Preparation and Properties of GO/ZnO/nHAp Composite Microsphere Bone Regeneration Material
Abstract
:1. Introduction
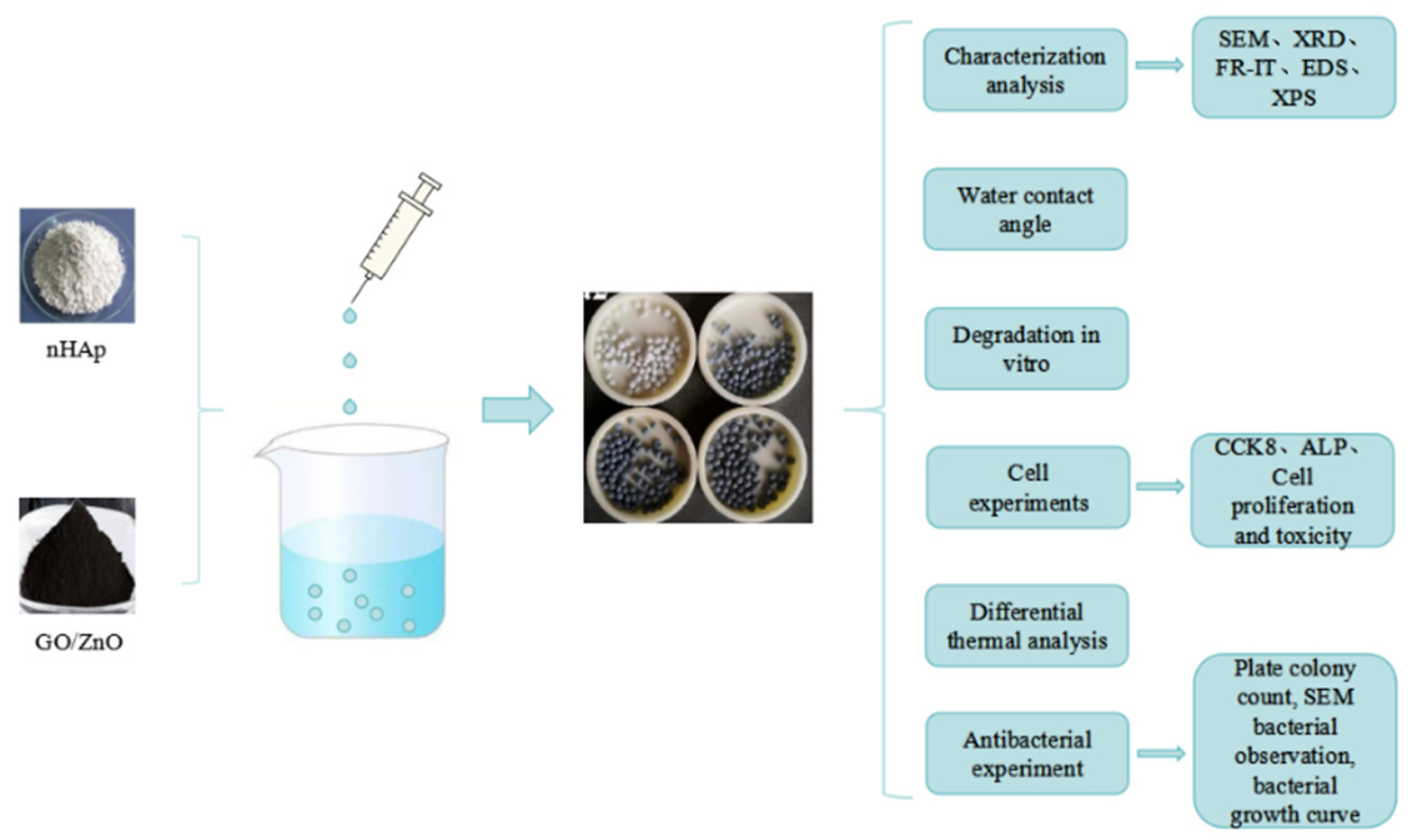
2. Materials and Methods
2.1. Preparation of Hydroxyapatite Nanoparticles (nHAps)
2.2. Preparation of Graphene Oxide, Zinc Oxide, and Hydroxyapatite (GO-ZnO-nHAp) Composite Microspheres
2.3. Analysis and Characterization
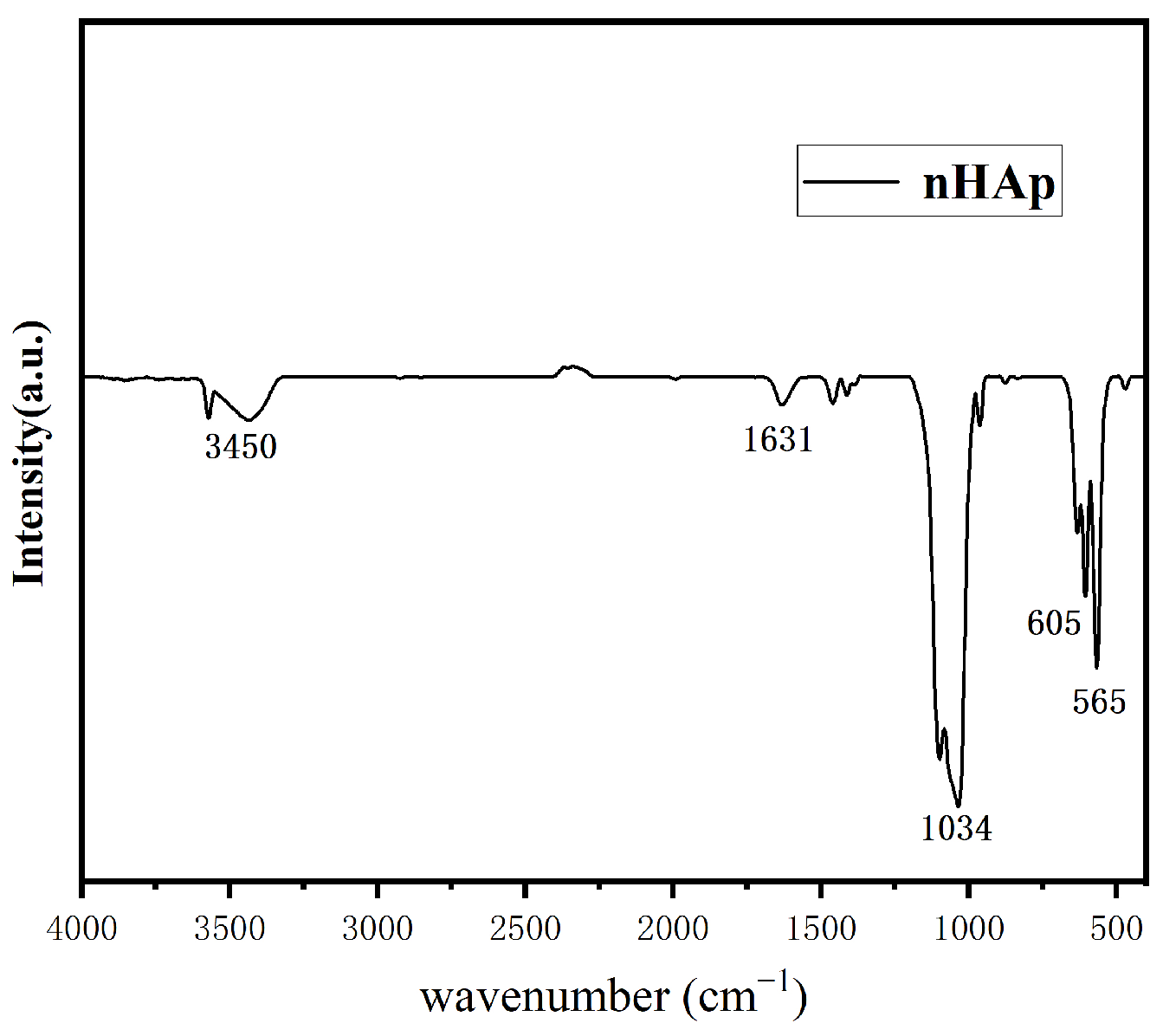
2.3.1. nHAp Characterization
Field Emission Scanning Electron Microscope (FE-SEM)
X-ray Diffraction (XRD)
Fourier Transform Infrared (FT-IR)
2.3.2. Characterization of GO-ZnO-nHAp Composite Microspheres
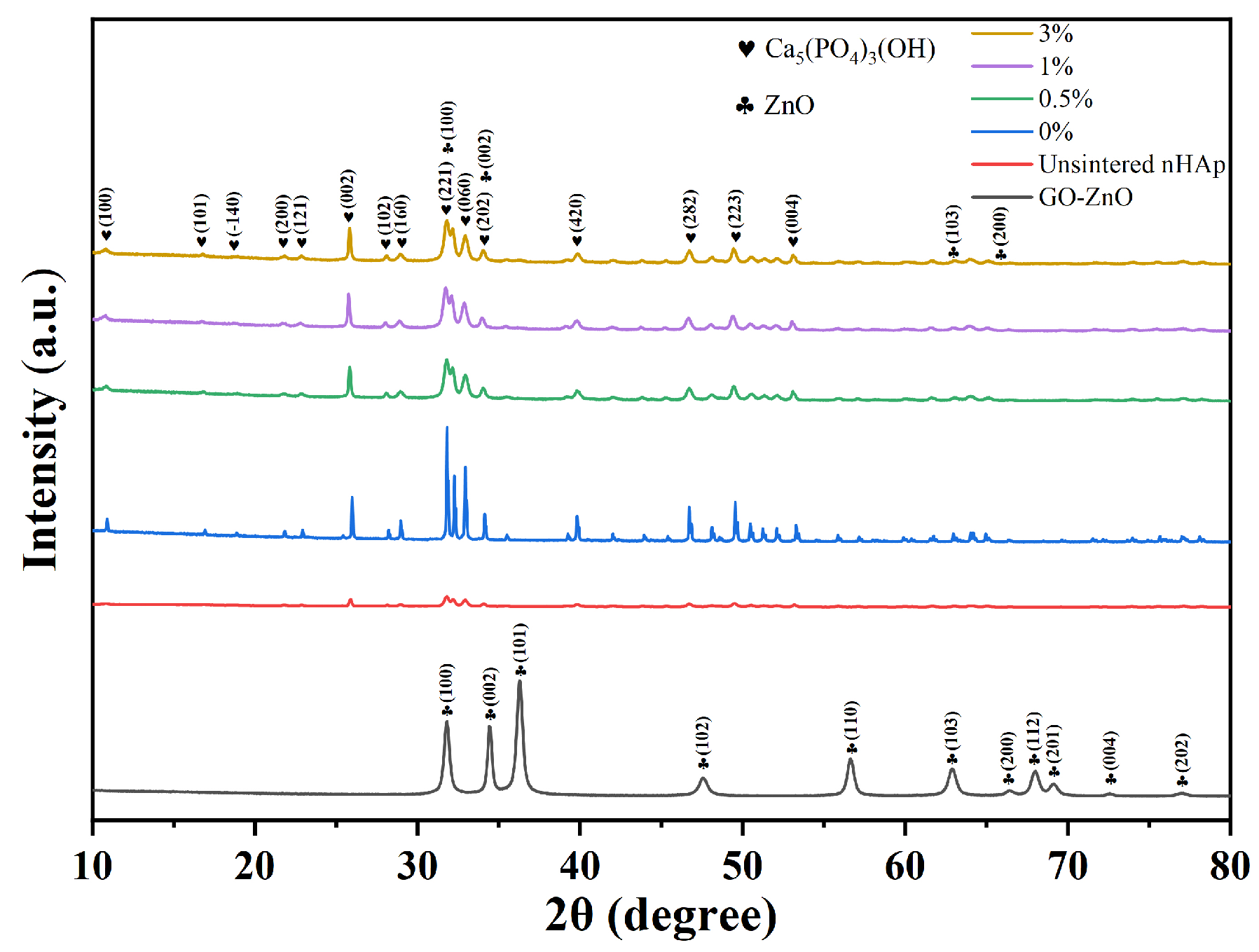
X-ray Diffraction (XRD)
Field Emission Scanning Electron Microscope (FE-SEM)
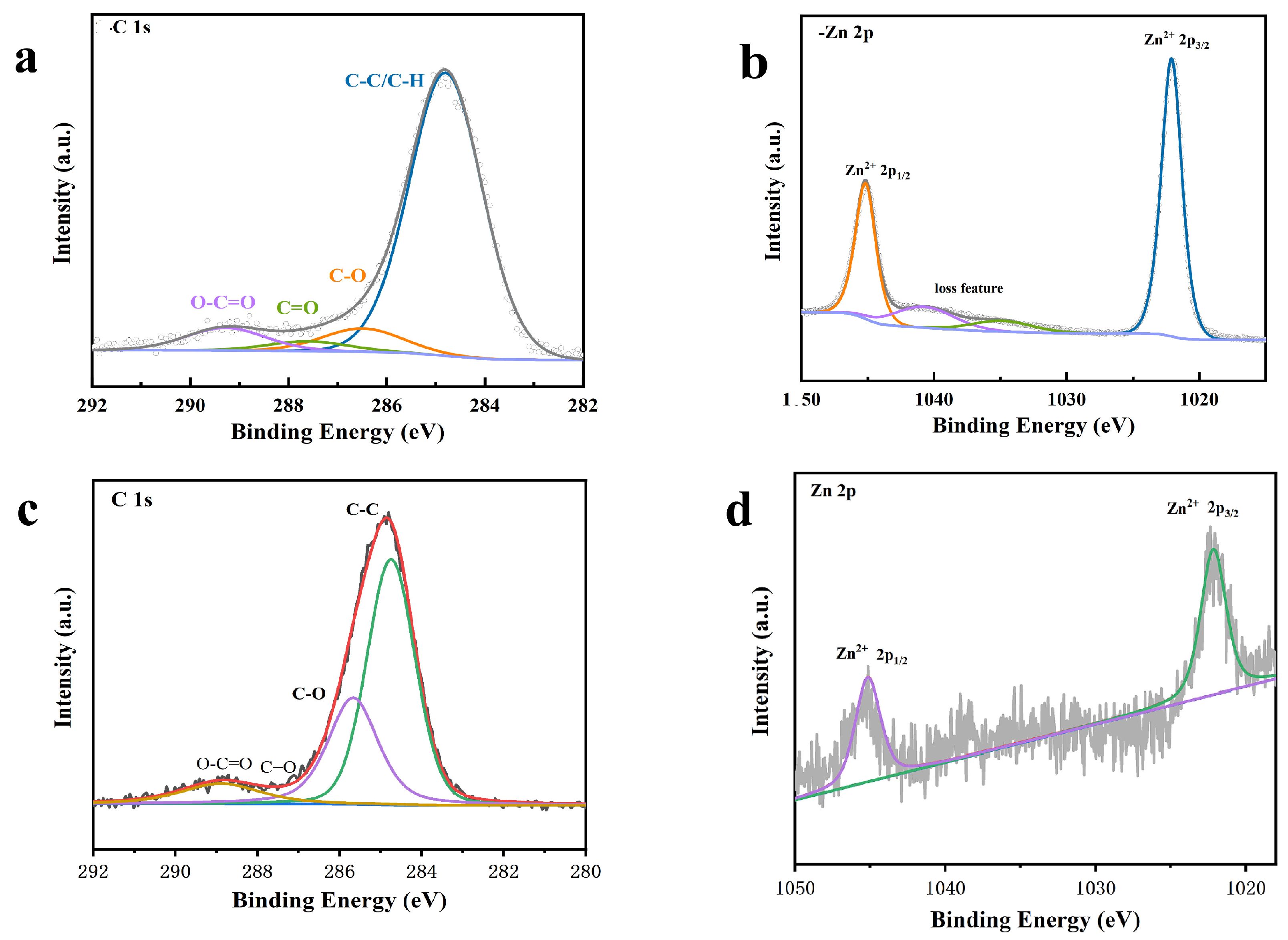
X-ray Photoelectron Spectroscopy (XPS)
Energy Dispersive Spectrometer (EDS)
Water Contact Angle Measurement
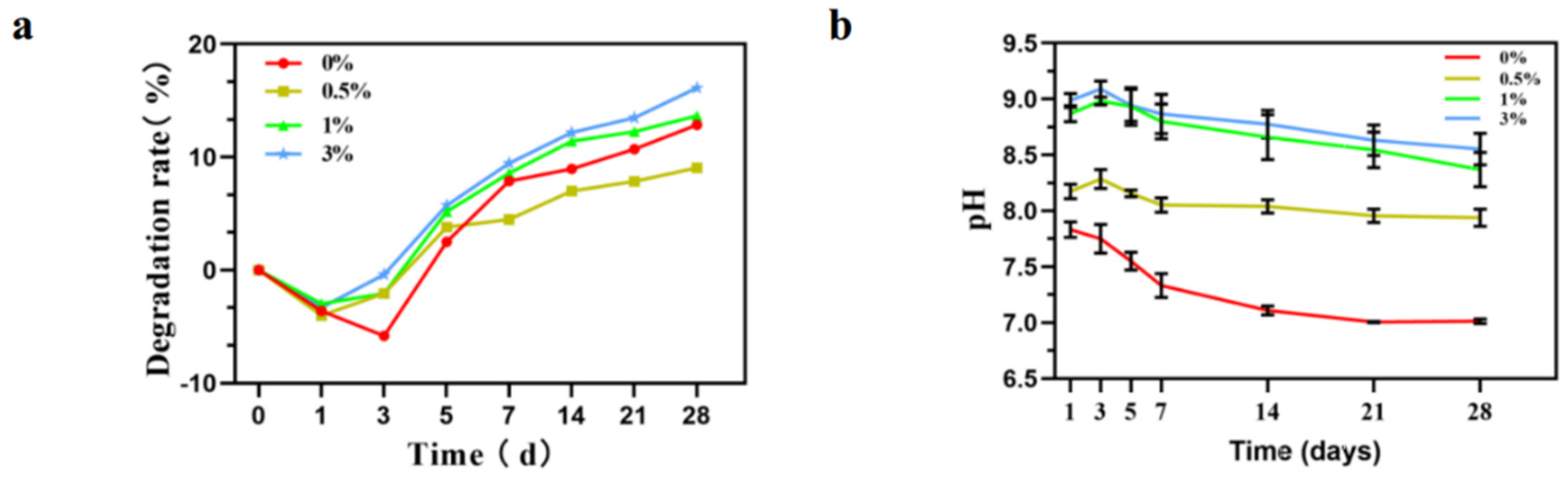
Determination of Degradation Performance and pH Value
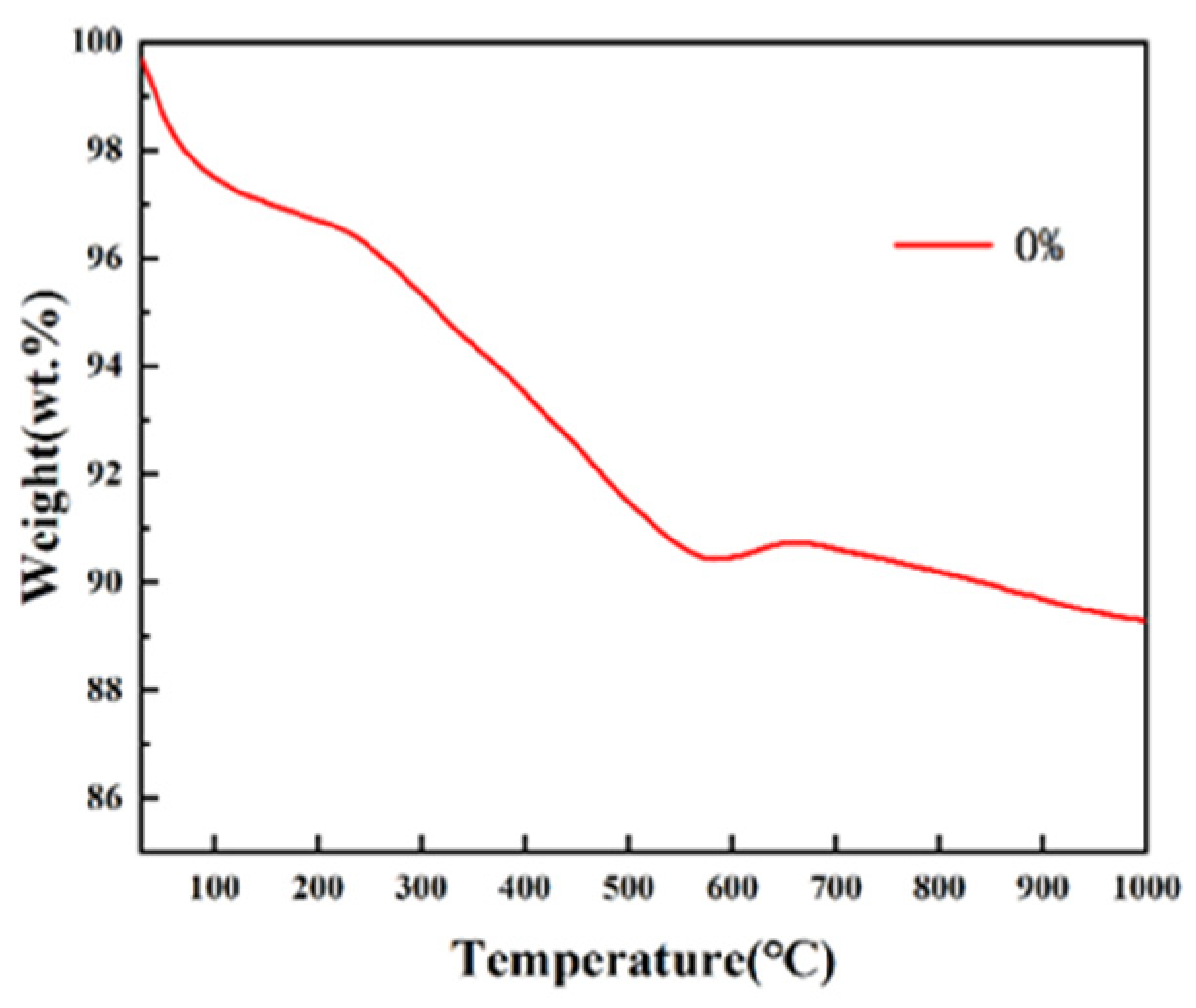
Differential Thermal Analysis (TG-DSC)
2.4. Cell Experiments
2.4.1. Cell Culture
2.4.2. Preparation of the Extract
2.4.3. Preparation of Control Group Solution
2.4.4. DMEM Osteogenic Induction Medium
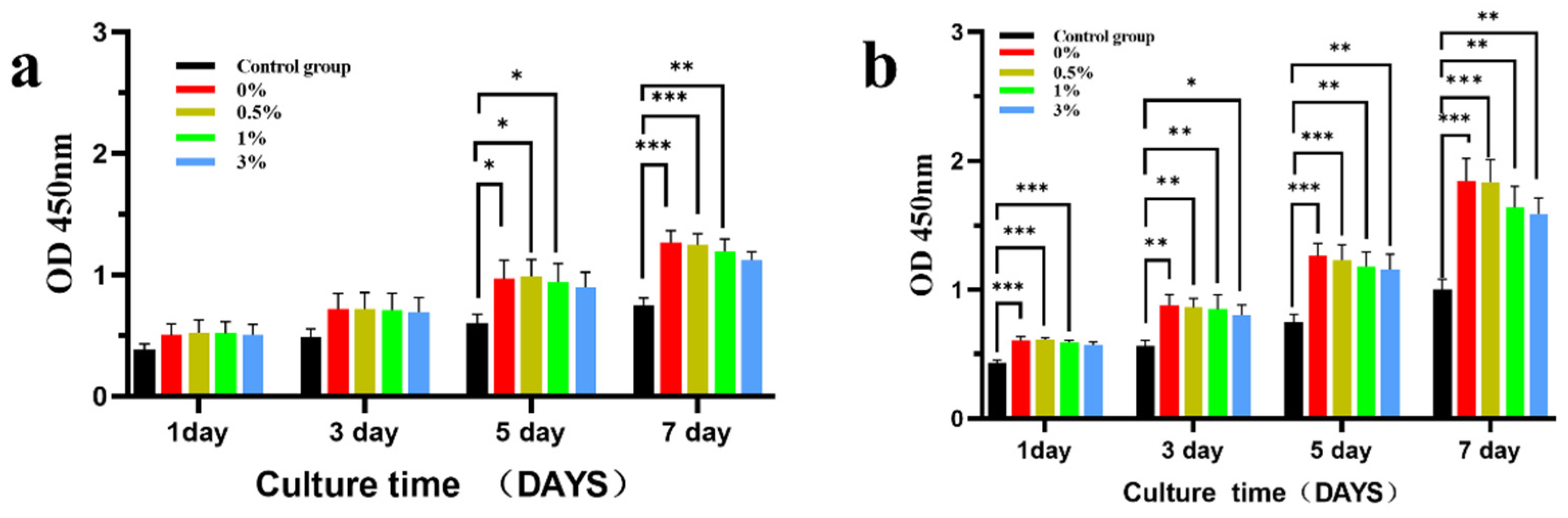
2.4.5. Cytotoxicity Was Detected by CCK-8 Method
2.4.6. Alkaline Phosphatase (ALP) Detection in Cells Cultured with the Material Extract
2.5. Antibacterial Performance Test
