1. Introduction
According to statistical data from 2018, breast cancers, cancers of the female reproductive system, and female genital organs caused the death of over a million women [
1]. Listed diseases are characterized by hormone dependence, phenotypic and molecular heterogeneity, and adaptive mechanisms, making them among diseases with high resistance to treatment [
2,
3]. Breast cancer is the most frequently diagnosed cancer, and there are two main types of breast cancer: ductal carcinoma (originating in the cells that line the milk duct) and lobular carcinoma (originating in the cells of the mammary glands of the breast lobules) [
4].
Breast cancer diagnostics are based on pathomorphological examinations of the collected tissue material and hormonal and molecular analyses [
5]. The treatment of breast cancer is complex. Depending on the type of cancer diagnosed, the stage of disease advancement, and the general health of the patient, several therapeutic methods are used in the treatment of breast cancer. These include surgery, radiotherapy, hormone therapy, chemotherapy, molecular therapies, and photodynamic therapy as a relatively new therapeutic approach. Combination therapy, based on the usage of several therapeutic approaches together, deserves special attention because it has been the most effective in the treatment of breast cancer for many years [
6].
In most cases, the selection of a therapeutic strategy for breast cancer depends on the tumor’s molecular characteristics. Chemotherapy is always used to treat the so-called “triple-negative” breast cancers, i.e., when the presence of estrogen receptors in the cancer cells is not diagnosed and the expression of the HER2 receptor is not confirmed [
7]. Both preoperative and postoperative treatment involve combination chemotherapy, which usually combines the effects of two or three anthracyclines. Less commonly, antimetabolites or alkylating drugs are used [
8]. In turn, photodynamic therapy (PDT), i.e., treatment method of cancerous changes based on the use of a photosensitizer (PS), light, and oxygen dissolved in the tissue, is an approach currently rarely used clinically in the treatment of breast cancer. Nevertheless, PDT has great potential in the treatment of breast cancer due to the high selectivity of photosensitizers and the low invasiveness of this therapy [
9,
10,
11]. It is worth investigating the effectiveness of PDT as a supportive therapy in the treatment of breast cancer.
Combination therapy is widely used in many medical fields, and it is based on the use of more than one drug or more than one type of therapy to treat a disease. The use of combination therapy in the treatment of breast cancers can significantly increase the therapeutic effectiveness and reduce the side effects of treatment, e.g., due to the possibility of reducing the doses of drugs [
12]. The effect of combination therapy may be additive when the treatment result is the sum of the two individual therapies, or synergistic when the treatment result is greater than the sum of their individual therapies [
12]. Nowadays, combining chemotherapy with other treatment methods or the use of combination chemotherapy is the basis of clinical cancer treatment. Chemotherapy can be combined with gene therapy [
13], immunotherapy [
14], or phototherapy [
15]. The synergy achieved thanks to combination therapy allows for a reduction in the dose of the drug and minimizes the side effects of treatment. In addition, it also gives a chance for effective treatment of cancers with a heterogeneous structure such as breast cancer. It was also proven that combination therapy can reverse multi-drug resistance that occurred in cancer cells and was caused by monochemotherapy [
16]. However, additional research is needed to optimize the procedures of combination therapies, especially in the case of sequential therapies in which compounds are administered one after the other. It is very important because the sequence of the component drugs administration to the cells may influence the overall therapeutic effect [
17].
Three-dimensional (3D) cell culture models that mimic the structure of cancer tissue are essential tools in modern cell engineering. The use of lab-on-a-chip microfluidic systems for cancer research allows cancer cells to be cultured in dynamic conditions, similar to conditions in a living organism [
18]. The use of microfluidic systems also gives the opportunity to conduct spatial cell co-cultures in which the interactions between non-malignant and cancer cells, and the interactions of the cells with the extracellular matrix (ECM) are preserved. Many studies indicate that the use of co-culture in in vitro cancer modeling studies is of great importance and advantage over the use of monoculture. Under physiological conditions, cancer tissue consists of both cancer cells and non-malignant cells of the same type: fibroblasts, adipocytes, inflammatory cells including lymphocytes and macrophages, as well as capillaries, and lymphatic vessels. Communication between these cells plays a crucial role in cancer development and progression. In particular, the interaction of cancer cells with fibroblasts capable of differentiating into CAF (cancer-associated fibroblasts) is important [
19]. Under in vivo conditions, fibroblasts are an important building element of the cancer microenvironment. Studies have shown that the content of these cells in cancer tissue of patients ranges from 20% to 90% [
20]. Many research groups are using co-culture in cancer research. Different ratios of normal and cancer cells in culture are used, between 1:1 and 1:10, to mimic the pathological cancer state [
21,
22].
A cancer-on-a-chip system can partially mimic tumor physiology in laboratory conditions [
23,
24]. The use of this type of microsystem may be of crucial importance in research into the effectiveness of combination anti-cancer therapies [
25]. In vitro 3D cancer models may also allow the study of sequential therapies, especially how the sequence of delivered therapeutic agents to cancer cells influences the efficiency of the used treatment.
In these studies, we propose a microfluidic model of a three-dimensional cell multilayer that can mimic the structure and microenvironment of breast cancer under in vitro conditions. To develop a 3D cellular model, we used a previously designed microfluidic system with a universal design [
26]. In this work, we proved the universality of the developed microfluidic technology, which has the potential to be used in the future in modeling other types of tissues characterized by the layered arrangement of cells (e.g., skin or bladder cancer). We used the developed breast cancer-on-a-chip model to analyze the effectiveness of anti-cancer therapies. We hypothesized that thanks to the use of flow conditions in the microsystem, it would be possible to create a cell multilayer composed of stromal cells and breast cancer cells. We assumed that the developed 3D cell model would imitate the structure of the stroma and parenchyma of cancer, and thus (to some extent) it would reflect the structure of a fragment of cancer tissue. We also assumed that the cell multilayer model would be used to test the effectiveness of chemotherapy, PDT, and combined sequential photochemotherapy in breast cancer treatment. The crucial stage of our work was to examine the influence of the sequence of administration of the component drugs during the combination therapy procedure on its effectiveness.
