Green Anisole as Antisolvent in Planar Triple-Cation Perovskite Solar Cells with Varying Cesium Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solutions
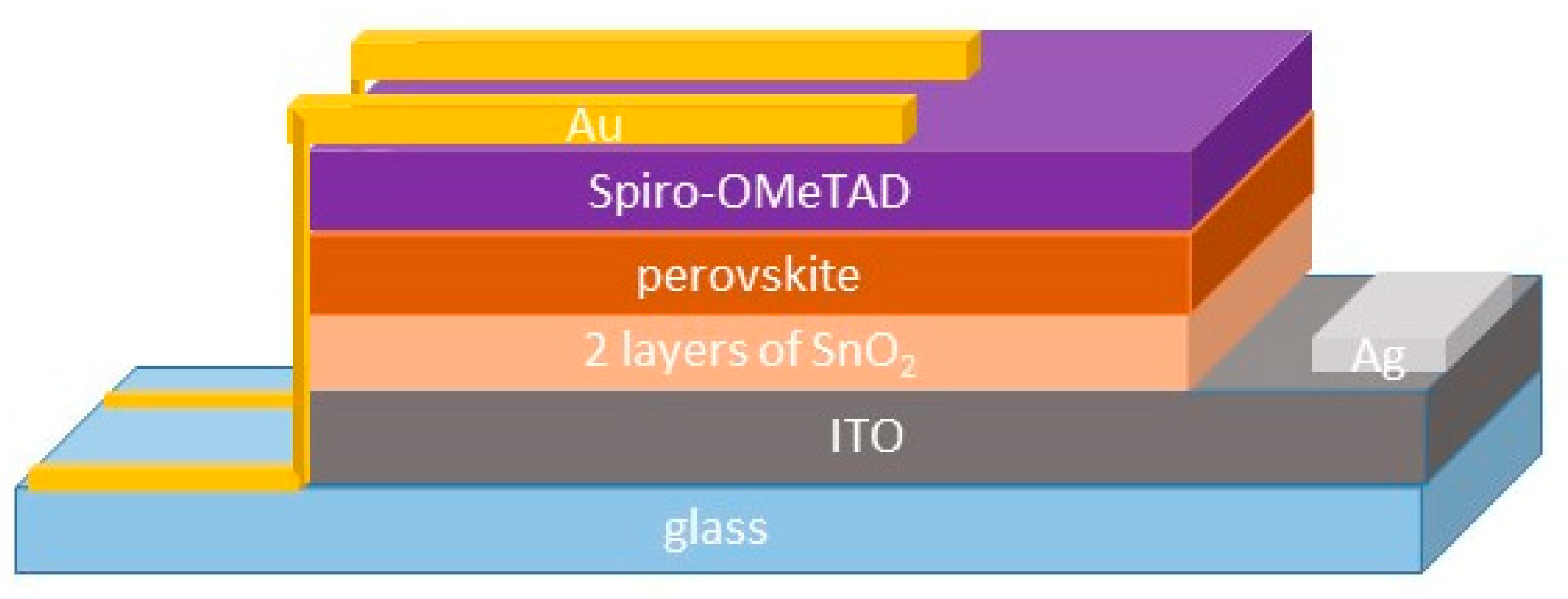
2.3. Device Fabrication
2.4. Device Characterization
3. Results and Discussion
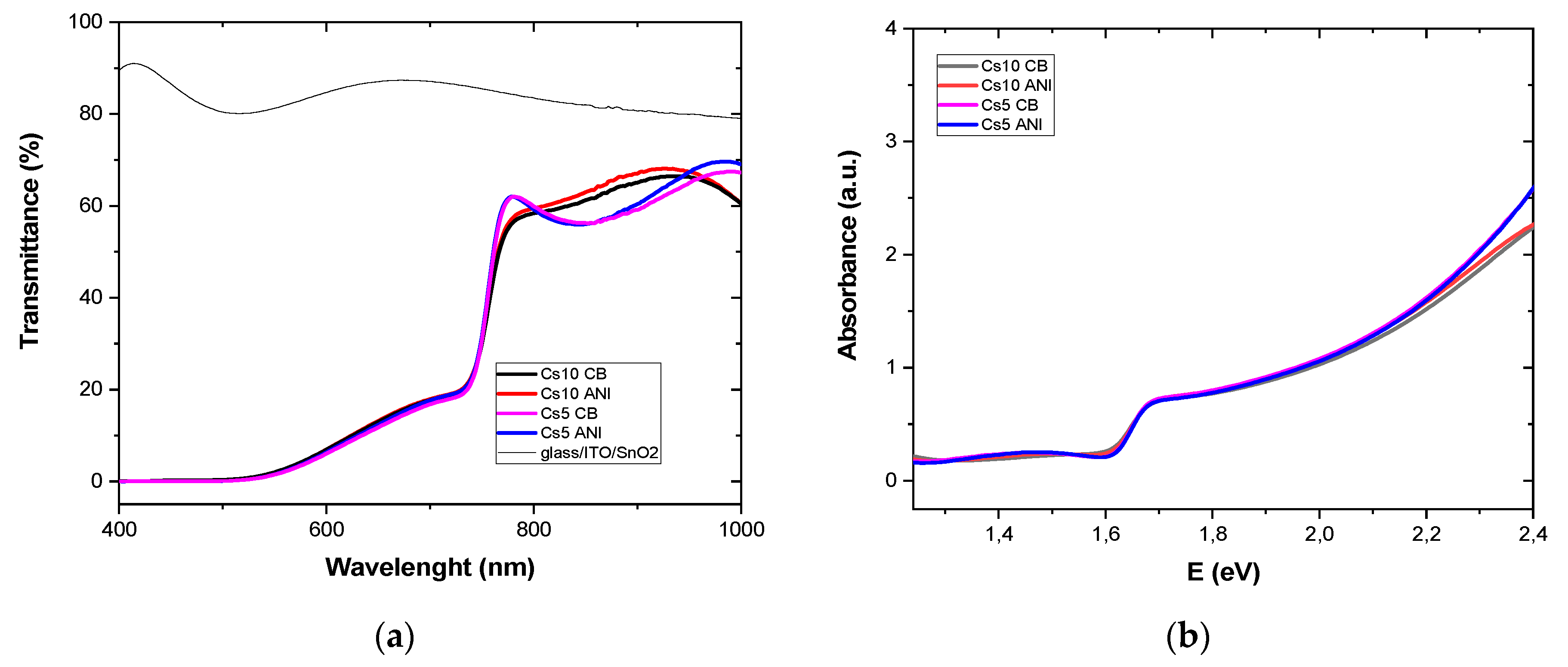
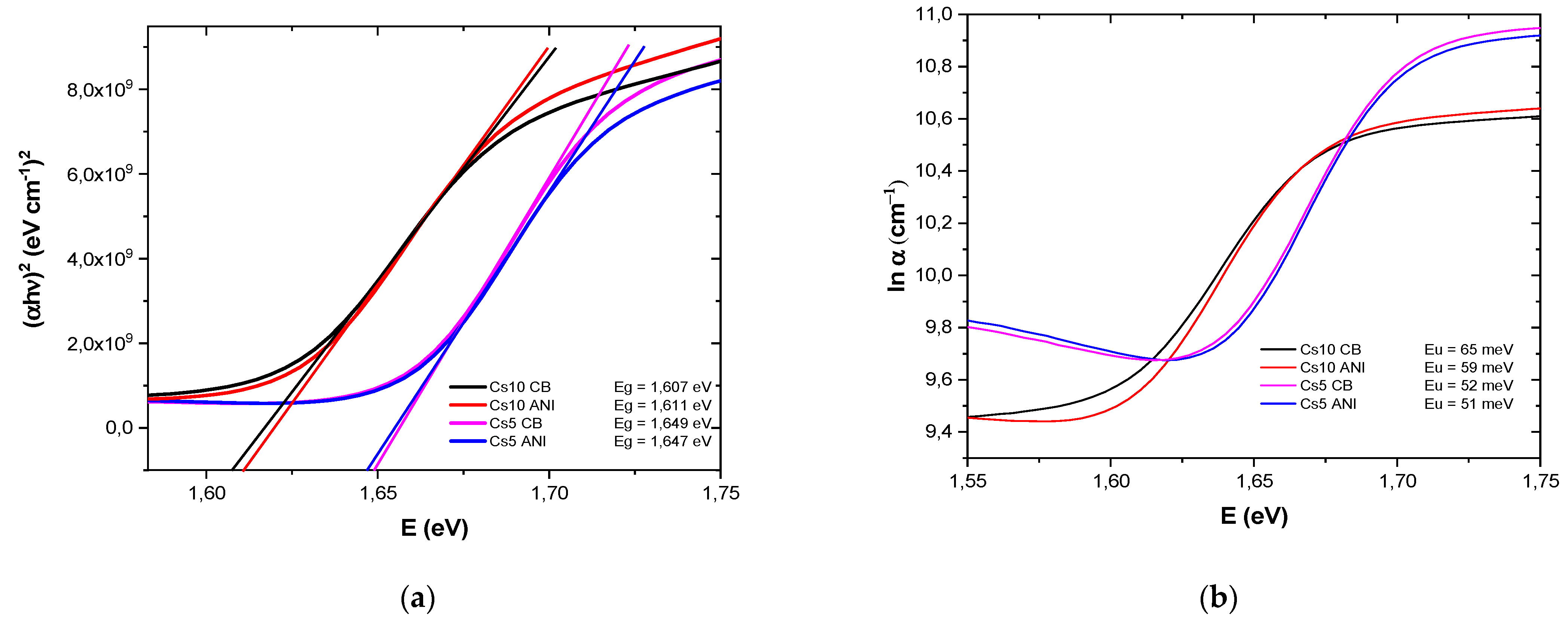
3.1. Perovskite Bandgap and Urbach Energy
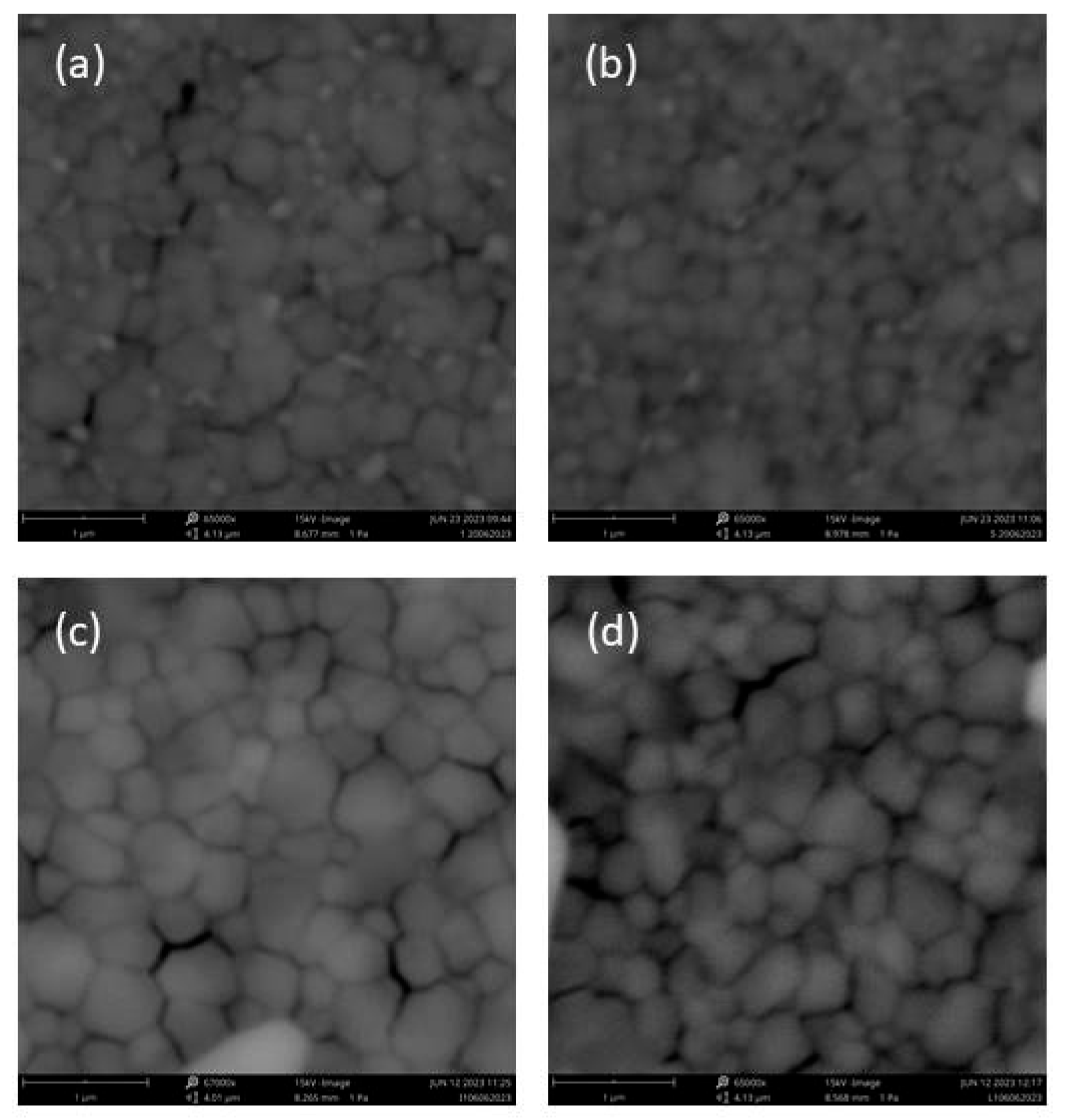
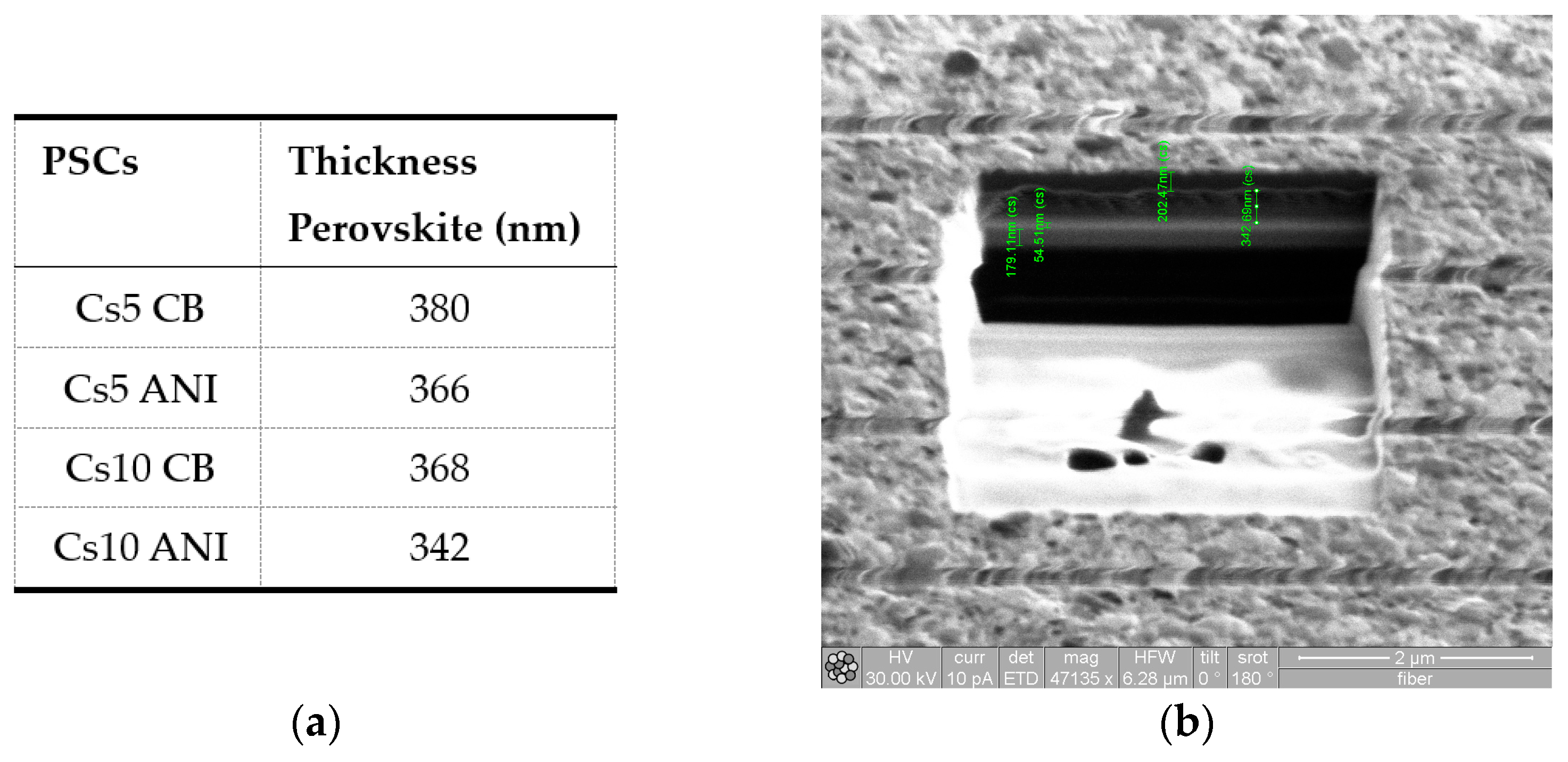
3.2. SEM and FIB Analyses
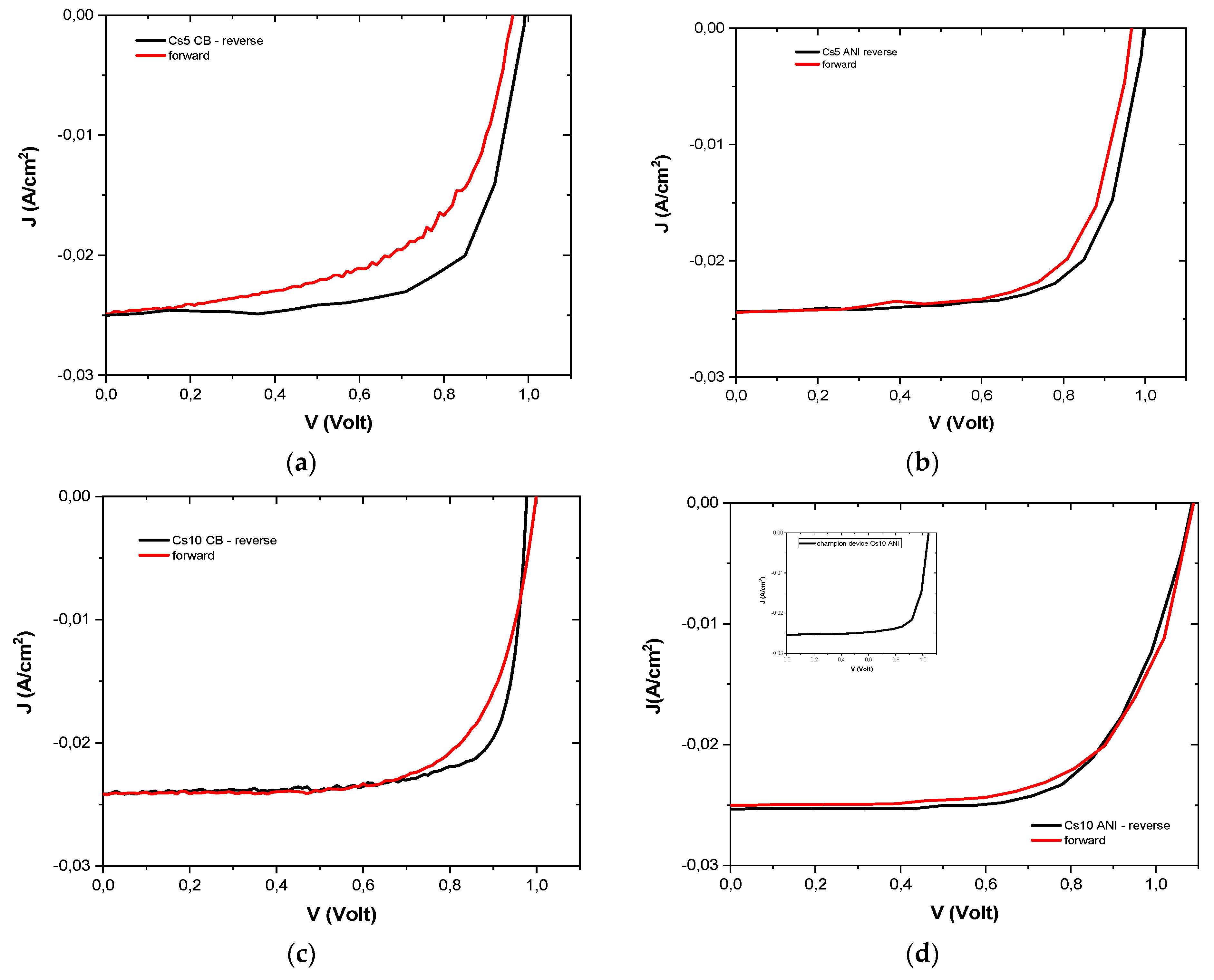
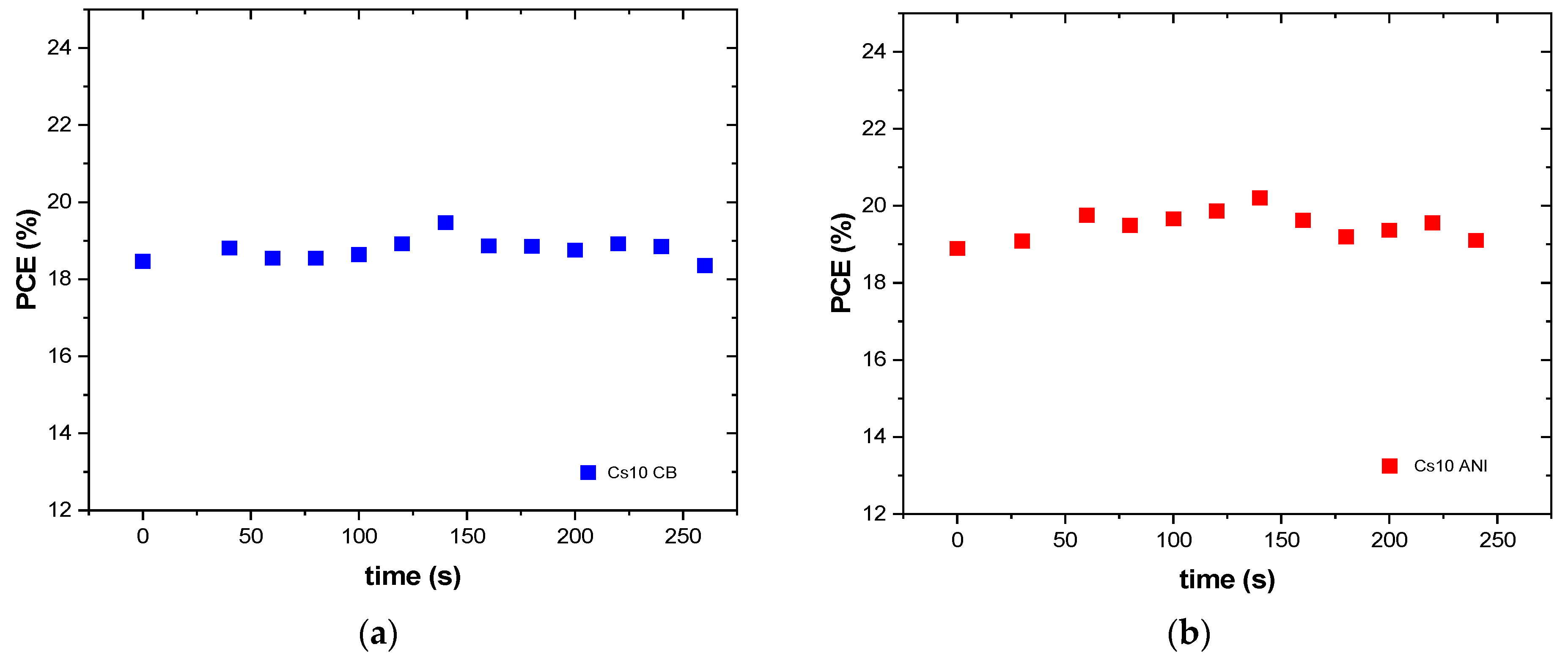
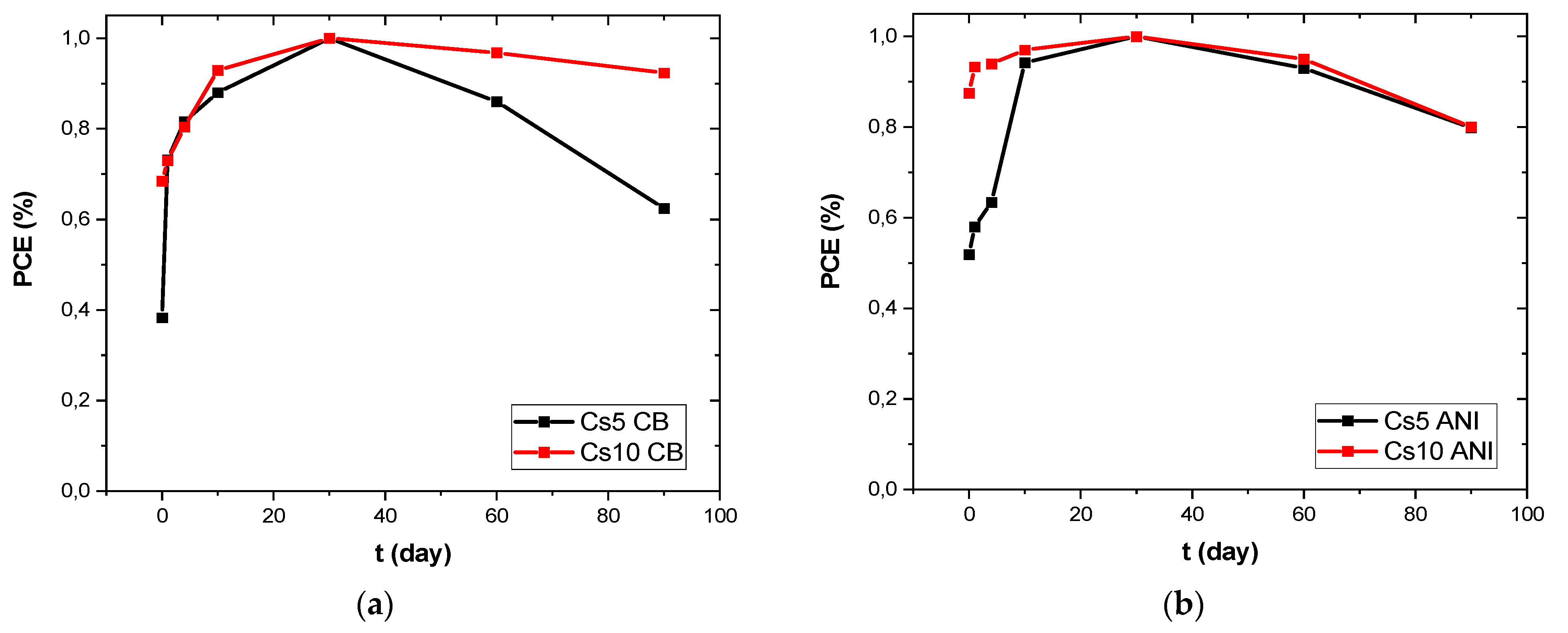
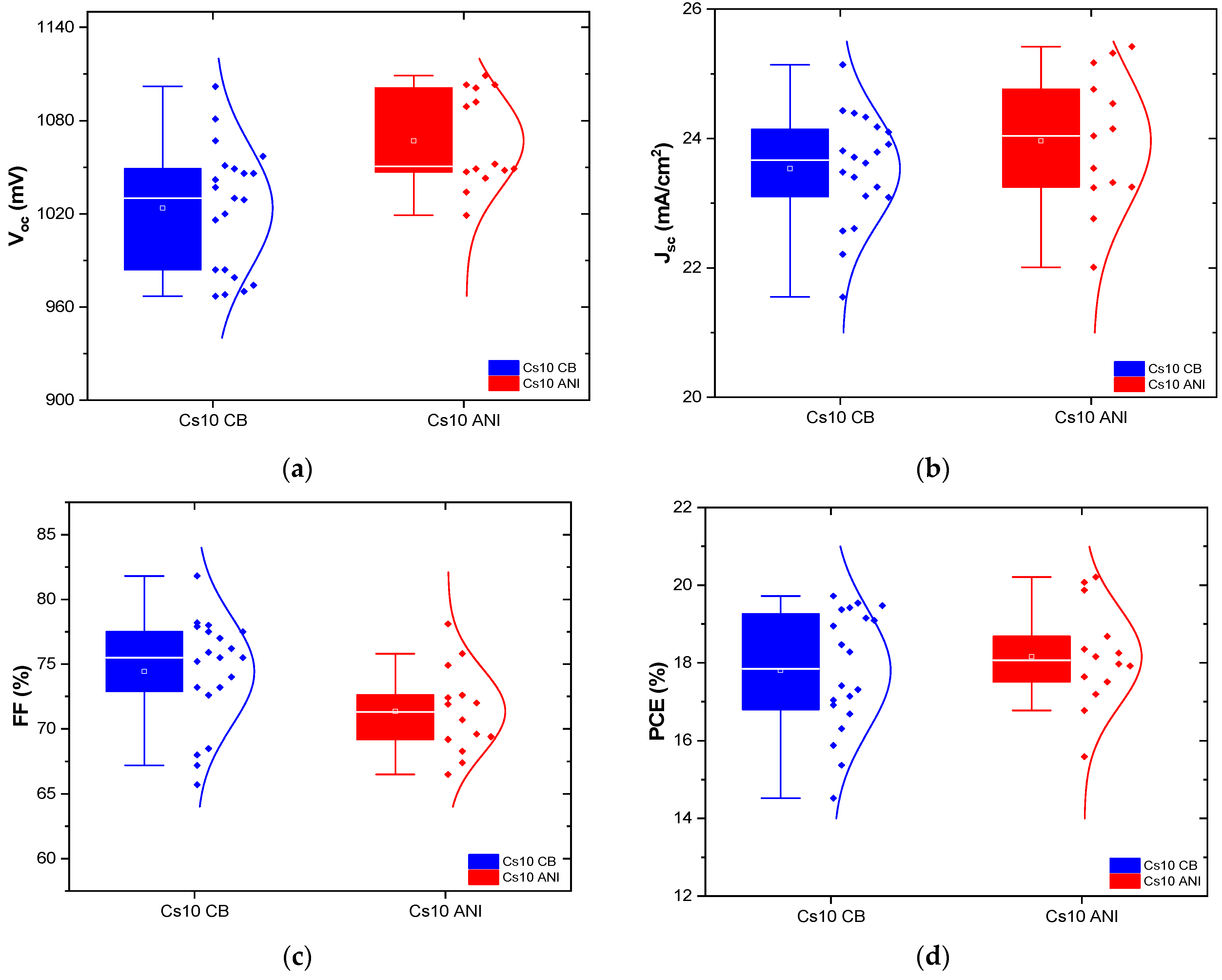
3.3. J-V Characterization
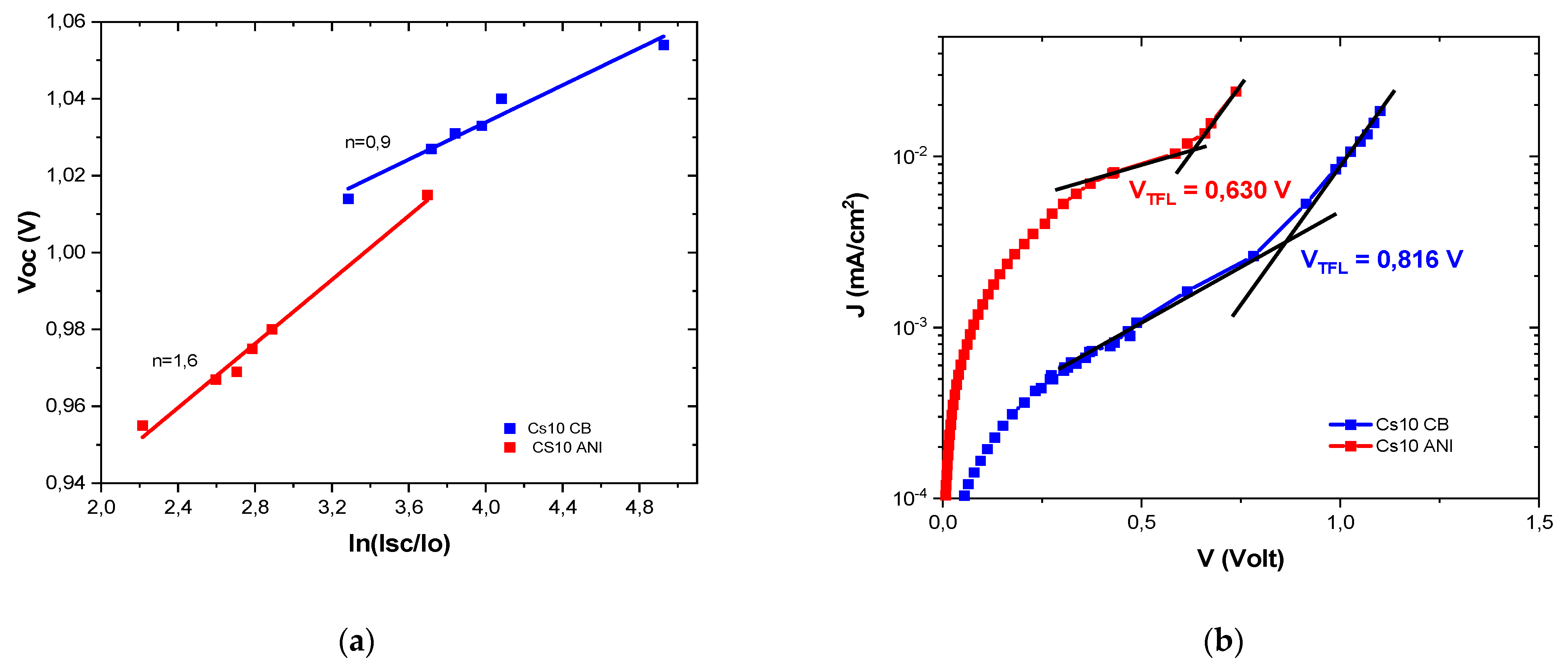
3.4. Ideality Factor and Dark J-V
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Deng, L.-L.; Leng, S.; Guo, X.; Tan, C.-H.; Choy, W.C.H.; Chen, C.-C. The mechanism of universal green antisolvents for intermediate phase controlled high-efficiency formamidinium-based perovskite solar cells. Mater. Horizons 2020, 7, 934–942. [Google Scholar] [CrossRef]
- Kwon, N.; Lee, J.; Ko, M.J.; Kim, Y.Y.; Seo, J. Recent progress of eco-friendly manufacturing process of efficient perovskite solar cells. Nano Converg. 2023, 10, 28. [Google Scholar] [CrossRef]
