A Comparative Study on the Effect of Substrate Structure on Electrochemical Performance and Stability of Electrodeposited Platinum and Iridium Oxide Coatings for Neural Electrodes
Abstract
:1. Introduction
2. Materials and Methods
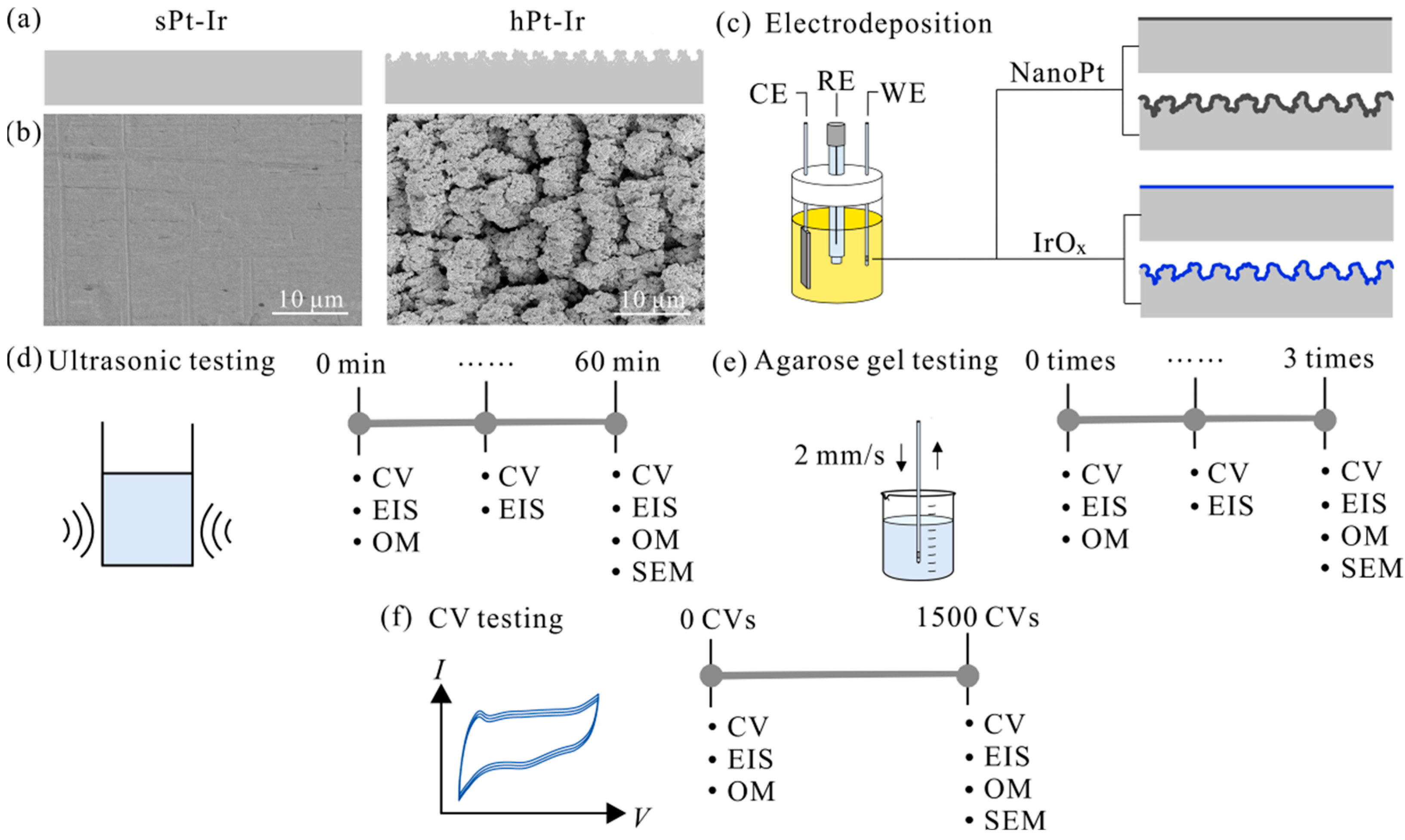
2.1. Electrode Fabrication
2.2. NanoPt and IrOx Coating Preparation
2.3. Electrochemical Performance Characterization
2.4. Surface Morphology Characterization
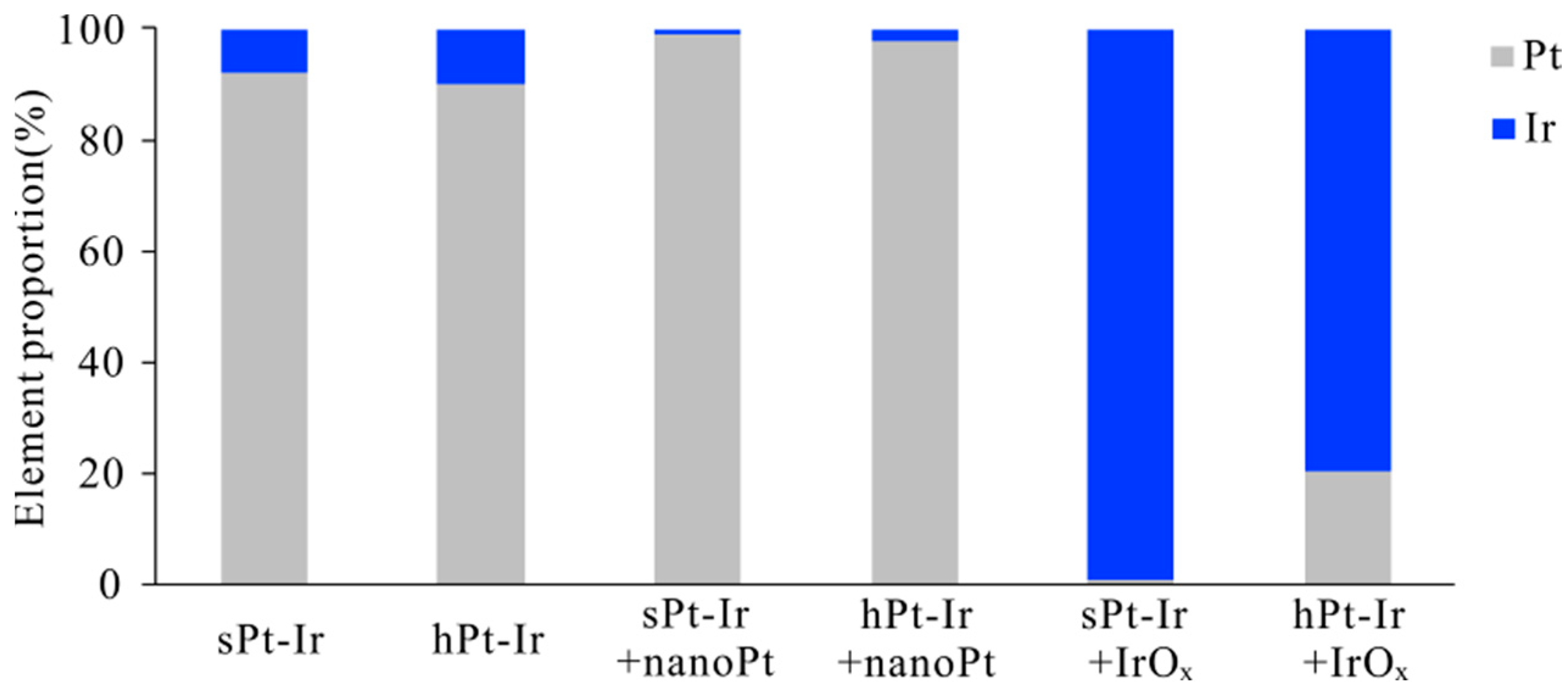
2.5. Surface Element Analysis
2.6. Ultrasonic Testing
2.7. Agarose Gel Testing
2.8. Cyclic Voltammetry Testing
3. Results
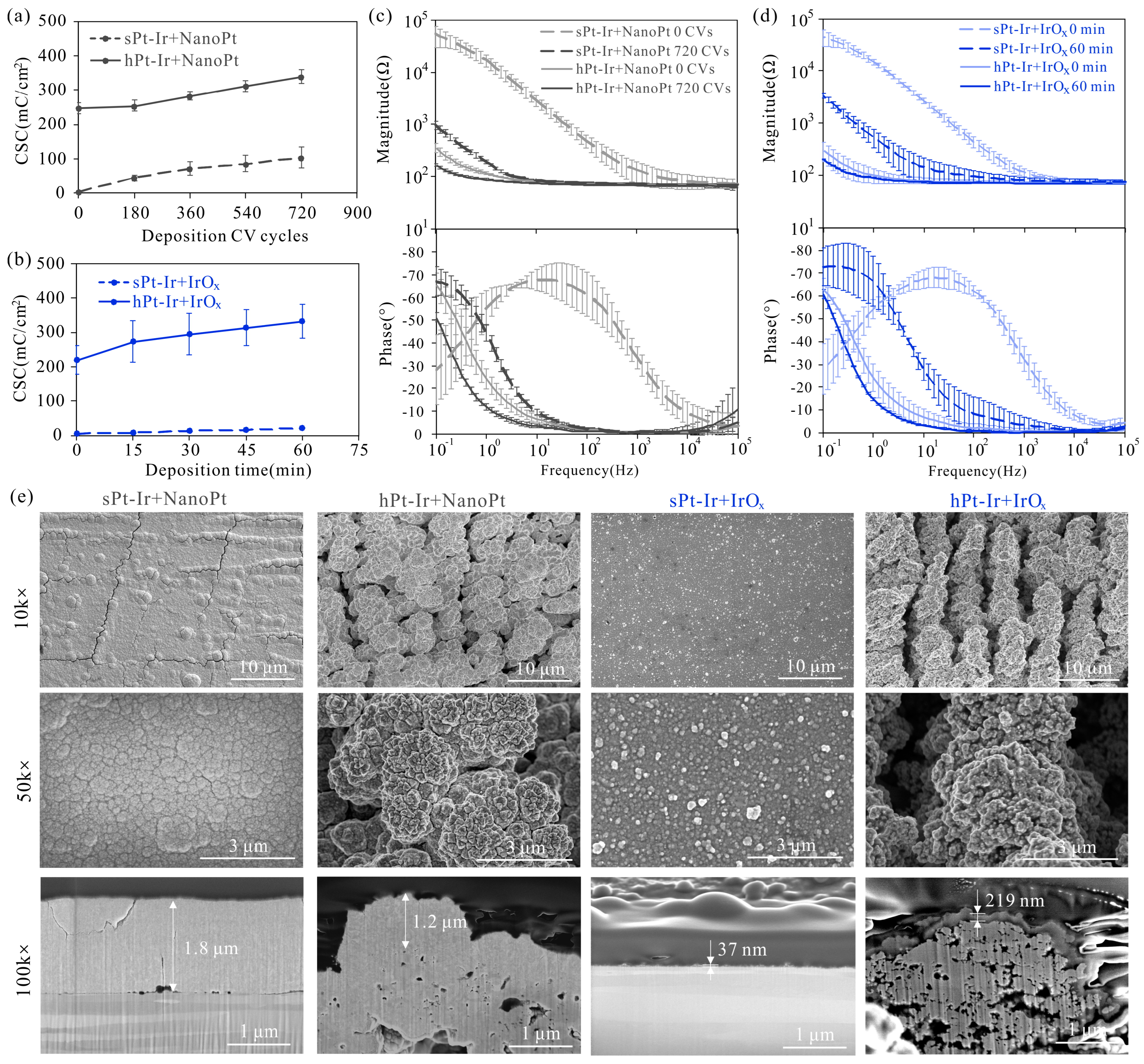
3.1. Electrodeposition of NanoPt and IrOx Coatings
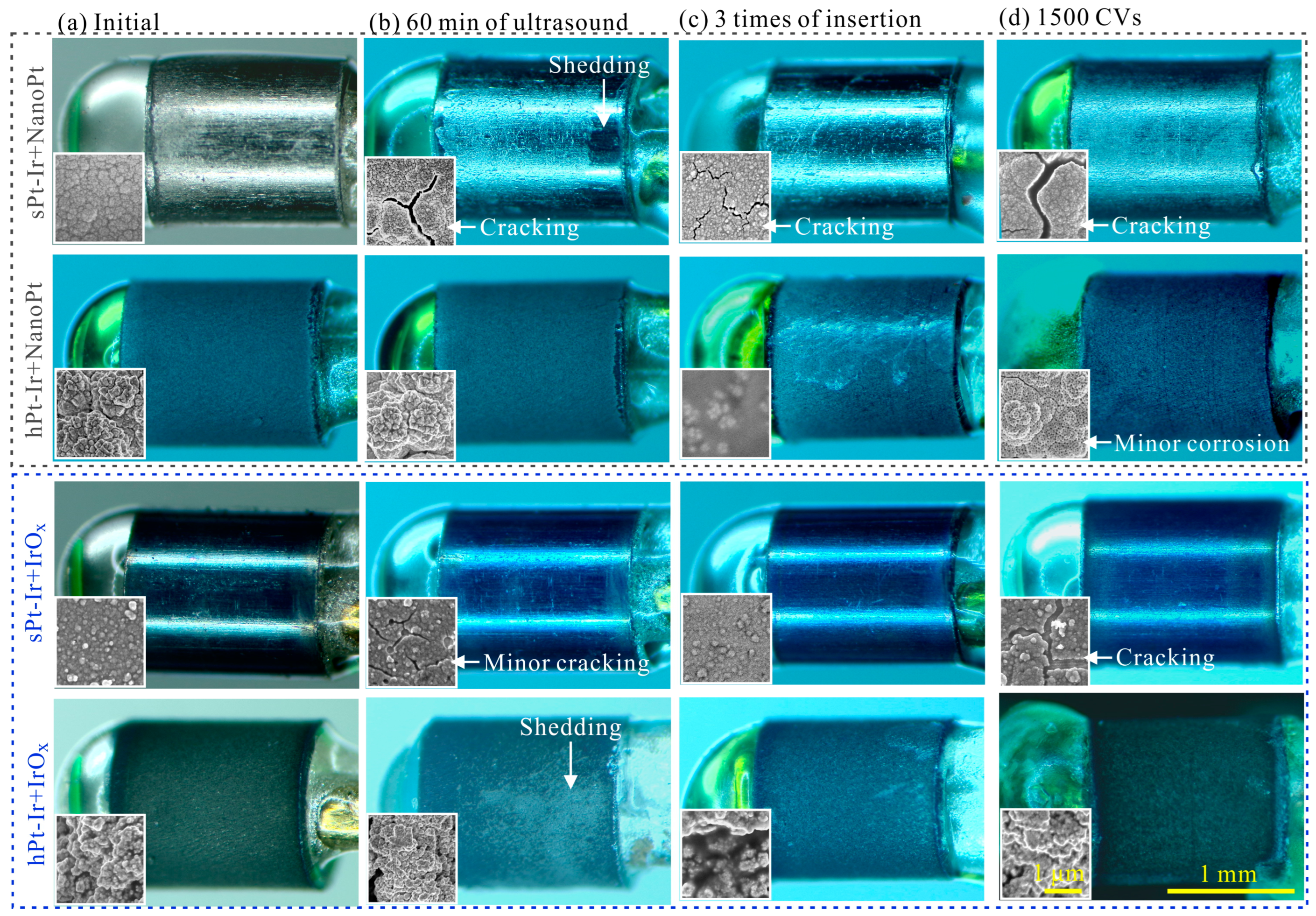
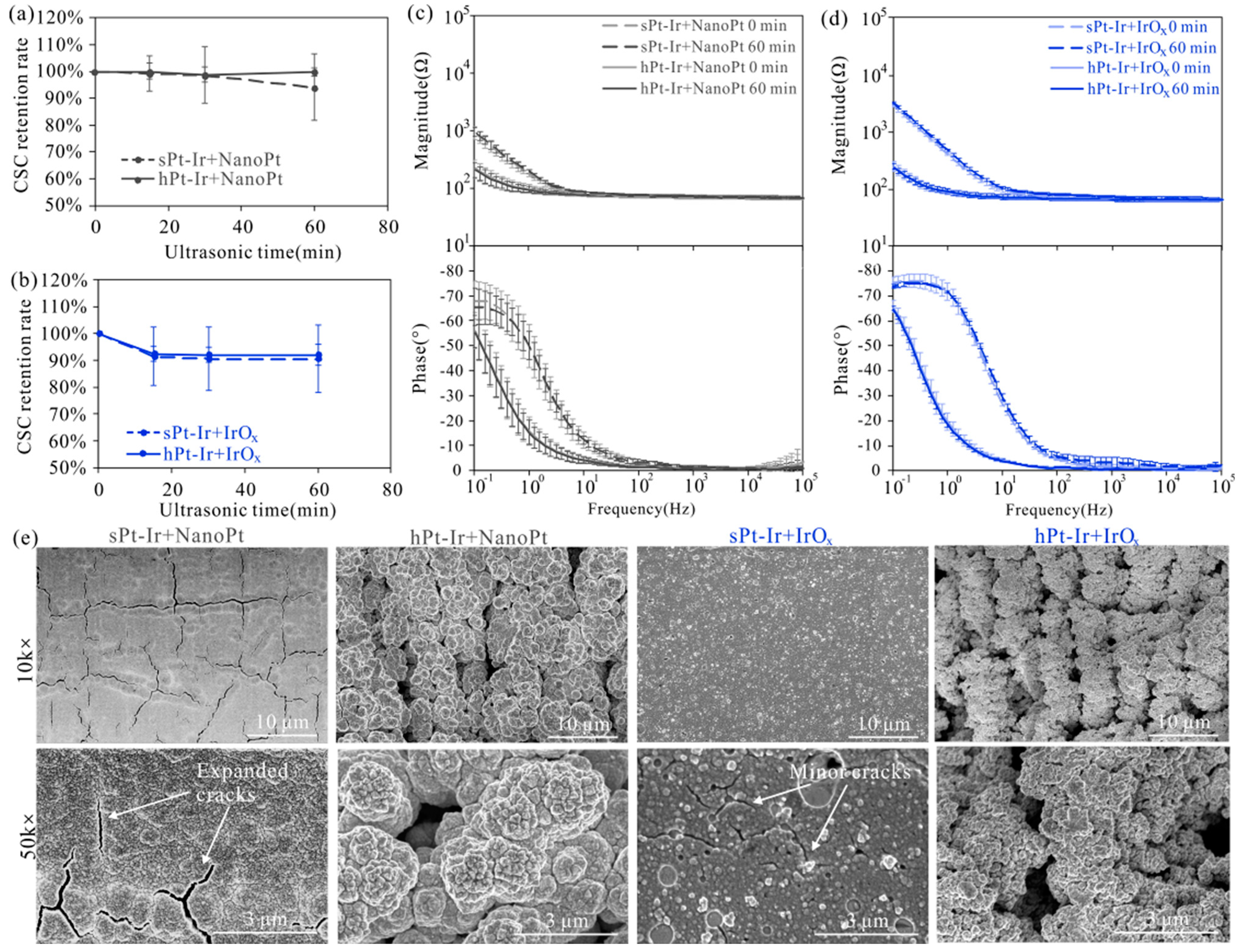
3.2. Mechanical Stability
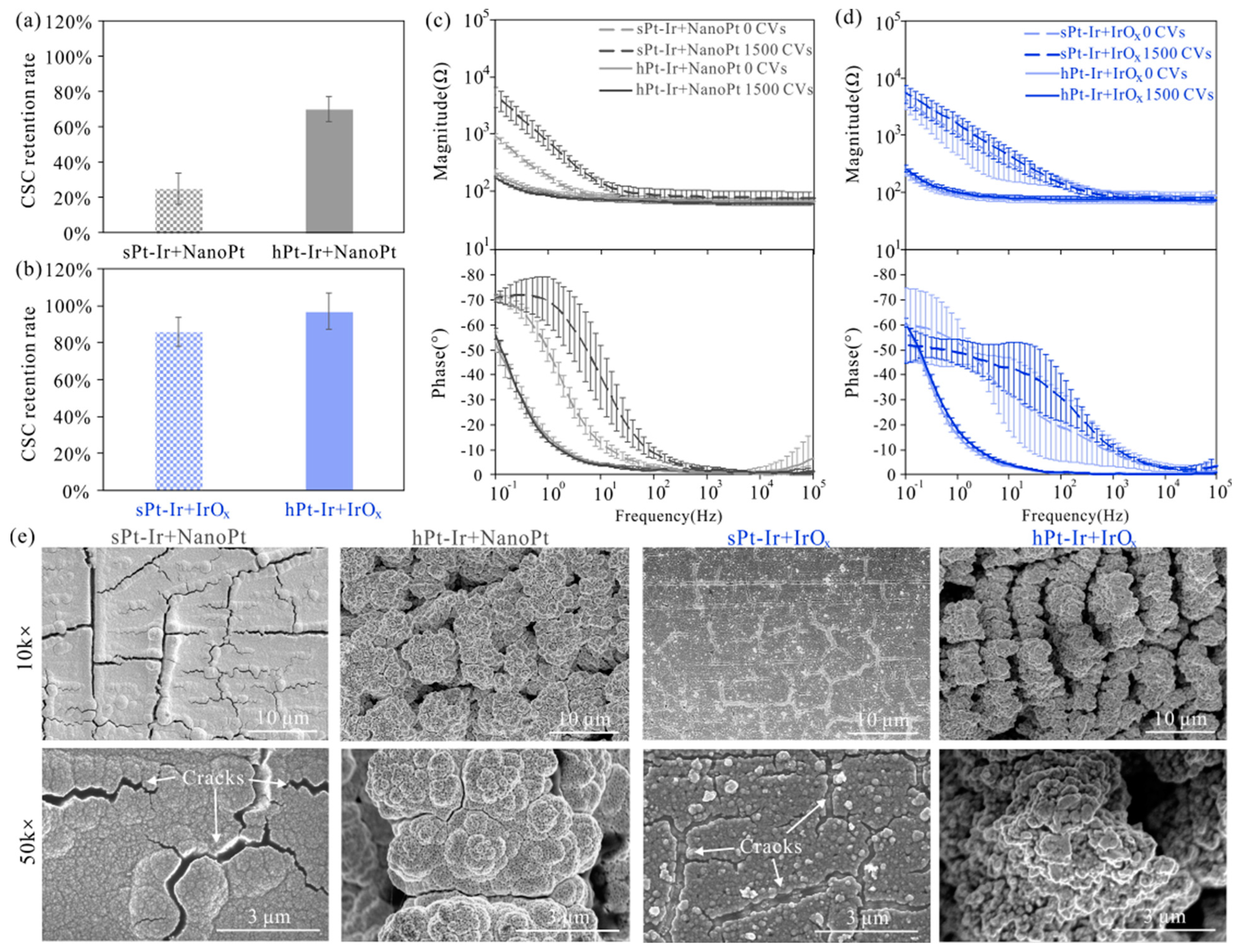
3.3. Electrochemical Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, J.K.; Mayberg, H.S.; Wang, D.D.; Richardson, R.M.; Halpern, C.H.; Krinke, L.; Arlotti, M.; Rossi, L.; Priori, A.; Marceglia, S.; et al. Proceedings of the 10th annual deep brain stimulation think tank: Advances in cutting edge technologies, artificial intelligence, neuromodulation, neuroethics, interventional psychiatry, and women in neuromodulation. Front. Hum. Neurosci. 2022, 16, 1084782. [Google Scholar] [CrossRef] [PubMed]
- Carron, R.; Roncon, P.; Lagarde, S.; Dibué, M.; Zanello, M.; Bartolomei, F. Latest views on the mechanisms of action of surgically implanted cervical vagal nerve stimulation in epilepsy. Neuromodul. Technol. Neural Interface 2023, 26, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Lorach, H.; Galvez, A.; Spagnolo, V.; Martel, F.; Karakas, S.; Intering, N.; Vat, M.; Faivre, O.; Harte, C.; Komi, S.; et