B.; Huang, G.; Chen, L.; Zhang, Y. Nitrogen-rich graphene aerogel with interconnected thousand-layer pancake structure as anode for high performance of lithium-ion batteries. J. Solid State Chem. 2021, 294, 121859. [Google Scholar] [CrossRef]
- Yao, L.; Deng, H.; Huang, Q.-A.; Su, Q.; Du, G. Three-dimensional carbon-coated ZnFe2O4 nanospheres/nitrogen-doped graphene aerogels as anode for lithium-ion batteries. Ceram. Int. 2017, 43, 1022–1028. [Google Scholar] [CrossRef]
- Tian, L.; Xie, Y.; Lu, J.; Hu, Q.; Xiao, Y.; Liu, T.; Davronbek, B.; Zhu, X.; Su, X. Self-assembled 3D Fe3O4/N-Doped graphene aerogel composite for large and fast lithium storage with an excellent cycle performance. J. Electroanal. Chem. 2022, 922, 116763. [Google Scholar] [CrossRef]
- Wang, G.; Xu, X.; Kou, X.; Liu, X.; Dong, X.; Ma, H.; Wang, D. N-Doping of Graphene Aerogel as a Multifunctional Air Cathode for Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2021, 13, 51312–51320. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Hao, S.; Di, S.; Su, L.; Qin, X.; Shao, G. In situ fabrication of hierarchical iron oxide spheres@N-doped 3D porous graphene aerogel for superior lithium storage. Ionics 2020, 26, 2303–2314. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Zhang, Q.-Q.; Li, Y.-H.; Li, L. Three-dimensional network graphene aerogel for enhancing adsorption and visible light photocatalysis of nitrogen-doped TiO2. Mater. Lett. 2019, 234, 298–301. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, H.; Wang, Q.; Zhang, X.; Li, Y. Well controlled reduction, N-doping and cross-linking of graphene hydrogel: As good template for photocatalytic reaction. Appl. Surf. Sci. 2020, 528, 146945. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, K.-W.; He, J.-W.; Li, F.-M.; Huang, H.; Chen, P.; Chen, Y. Nitrogen-doped graphene aerogel-supported ruthenium nanocrystals for pH-universal hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 1535–1543. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Cai, R.; Chen, C.; Xia, Y.; Zhang, H.; Yang, D.; Yao, X. Air cathode of zinc–air batteries: A highly efficient and durable aerogel catalyst for oxygen reduction. Nanoscale 2019, 11, 826–832. [Google Scholar] [CrossRef]
- Hu, J.; Shi, Z.; Wang, X.; Qiao, H.; Huang, H. Silver-modified porous 3D nitrogen-doped graphene aerogel: Highly efficient oxygen reduction electrocatalyst for Zn−Air battery. Electrochimica Acta 2019, 302, 216–224. [Google Scholar] [CrossRef]
- Cao, K.-W.; Huang, H.; Li, F.-M.; Yao, H.-C.; Bai, J.; Chen, P.; Jin, P.-J.; Deng, Z.-W.; Zeng, J.-H.; Chen, Y. Co nanoparticles supported on three-dimensionally N-doped holey graphene aerogels for electrocatalytic oxygen reduction. J. Colloid Interface Sci. 2020, 559, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.-L.; Zhang, L.-M.; Zhao, L.; Gu, D.-M.; Huang, G.-S.; Wang, Z.-B. Nitrogen-doped graphene aerogel with an open structure assisted by in-situ hydrothermal restructuring of ZIF-8 as excellent Pt catalyst support for methanol electro-oxidation. Int. J. Hydrogen Energy 2018, 43, 21899–21907. [Google Scholar] [CrossRef]