- Numata, Y.; Sanehira, Y.; Miyasaka, T. Drastic Change of Surface Morphology of Cesium–Formamidinium Perovskite Solar Cells by Antisolvent Processing. ACS Appl. Energy Mater. 2021, 4, 1069–1077. [Google Scholar] [CrossRef]
- Tian, S.; Li, J.; Li, S.; Bu, T.; Mo, Y.; Wang, S.; Li, W.; Huang, F. A facile green solvent engineering for up-scaling perovskite solar cell modules. Sol. Energy 2019, 183, 386–391. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Liu, X.; Tang, D.; Yuan, X. Ethyl acetate green antisolvent process for high-performance planar low-temperature SnO2-based perovskite solar cells made in ambient air. Chem. Eng. J. 2020, 379, 122298. [Google Scholar] [CrossRef]
- Taylor, A.D.; Sun, Q.; Goetz, K.P.; An, Q.; Schramm, T.; Hofstetter, Y.; Litterst, M.; Paulus, F.; Vaynzof, Y. A general approach to high-efficiency perovskite solar cells by any antisolvent. Nat. Commun. 2021, 12, 1878. [Google Scholar] [CrossRef]
- Zhao, P.; Kim, B.J.; Ren, X.; Lee, D.G.; Bang, G.J.; Jeon, J.B.; Bin Kim, W.; Jung, H.S. Antisolvent with an Ultrawide Processing Window for the One-Step Fabrication of Efficient and Large-Area Perovskite Solar Cells. Adv. Mater. 2018, 30, e1802763. [Google Scholar] [CrossRef]
- Yavari, M.; Mazloum-Ardakani, M.; Gholipour, S.; Tavakoli, M.M.; Turren-Cruz, S.; Taghavinia, N.; Grätzel, M.; Hagfeldt, A.; Saliba, M. Greener, Nonhalogenated Solvent Systems for Highly Efficient Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1800177. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Wenger, B.; Snaith, H.J.; Nicholas, R.J. Dopant-Free Planar n–i–p Perovskite Solar Cells with Steady-State Efficiencies Exceeding 18%. ACS Energy Lett. 2017, 2, 622–628. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Zhou, B.; Jia, X.; Ma, Q.; Yuan, N.; Zheng, X.; Ding, J.; Zhang, W. Green Anti-Solvent Processed Planar Perovskite Solar Cells with Efficiency Beyond 19%. Sol. RRL 2018, 2, 1700213. [Google Scholar] [CrossRef]
- Pellaroque, A.; Noel, N.K.; Habisreutinger, S.N.; Zhang, Y.; Barlow, S.; Marder, S.R.; Snaith, H.J. Efficient and Stable Perovskite Solar Cells Using Molybdenum Tris(dithiolene)s as p-Dopants for Spiro-OMeTAD. ACS Energy Lett. 2017, 2, 2044–2050. [Google Scholar] [CrossRef]
- Podapangi, S.K.; Mancini, L.; Xu, J.; Reddy, S.H.; Di Carlo, A.; Brown, T.M.; Zanotti, G. Green Anisole Solvent-Based Synthesis and Deposition of Phthalocyanine Dopant-Free Hole-Transport Materials for Perovskite Solar Cells. Energies 2023, 16, 3643. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Ašmontas, S.; Čerškus, A.; Gradauskas, J.; Grigucevičienė, A.; Leinartas, K.; Lučun, A.; Petrauskas, K.; Selskis, A.; Sužiedėlis, A.; Širmulis, E.; et al. Cesium-Containing Triple Cation Perovskite Solar Cells. Coatings 2021, 11, 279. [Google Scholar] [CrossRef]