al. Walking naturally after spinal cord injury using a brain-spine interface. Nature 2023, 618, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Mina, M.; Sahyoun, J.; Kalevar, A.; Tran, S.D. Retinal prostheses: Engineering and clinical perspectives for vision restoration. Sensors 2023, 23, 5782. [Google Scholar] [CrossRef] [PubMed]
- Borda, E.; Ghezzi, D. Advances in visual prostheses: Engineering and biological challenges. Prog. Biomed. Eng. 2022, 4, 32003. [Google Scholar] [CrossRef]
- Eggers, T.; Kilgore, J.; Green, D.; Vrabec, T.; Kilgore, K.; Bhadra, N. Combining direct current and kilohertz frequency alternating current to mitigate onset activity during electrical nerve block. J. Neural Eng. 2021, 18, 46010. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.G.; Rogers, E.R.; Harris, J.P.; Sullivan, A.; Ackermann, D.M.; Russo, M.; Lempka, S.F.; Mcmahon, S.B. Neuromodulation using ultra low frequency current waveform reversibly blocks axonal conduction and chronic pain. Sci. Transl. Med. 2021, 13, g9890. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, Z. Challenges and opportunities of implantable neural interfaces: From material, electrochemical and biological perspectives. Adv. Funct. Mater. 2023, 33, 2301223. [Google Scholar] [CrossRef]
- Zheng, X.S.; Tan, C.; Castagnola, E.; Cui, X.T. Electrode Materials for Chronic Electrical Microstimulation. Adv. Healthc. Mater. 2021, 10, 2100119. [Google Scholar] [CrossRef]
- Dalrymple, A.N.; Robles, U.A.; Huynh, M.; Nayagam, B.A.; Green, R.A.; Poole-Warren, L.A.; Fallon, J.B.; Shepherd, R.K. Electrochemical and biological performance of chronically stimulated conductive hydrogel electrodes. J. Neural Eng. 2020, 17, 26018. [Google Scholar] [CrossRef]
