NiFe2O4 Material on Carbon Paper as an Electrocatalyst for Alkaline Water Electrolysis Module
Abstract
:1. Introduction
2. Experimental
2.1. Electrolyzer
2.2. Preparation of NiFe Solution and Ru Solution for Hydrothermal Process
2.3. Deposition of Electrocatalysts on CP
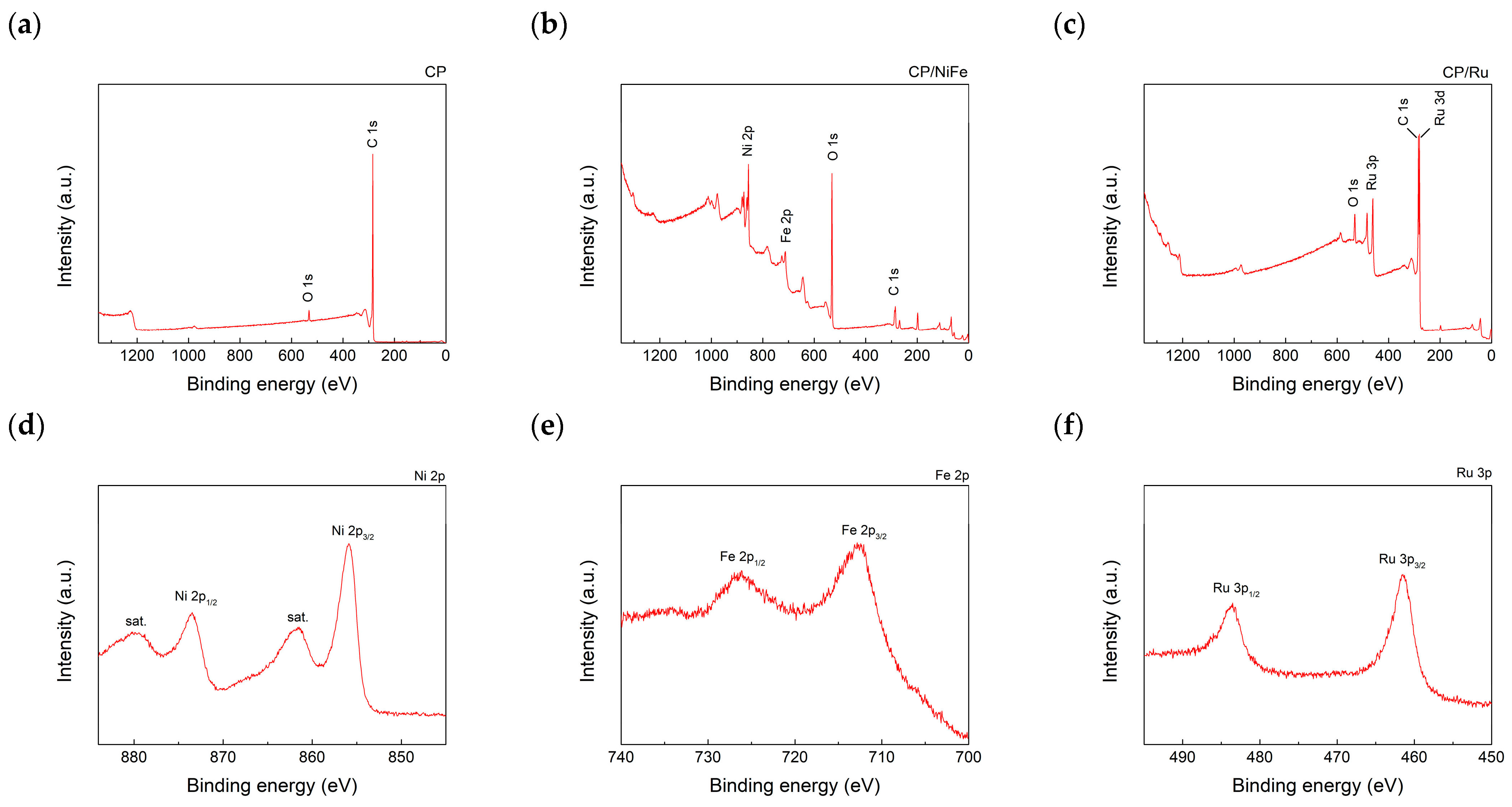
2.4. Characterization of Electrocatalysts on CP
3. Results and Discussion
3.1. Performance of Alkaline Water Electrolyzer
3.2. Material Loading
3.3. SEM Inspection of Electrocatalysts
3.4. XRD Results of Electrocatalysts
3.5. XPS Results of Electrocatalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perera, F.; Ashrafi, A.; Kinney, P.; Mills, D. Towards a fuller assessment of benefits to children’s health of reducing air pollution and mitigating climate change due to fossil fuel combustion. Environ. Res. 2019, 172, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, D.; Qin, Y.; Xie, Y.; Ling, Y.; Ye, H.; Zhang, Y. Facile construction of BiOBr/CoAl-LDH heterojunctions with suppressed Z-axis growth for efficient photoreduction of CO2. Sep. Purif. Technol. 2022, 302, 122090. [Google Scholar] [CrossRef]
- Tang, W.; Ye, H.; Xie, Y.; Chen, P.; Luo, L.; Zhang, Y. Transition metal bismuth spheres dispersed and anchored in benzene-ring-grafted porous g-C3N4 nanosheets for photocatalytic reduction of CO2. Chem. Eng. J. 2023, 478, 147350. [Google Scholar] [CrossRef]
- Ahmed, Z.; Ahmad, M.; Murshed, M.; Shah, M.I.; Mahmood, H.; Abbas, S. How do green energy technology investments, technological innovation, and trade globalization enhance green energy supply and stimulate environmental sustainability in the G7 countries? Gondwana Res. 2022, 112, 105–115. [Google Scholar] [CrossRef]
- Zaik, K.; Werle, S. Solar and wind energy in Poland as power sources for electrolysis process-A review of studies and experimental methodology. Int. J. Hydrogen Energy 2022, 48, 11628–11639. [Google Scholar] [CrossRef]
- Gopinath, M.; Marimuthu, R. A review on solar energy-based indirect water-splitting methods for hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 37742–37759. [Google Scholar] [CrossRef]
- Mikovits, C.; Wetterlund, E.; Wehrle, S.; Baumgartner, J.; Schmidt, J. Stronger together: Multi-annual variability of hydrogen production supported by wind power in Sweden. Appl. Energy 2021, 282, 116082. [Google Scholar] [CrossRef]
- Gazey, R.; Salman, S.; Aklil-D’Halluin, D. A field application experience of integrating hydrogen technology with wind power in a remote island location. J. Power Sources 2006, 157, 841–847. [Google Scholar] [CrossRef]