2.5.1. E. coli Used as an Example
2.5.2. Plate Colony Counting Method
2.5.3. Observation of Bacterial Morphology
2.5.4. Effects of Composite Microspheres on the Growth State of Bacteria
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nano-Hydroxyapatite Particles (nHAps)
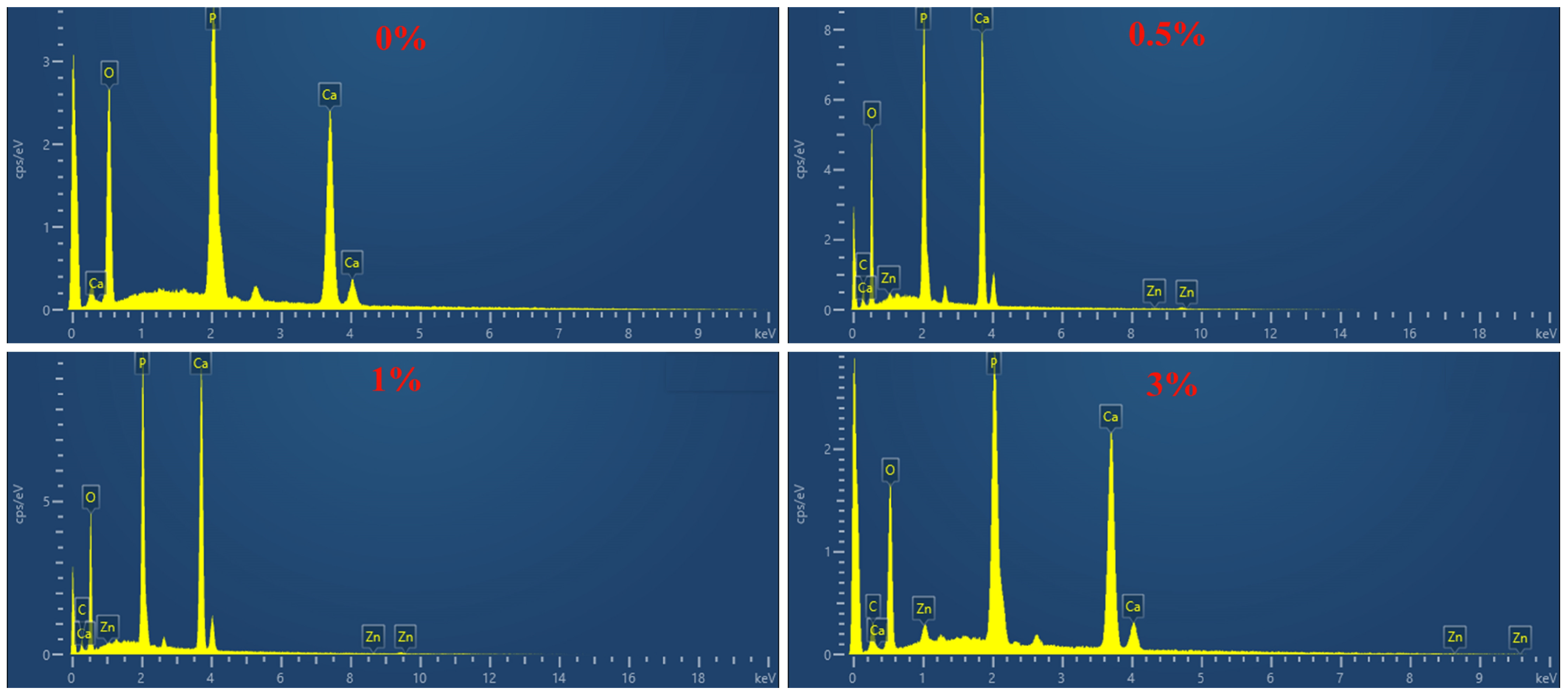
3.2. Characterization of GO-ZnO-nHAp Composite Microspheres
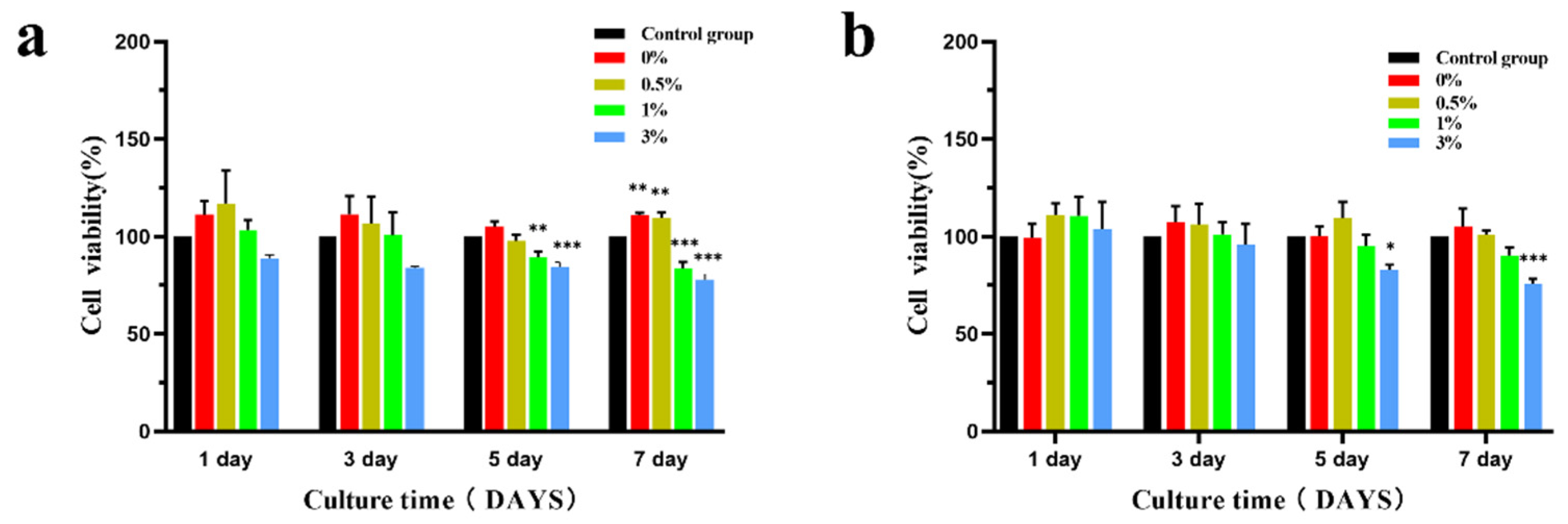
3.3. Cytological Test Results
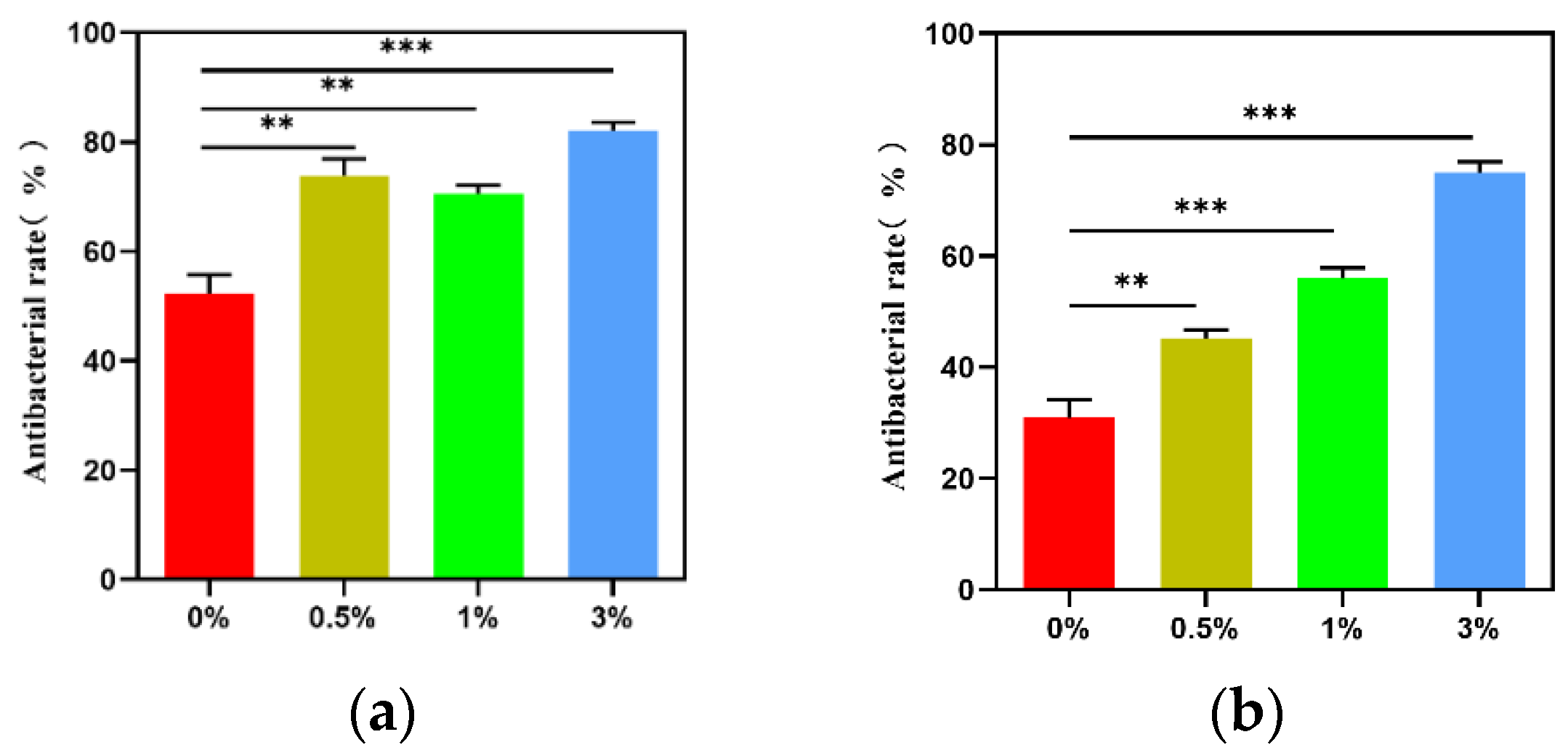
3.4. Results of Antibacterial Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Sun, K.; Wang, J.; He, Y.; Song, P.; Xiong, Y. Preparation and application of natural polymer composite hydroxyapatite material. Adv. Chem. 2016, 28, 885–895. [Google Scholar] [CrossRef]
- Chopra, V.; Thomas, J.; Sharma, A.; Panwar, V.; Kaushik, S.; Sharma, S.; Porwal, K.; Kulkarni, C.; Rajput, S.; Singh, H.; et al. Synthesis and Evaluation of a Zinc Eluting rGO/Hydroxyapatite Nanocomposite Optimized for Bone Augmentation. ACS Biomater. Sci. Eng. 2020, 6, 6710–6725. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Izanloo, S. Development of HAp/GO/Ag coating on 316 LVM implant for medical applications. J. Mech. Behav. Biomed. Mater. 2022, 126, 105075. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, Y.; Rashkow, J.T.; Lalwani, G.; Kanakia, S.; Sitharaman, B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials 2014, 35, 4863–4877. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, H.; Liu, E.; Hu, X.; Zhang, K.; Fan, J. Preparation of polycrystalline ZnO nanoparticles loaded onto graphene oxide and their antibacterial properties. Mater. Today Commun. 2021, 28, 102531. [Google Scholar] [CrossRef]
- Ferrone, E.; Araneo, R.; Notargiacomo, A.; Pea, M.; Rinaldi, A. ZnO Nanostructures and Electrospun ZnO-Polymeric Hybrid Nanomaterials in Biomedical, Health, and Sustainability Applications. Nanomaterials 2019, 9, 1449. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, X.M.; Lv, Y.G.; Li, Y.J.; Zhang, G.L. Preparation and characterization of GO/ZnO/Ag nanocomposites and their synergistic antibacterial effect on Streptococcus mutans. AIP Adv. 2023, 13, 035313. [Google Scholar] [CrossRef]
- He, X.L.; Yang, Y.; Zhang, Z.F.; Wang, S.L.; Jin, X.X. Microstructure and mechanical properties of Al2O3 porous ceramic spheres prepared by gel-drop method. J. Silic. 2022, 50, 729–734. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation Of Medical Devices-Part 12: Sample Preparation And Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021.
- Sang, R.; Chen, M.; Yang, Y.; Li, Y.; Shi, J.; Deng, Y.; Chen, X.; Yang, W. HAp@GO drug delivery vehicle with dual-stimuli-triggered drug release property and efficient synergistic therapy function against cancer. J. Biomed. Mater. Res. Part A 2019, 107, 2296–2309. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release Off. J. Control. Release Soc. 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sun, M.; Ren, Q.Q.; Wu, S.; Guo, W.; Wang, W.J. Progress in the application of ceramic nanoparticles in biomedicine. Jiangsu Ceram. 2021, 54, 19–21, 24. [Google Scholar]