- Basova, T.V.; Vikulova, E.S.; Dorovskikh, S.I.; Hassan, A.; Morozova, N.B. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Boehler, C.; Vieira, D.M.; Egert, U.; Asplund, M. NanoPt—A nanostructured electrode coating for neural recording and microstimulation. ACS Appl. Mater. Interfaces 2020, 12, 14855–14865. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Yu, S.; Fan, Z.; Huang, Y.; Song, B.; Zhou, T. Nanocone-array-based platinum-iridium oxide neural microelectrodes: Structure, electrochemistry, durability and biocompatibility study. Nanomaterials 2022, 12, 3445. [Google Scholar] [CrossRef]
- Zeng, Q.; Xia, K.; Zhang, Y.; Wu, T. Well controlled 3D iridium oxide/platinum nanocomposites with greatly enhanced electrochemical performances. Adv. Mater. Interfaces 2019, 6, 1900356. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, Z.; Cai, G.; Wu, T. Platinum nanocrystal assisted by low-content iridium for high-performance flexible electrode: Applications on neural interface, water oxidation, and anti-microbial contamination. Adv. Mater. Interfaces 2021, 8, 2100965. [Google Scholar] [CrossRef]
- Kim, R.; Nam, Y. Electrochemical layer-by-layer approach to fabricate mechanically stable platinum black microelectrodes using a mussel-inspired polydopamine adhesive. J. Neural Eng. 2015, 12, 26010. [Google Scholar] [CrossRef]
- Ivanovskaya, A.N.; Belle, A.M.; Yorita, A.M.; Qian, F.; Chen, S.; Tooker, A.; Lozada, R.G.I.; Dahlquist, D.; Tolosa, V. Electrochemical roughening of thin-film platinum for neural probe arrays and biosensing applications. J. Electrochem. Soc. 2018, 165, G3125. [Google Scholar] [CrossRef]