2. Materials and Methods
2.1. The Fabrication and Geometry of the Microfluidic System
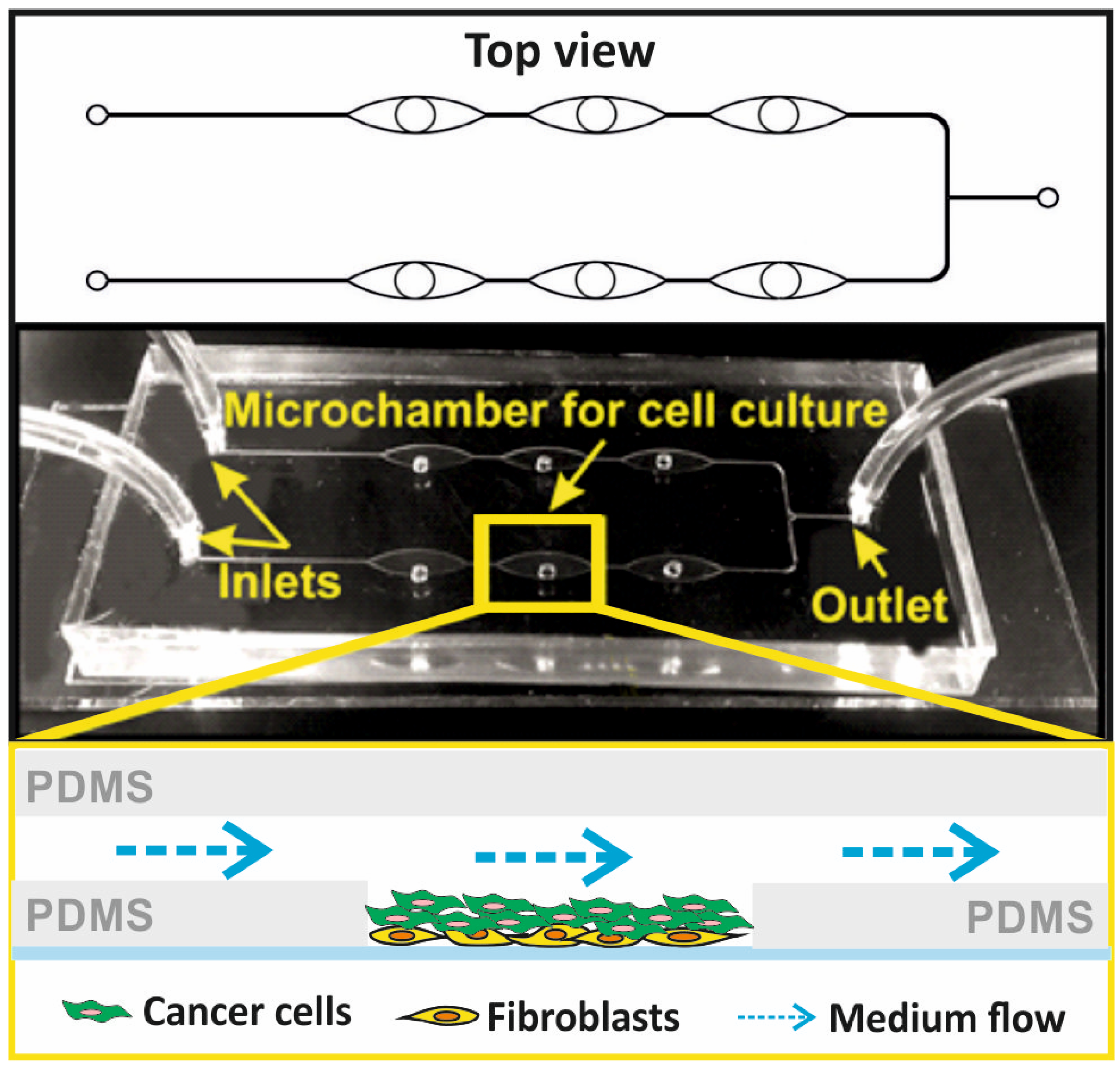
We designed a hybrid, three-layer (PDMS-PDMS-glass) microsystem for the culture of non-malignant and cancer cells in the form of a three-dimensional (3D) cell multilayer. The microsystem consisted of six culture microchambers, allowing several independent analyses to be performed simultaneously. The microchambers in the microsystem were arranged linearly in two rows and connected by a network of microchannels. The microsystem was made of three layers of transparent materials: the first layer of PDMS with a thickness of about 5 mm, equipped with a longitudinal microchamber and channels with a width of 100 µm; the second layer of PDMS with a thickness of 100 µm, equipped with six holes with a diameter of 2 mm of each; and the third layer of sodium glass with dimensions 76 mm × 25 mm × 1 mm. In order to make a thin layer of PDMS, two PMMA plates with dimensions of 100 mm × 100 mm each were used, one of which had a cavity with dimensions of 60 mm × 40 mm and a depth of 100 µm. The cavity between the plates was filled with a mixture of the non-cross-linked PDMS prepolymer with a cross-linking agent (Dow, Sylgard 184) in a weight ratio of 10:1, using a syringe. PDMS was cross-linked at a high temperature (75 °C, 90 min). The plates were then separated to obtain a 100 µm thick polymer membrane. Holes with a diameter of 2 mm each were made in the membrane with a precision Uni-Core punch, in accordance with the geometry of the pattern. All layers of the microsystem were connected using oxygen plasma, which allowed for the creation of a space for cell culture inside the microsystem. The microsystem was used for the culture of human mammary fibroblast and breast cancer cells. To mimic in vivo cancer conditions, mammary fibroblasts were used as the stroma for cancer cells (
Figure 1).
The microfluidic system for the 3D culture of non-malignant and cancer breast cells was produced using micromilling techniques and the PDMS casting technique. A detailed description of the microsystem fabrication was presented in our previous works [
26,
27]. The distribution of culture microchambers in the designed microsystem is consistent with the distribution of wells on a standard 384-well plate. Thanks to that, it was possible to perform spectrofluorometric measurements in the microsystem using a standard multi-well plate reader.