- Subedi, B.; Subedi, B.; Li, C.; Li, C.; Chen, C.; Chen, C.; Liu, D.; Liu, D.; Junda, M.M.; Junda, M.M.; et al. Urbach Energy and Open-Circuit Voltage Deficit for Mixed Anion–Cation Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 7796–7804. [Google Scholar] [CrossRef] [PubMed]
- Brockway, L.; Pendyala, C.; Jasinski, J.; Sunkara, M.K.; Vaddiraju, S. A Postsynthesis Decomposition Strategy for Group III–Nitride Quantum Wires. Cryst. Growth Des. 2011, 11, 4559–4564. [Google Scholar] [CrossRef]
- Melvin, A.A.; Stoichkov, V.D.; Kettle, J.; Mogilyansky, D.; Katz, E.A.; Visoly-Fisher, I. Lead iodide as a buffer layer in UV-induced degradation of CH3NH3PbI3 films. Sol. Energy 2018, 159, 794–799. [Google Scholar] [CrossRef]
- Mahon, N.S.; Korolik, O.V.; Khenkin, M.V.; Arnaoutakis, G.E.; Galagan, Y.; Soriūtė, V.; Litvinas, D.; Ščajev, P.; Katz, E.A.; Mazanik, A.V. Photoluminescence kinetics for monitoring photoinduced processes in perovskite solar cells. Sol. Energy 2020, 195, 114–120. [Google Scholar] [CrossRef]
- Anizelli, H.S.; Stoichkov, V.; Fernandes, R.V.; Duarte, J.L.; Laureto, E.; Kettle, J.; Visoly-Fisher, I.; Katz, E.A. Application of luminescence downshifting materials for enhanced stability of CH3NH3PbI3(1-x)Cl3x perovskite photovoltaic devices. Org. Electron. 2017, 49, 129–134. [Google Scholar] [CrossRef]