- Zhang, K.; Deng, J.; Guo, X.; Sun, L.; Lei, S. Study on the adhesion and tribological behavior of PVD TiAlN coatings with a multi-scale textured substrate surface. Int. J. Refract. Hard Met. 2018, 72, 292–305. [Google Scholar] [CrossRef]
- Park, J.; Park, B.; Son, Y.J.; Lee, S.H.; Um, S.; Kim, Y.; Ok, M.; Sun, J.; Han, H.; Jeon, H. Femtosecond laser-mediated anchoring of polymer layers on the surface of a biodegradable metal. J. Magnes. Alloys 2021, 9, 1373–1381. [Google Scholar] [CrossRef]
- Zhang, P.; Zou, X.; Zhang, S.; Xia, C.; Liang, C.; Liu, N.; Wang, H. Improve the binding force of PEEK coating with Mg surface by femtosecond lasers induced micro/nanostructures. J. Mater. Sci. 2021, 56, 13313–13322. [Google Scholar] [CrossRef]
- Li, L.; Jiang, C.; Duan, W.; Wang, Z.; Zhang, F.; He, C.; Long, T.; Li, L. Electrochemical and biological performance of hierarchical platinum-iridium electrodes structured by a femtosecond laser. Microsyst. Nanoeng. 2022, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, C.; Li, L. Hierarchical platinum-iridium neural electrodes structured by femtosecond laser for superwicking interface and superior charge storage capacity. Bio-Des. Manuf. 2022, 5, 163–173. [Google Scholar] [CrossRef]
- Ramesh, V.; Stratmann, N.; Schaufler, V.; Angelov, S.D.; Nordhorn, I.D.; Heissler, H.E.; Martínez-Hincapié, R.; Čolić, V.; Rehbock, C.; Schwabe, K.; et al. Mechanical stability of nano-coatings on clinically applicable electrodes, generated by electrophoretic deposition. Adv. Healthc. Mater. 2022, 11, 2102637. [Google Scholar] [CrossRef] [PubMed]