2.2. Cell Lines
Non-malignant and cancer breast cell lines were used in our study. The non-malignant cell line was human mammary fibroblasts (HMF), obtained from ScienCell Research. HMF are connective tissue cells that derive from mesoderm. The HMF cells were cultured using 75 cm2 standard culture flasks, with surface modified with poly-l-lysine in a concentration of 2 µg/cm2 (ScienCell, Carlsbad, CA, USA) in Fibroblast Medium (ScienCell) supplemented with 10% vol. fetal bovine serum (ScienCell), 1% vol. streptomycin and penicillin (ScienCell), and 1% vol. Fibroblast Growth Supplement (ScienCell). The cancer cell line used in this research was human breast adenocarcinoma (MCF-7), obtained from the American Type Culture Collection. The cancer cells were cultured using 25 cm2 standard culture flasks in DMEM medium (Biowest, Nuaillé, France), supplemented with 10% vol. fetal bovine serum (Biowest), 1% vol. streptomycin and penicillin (Biowest), and 2 mM L-glutamine (Biowest). Both cell lines were cultured at 37 °C in a humidified atmosphere, including 5% CO2 (HeraCell 150, Thermo Scientific, Waltham, MA, USA).
In the microfluidic system, two types of cells were cultured simultaneously in the form of a co-culture. In all studies in the microsystem, the Fibroblast Medium (ScienCell) supplemented with 10% vol. fetal bovine serum (ScienCell), 1% vol. streptomycin and penicillin (ScienCell), and 1% vol. Fibroblast Growth Supplement (ScienCell) was used. A culture medium with a richer composition was used for cell co-culture.
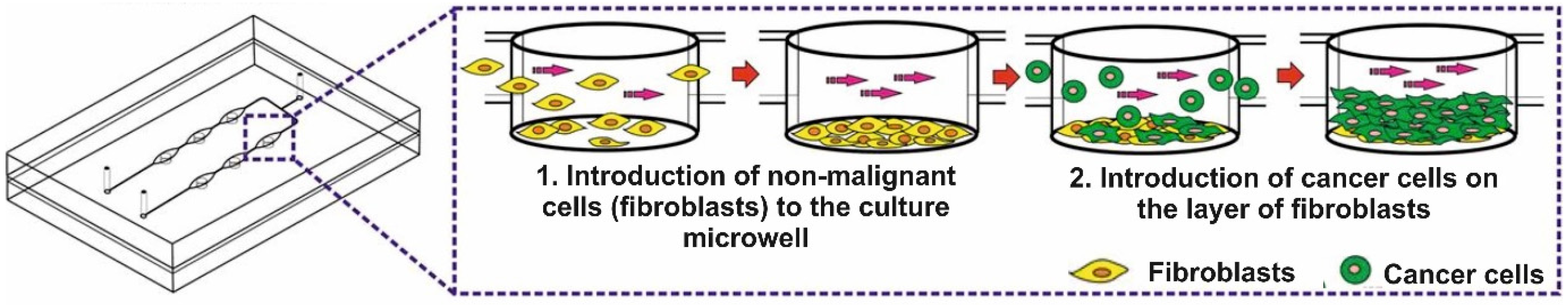
2.3. The Cell Introduction into the Microsystem and 3D Cell Culture Creation
Before starting cell culture, the microsystem was sterilized. For this purpose, an ethyl alcohol solution with a concentration of 70% vol. was introduced into the microsystem using a peristaltic pump for 20 min (flow rate = 5 µL/min). At the same time, the microsystem was exposed to UV radiation (UV Black Ray lamp, time = 20 min, a distance of the microsystem from the light source = 20 cm). The microsystem was filled with the medium and incubated for 3 h (37 °C, 5% CO2).
To prepare the cell suspension for examination in the microsystem, fibroblasts and cancer cells were washed with DPBS solution (Biowest) and then detached from the culture bottle with trypsin (Biowest). The cells were centrifuged and then resuspended in 1 mL of medium. Next, the cell suspension density was established based on calculations in Thoma chambers and diluted to obtain the appropriate cell density. Cell suspensions with a density of 10
6 cells/mL (in the case of non-malignant cells) or 3 × 10
6 cells/mL (in the case of cancer cells) were introduced into the microsystem. The ratio of the seeding density of fibroblasts and cancer cells in the microsystem was optimized. The results of the optimization tests are presented in the
Supplementary Materials (Figure S1).
A fibroblast suspension was prepared and introduced into the microsystem via a peristaltic pump at a flow rate of 10 µL/min. The regular cell distribution in the microchambers was monitored with an inverted microscope. Non-malignant cells in the microsystem were incubated for 24 h (37 °C, 5% CO
2). Once the fibroblasts adhered to the substrate, a cancer cell suspension was prepared. Breast cancer cells were introduced directly onto the layer made of non-malignant cells (flow rate = 10 µL/min) (
Figure 2). Some of the cancer cells adhered to the substrate, and some to the layer of non-malignant cells. In this way, a multilayer cell culture was obtained in the microsystem. In the following days of culture, the proliferation and changes in the morphology of the cells in the microsystem were monitored. The medium was changed every 24 h (max. flow rate = 2 µL/min). The obtained cell multilayers were fluorescently stained and imaged using confocal microscopy to confirm the uniform and reproducible cell distribution in all culture microwells in the microsystem. The imaging results are presented in the
Supplementary Materials (Figure S2).
The geometry of the microsystem allowed the cells to be placed in the culture microwell. The non-malignant cells introduced into the microsystem stayed at the bottom of the microwells and adhered to the substrate there. The cancer cells also stopped inside the microwells and adhered to a monolayer made of fibroblasts (
Figure 2). The cancer cells in the microsystem could proliferate and grow on top of each other.