- La Ferrara, V.; De Maria, A.; Rametta, G.; Veneri, P.D. The effect of storage cycle on improvement in the photovoltaic parameters of planar triple cation perovskite solar cells. Mater. Adv. 2021, 2, 5396–5405. [Google Scholar] [CrossRef]
- Li, N.; Niu, X.; Li, L.; Wang, H.; Huang, Z.; Zhang, Y.; Chen, Y.; Zhang, X.; Zhu, C.; Zai, H.; et al. Liquid medium annealing for fabricating durable perovskite solar cells with improved reproducibility. Science 2021, 373, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Rühle, S. Tabulated values of the Shockley–Queisser limit for single junction solar cells. Sol. Energy 2016, 130, 139–147. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Noel, N.K.; Snaith, H.J. Hysteresis Index: A Figure without Merit for Quantifying Hysteresis in Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 2472–2476. [Google Scholar] [CrossRef]
- Lee, G.W.; Shim, J.-I.; Shin, D.-S. On the ideality factor of the radiative recombination current in semiconductor light-emitting diodes. Appl. Phys. Lett. 2016, 109, 031104. [Google Scholar] [CrossRef]
- Courtier, N. Interpreting Ideality Factors for Planar Perovskite Solar Cells: Ectypal Diode Theory for Steady-State Operation. Phys. Rev. Appl. 2020, 14, 024031. [Google Scholar] [CrossRef]
- Caprioglio, P.; Wolff, C.M.; Sandberg, O.J.; Armin, A.; Rech, B.; Albrecht, S.; Neher, D.; Stolterfoht, M. On the Origin of the Ideality Factor in Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 2000502. [Google Scholar] [CrossRef]