- Hyakumura, T.; Aregueta-Robles, U.; Duan, W.; Villalobos, J.; Adams, W.K.; Poole-Warren, L.; Fallon, J.B. Improving deep brain stimulation electrode performance in vivo through use of conductive hydrogel coatings. Front. Neurosci. 2021, 15, 761525. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.; Oberueber, F.; Stieglitz, T.; Asplund, M. Nanostructured platinum as an electrochemically and mechanically stable electrode coating. In Proceedings of the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 1058–1061. [Google Scholar]
- Plyasova, L.M.; Molina, I.Y.; Gavrilov, A.N.; Cherepanova, S.V.; Cherstiouk, O.V.; Rudina, N.A.; Savinova, E.R.; Tsirlina, G.A. Electrodeposited platinum revisited: Tuning nanostructure via the deposition potential. Electrochim. Acta 2006, 51, 4477–4488. [Google Scholar] [CrossRef]
- Obradović, M.D.; Balanč, B.D.; Lačnjevac, U.; Gojković, S.L. Electrochemically deposited iridium-oxide: Estimation of intrinsic activity and stability in oxygen evolution in acid solution. J. Electroanal. Chem. 2021, 881, 114944. [Google Scholar] [CrossRef]
- Kötz, R.; Neff, H.; Stucki, S. Anodic iridium oxide films: XPS-studies of oxidation state changes and O2-Evolution. J. Electrochem. Soc. 1984, 131, 72. [Google Scholar] [CrossRef]
- Boehler, C.; Stieglitz, T.; Asplund, M. Nanostructured platinum grass enables superior impedance reduction for neural microelectrodes. Biomaterials 2015, 67, 346–353. [Google Scholar] [CrossRef]
- Weremfo, A.; Carter, P.; Hibbert, D.B.; Zhao, C. Investigating the interfacial properties of electrochemically roughened platinum electrodes for neural stimulation. Langmuir 2015, 31, 2593–2599. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Pranti, A.S.; Schander, A.; Bödecker, A.; Lang, W. PEDOT: PSS coating on gold microelectrodes with excellent stability and high charge injection capacity for chronic neural interfaces. Sens. Actuators B Chem. 2018, 275, 382–393. [Google Scholar] [CrossRef]