It was assumed that the formation of a cell multilayer in the microsystem was possible due to interactions between cancer cells and stromal cells (fibroblasts). The fibroblasts used in the experiments are the primary cell lines that are sensitive to some mechanical factors. Direct exposure of the monolayer of non-malignant cells to the flow of the cancer cell suspension could cause fibroblasts to detach from the substrate (due to their strong affinity for cancer cells) [
27]. The cell culture in the microwells could reduce the effect of the detachment of the cells. This was an additional benefit resulting from the 3D geometry of the microsystem. The longitudinal shape of the microchambers (above the microwells) allowed the internal volume of the microsystem to be increased. As a result, additional space above the cell multilayer, filled with the culture medium was obtained. Thus, the geometry of the microsystem was designed to provide the cells with a sufficient culture medium to maintain the normal vital functions of the cells in a multilayer culture.
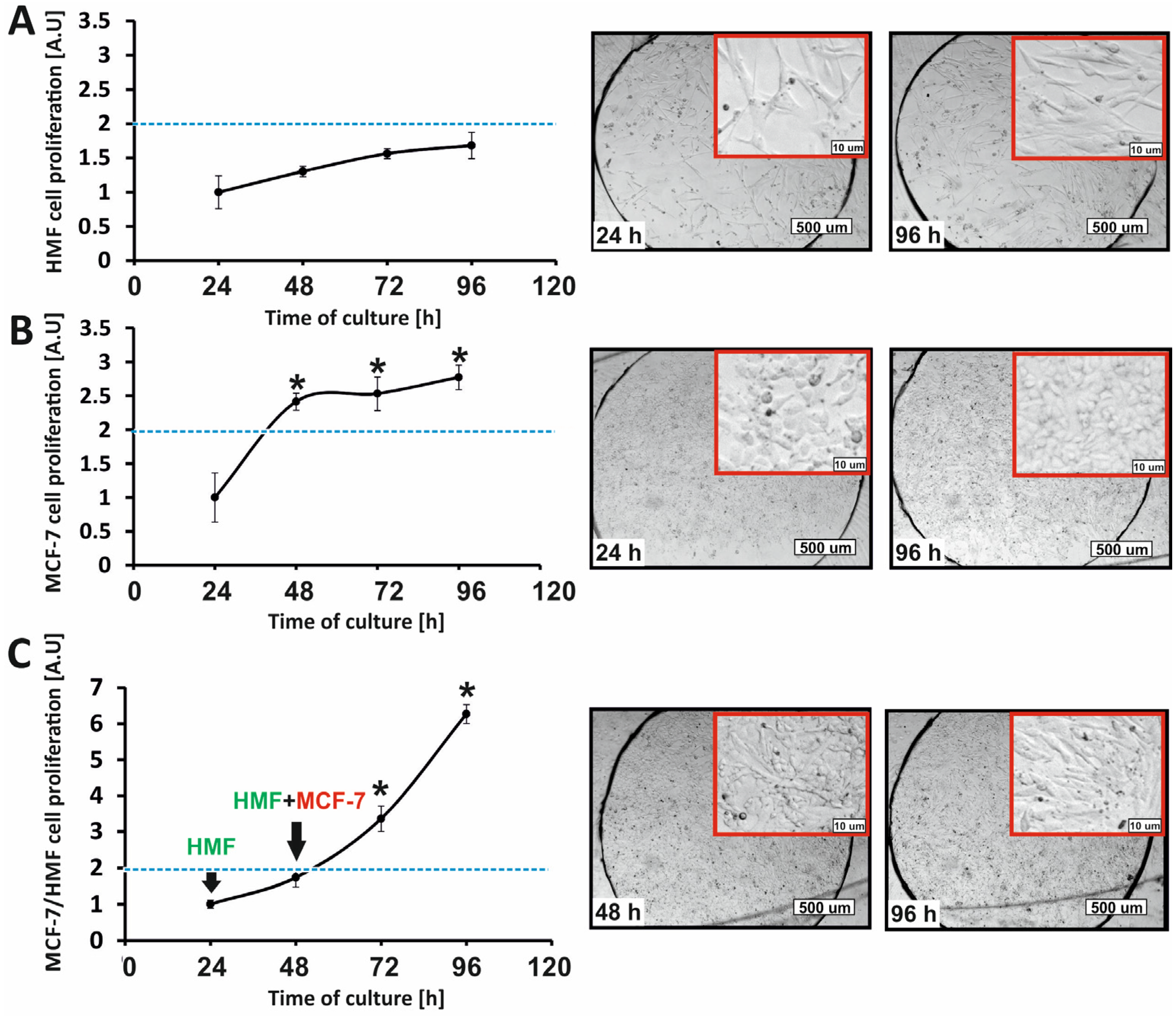
Studies on the proliferation of normal cells and cancer breast cells growing in the form of cell monolayers were also performed in the developed microsystem. In order to obtain monolayers of MCF-7 and HMF cells, cell suspensions with densities of 3 × 106 cells/mL and 106 cells/mL, respectively, were introduced into the microsystem. Then, the microsystem with cells was incubated for 24 h.
2.4. Analysis of 3D Cell Culture Formation under Microfluidic Conditions
In order to confirm the three-dimensional, spatial, and multilayered arrangement of cells grown in the microsystem, the non-malignant and cancer cells were stained with fluorescent dyes (CellTrackers, Thermo Fisher Scientific, Waltham, MA, USA). The non-malignant cells (HMF) were stained with red CMTPX dye. For this purpose, the cells in the culture vessel were incubated with 1 mL of CMTPX solution at a concentration of 6.25 µg/mL for 45 min (37 °C, 5% CO2). MCF-7 cells were stained with CMFDA, which showed green fluorescence. For this purpose, the cancer cells were incubated with 1 mL of CMFDA dye solution at a concentration of 5 µg/mL, also for 45 min (37 °C, 5% CO2). CellTracker dye working solutions were prepared via 1000-times dilution of the stock of CMFDA and CMTPX solutions in a culture medium without FBS and phenol red. The stained cells were detached from the surface, then introduced into a microsystem and cultured for 96 h. After incubation, a Z-axis scan of cell culture was performed using a confocal microscope (Zeiss Axio Observer 7 with LSM 900). Image acquisition was performed using ZEN Blue 3.6 software.