- Lin, P.; Lin, J.; Yu, D.; Lin, T.; He, P. Application status of spark plasma sintering technology in the field of material bonding. Weld. J. 2022, 43, 15–21. [Google Scholar] [CrossRef]
- Wang, G.Z.; Zhang, M.T.; Guo, P.Y. Study on preparation of Co-Mn composites by spark plasma sintering (SPS). Powder Metall. Ind. 2014, 24, 28–32. [Google Scholar] [CrossRef]
- Zhu, B.; He, Y.H.; Meng, Z.D.; Zhang, Y. Preparation and properties of porous ZnO/HA biological composites. J. Compos. Mater. 2019, 36, 2637–2643. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Uddin, M.T.; Islam, M.A.; Rahman, M.M. Wet-chemically prepared low-dimensional ZnO/Al2O3/Cr2O3 nanoparticles for xanthine sensor development using an electrochemical method. RSC Adv. 2018, 8, 12562–12572. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.W. Synthesis and Modification of Hydroxyapatite Nano-Powder by Microwave Hydrothermal Control. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2019. [Google Scholar] [CrossRef]
- ISO 10993-5:2009(E); Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cyto-Toxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Christenson, E.M.; Anseth, K.S.; van der Beucken, J.J.J.P.; Chan, C.K.; Ercan, B.; Jansen, J.A.; Laurencin, C.T.; Li, W.-J.; Murugan, R.; Nair, L.S.; et al. Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11–22. [Google Scholar] [CrossRef]