- Chaparro, A.; Soler, J.; Escudero, M.; De Ceballos, E.; Wittstadt, U.; Daza, L. Data results and operational experience with a solar hydrogen system. J. Power Sources 2005, 144, 165–169. [Google Scholar] [CrossRef]
- Zou, W.-J.; Kim, Y.-B. Temperature control for a 5 kW water-cooled PEM fuel cell system for a household application. IEEE Access 2019, 7, 144826–144835. [Google Scholar] [CrossRef]
- Noh, C.; Shin, M.; Kwon, Y. A strategy for lowering cross-contamination of aqueous redox flow batteries using metal-ligand complexes as redox couple. J. Power Sources 2022, 520, 230810. [Google Scholar] [CrossRef]
- Taherian, R.; Golikand, A.N.; Hadianfard, M.J. Preparation and properties of a phenolic/graphite nanocomposite bipolar plate for proton exchange membrane fuel cell. ECS J. Solid State Sci. Technol. 2012, 1, M39. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Chapman, A.; Itaoka, K.; Farabi-Asl, H.; Fujii, Y.; Nakahara, M. Societal penetration of hydrogen into the future energy system: Impacts of policy, technology and carbon targets. Int. J. Hydrogen Energy 2020, 45, 3883–3898. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Deng, X.; Liu, B.; Ma, J.; Yang, F.; Ouyang, M. Active pressure and flow rate control of alkaline water electrolyzer based on wind power prediction and 100% energy utilization in off-grid wind-hydrogen coupling system. Appl. Energy 2022, 328, 120172. [Google Scholar] [CrossRef]
- Arthur, T.; Millar, G.J.; Sauret, E.; Love, J. Renewable hydrogen production using non-potable water: Thermal integration of membrane distillation and water electrolysis stack. Appl. Energy 2023, 333, 120581. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, J.; Liu, N.; Wu, J.; Kong, L. The improvement in hydrogen storage performance of MgH2 enabled by multilayer Ti3C2. Micromachines 2021, 12, 1190. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Yang, C.-W.; Lee, J.-Y. Implementation and evaluation for anode purging of a fuel cell based on nitrogen concentration. Appl. Energy 2014, 113, 1519–1524. [Google Scholar] [CrossRef]
- Jienkulsawad, P.; Patcharavorachot, Y.; Chen, Y.-S.; Arpornwichanop, A. Energy and exergy analyses of a hybrid system containing solid oxide and molten carbonate fuel cells, a gas turbine, and a compressed air energy storage unit. Int. J. Hydrogen Energy 2021, 46, 34883–34895. [Google Scholar] [CrossRef]
- Millet, P. Fundamentals of water electrolysis. Hydrog. Prod. 2015, 37–62. [Google Scholar] [CrossRef]