- Bube, R.H. Trap Density Determination by Space-Charge-Limited Currents. J. Appl. Phys. 1962, 33, 1733–1737. [Google Scholar] [CrossRef]
- Tress, W.; Yavari, M.; Domanski, K.; Yadav, P.; Niesen, B.; Baena, J.P.C.; Hagfeldt, A.; Graetzel, M. Interpretation and evolution of open-circuit voltage, recombination, ideality factor and subgap defect states during reversible light-soaking and irreversible degradation of perovskite solar cells. Energy Environ. Sci. 2017, 11, 151–165. [Google Scholar] [CrossRef]
| Antisolvent | Molecular Weight (g/mol) | Boiling Point (°C) | Viscosity (cP) | Solubility in H2O (g/100 g) | Relative Polarity | ICH Class |
|---|---|---|---|---|---|---|
| 1-Butanol | 74 | 118 | 3.006 | 7.7 | 0.586 | 3 |
| Ethyl Acetate | 88 | 77 | 0.443 | 8.7 | 0.228 | 3 |
| Anisole | 108 | 154 | 0.789 | 0.10 | 0.198 | 3 |
| Chlorobenzene | 113 | 131 | 0.760 | 0.05 | 0.188 | 2 |
| Architecture (Glass/TCO) | J-V Parameters | Storage and Aging Time | Final Relative PCE | Ref. |
|---|---|---|---|---|
| compactTiO2/mesoTiO2/ Cs0.05(MA0.17FA0.83)(0.95)Pb(I0.83Br0.17)3 | Voc = 1.15 V | <20% RH @ RT–1000 h | 93% | Zhao et al. [8] |
| Jsc = 21.98 mA/cm2 | ||||
| FF = 78% | ||||
| PCE = 19.76% | ||||
| compactTiO2/meso TiO2/Cs0.05(MA0.17FA0.83)(0.95)Pb(I0.83Br0.17)3 | Voc = 1.12 V | MPPT–60 s | Yavari et al. [9] | |