- Fan, P.; Song, Y.; Lu, B.; Wang, Y.; Dai, Y.; Xie, J.; He, E.; Xu, Z.; Yang, G.; Mo, F.; et al. PtNPs/PEDOT:PSS-modified microelectrode arrays reveal electrophysiological activities of different neurons in medial amygdala of mice under innate fear. Front. Neurosci. 2022, 16, 868235. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Newbold, C.; Stathopoulos, D.; Carter, P.; Cowan, R.; Wallace, G.G. Comparison of the in vitro and in vivo electrochemical performance of bionic electrodes. Micromachines 2022, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Schatmeyer, B.; Landazuri, P.; Uysal, U.; Nazzaro, J.; Kinsman, M.J.; Camarata, P.J.; Ulloa, C.M.; Hammond, N.; Pearson, C.; et al. SEEG for expansion of a surgical epilepsy program: Safety and efficacy in 152 consecutive cases. Epilepsia Open 2021, 6, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Bove, F.; Mulas, D.; Cavallieri, F.; Castrioto, A.; Chabardès, S.; Meoni, S.; Schmitt, E.; Bichon, A.; Di Stasio, E.; Kistner, A.; et al. Long-term outcomes (15 years) after subthalamic nucleus deep brain stimulation in patients with Parkinson disease. Neurology 2021, 97, e254–e262. [Google Scholar] [CrossRef] [PubMed]
- Ganji, M.; Hossain, L.; Tanaka, A.; Thunemann, M.; Halgren, E.; Gilja, V.; Devor, A.; Dayeh, S.A. Monolithic and scalable Au nanorod substrates improve PEDOT-metal adhesion and stability in neural electrodes. Adv. Healthc. Mater. 2018, 7, 1800923. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.; Oberueber, F.; Schlabach, S.; Stieglitz, T.; Asplund, M. Long-term stable adhesion for conducting polymers in biomedical applications: IrOx and nanostructured platinum solve the chronic challenge. ACS Appl. Mater. Inter. 2017, 9, 189–197. [Google Scholar] [CrossRef]