- Xiang, R.; Peng, L.; Wei, Z. Tuning interfacial structures for better catalysis of water electrolysis. Chem.—Eur. J. 2019, 25, 9799–9815. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Fang, J.; Ai, X.; Huang, D.; Zhong, Z.; Yang, X.; Wang, L. Comparative study of alkaline water electrolysis, proton exchange membrane water electrolysis and solid oxide electrolysis through multiphysics modeling. Appl. Energy 2022, 312, 118788. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Murthy, A.P.; Lee, S.J.; Karuppasamy, K.; Arumugam, S.R.; Yu, Y.; Hanafiah, M.M.; Kim, H.-S.; Mittal, V.; Choi, M.Y. Recent progress on synthetic strategies and applications of transition metal phosphides in energy storage and conversion. Ceram. Int. 2021, 47, 4404–4425. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Song, Q.; Xu, X.; Hou, W. Sulfur and molybdenum Co-doped graphitic carbon nitride as a superior water dissociation electrocatalyst for alkaline hydrogen evolution reaction. Ceram. Int. 2020, 46, 14178–14187. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Doan, T.L.; Lee, H.E.; Kim, M.; Cho, W.C.; Cho, H.S.; Kim, T. Influence of IrO2/TiO2 coated titanium porous transport layer on the performance of PEM water electrolysis. J. Power Sources 2022, 533, 231370. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Y.; Wang, K.; Tang, Z.; Chen, S. High-performance Ru-based electrocatalyst composed of Ru nanoparticles and Ru single atoms for hydrogen evolution reaction in alkaline solution. Int. J. Hydrogen Energy 2020, 45, 18840–18849. [Google Scholar] [CrossRef]
- Gao, L.; Cui, X.; Sewell, C.D.; Li, J.; Lin, Z. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 8428–8469. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Šljukić, B.; Sequeira, C.; Macciò, D.; Saccone, A.; Figueiredo, J. Electrocatalytic approach for the efficiency increase of electrolytic hydrogen production: Proof-of-concept using platinum–dysprosium alloys. Energy 2013, 50, 486–492. [Google Scholar] [CrossRef]
- Talluri, B.; Yoo, K.; Kim, J. Lanthanum oxide rods as a novel and efficient bifunctional hydrogen and oxygen evolution electrocatalyst for overall water splitting. Ceram. Int. 2022, 48, 18645–18650. [Google Scholar] [CrossRef]
- Naeem, S.; Naeem, F.; Mujtaba, J.; Shukla, A.K.; Mitra, S.; Huang, G.; Gulina, L.; Rudakovskaya, P.; Cui, J.; Tolstoy, V. Oxygen generation using catalytic nano/micromotors. Micromachines 2021, 12, 1251. [Google Scholar] [CrossRef] [PubMed]
- Levene, J.I.; Mann, M.K.; Margolis, R.M.; Milbrandt, A. An analysis of hydrogen production from renewable electricity sources. Sol. Energy 2007, 81, 773–780. [Google Scholar] [CrossRef]
- Aulakh, D.J.S.; Boulama, K.G.; Pharoah, J.G. On the reduction of electric energy consumption in electrolysis: A thermodynamic study. Int. J. Hydrogen Energy 2021, 46, 17084–17096. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Jafari-Asl, M.; Nabiyan, A.; Rezaei, B. Ni3S2/ball-milled silicon flour as a bi-functional electrocatalyst for hydrogen and oxygen evolution reactions. Energy 2016, 116, 392–401. [Google Scholar] [CrossRef]