- Rao, S.H.; Harini, B.; Catamaran, R.P.K.; Balagangadharan, K.; Selvamurugan, N. Natural and synthetic poly-meres/bio ceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macrom. 2018, 110, 88–96. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Wang, C.; Sun, Y.; Ma, Y.; Dong, X.; Qian, J.; Yuan, Y.; Liu, C. Controlled synthesis and transformation of nano-hydroxyapatite with tailored morphologies for biomedical applications. J. Mater. Chem. B 2017, 5, 9148–9156. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, S.; Lin, Z.; Li, E.N.; Yagi, H.; Cao, G.; Yocum, L.; Li, L.; Hao, T.; Bruce, K.K. Graphene oxide-functionalized nanocomposites promote osteogenesis of human mesenchymal stem cells via enhancement of BMP-SMAD1/5 signaling pathway. Biomaterials 2021, 277, 121082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yu, P.; Shi, X.; Ling, T.; Zeng, W.; Chen, A.; Yang, W.; Zhou, Z. Hierarchically Porous Hydroxyapatite Hybrid Scaffold Incorporated with Reduced Graphene Oxide for Rapid Bone Ingrowth and Repair. ACS Nano 2019, 13, 9595–9606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, J.; Li, H. Synthesis of hydroxyapatite–reduced graphite oxide nanocomposites for biomedical applications: Oriented nucleation and epitaxial growth of hydroxyapatite. J. Mater. Chem. B 2013, 1, 1826–1834. [Google Scholar] [CrossRef]