- Wissel, K.; Brandes, G.; Pütz, N.; Angrisani, G.L.; Thieleke, J.; Lenarz, T.; Durisin, M. Platinum corrosion products from electrode contacts of human cochlear implants induce cell death in cell culture models. PLoS ONE 2018, 13, e196649. [Google Scholar] [CrossRef]
- Shepherd, R.K.; Carter, P.M.; Enke, Y.L.; Wise, A.K.; Fallon, J.B. Chronic intracochlear electrical stimulation at high charge densities results in platinum dissolution but not neural loss or functional changes in vivo. J. Neural Eng. 2019, 16, 26009. [Google Scholar] [CrossRef]
- Clark, G.M.; Clark, J.; Cardamone, T.; Clarke, M.; Nielsen, P.; Jones, R.; Arhatari, B.; Birbilis, N.; Curtain, R.; Xu, J.; et al. Biomedical studies on temporal bones of the first multi-channel cochlear implant patient at the University of Melbourne. Cochlear Implants Int. 2014, 15, S1–S15. [Google Scholar] [CrossRef] [PubMed]
- Mccormick, W.; Mccrudden, D. Development of a highly nanoporous platinum screen-printed electrode and its application in glucose sensing. J. Electroanal. Chem. 2020, 860, 113912. [Google Scholar] [CrossRef]
- Weltin, A.; Kieninger, J.; Urban, G.A.; Arndt, S.; Rosskothen-Kuhl, N. Cochlear implant electrodes as electrochemical sensors in vivo. In Proceedings of the 10th International IEEE/EMBS Conference on Neural Engineering (NER), Virtual, Italy, 4–6 May 2021; pp. 617–620. [Google Scholar]
- Dong, Q.; Sun, X.; He, S. Iridium oxide enabled sensors applications. Catalysts 2021, 11, 1164. [Google Scholar] [CrossRef]