| Jsc = 23.26 mA/cm2 | ||||
| FF = 76% | ||||
| PCE = 19.88% | ||||
| compactTiO2/meso TiO2/Cs0.05(MA0.17FA0.83)(0.95)Pb(I0.83Br0.17)3 | Voc = 1.10 V | MPPT–45 s | Wang et al. [2] | |
| Jsc = 22.23 mA/cm2 | ||||
| FF = 75% | ||||
| PCE = 18.43% | ||||
| compact TiO2/Cs0.05(MA0.17FA0.83)(0.95)Pb(I0.83Br0.17)3 | Voc = 1.10 V | MPPT–100 s | Zhang et al. [11] | |
| Jsc = 22.78 mA/cm2 | ||||
| FF = 77% | ||||
| PCE = 19.42% | ||||
| SnO2/FA0.83MA0.117Pb(I0.87Br0.17)3et alet | Voc = 1.14 V | MPPT–45 s | Habisreutinger et al. [10] | |
| Jsc = 22.07 mA/cm2 | ||||
| FF = 75% | ||||
| PCE = 18.9% | ||||
| SnO2/FA0.83MA0.117Pb(I0.87Br0.17)3 | Voc = 1.11 V | 30% humidity @ 85 °C–500 h | 50% | Pellaroque et al. [12] |
| Jsc = 21.96 mA/cm2 | ||||
| FF = 73% | ||||
| PCE = 18.2% |
| Glass/ITO/SnO2/Perovskite | Eg (eV) | Eu (eV) |
|---|---|---|
| Cs10 CB | 1.607 | 0.065 |
| Cs 10 ANI | 1.611 | 0.059 |
| Cs5 CB | 1.649 | 0.052 |
| Cs5 ANI | 1.647 | 0.051 |
| Months | Temperature Range (°C) | Relative Humidity (%) |
|---|---|---|
| April | 10–17 | 55–85 |
| May | 17–28 | 62–93 |
| June | 24–31 | 52–87 |
| July | 29–37 | 45–73 |
| September | 26–37 | 41–76 |
| Device | Voc (mV) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Cs5 CB | 1005 | 26.04 | 70.9 | 18.58 |
| Cs5 CB * | 1121 | 24.07 | 77.3 | 20.9 |
| Cs5 ANI | 1006 | 25.54 | 72.3 | 18.58 |