- Ahtzaz, S.; Nasir, M. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017, 2, 409–418. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Q.; Liu, X.; Yang, X.; Yan, H.; Xiong, Z.; Xu, N.; Ma, J.; Feng, Q.; Shen, Z. ZnO nanostructures enhance the osteogenic capacity of SaOS-2 cells on acid-etched pure Ti. Mater. Lett. 2017, 215, 173–175. [Google Scholar] [CrossRef]
- Saha, N.; Dubey, A.K.; Basu, B. Cellular proliferation, cellular viability, and biocompatibility of HA-ZnO composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 256–264. [Google Scholar] [CrossRef]
- Bhowmick, A.; Pramanik, N.; Manna, P.J.; Mitra, T.; Selvaraj, T.K.R.; Gnanamani, A.; Das, M.; Kundu, P.P. Development of porous and antimicrobial CTS–PEG–HAP–ZnO nano-composites for bone tissue engineering. RSC Adv. 2015, 5, 99385–99393. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Pan, C.; Hu, Y.; Gong, Z.; Yang, Y.; Liu, S.; Quan, L.; Yang, Z.; Wei, Y.; Ye, W. Improved Blood Compatibility and Endothelialization of Titanium Oxide Nanotube Arrays on Titanium Surface by Zinc Doping. ACS Biomater. Sci. Eng. 2020, 6, 2072–2083. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Sahu, S.N.; Khadeer, M.A.; Bai, G.; Franklin, R.B.; Gupta, A. Overexpression of the ZIP1 zinc transporter induces an osteogenic phenotype in mesenchymal stem cells. Bone 2006, 38, 181–198. [Google Scholar] [CrossRef]