- Mahale, N.K.; Ingle, S.T. Electrocatalytic hydrogen evolution reaction on nano-nickel decorated graphene electrode. Energy 2017, 119, 872–878. [Google Scholar] [CrossRef]
- Liu, Z.; Corva, M.; Amin, H.M.; Blanc, N.; Linnemann, J.; Tschulik, K. Single Co3O4 nanocubes electrocatalyzing the oxygen evolution reaction: Nano-impact insights into intrinsic activity and support effects. Int. J. Mol. Sci. 2021, 22, 13137. [Google Scholar] [CrossRef]
- Parkash, A.; Seehar, T.H.; Pirzada, A.M.; Islam, M.; Larik, R. Evaluation of Novel Fuel Cell Catalysts with Ultra-Low Noble Metal Contents towards Electrochemical Catalysis. ECS J. Solid State Sci. Technol. 2022, 11, 091009. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Xu, W.; Jawaid, S.; Mohammed-Ibrahim, J.; Liu, W.; Kuang, Y.; Sun, X. Recent advances in non-precious metal-based electrodes for alkaline water electrolysis. ChemNanoMat 2020, 6, 336–355. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Zhang, T.; Liu, S.; Song, L.; Yang, F.; Ouyang, M.; Shen, X. Exploration of the configuration and operation rule of the multi-electrolyzers hybrid system of large-scale alkaline water hydrogen production system. Appl. Energy 2023, 331, 120413. [Google Scholar] [CrossRef]
- Kim, S.; Han, J.H.; Yuk, J.; Kim, S.; Song, Y.; So, S.; Lee, K.T.; Kim, T.-H. Highly selective porous separator with thin skin layer for alkaline water electrolysis. J. Power Sources 2022, 524, 231059. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2014, 8, 23–39. [Google Scholar] [CrossRef]
- Navadeepthy, D.; Rebekah, A.; Viswanthan, C.; Ponpandian, N. Boosting the kinetics of oxygen and hydrogen evolution in alkaline water splitting using nickel ferrite/N-graphene nanocomposite as a bifunctional electrocatalyst. Int. J. Hydrogen Energy 2021, 46, 21512–21524. [Google Scholar] [CrossRef]
- Wang, D.; Watanabe, F.; Zhao, W. Reduced graphene oxide-NiO/Ni nanomembranes as oxygen evolution reaction electrocatalysts. ECS J. Solid State Sci. Technol. 2017, 6, M3049. [Google Scholar] [CrossRef]
- Raja, D.S.; Lin, H.-W.; Lu, S.-Y. Synergistically well-mixed MOFs grown on nickel foam as highly efficient durable bifunctional electrocatalysts for overall water splitting at high current densities. Nano Energy 2019, 57, 1–13. [Google Scholar] [CrossRef]
- Todoroki, N.; Wadayama, T. Heterolayered Ni–Fe hydroxide/oxide nanostructures generated on a stainless-steel substrate for efficient alkaline water splitting. ACS Appl. Mater. Interfaces 2019, 11, 44161–44169. [Google Scholar] [CrossRef]