| Cs10 CB | 984 | 23.79 | 81.8 | 19.15 |
| Cs10 ANI | 1049 | 25.42 | 75.8 | 20.21 |
| Glass/ITO/SnO2/Perovskite | PCE Reverse (%) | PCE Forward (%) | HI | Relative %PCE over 90 Days |
|---|---|---|---|---|
| Cs5 CB | 17.03 | 13.85 | 0.18 | 62 |
| Cs 5 ANI | 17.10 | 16.10 | 0.06 | 80 |
| Cs10 CB | 18.28 | 16.64 | 0.09 | 92 |
| Cs10 ANI | 18.20 | 17.76 | 0.02 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Ferrara, V.; De Maria, A.; Rametta, G. Green Anisole as Antisolvent in Planar Triple-Cation Perovskite Solar Cells with Varying Cesium Concentrations. Micromachines 2024, 15, 136. https://doi.org/10.3390/mi15010136
La Ferrara V, De Maria A, Rametta G. Green Anisole as Antisolvent in Planar Triple-Cation Perovskite Solar Cells with Varying Cesium Concentrations. Micromachines. 2024; 15(1):136. https://doi.org/10.3390/mi15010136
Chicago/Turabian StyleLa Ferrara, Vera, Antonella De Maria, and Gabriella Rametta. 2024. "Green Anisole as Antisolvent in Planar Triple-Cation Perovskite Solar Cells with Varying Cesium Concentrations" Micromachines 15, no. 1: 136. https://doi.org/10.3390/mi15010136
APA StyleLa Ferrara, V., De Maria, A., & Rametta, G. (2024). Green Anisole as Antisolvent in Planar Triple-Cation Perovskite Solar Cells with Varying Cesium Concentrations. Micromachines, 15(1), 136. https://doi.org/10.3390/mi15010136