2.5. Analysis of Cell Proliferation in Co-Culture
2.5.1. AlamarBlue Assay
The AlamarBlue assay was used to test the proliferation and determine the metabolic activity of cells grown in the microfluidic system. For this purpose, a 10% vol. AlamarBlue reagent solution was prepared in a culture medium. The solution was loaded into the microsystem using a peristaltic pump (for 15 min, flow rate = 2 µL/min). The microsystem with the cells was incubated for 45 min (37 °C, 5% CO2), and then the fluorescence intensity (λex = 552 nm, λem = 582 nm) was measured with a plate reader (Varian, Palo Alto, CA, USA). The time of cell incubation with the AlamarBlue reagent solution was selected experimentally. The measured fluorescence intensity was proportional to the number of metabolically active (viable) cells in the population.
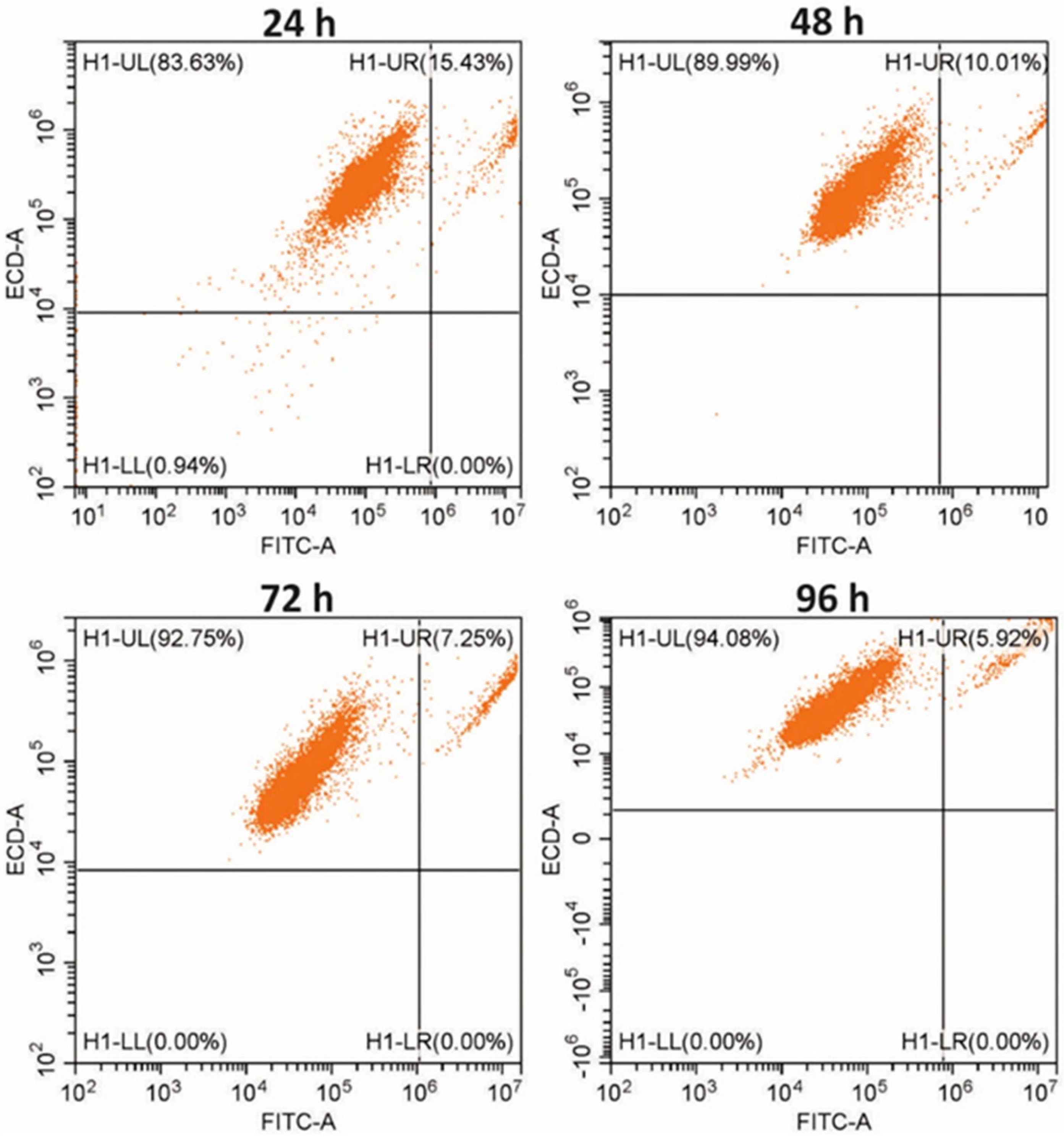
2.5.2. Flow Cytometry
Changes in the number of non-malignant and cancer cells co-cultured in the macroscale for 4 following days were analyzed via flow cytometry. For analysis, the cells were stained with vital fluorescent dyes (CellTrackers, Thermo Fisher Scientific) according to the manufacturer’s instructions. Fibroblasts were stained with CMFDA (green fluorescence). For this purpose, the fibroblasts in the culture vessel were incubated with 1 mL of CMFDA dye solution at a concentration of 5 µg/mL for 45 min (37 °C, 5% CO2). The cancer cells were stained with red CMTPX dye. For this purpose, the cells in the culture vessel were incubated with 1 mL of CMTPX solution at a concentration of 6.25 µg/mL, also for 45 min (37 °C, 5% CO2). The stained cells were seeded in a 12-well plate. Only non-malignant cells (fibroblasts monoculture) were seeded in the first four wells, only cancer cells (cancer monoculture) were plated in the next four wells, and both non-malignant and cancer cells (co-culture) were co-cultured in the last four wells of the plate. Three different samples were prepared daily for analysis: fibroblast monoculture, cancer cell monoculture, and non-malignant and cancer cell co-culture. The suspensions of the cells detached from the substrate were centrifuged (3 min, 2000 rpm), and each of the obtained cell pellets was resuspended in 300 µL of the medium. Changes in the number of cells in the non-malignant, cancer, and co-culture populations were analyzed using a flow cytometer (CytoFLEX). Sample preparation procedures and analysis were repeated for four following days.