- Seaton, B.T.; Heien, M.L. Biocompatible reference electrodes to enhance chronic electrochemical signal fidelity in vivo. Anal. Bioanal. Chem. 2021, 413, 6689–6701. [Google Scholar] [CrossRef]
- Seaton, B.T.; Hill, D.F.; Cowen, S.L.; Heien, M.L. Mitigating the effects of electrode biofouling-induced impedance for improved long-term electrochemical measurements in vivo. Anal. Chem. 2020, 92, 6334–6340. [Google Scholar] [CrossRef]
- Robbins, E.M.; Castagnola, E.; Cui, X.T. Accurate and stable chronic in vivo voltammetry enabled by a replaceable subcutaneous reference electrode. iScience 2022, 25, 104845. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Jiang, C.; Li, L. A Comparative Study on the Effect of Substrate Structure on Electrochemical Performance and Stability of Electrodeposited Platinum and Iridium Oxide Coatings for Neural Electrodes. Micromachines 2024, 15, 70. https://doi.org/10.3390/mi15010070
Li L, Jiang C, Li L. A Comparative Study on the Effect of Substrate Structure on Electrochemical Performance and Stability of Electrodeposited Platinum and Iridium Oxide Coatings for Neural Electrodes. Micromachines. 2024; 15(1):70. https://doi.org/10.3390/mi15010070
Chicago/Turabian StyleLi, Linze, Changqing Jiang, and Luming Li. 2024. "A Comparative Study on the Effect of Substrate Structure on Electrochemical Performance and Stability of Electrodeposited Platinum and Iridium Oxide Coatings for Neural Electrodes" Micromachines 15, no. 1: 70. https://doi.org/10.3390/mi15010070
APA StyleLi, L., Jiang, C., & Li, L. (2024). A Comparative Study on the Effect of Substrate Structure on Electrochemical Performance and Stability of Electrodeposited Platinum and Iridium Oxide Coatings for Neural Electrodes. Micromachines, 15(1), 70. https://doi.org/10.3390/mi15010070