- Cai, A.; He, H.; Zhang, Q.; Xu, Y.; Li, X.; Zhang, F.; Fan, X.; Peng, W.; Li, Y. Synergistic Effect of N-Doped sp2 Carbon and Porous Structure in Graphene Gels toward Selective Oxidation of C–H Bond. ACS Appl. Mater. Interfaces 2021, 13, 13087–13096. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Ding, J.; Xu, N.; Bernards, M.T.; He, Y.; Shi, Y. Three-Dimensional Nitrogen-Doped Graphene Aerogel-Supported MnO Nanoparticles as Efficient Electrocatalysts for CO2 Reduction to CO. ACS Sustain. Chem. Eng. 2020, 8, 4983–4994. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, L.; Yang, K.; Wang, Y.; Wang, Z.; Kong, Y.; Xue, H. Electrosorption of Toxic Heavy Metal Ions by Mono S- or N-Doped and S, N-Codoped 3D Graphene Aerogels. J. Electrochem. Soc. 2017, 164, E17. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; Xiang, Y.; Zhang, Q. Enhanced electrochemical capacitance and oil-absorbability of N-doped graphene aerogel by using amino-functionalized silica as template and doping agent. J. Power Sources 2018, 379, 240–248. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, J.; Duan, X.; Tan, X.; Liu, S.; Wang, S. Self-assembly of 3D MnO2/N-doped graphene hybrid aerogel for catalytic degradation of water pollutants: Structure-dependent activity. Chem. Eng. J. 2019, 369, 1049–1058. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Liu, Y.; Fang, T.; Zhang, L.; Xing, B. Ring defects-rich and pyridinic N-doped graphene aerogel as floating adsorbent for efficient removal of tetracycline: Evidence from NEXAFS measurements and theoretical calculations. J. Hazard. Mater. 2022, 435, 128940. [Google Scholar] [CrossRef]
- Fernández-Sáez, N.; Villela-Martinez, D.E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Pastrana-Martínez, L.M. Heteroatom-doped graphene aerogels and carbon-magnetite catalysts for the heterogeneous electro-Fenton degradation of acetaminophen in aqueous solution. J. Catal. 2019, 378, 68–79. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Lyu, C.; Niu, S.; Dong, Z.; Lyu, C. Enhanced catalytic oxidation of benzotriazole via peroxymonosulfate activated by CoFe2O4 supported onto nitrogen-doped three-dimensional graphene aerogels. Chem. Eng. J. 2020, 400, 125897. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, W.; Ma, J.; Yang, Y.; Tang, B. Carbon fibers assisted 3D N-doped graphene aerogel on excellent adsorption capacity and mechanical property. Colloids Surf. Physicochem. Eng. Asp. 2021, 608, 125602. [Google Scholar] [CrossRef]
- Chen, Y.; Li, G.; Mu, W.; Wan, X.; Lu, D.; Gao, J.; Wen, D. Nonenzymatic Sweat Wearable Uric Acid Sensor Based on N-Doped Reduced Graphene Oxide/Au Dual Aerogels. Anal. Chem. 2023, 95, 3864–3872. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tan, W.; Chen, C.; Tao, Y.; Qin, Y.; Kong, Y. Nonenzymatic glucose sensing by CuO nanoparticles decorated nitrogen-doped graphene aerogel. Mater. Sci. Eng. C 2017, 78, 210–217. [Google Scholar] [CrossRef]
- Ruiyi, L.; Ling, L.; Hongxia, B.; Zaijun, L. Nitrogen-doped multiple graphene aerogel/gold nanostar as the electrochemical sensing platform for ultrasensitive detection of circulating free DNA in human serum. Biosens. Bioelectron. 2016, 79, 457–466. [Google Scholar] [CrossRef]
- Shu, R.; Yang, X.; Zhao, Z. Fabrication of core-shell structure NiFe2O4@SiO2 decorated nitrogen-doped graphene composite aerogels towards excellent electromagnetic absorption in the Ku band. Carbon 2023, 210, 118047. [Google Scholar] [CrossRef]
- Shu, R.; Zhao, Z.; Yang, X. Fabrication of nitrogen-doped reduced graphene oxide/porous magnesium ferrite@silica dioxide composite with excellent dual-band electromagnetic absorption performance. Chem. Eng. J. 2023, 473, 145224. [Google Scholar] [CrossRef]