- Ragab, H.S.; Ibrahim, F.A.; Abdallah, F.; Al-Ghamdi, A.A.; El-Tantawy, F.; Radwan, N.; Yakuphanoglu, F. Synthesis and In Vitro Antibacterial Properties of Hydroxyapatite Nanoparticles. IOSR J. Pharm. Biol. Sci. 2014, 9, 77–85. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, M.; Wang, Y.; Shao, Y.; Zhu, Y.; Yang, S. Efficient antibacterial activity of hydroxyapatite through ROS generation motivated by trace Mn(iii) coupled H vacancies. J. Mater. Chem. B 2021, 9, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Werber, J.R.; Chu, C.; Zucker, I.; Kim, J.-H.; Osuji, C.O.; Elimelech, M. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc. Natl. Acad. Sci. USA 2017, 114, E9793–E9801. [Google Scholar] [CrossRef]
- Dhandapani, P.; AlSalhi, M.S.; Karthick, R.; Chen, F.; Devanesan, S.; Kim, W.; Rajasekar, A.; Ahmed, M.; Aljaafreh, M.J.; Muhammad, A. Biological mediated synthesis of RGO-ZnO composites with enhanced photocatalytic and antibacterial activity. J. Hazard. Mater. 2021, 409, 124661. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kim, J.-H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, 11, 1927–1945. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, A.; Liu, Y.; Jiao, D.; Zeng, D.; Wang, X.; Cao, L.; Jiang, X. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by BMP-2 peptide. Int. J. Nanomed. 2019, 14, 733–751. [Google Scholar] [CrossRef]