- Loh, A.; Li, X.; Taiwo, O.O.; Tariq, F.; Brandon, N.P.; Wang, P.; Xu, K.; Wang, B. Development of Ni–Fe based ternary metal hydroxides as highly efficient oxygen evolution catalysts in AEM water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 24232–24247. [Google Scholar] [CrossRef]
- Thangavel, P.; Kim, G.; Kim, K.S. Electrochemical integration of amorphous NiFe (oxy) hydroxides on surface-activated carbon fibers for high-efficiency oxygen evolution in alkaline anion exchange membrane water electrolysis. J. Mater. Chem. A 2021, 9, 14043–14051. [Google Scholar] [CrossRef]
- Thangavel, P.; Ha, M.; Kumaraguru, S.; Meena, A.; Singh, A.N.; Harzandi, A.M.; Kim, K.S. Graphene-nanoplatelets-supported NiFe-MOF: High-efficiency and ultra-stable oxygen electrodes for sustained alkaline anion exchange membrane water electrolysis. Energy Environ. Sci. 2020, 13, 3447–3458. [Google Scholar] [CrossRef]
- Tseng, C.-Y.; Cheng, I.-C.; Chen, J.-Z. Low-pressure-plasma-processed NiFe-MOFs/nickel foam as an efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 35990–35998. [Google Scholar] [CrossRef]
- Liu, C.; Tseng, C.-Y.; Wang, Y.-C.; Cheng, I.-C.; Chen, J.-Z. Low-Pressure Plasma-Processed Ruthenium/Nickel Foam Electrocatalysts for Hydrogen Evolution Reaction. Materials 2022, 15, 2603. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Meng, H.; Yan, S.; Zhu, H.; Ma, W.; Wang, C.; Ma, F.; Hu, Z. The in-situ construction of NiFe sulfide with nanoarray structure on nickel foam as efficient bifunctional electrocatalysts for overall water splitting. J. Alloys Compd. 2021, 874, 159874. [Google Scholar] [CrossRef]
- Ram Kumar, K.; Maiyalagan, T. Iron nickel sulphide embedded on multi-walled carbon nanotubes as efficient electrocatalysts for oxygen evolution reaction in alkaline medium. Ceram. Int. 2023, 49, 1195–1202. [Google Scholar] [CrossRef]
- Caprì, A.; Gatto, I.; Lo Vecchio, C.; Trocino, S.; Carbone, A.; Baglio, V. Anion Exchange Membrane Water Electrolysis Based on Nickel Ferrite Catalysts. ChemElectroChem 2023, 10, e202201056. [Google Scholar] [CrossRef]
- Tetzlaff, D.; Pellumbi, K.; Baier, D.M.; Hoof, L.; Barkur, H.S.; Smialkowski, M.; Amin, H.M.; Grätz, S.; Siegmund, D.; Borchardt, L. Sustainable and rapid preparation of nanosized Fe/Ni-pentlandite particles by mechanochemistry. Chem. Sci. 2020, 11, 12835–12842. [Google Scholar] [CrossRef]
- Amin, H.M.; Apfel, U.P. Metal-Rich Chalcogenides as Sustainable Electrocatalysts for Oxygen Evolution and Reduction: State of the Art and Future Perspectives. Eur. J. Inorg. Chem. 2020, 2020, 2679–2690. [Google Scholar] [CrossRef]