- Shu, R.; Xu, J.; Shi, J. Construction of nitrogen-doped graphene-based ternary magnetic composite aerogel towards excellent electromagnetic absorption in the Ku-band. J. Alloys Compd. 2023, 956, 170349. [Google Scholar] [CrossRef]
- Shu, R.; Xu, J.; Shi, J. Synthesis of nitrogen-doped graphene-based binary composite aerogels for ultralightweight and broadband electromagnetic absorption. Ceram. Int. 2023, 49, 30214–30223. [Google Scholar] [CrossRef]
- Shu, R.; Wan, Z.; Zhang, J.; Wu, Y.; Shi, J. Synergistically assembled nitrogen-doped reduced graphene oxide/multi-walled carbon nanotubes composite aerogels with superior electromagnetic wave absorption performance. Compos. Sci. Technol. 2021, 210, 108818. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, G.; Zhang, C.; Wu, Y.; Zhang, J. Nitrogen-Doping-Regulated Electromagnetic Wave Absorption Properties of Ultralight Three-Dimensional Porous Reduced Graphene Oxide Aerogels. Adv. Electron. Mater. 2021, 7, 2001001. [Google Scholar] [CrossRef]
- Tang, J.; Liang, N.; Wang, L.; Li, J.; Tian, G.; Zhang, D.; Feng, S.; Yue, H. Three-dimensional nitrogen-doped reduced graphene oxide aerogel decorated with Ni nanoparticles with tunable and unique microwave absorption. Carbon 2019, 152, 575–586. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Yan, J.; Huang, Y.; Xia, L.; Guang, Z. Synthesis of lightweight N-doped graphene foams with open reticular structure for high-efficiency electromagnetic wave absorption. Chem. Eng. J. 2019, 368, 285–298. [Google Scholar] [CrossRef]
- Wang, X.-X.; Ma, T.; Shu, J.-C.; Cao, M.-S. Confinedly tailoring Fe3O4 clusters-NG to tune electromagnetic parameters and microwave absorption with broadened bandwidth. Chem. Eng. J. 2018, 332, 321–330. [Google Scholar] [CrossRef]
- Xu, J.; Shi, Y.; Zhang, X.; Yuan, H.; Li, B.; Zhu, C.; Zhang, X.; Chen, Y. General strategy for fabrication of N-doped carbon nanotube/reduced graphene oxide aerogels for dissipation and conversion of electromagnetic energy. J. Mater. Chem. C 2020, 8, 7847–7857. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Lv, C.; Zhu, A.; Zhu, X.; Guo, S.; Chen, C.; Yang, D. Seaweed-Derived Route to Fe2O3 Hollow Nanoparticles/N-Doped Graphene Aerogels with High Lithium Ion Storage Performance. ACS Appl. Mater. Interfaces 2016, 8, 7047–7053. [Google Scholar] [CrossRef]
- Shu, X.; Yan, S.; Fang, B.; Song, Y.; Zhao, Z. A 3D multifunctional nitrogen-doped RGO-based aerogel with silver nanowires assisted self-supporting networks for enhanced electromagnetic wave absorption. Chem. Eng. J. 2023, 451, 138825. [Google Scholar] [CrossRef]
- Pruna, A.I.; Cárcel, A.C.; Benedito, A.; Giménez, E. The Effect of Solvothermal Conditions on the Properties of Three-Dimensional N-Doped Graphene Aerogels. Nanomaterials 2019, 9, 350. [Google Scholar] [CrossRef]
- Yu, W.; Shao, G. Morphology engineering of defective graphene for microwave absorption. J. Colloid Interface Sci. 2023, 640, 680–687. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, L.; Yu, G.; Wei, J.; Shao, G. Polarization genes dominated heteroatom-doped graphene aerogels toward super-efficiency microwave absorption. J. Mater. Chem. C 2023, 11, 9804–9814. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Li, H.; Dugnani, R.; Du, Q.; UrRehman, H.; Kang, H.; Liu, H. Facile synthesis of three-dimensional lightweight nitrogen-doped graphene aerogel with excellent electromagnetic wave absorption properties. J. Mater. Sci. 2018, 53, 4067–4077. [Google Scholar] [CrossRef]