- Weng, W.; Li, X.; Nie, W.; Liu, H.; Liu, S.; Huang, J.; Zhou, Q.; He, J.; Su, J.; Dong, Z.; et al. One-Step Preparation of an AgNP-nHA@RGO Three-Dimensional Porous Scaffold and Its Application in Infected Bone Defect Treatment. Int. J. Nanomed. 2020, 15, 5027–5042. [Google Scholar] [CrossRef] [PubMed]











| Level | Relative Growth Rate |
|---|---|
| 0 | ≥100 |
| 1 | 80–99 |
| 2 | 50–79 |
| 3 | 30–49 |
| 4 | 0–29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, C.; Zhang, S.; Zhang, L.; Hao, J.; Jia, Z.; Zheng, X.; Lv, Y.; Fu, S.; Zhang, G. Preparation and Properties of GO/ZnO/nHAp Composite Microsphere Bone Regeneration Material. Micromachines 2024, 15, 122. https://doi.org/10.3390/mi15010122
Wu J, Wang C, Zhang S, Zhang L, Hao J, Jia Z, Zheng X, Lv Y, Fu S, Zhang G. Preparation and Properties of GO/ZnO/nHAp Composite Microsphere Bone Regeneration Material. Micromachines. 2024; 15(1):122. https://doi.org/10.3390/mi15010122
Chicago/Turabian StyleWu, Jiang, Chunmei Wang, Shuangsheng Zhang, Ling Zhang, Jingshun Hao, Zijian Jia, Xiaomei Zheng, Yuguang Lv, Shuang Fu, and Guoliang Zhang. 2024. "Preparation and Properties of GO/ZnO/nHAp Composite Microsphere Bone Regeneration Material" Micromachines 15, no. 1: 122. https://doi.org/10.3390/mi15010122
APA StyleWu, J., Wang, C., Zhang, S., Zhang, L., Hao, J., Jia, Z., Zheng, X., Lv, Y., Fu, S., & Zhang, G. (2024). Preparation and Properties of GO/ZnO/nHAp Composite Microsphere Bone Regeneration Material. Micromachines, 15(1), 122. https://doi.org/10.3390/mi15010122