- Chen, Z.; Qing, H.; Zhou, K.; Sun, D.; Wu, R. Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Prog. Mater. Sci. 2020, 108, 100618. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Metal-organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lazaro, A.; Caprì, A.; Gatto, I.; Ledesma-García, J.; Rey-Raap, N.; Arenillas, A.; Espinosa-Lagunes, F.; Baglio, V.; Arriaga, L. NiFe2O4 hierarchical nanoparticles as electrocatalyst for anion exchange membrane water electrolysis. J. Power Sources 2023, 556, 232417. [Google Scholar] [CrossRef]
- Li, T.-T.; Shi, B.-Y.; Jiang, L.-W.; Zheng, J.-F.; Wang, J.-J. Design and Preparation of NiFe2O4@ FeOOH Composite Electrocatalyst for Highly Efficient and Stable Oxygen Evolution Reaction. Molecules 2022, 27, 7438. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhu, W.; Yu, X.; Zhang, H.; Li, Y.; Sun, X.; Wang, X.; Wang, H.; Wang, J.; Luo, J. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes. Adv. Mater. 2014, 26, 2683–2687. [Google Scholar] [CrossRef]
- Zou, X.; Wu, Y.; Liu, Y.; Liu, D.; Li, W.; Gu, L.; Liu, H.; Wang, P.; Sun, L.; Zhang, Y. In situ generation of bifunctional, efficient Fe-based catalysts from mackinawite iron sulfide for water splitting. Chem 2018, 4, 1139–1152. [Google Scholar] [CrossRef]
- Gao, Y.; Montana, A.; Chen, F. Evaluation of porosity and thickness on effective diffusivity in gas diffusion layer. J. Power Sources 2017, 342, 252–265. [Google Scholar] [CrossRef]
- Waseem, S.; Maheshwari, P.H.; Maheshwari, P.; Sahu, A.K.; Saini, A.; Dhakate, S.R. Configuring the Porosity and Microstructure of Carbon Paper Electrode Using Pore Formers and Its Influence on the Performance of PEMFC. Energy Fuels 2020, 34, 16736–16745. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, H.; Liu, T.; Li, X.; Liu, Z. Carbon paper coated with supported tungsten trioxide as novel electrode for all-vanadium flow battery. J. Power Sources 2012, 218, 455–461. [Google Scholar] [CrossRef]
- Lopez-Fernandez, E.; Sacedon, C.G.; Gil-Rostra, J.; Yubero, F.; Gonzalez-Elipe, A.R.; de Lucas-Consuegra, A. Recent Advances in Alkaline Exchange Membrane Water Electrolysis and Electrode Manufacturing. Molecules 2021, 26, 6326. [Google Scholar] [CrossRef]
- Razmjooei, F.; Morawietz, T.; Taghizadeh, E.; Hadjixenophontos, E.; Mues, L.; Gerle, M.; Wood, B.D.; Harms, C.; Gago, A.S.; Ansar, S.A.; et al. Increasing the performance of an anion-exchange membrane electrolyzer operating in pure water with a nickel-based microporous layer. Joule 2021, 5, 1776–1799. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Phillips, R.; Dunnill, C.W. Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas. RSC Adv. 2016, 6, 100643–100651. [Google Scholar] [CrossRef]