- Cao, M.; Wang, X.; Cao, W.; Fang, X.; Wen, B.; Yuan, J. Thermally Driven Transport and Relaxation Switching Self-Powered Electromagnetic Energy Conversion. Small 2018, 14, 1800987. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Gu, W.; Wu, Y.; Zhang, B.; Wang, G.; Yang, Y.; Ji, G. Heterointerface Engineering in Electromagnetic Absorbers: New Insights and Opportunities. Adv. Mater. 2022, 34, 2106195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fang, X.; Zhang, Y.; Guo, J.; Gong, C.; Estevez, D.; Qin, F.; Zhang, J. Ultralight reduced graphene oxide aerogels prepared by cation-assisted strategy for excellent electromagnetic wave absorption. Nanotechnology 2020, 31, 275707. [Google Scholar] [CrossRef]
- Suo, B.; Zhang, X.; Jiang, X.; Yan, F.; Luo, Z.; Chen, Y. Atomically Dispersed Ni Single-Atoms Anchored on N-Doped Graphene Aerogels for Highly Efficient Electromagnetic Wave Absorption. Chin. Phys. Lett. 2022, 39, 045201. [Google Scholar] [CrossRef]
- Xu, J.; Liu, M.; Zhang, X.; Li, B.; Zhang, X.; Zhang, X.; Zhu, C.; Chen, Y. Atomically dispersed cobalt anchored on N-doped graphene aerogels for efficient electromagnetic wave absorption with an ultralow filler ratio. Appl. Phys. Rev. 2022, 9, 011402. [Google Scholar] [CrossRef]
- Gao, Y.; Lei, Z.; Pan, L.; Wu, Q.; Zhuang, X.; Tan, G.; Ning, M.; Man, Q. Lightweight chitosan-derived carbon/rGO aerogels loaded with hollow Co1−xNixO nanocubes for superior electromagnetic wave absorption and heat insulation. Chem. Eng. J. 2023, 457, 141325. [Google Scholar] [CrossRef]
- Zhou, J.; Shu, X.; Wang, Z.; Liu, Y.; Wang, Y.; Zhou, C.; Kong, L. Hydrothermal synthesis of polyhedral FeCo alloys with enhanced electromagnetic absorption performances. J. Alloys Compd. 2019, 794, 68–75. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.; Ma, H.; Zhao, Y.; Li, L.; Li, P.; Yan, J.; Yun, J.; Zhao, W.; Zhang, H.; et al. Hollow spherical NiFe-MOF derivative and N-doped rGO composites towards the tunable wideband electromagnetic wave absorption: Experimental and theoretical study. Carbon 2023, 201, 347–361. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Fu, R.; Zhu, H.; Jiao, Q.; Feng, T.; Feng, C.; Shi, D.; Li, H.; Zhao, Y. Rational Construction of Hierarchically Porous Fe–Co/N-Doped Carbon/rGO Composites for Broadband Microwave Absorption. Nano-Micro Lett. 2019, 11, 76. [Google Scholar] [CrossRef]
- Wei, J.; Shao, G.; Zhang, L.; Huang, X. Highly effective and tunable microwave absorber integrating multiscale attenuation behaviours derived from prussian blue analogue/graphene oxide aerogel. J. Colloid Interface Sci. 2023, 631, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Klencsár, Z.; Németh, P.; Sándor, Z.; Horváth, T.; Sajó, I.E.; Mészáros, S.; Mantilla, J.; Coaquira, J.A.H.; Garg, V.K.; Kuzmann, E.; et al. Structure and magnetism of Fe–Co alloy nanoparticles. J. Alloys Compd. 2016, 674, 153–161. [Google Scholar] [CrossRef]
- Sun, F.; Liu, Q.; Xu, Y.; Xin, X.; Wang, Z.; Song, X.; Zhao, X.; Xu, J.; Liu, J.; Zhao, L.; et al. Attapulgite modulated thorny nickel nanowires/graphene aerogel with excellent electromagnetic wave absorption performance. Chem. Eng. J. 2021, 415, 128976. [Google Scholar] [CrossRef]