2.6. Combined Therapy Procedure in the Microsystem
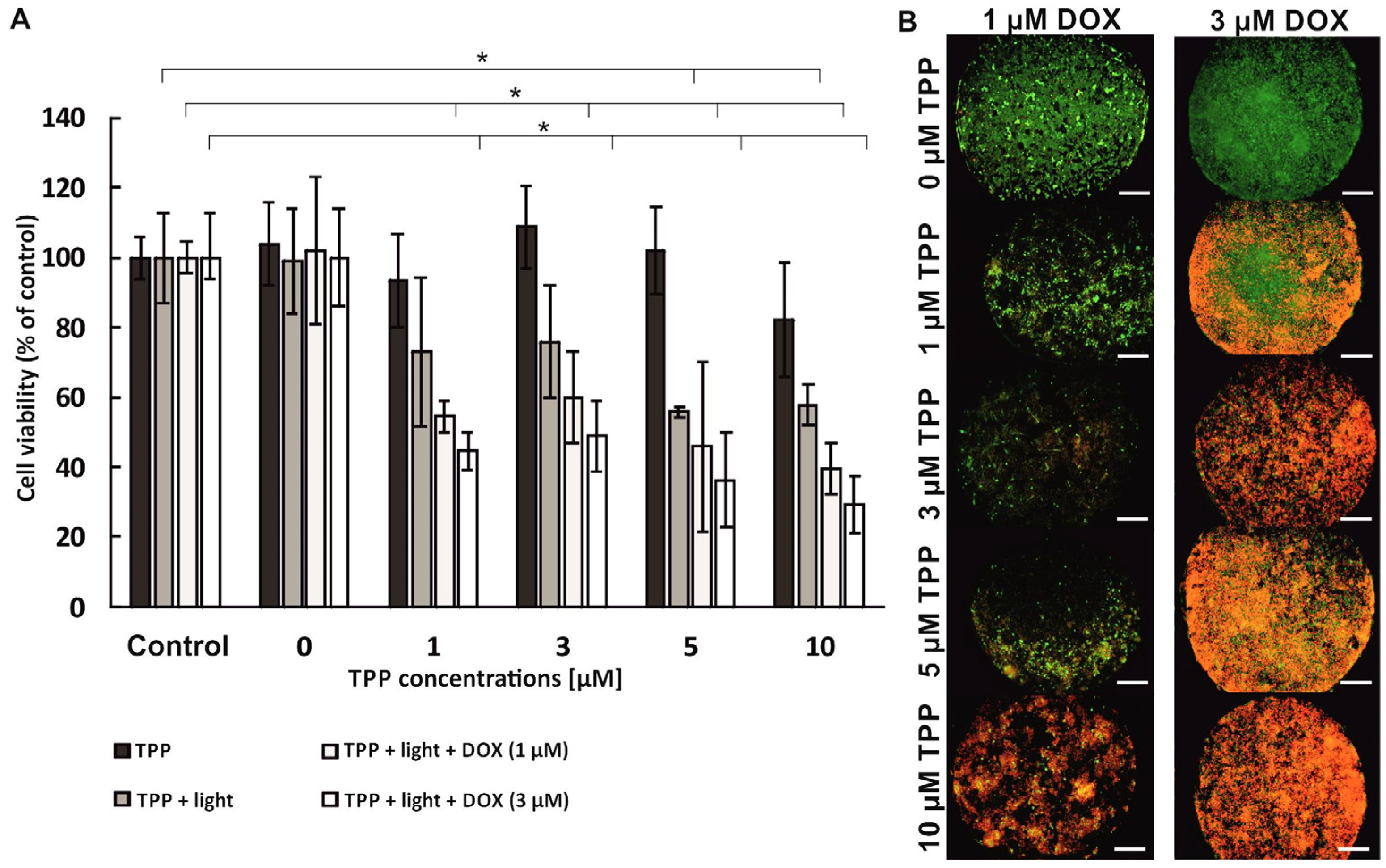
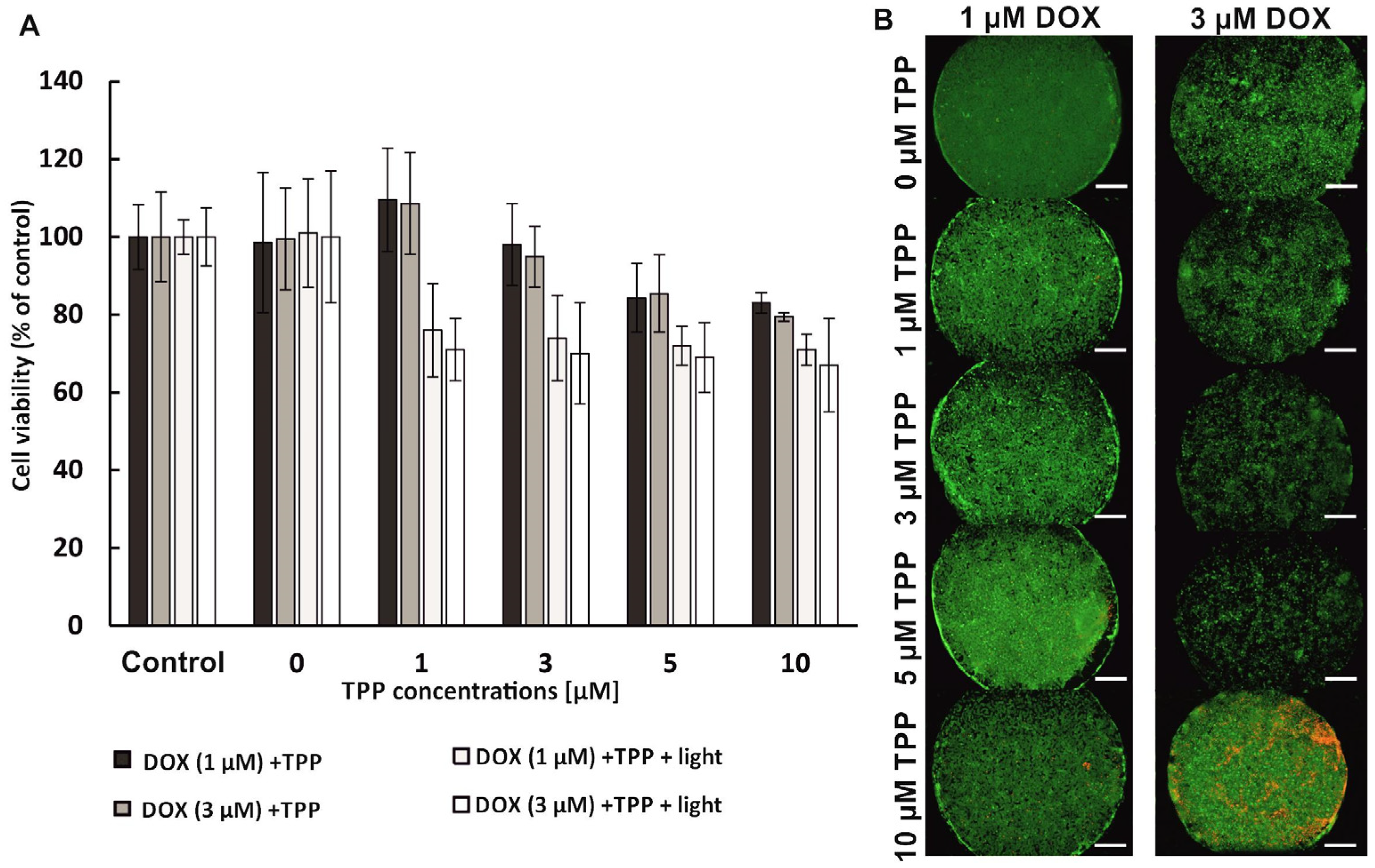
The combination therapy was performed in sequential mode, which means that the anti-tumor compounds were administered to the cells one after the other. Two sequences of compound administration were used in the research: (1) PDT→DOX, which means that in the first stage the PDT procedure with the use of free meso-tetraphenylporphyrin (as photosensitizer—PS) was carried out, and next the chemotherapy procedure with the use of doxorubicin was performed; (2) DOX→PDT, which means that the chemotherapy procedure was performed first, and then the administration of PS solutions and irradiation were performed (PDT procedure).
To perform the combination therapy in the PDT→DOX sequence, photosensitizer solutions with concentrations of 0 µM–10 µM were prepared in the culture medium without FBS and introduced into the microsystems (for 15 min, at a flow rate of 2 µL/min). The cells were incubated with PS for 24 h (37 °C, 5% CO2). Next, the medium with the non-accumulated photosensitizer was removed by introducing PBS into the microsystem. Then, a fresh medium was introduced into the microsystem (5 min, 2 µL/min). The cells in the microsystem were irradiated (10 min, λ = 640 nm, 40 mW/cm2), and then re-incubated for 24 h (37 °C, 5% CO2). In the next stage, doxorubicin solutions with concentrations 0 µM, 1 µM, and 3 µM were prepared in the culture medium without phenol red. Then, the solutions of DOX were introduced into the microsystems (15 min, 2 µL/min). The cells in the microsystem were incubated with doxorubicin solutions for 24 h (37 °C, 5% CO2). For the combined therapy in the DOX→PDT sequence, first doxorubicin solutions were introduced into the microsystem (15 min, 2 µL/min), and then incubated for 24 h (37 °C, 5% CO2). After this time, PS solutions were introduced (15 min, 2 µL/min). After 24 h incubation (37 °C, 5% CO2) the cells were irradiated (10 min, λ = 640 nm, 40 mW/cm2). Finally, the cells were re-incubated for 24 h (37 °C, 5% CO2). After the combination therapy procedure in the microsystem (PDT→DOX or DOX→PDT), the AlamarBlue test and the CAM/PI differential staining were performed. The control samples were cells untreated with any compound (no TPP, no DOX) and irradiated or non-irradiated.
2.7. Differential Staining with Calcein-AM and Propidium Iodide (CAM/PI)