- Abbasi, R.; Setzler, B.P.; Lin, S.; Wang, J.; Zhao, Y.; Xu, H.; Pivovar, B.; Tian, B.; Chen, X.; Wu, G. A roadmap to low-cost hydrogen with hydroxide exchange membrane electrolyzers. Adv. Mater. 2019, 31, 1805876. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials. Acs Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 946. [Google Scholar]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and affordable hydrogen fuel from alkaline water splitting: Past, recent progress, and future prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2019, 120, 851–918. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, Z.; Xie, C.; Feng, S.; Liu, D.; Shao, M.; Wang, S. Layered double hydroxide nanosheets with multiple vacancies obtained by dry exfoliation as highly efficient oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, C.; Cho, Y.; Hyun, G.; Jeon, S.; Park, J.H. Suppressing buoyant force: New avenue for long-term durability of oxygen evolution catalysts. Nano Energy 2018, 54, 184–191. [Google Scholar] [CrossRef]
- Jiménez, J.M.O.; González, R.L.; Mendoza, C.G.; Cuauhtémoc-López, I.; Lemus, M.A.A.; Mendoza, G.M. Comparative Effect of Adsorption and Photodegradation on Benzene and Naphthalene Using Bismuth Oxide Modify Graphene Oxide. J. Mex. Chem. Soc. 2022, 66, 371–384. [Google Scholar]
- Cui, W.; Bai, H.; Shang, J.; Wang, F.; Xu, D.; Ding, J.; Fan, W.; Shi, W. Organic-inorganic hybrid-photoanode built from NiFe-MOF and TiO2 for efficient PEC water splitting. Electrochim. Acta 2020, 349, 136383. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.-H. Preparation and electrochemical properties of mesoporous NiFe2O4/N-doped carbon nanocomposite as an anode for lithium ion battery. Front. Mater. 2020, 7, 178. [Google Scholar] [CrossRef]
- Gopinath, K.; Karthika, V.; Gowri, S.; Senthilkumar, V.; Kumaresan, S.; Arumugam, A. Antibacterial activity of ruthenium nanoparticles synthesized using Gloriosa superba L. leaf extract. J. Nanostruct. Chem. 2014, 4, 83. [Google Scholar] [CrossRef]
- Kotsugi, Y.; Han, S.-M.; Kim, Y.-H.; Cheon, T.; Nandi, D.K.; Ramesh, R.; Yu, N.-K.; Son, K.; Tsugawa, T.; Ohtake, S. Atomic layer deposition of Ru for replacing Cu-interconnects. Chem. Mater. 2021, 33, 5639–5651. [Google Scholar] [CrossRef]
- Cai, M.; Cao, S.; Zhuo, Z.; Wang, X.; Shi, K.; Cheng, Q.; Xue, Z.; Du, X.; Shen, C.; Liu, X. Fabrication of Ni2P Cocatalyzed CdS Nanorods with a Well-Defined Heterointerface for Enhanced Photocatalytic H2 Evolution. Catalysts 2022, 12, 417. [Google Scholar] [CrossRef]