- Yuan, H.; Li, B.; Zhu, C.; Xie, Y.; Jiang, Y.; Chen, Y. Dielectric behavior of single iron atoms dispersed on nitrogen-doped nanocarbon. Appl. Phys. Lett. 2020, 116, 153101. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Z.; Huang, Y.; Zhang, Y.; Ge, Z.; Ma, W.; Zhang, T.; Wu, M.; Xu, S.; Fan, F.; et al. Consecutively Strong Absorption from Gigahertz to Terahertz Bands of a Monolithic Three-Dimensional Fe3O4/Graphene Material. ACS Appl. Mater. Interfaces 2019, 11, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, H. Highly enhanced electromagnetic wave absorption bandwidth based on reduced graphene oxide-Fe aerogel composites. Nanotechnology 2019, 31, 095711. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zong, Y.; Tan, G.; Sun, Y.; Lan, Y.; He, M.; Ren, Z.; Zheng, X. Solvothermal synthesis of nitrogen-doped graphene decorated by superparamagnetic Fe3O4 nanoparticles and their applications as enhanced synergistic microwave absorbers. Carbon 2017, 115, 493–502. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Zhu, T.; Chang, S.; Wang, W. CoFe2O4/N-doped reduced graphene oxide aerogels for high-performance microwave absorption. Chem. Eng. J. 2020, 388, 124317. [Google Scholar] [CrossRef]
- Deng, L.; Shu, R.; Zhang, J. Fabrication of ultralight nitrogen-doped reduced graphene oxide/nickel ferrite composite foams with three-dimensional porous network structure as ultrathin and high-performance microwave absorbers. J. Colloid Interface Sci. 2022, 614, 110–119. [Google Scholar] [CrossRef]
- Xu, J.; Shu, R.; Wan, Z.; Shi, J. Construction of three-dimensional hierarchical porous nitrogen-doped reduced graphene oxide/hollow cobalt ferrite composite aerogels toward highly efficient electromagnetic wave absorption. J. Mater. Sci. Technol. 2023, 132, 193–200. [Google Scholar] [CrossRef]
- Wang, X.; Liao, J.; Du, R.; Wang, G.; Tsidaeva, N.; Wang, W. Achieving super-broad effective absorption bandwidth with low filler loading for graphene aerogels/raspberry-like CoFe2O4 clusters by N doping. J. Colloid Interface Sci. 2021, 590, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.; Zhang, J.; Wu, Y.; Wan, Z.; Li, X. Synthesis of nitrogen-doped reduced graphene oxide/cobalt–zinc ferrite composite aerogels with superior compression recovery and electromagnetic wave absorption performance. Nanoscale 2021, 13, 4485–4495. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.; Zhang, J.; Guo, C.; Wu, Y.; Wan, Z.; Shi, J.; Liu, Y.; Zheng, M. Facile synthesis of nitrogen-doped reduced graphene oxide/nickel-zinc ferrite composites as high-performance microwave absorbers in the X-band. Chem. Eng. J. 2020, 384, 123266. [Google Scholar] [CrossRef]
- Fu, K.; Zhao, J.; Liu, F.; Wu, L.; Jin, Z.; Yang, Y.; Qiao, J.; Wang, Z.; Wang, F.; Liu, J. Enhanced electromagnetic wave absorption of nitrogen-doped reduced graphene oxide aerogels with LaFeO3 cluster modifications. Carbon 2023, 210, 118071. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Zhao, Y.; Zhao, X.; You, W.; Li, X.; Che, R. “Matryoshka Doll”-Like CeO2 Microspheres with Hierarchical Structure to Achieve Significantly Enhanced Microwave Absorption Performance. ACS Appl. Mater. Interfaces 2018, 10, 27540–27547. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, X.; Jia, Z.; Wang, B.; Wu, X.; Wu, G. Synthesis of 3D cerium oxide/porous carbon for enhanced electromagnetic wave absorption performance. Adv. Compos. Hybrid Mater. 2021, 4, 1398–1412. [Google Scholar] [CrossRef]