To determine the viability of the cells cultured in the microsystems, a solution containing PI (final concentration: 2 µg/mL) and CAM (final concentration: 4 µM) was introduced into the microsystem with cultured cells. The prepared dye solution was introduced into the microsystem at a flow rate of 2 µL/min. The cells were incubated with fluorescent dyes for 10 min (37 °C, 5% CO2). After incubation, the cells were observed with an inverted fluorescence microscope (Olympus IX71). Live cells showed green fluorescence and dead cells showed red fluorescence.
2.8. Statistical Analysis
A minimum of three independent repetitions were performed for each tested concentration of compounds (nmin = 3). One repetition means performing the tests in one microsystem (in six culture microwells). The obtained results were averaged, and the standard deviation (SD) was calculated. The ANOVA test was performed, which consisted of a one-step analysis of variance. The level of statistical significance α was set at 0.05 (95% confidence interval). Data marked with an asterisk in the graphs indicate statistically significant differences. All results shown in the graphs are expressed as a percentage of cell viability relative to the control.
4. Discussion
In our research, we developed a 3D cancer-on-a-chip model that could be used to analyze the effectiveness of anti-cancer therapies. A multilayer cell model was used to test the efficacy of combined photochemotherapy in the treatment of breast cancer. The proliferation of co-cultures of non-malignant and cancer breast cells under microfluidic conditions was investigated in the first stage of this research. Fibroblasts are physiological components of the stroma of a tumor. In our study, it was proven that the presence of fibroblasts in cell culture was a factor that stimulated the division of cancer cells. As a result, cell co-culture under microfluidic conditions allowed us to obtain a spatial cell model similar to the structure of in vivo cancer tissue. The developed microfluidic system combines three features that determine the creation of a 3D multilayer cell culture:
Specific geometry that allows for cell culture in a microwell with indirect exposure to flow;
Possibility of generating laminar flow (imitating blood flow) and controlling it;
Possibility of seeding layers of stroma cells (fibroblasts) and cells imitating tumor parenchyma (cancer cells) in microchambers.