- Zhang, F.-S.; Wang, J.-W.; Luo, J.; Liu, R.-R.; Zhang, Z.-M.; He, C.-T.; Lu, T.-B. Extraction of nickel from NiFe-LDH into Ni 2 P@ NiFe hydroxide as a bifunctional electrocatalyst for efficient overall water splitting. Chem. Sci. 2018, 9, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]

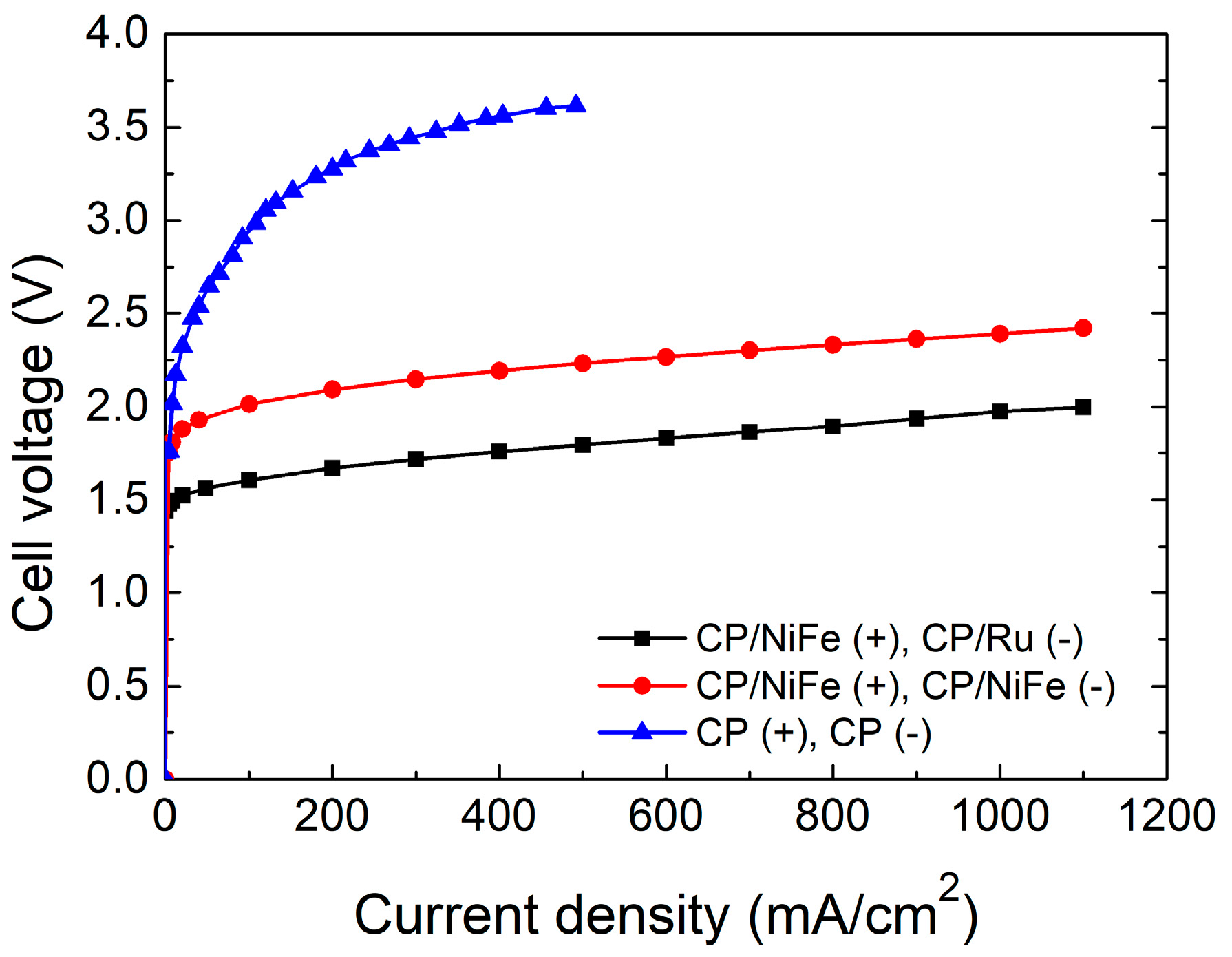
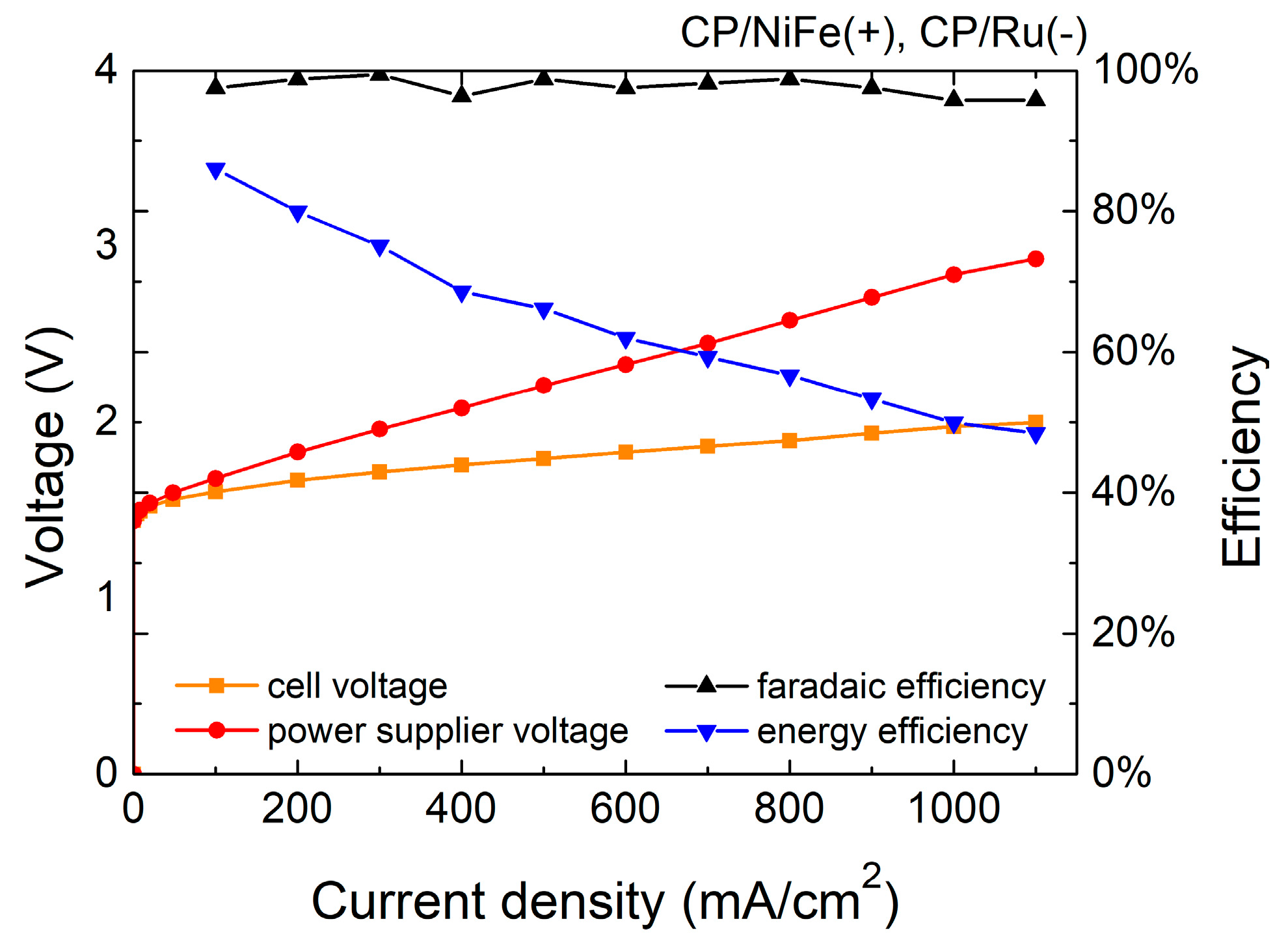

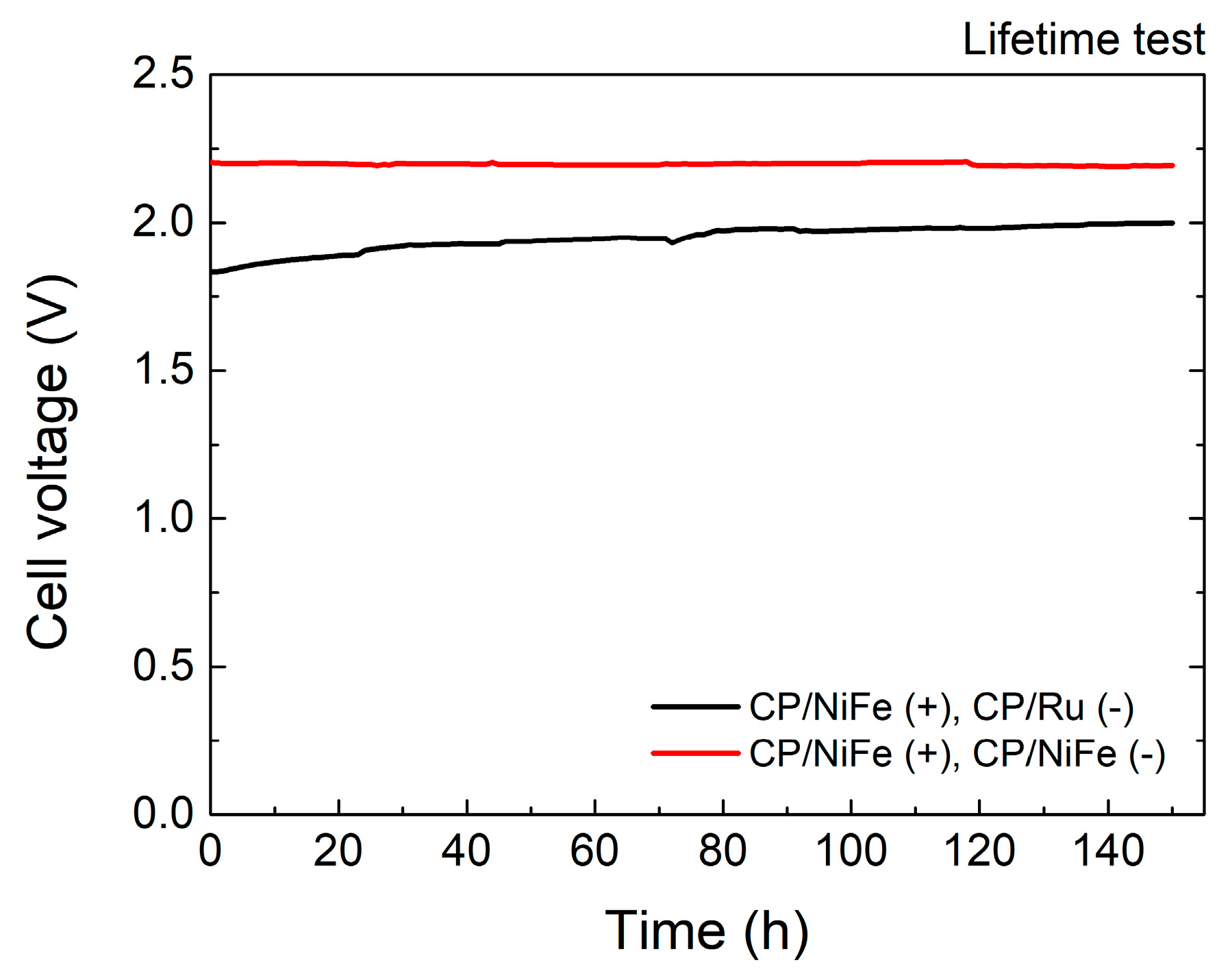
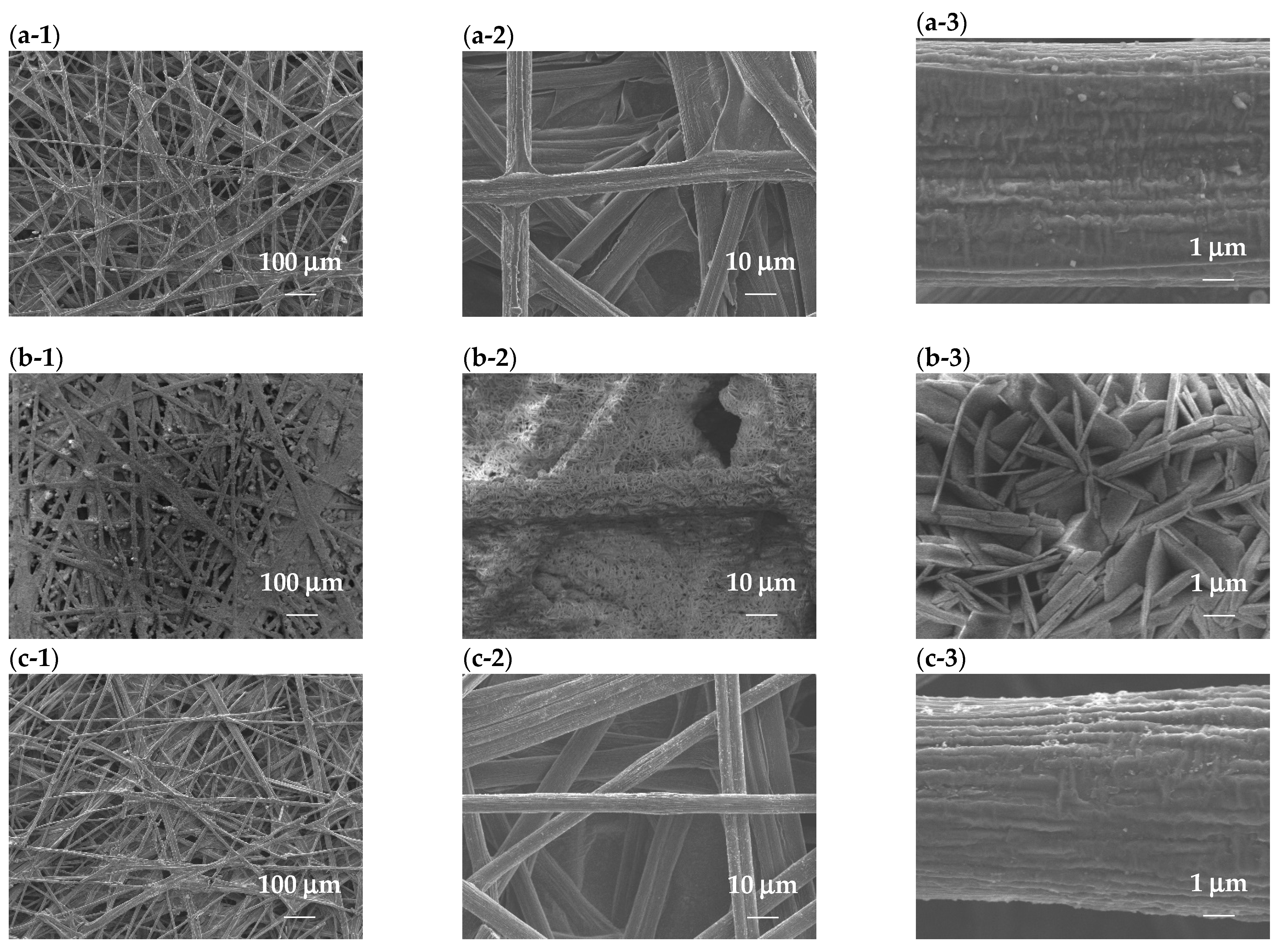

| Electrocatalysts | Current Density | Cell Voltage | Power Supply Voltage | H2 Production Rate (Theoretical) | H2 Production Rate (Experimental) | Specific Energy Consumption | Faradaic Efficiency (FE) | Energy Efficiency (η) |
|---|---|---|---|---|---|---|---|---|
| unit | mA/cm2 | V | V | mL/min | mL/min | kWh/m3 | % | % |
| NiFe(+)/Ru(−) | 100 | 1.60 | 1.68 | 19.0 | 18.5 | 3.8 | 97.6 | 86.0 |
| 500 | 1.79 | 2.21 | 94.9 | 93.8 | 4.9 | 98.8 | 66.2 | |
| 1000 | 1.98 | 2.84 | 189.8 | 181.8 | 6.5 | 95.8 | 49.9 | |
| NiFe(+)/NiFe(−) | 100 | 2.02 | 2.08 | 19.0 | 18.5 | 4.7 | 97.5 | 69.4 |
| 500 | 2.23 | 2.59 | 94.9 | 94.0 | 5.7 | 99.1 | 56.6 | |
| 1000 | 2.39 | 3.11 | 189.8 | 188.0 | 6.9 | 99.1 | 47.2 |
| Electrocatalysts | Time | Cell Voltage | Increment |
|---|---|---|---|
| unit | h | V | V |
| NiFe(+)/Ru(−) | 0 | 1.833 | |
| 50 | 1.936 | 0.103 (+5.6%) | |
| 100 | 1.975 | 0.142 (+7.7%) | |
| 150 | 2.000 | 0.167 (+9.1%) | |
| NiFe(+)/NiFe(−) | 0 | 2.203 | |
| 50 | 2.197 | −0.006 (−0.3%) | |
| 100 | 2.201 | −0.002 (−0.1%) | |
| 150 | 2.193 | −0.010 (−0.5%) |
| Electrocatalysts | Material Loading | Standard Deviation |
|---|---|---|
| CP/NiFe | 2.03 mg/cm2 | 0.37 mg/cm2 |
| CP/Ru | 1.19 mg/cm2 | 0.14 mg/cm2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Yu, S.-E.; Su, Y.-L.; Cheng, I.-C.; Chuang, Y.-C.; Chen, Y.-S.; Chen, J.-Z. NiFe2O4 Material on Carbon Paper as an Electrocatalyst for Alkaline Water Electrolysis Module. Micromachines 2024, 15, 62. https://doi.org/10.3390/mi15010062
Wang Y-C, Yu S-E, Su Y-L, Cheng I-C, Chuang Y-C, Chen Y-S, Chen J-Z. NiFe2O4 Material on Carbon Paper as an Electrocatalyst for Alkaline Water Electrolysis Module. Micromachines. 2024; 15(1):62. https://doi.org/10.3390/mi15010062
Chicago/Turabian StyleWang, Ying-Chyi, Shuo-En Yu, Yu-Lun Su, I-Chun Cheng, Yi-Cheng Chuang, Yong-Song Chen, and Jian-Zhang Chen. 2024. "NiFe2O4 Material on Carbon Paper as an Electrocatalyst for Alkaline Water Electrolysis Module" Micromachines 15, no. 1: 62. https://doi.org/10.3390/mi15010062
APA StyleWang, Y.-C., Yu, S.-E., Su, Y.-L., Cheng, I.-C., Chuang, Y.-C., Chen, Y.-S., & Chen, J.-Z. (2024). NiFe2O4 Material on Carbon Paper as an Electrocatalyst for Alkaline Water Electrolysis Module. Micromachines, 15(1), 62. https://doi.org/10.3390/mi15010062