- Gao, S.; Wang, G.-S.; Guo, L.; Yu, S.-H. Tunable and Ultraefficient Microwave Absorption Properties of Trace N-Doped Two-Dimensional Carbon-Based Nanocomposites Loaded with Multi-Rare Earth Oxides. Small 2020, 16, 1906668. [Google Scholar] [CrossRef]
- Zhao, P.-Y.; Wang, H.-Y.; Cai, B.; Sun, X.-B.; Hou, Z.-L.; Wu, J.-T.; Bai, M.; Wang, G.-S. Electrospinning fabrication and ultra-wideband electromagnetic wave absorption properties of CeO2/N-doped carbon nanofibers. Nano Res. 2022, 15, 7788–7796. [Google Scholar] [CrossRef]
- Wu, L.; Shu, R.; Zhang, J.; Chen, X. Synthesis of three-dimensional porous netlike nitrogen-doped reduced graphene oxide/cerium oxide composite aerogels towards high-efficiency microwave absorption. J. Colloid Interface Sci. 2022, 608, 1212–1221. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Zhou, M.; Tan, S.; Peymanfar, R.; Aslibeiki, B.; Ji, G. Ultrabroad Microwave Absorption Ability and Infrared Stealth Property of Nano-Micro CuS@rGO Lightweight Aerogels. Nano-Micro Lett. 2022, 14, 171. [Google Scholar] [CrossRef]
- Xu, J.; Liu, L.; Zhang, X.; Li, B.; Zhu, C.; Chou, S.; Chen, Y. Tailoring electronic properties and polarization relaxation behavior of MoS2 monolayers for electromagnetic energy dissipation and wireless pressure micro-sensor. Chem. Eng. J. 2021, 425, 131700. [Google Scholar] [CrossRef]
- Zhu, T.; Shen, W.; Wang, X.; Song, Y.-F.; Wang, W. Paramagnetic CoS2@MoS2 core-shell composites coated by reduced graphene oxide as broadband and tunable high-performance microwave absorbers. Chem. Eng. J. 2019, 378, 122159. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Jia, Z.; Wang, L.; Guo, X.; Zhao, B.; Zhang, R. Exceptionally porous three-dimensional architectural nanostructure derived from CNTs/graphene aerogel towards the ultra-wideband EM absorption. Compos. Part B Eng. 2020, 196, 108122. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Ji, H.; Bai, P.; Wang, S.; Fan, B.; Guo, X.; Zhang, R. Lightweight graphene aerogels by decoration of 1D CoNi chains and CNTs to achieve ultra-wide microwave absorption. Carbon 2021, 176, 411–420. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Yuan, H.; Zhang, S.; Zhu, C.; Zhang, X.; Chen, Y. N-doped reduced graphene oxide aerogels containing pod-like N-doped carbon nanotubes and FeNi nanoparticles for electromagnetic wave absorption. Carbon 2020, 159, 357–365. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yuan, H.; Li, K.; Ouyang, Q.; Zhu, C.; Zhang, S.; Chen, Y. CoNi nanoparticles encapsulated by nitrogen-doped carbon nanotube arrays on reduced graphene oxide sheets for electromagnetic wave absorption. Chem. Eng. J. 2020, 383, 123208. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Cheng, J.-B.; Zhu, J.-Y.; Wang, Y.-Z. Ultralight CoNi/rGO aerogels toward excellent microwave absorption at ultrathin thickness. J. Mater. Chem. C 2019, 7, 441–448. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, C.; Jia, Z.; Liu, X.; Xu, B.; Zhang, D.; Wu, G. FeNi nanoparticles embedded reduced graphene/nitrogen-doped carbon composites towards the ultra-wideband electromagnetic wave absorption. J. Colloid Interface Sci. 2021, 584, 382–394. [Google Scholar] [CrossRef]
- Shu, R.; Wan, Z.; Zhang, J.; Wu, Y.; Liu, Y.; Shi, J.; Zheng, M. Facile Design of Three-Dimensional Nitrogen-Doped Reduced Graphene Oxide/Multi-Walled Carbon Nanotube Composite Foams as Lightweight and Highly Efficient Microwave Absorbers. ACS Appl. Mater. Interfaces 2020, 12, 4689–4698. [Google Scholar] [CrossRef]