The developed microfluidic system allowed easy observation of reorganization and 3D cell structure (among others thanks to the use of transparent construction materials—PDMS/glass).
Photochemotherapy is a form of combined anti-cancer treatment and it includes the use of light and photosensitizers in therapy, as well as other anti-cancer drugs, e.g., cytostatics [
28,
29]. In our research, we used two types of compounds with anti-cancer potential and different mechanisms of action. Doxorubicin is a phase-specific anthracycline clinically used in monochemotherapy or combination chemotherapy for many types of cancer, including gynecological and breast cancers [
30]. Meso-tetraphenylporphyrin is a synthetic heterocyclic porphyrin compound and a photosensitizer clinically used in photodynamic anti-cancer therapy (PDT) [
31]. The PDT mechanism is based on the activation of the photosensitizer accumulated in cells by the light of appropriate power and wavelength. The activated photosensitizer undergoes a series of energy transformations, and as a result, reactive oxygen species are produced in the cancer cell. The effect of PDT is the death of cancer cells [
32].
The analysis of the results obtained in our study proved that using two drugs with varying mechanisms of action in the sequential combined therapy of breast cancer could be very effective. However, it was confirmed that the effect of sequential photochemotherapy with doxorubicin and meso-tetraphenylporphyrin significantly depends on the sequence of therapeutic procedures. A synergistic therapeutic effect was observed in the case of using the PDT→DOX sequence. The cumulative effect of the combination of the two therapies was higher than the efficacy of monochemotherapy or photodynamic therapy. It is probable that the accumulation of photosensitizer in breast cancer cells, followed by the action of intracellular oxidative stress induced by TPP activation, sensitized breast cells to the cytostatic effects. We supposed that breast cancer cells became susceptible to the action of doxorubicin, and the replication processes of cancer cells were inhibited in cell culture. However, no synergistic effect was observed when the reverse sequence of component procedures was used during photochemotherapy (DOX→PDT). In addition, the high viability of breast cells after photochemotherapy indicated that only the cytostatic compound influenced their viability. The uptake of the photosensitizer via doxorubicin-loaded breast cancer cells was probably reduced. The accumulation of cytostatics in the cancer cells could reduce the accumulation of the photosensitizer [
33]. Thus, the photodynamic therapy procedure did not enhance the therapeutic effect because the photocytotoxic reactions could not proceed correctly, with sufficient efficacy to reduce cell viability.
There are several reports in the literature that confirm that in vitro studies on the use of the combination of PDT with chemotherapy in the treatment of breast cancers, cancers of the female reproductive system, and female genital organs are conducted [
34,
35,
36,
37,
38]. Benito-Miguel et al. [
34] proposed the use of cytostatic nanoparticles to enhance the photodynamic effect during PDT on cervical cancer cells (HeLa). The therapy was performed with 5-fluorouracil encapsulated in biodegradable chitosan nanocapsules in combination with a photosensitizer and 5-aminolevulinic acid. Cancer cell viability, apoptotic processes, and ROS level were examined after sequential combination therapy. The authors proved a synergistic effect of the tested combination therapy. Sanchez-Ramirez et al. [
37] studied the efficacy of photochemotherapy on SCOV-3 ovarian cancer cells. Poly (lactide-glycol) nanoparticles combined with carboplatin (cytostatic) and indocyanine green (photosensitizer) were designed and synthesized. An increase in the photocytotoxic effect was observed after irradiation of ovarian tumor cells, which contained accumulated component drugs.
The dependence of the effectiveness of combination therapy on the sequence of the administered compounds was examined [
29,
39,
40,
41,
42,
43,
44]. Rizvi et al. [
29] studied the sequential combination of the benzoporphyrin monoacid as a photosensitizer with carboplatin action. They performed research using three-dimensional models of ovarian cancer formed in a hydrogel. Carboplatin was administered to the ovarian cells before or after PDT. A synergistic effect was achieved only for one of the used sequences. Erdem et al. [
39] conducted a similar study on SCOV-3 ovarian cells. They tested the effectiveness of the combination of verteporphyrin-based PDT with the action of cationic peptides. The obtained results confirmed that only the delivery of cationic peptides to the cells before the photosensitizer and irradiation allows the synergistic effect of the therapy to be achieved. In turn, Ali et al. [
40] performed photochemotherapy based on the combination of doxorubicin or methotrexate with PDT based on aluminum phthalocyanines. Various sequences of therapeutic procedures were tested on cancer cells from the cervix, breast, and brain. It was confirmed that the order of drug administration did not affect the effectiveness of the combination therapy.
In contrast to the literature [
29,
39,
40,
41,
42,
43,
44], we present for the first time, a study of the effectiveness of combined photochemotherapy on a three-dimensional, spatial, and heterogeneous cell model that mimics the structure of cancer tissue. To the best of our knowledge, a dependence of the efficacy of combined photochemotherapy on the sequence of the component drugs was observed under microfluidic conditions for the first time. In conclusion, the advanced cancer-on-a-chip cell multilayer model was successfully used to perform combined photochemotherapy on non-malignant and cancer breast cells. A synergistic therapeutic effect was obtained, but only in one of the two tested delivery sequences of the component compounds. It was investigated that the chemotherapy procedure can immunize cancer cells against the effects of photodynamic therapy, possibly as a result of reduced photosensitizer accumulation. Therefore, it was proven that the effect of sequential photochemotherapy is strongly dependent on the sequence of component procedures. Photochemotherapy is a method not widely used to treat breast cancers, cancers of the female reproductive system, and female genital organs. Nevertheless, our research shows that there is a potential for clinical use of photochemotherapy in the treatment of breast cancers and obtaining high treatment effectiveness.