- Wan, Z.; Shu, R.; Zhang, J.; Wu, Y. Synthesis of three-dimensional porous nitrogen-doped reduced graphene oxide/multi-walled carbon nanotubes composite aerogel as lightweight and high-performance electromagnetic wave absorbers. Diam. Relat. Mater. 2021, 112, 108245. [Google Scholar] [CrossRef]
- Ren, F.; Guo, Z.; Shi, Y.; Jia, L.; Qing, Y.; Ren, P.; Yan, D. Lightweight and highly efficient electromagnetic wave-absorbing of 3D CNTs/GNS@CoFe2O4 ternary composite aerogels. J. Alloys Compd. 2018, 768, 6–14. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Y.; Qi, N.; Wang, Q.; Zhang, X.; Li, Y. Preparation of Graphene Aerogel with High Mechanical Stability and Microwave Absorption Ability via Combining Surface Support of Metallic-CNTs and Interfacial Cross-Linking by Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 10409–10417. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xie, A.; Sun, M.; Wang, Y.; Wang, M. Reduced graphene oxide (RGO) modified spongelike polypyrrole (PPy) aerogel for excellent electromagnetic absorption. J. Mater. Chem. A 2015, 3, 14358–14369. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Wang, L.; Ren, J.; Xu, Y. Ultralight graphene aerogel enhanced with transformed micro-structure led by polypyrrole nano-rods and its improved microwave absorption properties. Compos. Part Appl. Sci. Manuf. 2017, 97, 141–150. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Li, D.; Jiang, L. Lightweight and recoverable ANF/rGO/PI composite aerogels for broad and high-performance microwave absorption. Compos. Part B Eng. 2021, 213, 108701. [Google Scholar] [CrossRef]
- Yu, Z.; Dai, T.; Yuan, S.; Zou, H.; Liu, P. Electromagnetic Interference Shielding Performance of Anisotropic Polyimide/Graphene Composite Aerogels. ACS Appl. Mater. Interfaces 2020, 12, 30990–31001. [Google Scholar] [CrossRef]
- Song, P.; Liu, B.; Liang, C.; Ruan, K.; Qiu, H.; Ma, Z.; Guo, Y.; Gu, J. Lightweight, Flexible Cellulose-Derived Carbon Aerogel@Reduced Graphene Oxide/PDMS Composites with Outstanding EMI Shielding Performances and Excellent Thermal Conductivities. Nano-Micro Lett. 2021, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fang, J.; Xu, C.; Cao, H.; Zhang, R.; Zhao, B.; Huang, M.; Wang, X.; Lv, H.; Che, R. One-Dimensional Magnetic FeCoNi Alloy Toward Low-Frequency Electromagnetic Wave Absorption. Nano-Micro Lett. 2022, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, Z.; Wang, M.; Cao, X.; Zhang, Y.; Song, P.; Zhang, T.; Ye, X.; Yang, Y.; Gu, W.; et al. Confining Tiny MoO2 Clusters into Reduced Graphene Oxide for Highly Efficient Low Frequency Microwave Absorption. Small 2020, 16, 2001686. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Nie, X.; Yang, W.; Wang, Y.; Yu, R.; Shui, J. Multifunctional Organic–Inorganic Hybrid Aerogel for Self-Cleaning, Heat-Insulating, and Highly Efficient Microwave Absorbing Material. Adv. Funct. Mater. 2019, 29, 1807624. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, X.; Yang, S.; Wang, D.; Liang, L.; Wang, S.; Ning, Y.; Yin, W.; Li, Y. Multifunctional elastic rGO hybrid aerogels for microwave absorption, infrared stealth and heat insulation. Chem. Eng. J. 2023, 452, 139376. [Google Scholar] [CrossRef]
- Shao, G.; Shen, X.; Huang, X. Multilevel Structural Design and Heterointerface Engineering of a Host–Guest Binary Aerogel toward Multifunctional Broadband Microwave Absorption. ACS Mater. Lett. 2022, 4, 1787–1797. [Google Scholar] [CrossRef]
- Shao, G.; Hanaor, D.A.H.; Shen, X.; Gurlo, A. Freeze Casting: From Low-Dimensional Building Blocks to Aligned Porous Structures—A Review of Novel Materials, Methods, and Applications. Adv. Mater. 2020, 32, 1907176. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhi, D.; Chen, Y.; Li, B.; Zhou, Z.; Meng, F. Multiaxial electrospun generation of hollow graphene aerogel spheres for broadband high-performance microwave absorption. Nano Res. 2020, 13, 477–484. [Google Scholar] [CrossRef]
- Zhi, D.; Li, T.; Qi, Z.; Li, J.; Tian, Y.; Deng, W.; Meng, F. Core-shell heterogeneous graphene-based aerogel microspheres for high-performance broadband microwave absorption via resonance loss and sequential attenuation. Chem. Eng. J. 2022, 433, 134496. [Google Scholar] [CrossRef]
- Huang, X.; Yu, G.; Zhang, Y.; Zhang, M.; Shao, G. Design of cellular structure of graphene aerogels for electromagnetic wave absorption. Chem. Eng. J. 2021, 426, 131894. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Xia, L.; Wang, R.; Cao, Y.; Cheng, Z.; Huang, Y. Much enhanced electromagnetic wave absorbing properties from the synergistic effect of graphene/γ-graphyne heterostructure in both gigahertz and terahertz band ranges. Nano Res. 2023, 16, 88–100. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Lu, Z.; Cheng, R.; Wang, N.; Li, Y. Construction of multi-dimensional NiCo/C/CNT/rGO aerogel by MOF derivative for efficient microwave absorption. Carbon 2023, 205, 411–421. [Google Scholar] [CrossRef]
- Huang, X.; Wei, J.; Zhang, Y.; Qian, B.; Jia, Q.; Liu, J.; Zhao, X.; Shao, G. Ultralight Magnetic and Dielectric Aerogels Achieved by Metal–Organic Framework Initiated Gelation of Graphene Oxide for Enhanced Microwave Absorption. Nano-Micro Lett. 2022, 14, 107. [Google Scholar] [CrossRef]

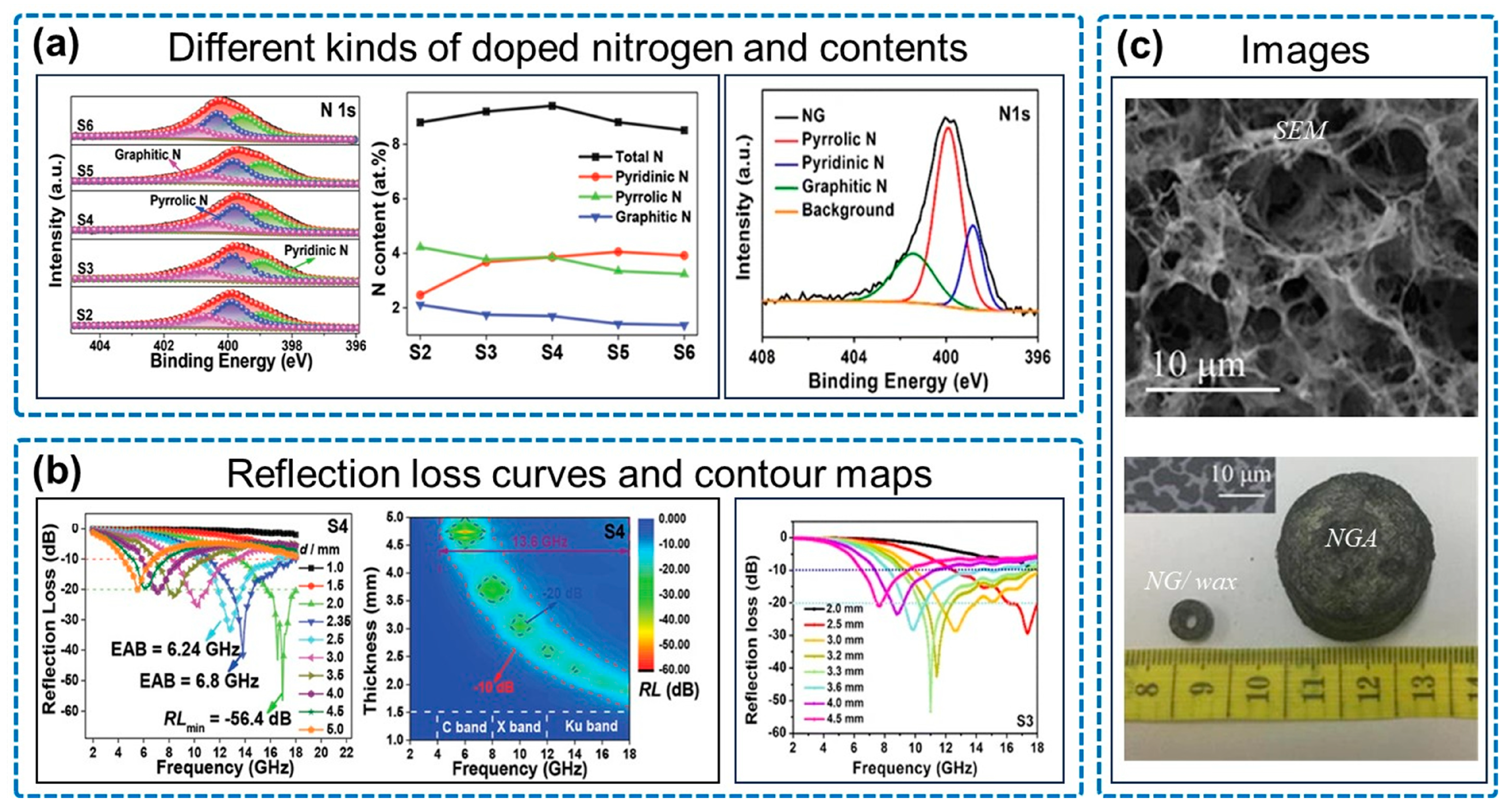

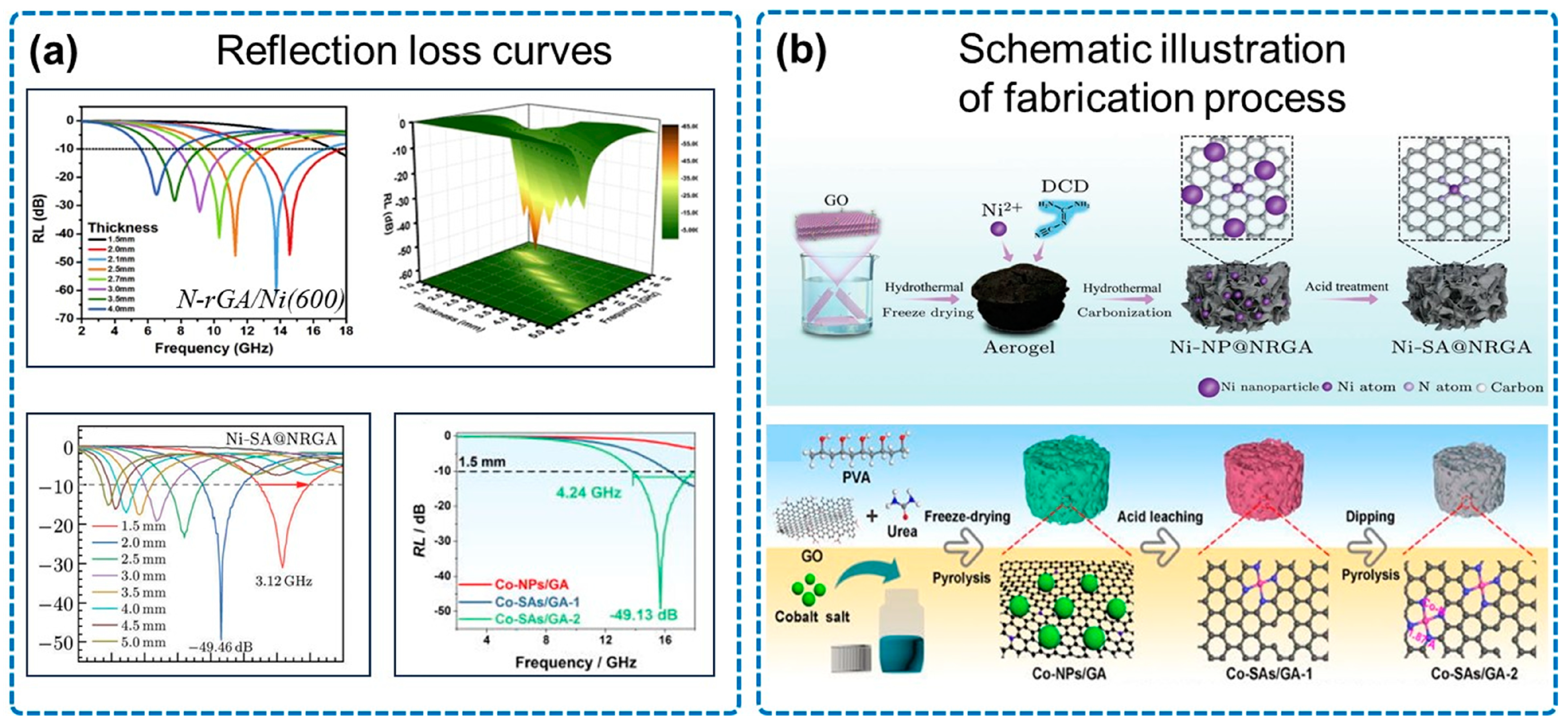

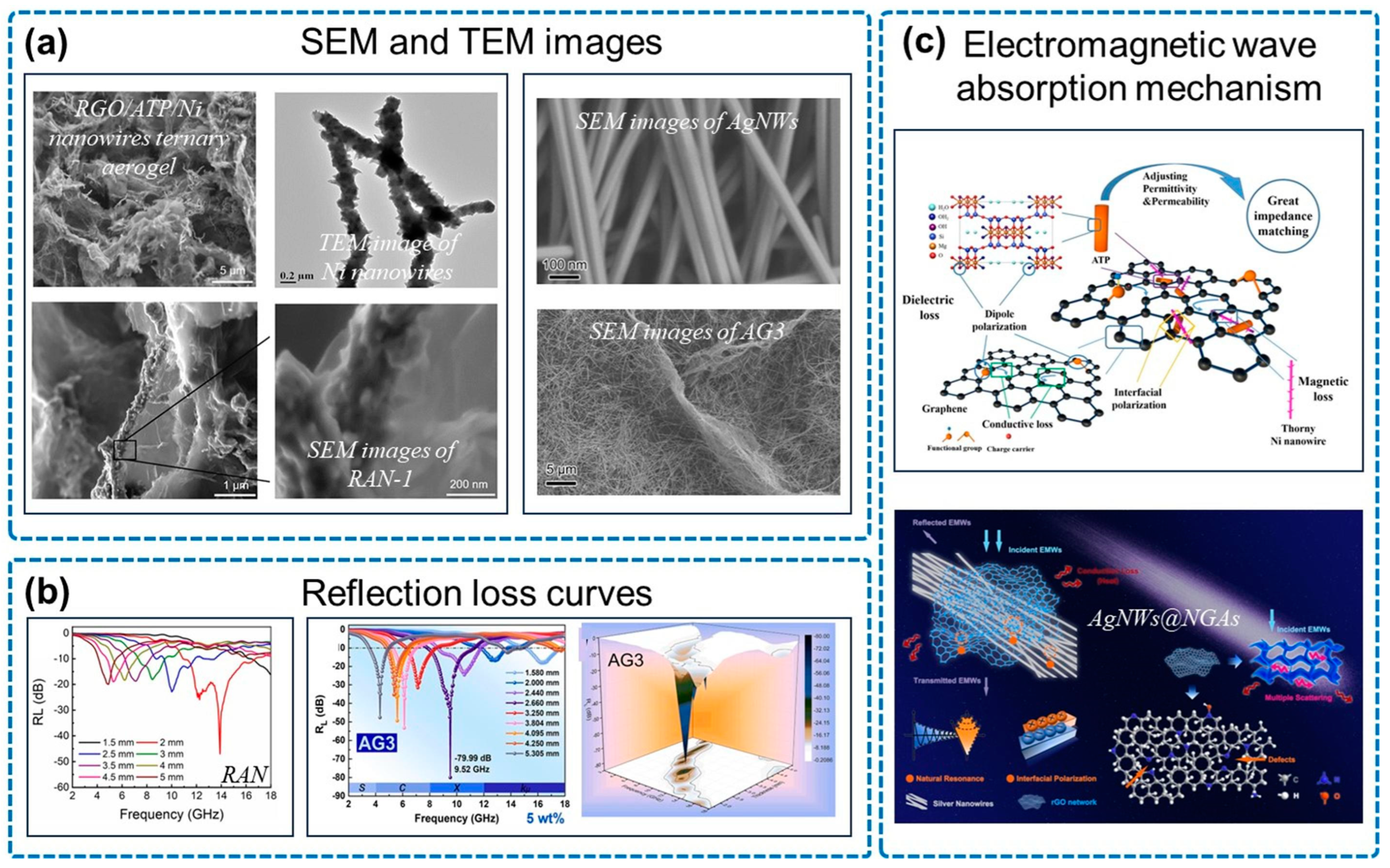
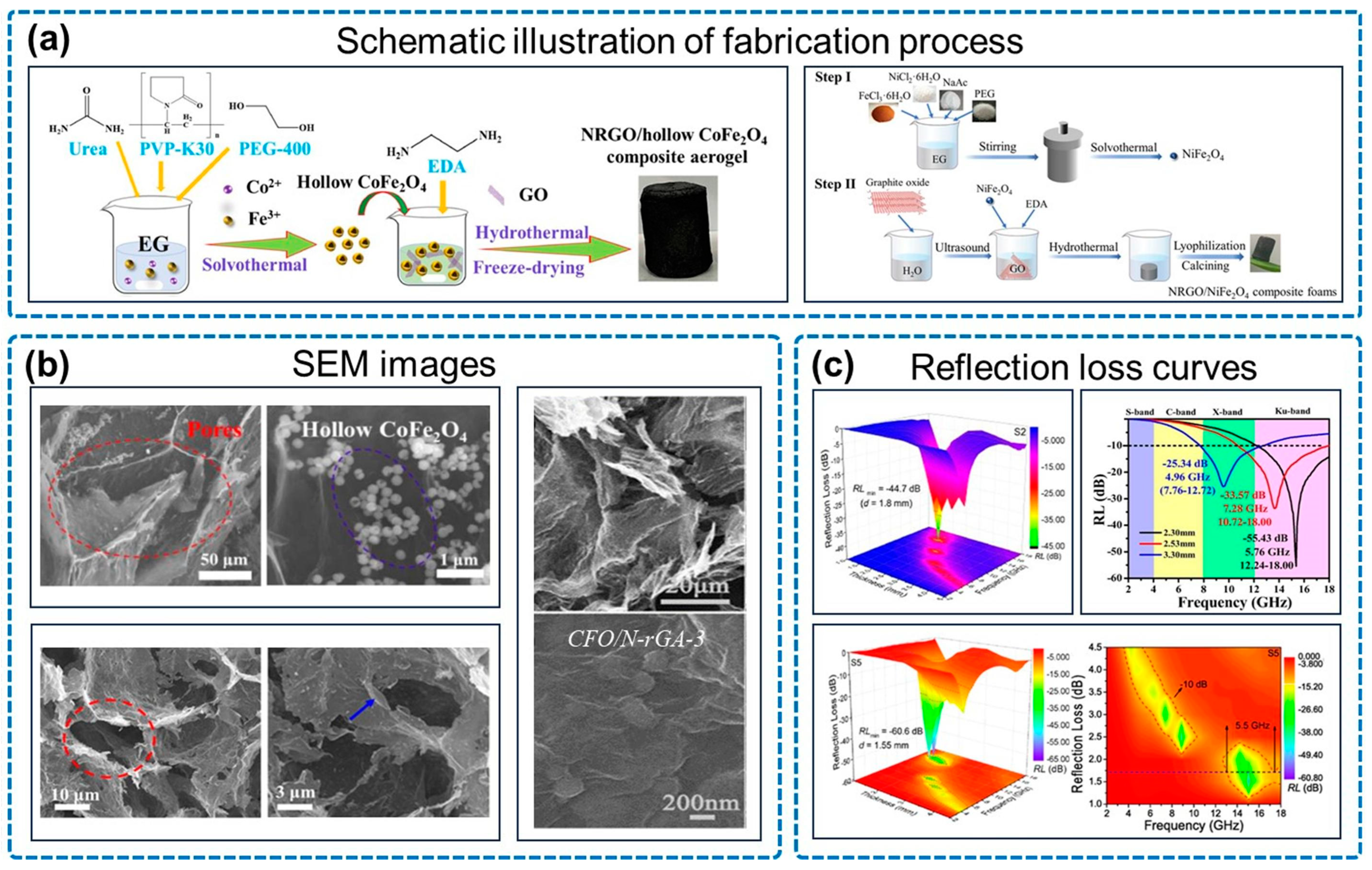
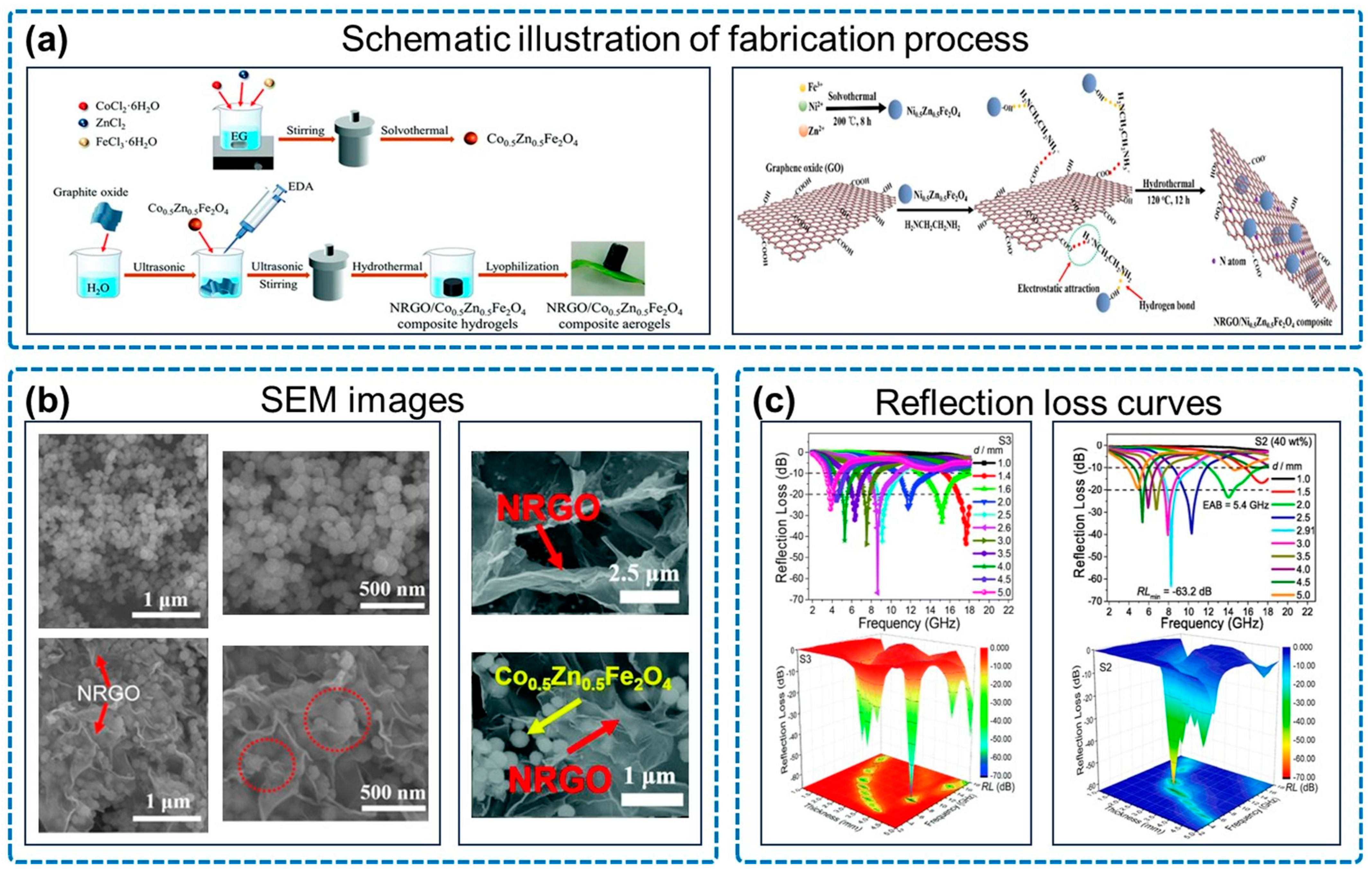
| Type | Sample | RLmin (dB) | EAB (GHz) | Thickness (mm) |
|---|---|---|---|---|
| Pure GA | GA [7] | −61.09 | 6.30 | 4.81 |
| Pure NGA | NGA [51] | −56.4 | 6.8 | 2.0 |
| NGA/magnetic metal | NGA/Ni [52] | −60.8 | 5.1 | 2.1 |
| AgNWs@NGA [57] | −79.99 | 3.5 | 2.66 | |
| CoNiFe-PBA/NGA [68] | −66.23 | 6.6 | 2.6 | |
| NGA/magnetic oxide | CFO/NGA [78] | −60.4 | 6.48 | 2.1 |
| Co0.5Zn0.5Fe2O4/NGA [82] | −66.8 | 5.0 | 2.6 | |
| CeO2/NGA [89] | −50.0 | 5.7 | 4.0 | |
| NGA/CNT | MWCNTs/NGA [100] | −46.3 | 4.2 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Yao, X.; Xing, Y. A Review of Nitrogen-Doped Graphene Aerogel in Electromagnetic Wave Absorption. Micromachines 2023, 14, 1762. https://doi.org/10.3390/mi14091762
Wu Z, Yao X, Xing Y. A Review of Nitrogen-Doped Graphene Aerogel in Electromagnetic Wave Absorption. Micromachines. 2023; 14(9):1762. https://doi.org/10.3390/mi14091762
Chicago/Turabian StyleWu, Ze, Xinke Yao, and Youqiang Xing. 2023. "A Review of Nitrogen-Doped Graphene Aerogel in Electromagnetic Wave Absorption" Micromachines 14, no. 9: 1762. https://doi.org/10.3390/mi14091762
APA StyleWu, Z., Yao, X., & Xing, Y. (2023). A Review of Nitrogen-Doped Graphene Aerogel in Electromagnetic Wave Absorption. Micromachines, 14(9), 1762. https://doi.org/10.3390/mi14091762







