Numerical Analysis of the Cell Droplet Loading Process in Cell Printing
Abstract
1. Introduction
2. Computational Procedures
2.1. Process Governing Equations
2.2. Contact Algorithms
2.3. Material Models
The Cell Droplet
3. Simulation Setup and Result
3.1. FEM Model Setup
3.2. Representative Simulation Results
3.2.1. Evolution of the Landing Process
3.2.2. The Effect of Different Jet Velocities
3.2.3. The Effect of Different Numbers of Layers
3.2.4. The Effect of Different Cell Droplet Diameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, F.; Cheng, J.; Zhang, J.; Yu, H.; Dai, W.; Yan, W.; Sun, M.; Ding, G.; Li, Q.; Meng, Q.; et al. Comparison of three different acidic solutions in tendon decellularized extracellular matrix bio-ink fabrication for 3D cell printing. Acta Biomater. 2021, 131, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, C.; Wang, Z.; Li, S.; Chen, Z.; Huang, J.; Liu, H.; Yan, Y. Fabrication of hydrogels with adjustable mechanical properties through 3D cell-laden printing technology. Colloids Surf. Physicochem. Eng. Asp. 2022, 646, 128980. [Google Scholar] [CrossRef]
- Panja, N.; Maji, S.; Choudhuri, S.; Ali, K.A.; Hossain, C.M. 3D Bioprinting of Human Hollow Organs. AAPS Pharmscitech 2022, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Qin, C.; Wu, C. 3D printing of cell-delivery scaffolds for tissue regeneration. Regen. Biomater. 2023, 10, rbad032. [Google Scholar] [CrossRef]
- Wang, C.; Park, M.J.; Choo, Y.W.; Huang, Y.; Phuntsho, S.; Shon, H.K. Inkjet printing technique for membrane fabrication and modification: A review. Desalination 2023, 565, 116841. [Google Scholar] [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Laidmäe, I.; Topelius, N.S.; Grytsyk, A.; Raal, A. Semi-solid extrusion 3D-printing of eucalypt extract-loaded polyethylene oxide gels intended for pharmaceutical applications. Ann. 3D Print. Med. 2023, 12, 100123. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Gregory, D.A.; Jimenez-Franco, A.; Tomeh, M.A.; Lv, S.; Wang, J.; Haycock, J.W.; Lu, J.R.; Zhao, X. Patterning the neuronal cells via inkjet printing of self-assembled peptides on silk scaffolds. Prog. Nat. Sci. Mater. Int. 2020, 30, 686–696. [Google Scholar] [CrossRef]
- Lin, X.; Fang, F.; Wang, C.; Kankala, R.K.; Zhou, S. Inkjet printing-assisted single-cell microarray on a hydrophobic surface chip for real-time monitoring of enzyme kinetics at single-cell level. Talanta 2021, 225, 122019. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.-S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.-B.; Lee, H.; Kim, J.H.; Cho, D.-W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Kim, B.S.; Cho, W.; Gao, G.; Ahn, M.; Kim, J.; Cho, D. Construction of Tissue-Level Cancer-Vascular Model with High-Precision Position Control via In Situ 3D Cell Printing. Small Methods 2021, 5, 2100072. [Google Scholar] [CrossRef]
- Singh, N.K.; Han, W.; Nam, S.A.; Kim, J.W.; Kim, J.Y.; Kim, Y.K.; Cho, D.-W. Three-dimensional cell-printing of advanced renal tubular tissue analogue. Biomaterials 2020, 232, 119734. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chu, H.; Cai, S.; Liang, W.; Yu, H.; Wang, Y.; Liu, L. 3D cell-printing of gradient multi-tissue interfaces for rotator cuff regeneration. Bioact. Mater. 2023, 19, 611–625. [Google Scholar]
- Yang, W.; Chu, H.; Cai, S.; Liang, W.; Yu, H.; Wang, Y.; Liu, L. Micropatterned Cell-Repellent Interface Using Femtosecond Laser Direct Writing to Engineer Controlled Cell Organization. Adv. Mater. Technol. 2021, 6, 2100178. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, G.; Huang, Y.; Jeremy Tzeng, T.R.; Chrisey, D. Effect of laser fluence in laser-assisted direct writing of human colon cancer cell. Rapid Prototyp. J. 2010, 16, 202–208. [Google Scholar] [CrossRef]
- He, P.; Liu, Y.; Qiao, R. Fluid dynamics of the droplet impact processes in cell printing. Microfluid. Nanofluid. 2015, 18, 569–585. [Google Scholar] [CrossRef]
- Ladjal, H.; Hanus, J.; Ferreira, A. Micro-to-nano biomechanical modeling for assisted biological cell injection. IEEE Trans. Biomed. Eng. 2013, 60, 2461–2471. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.; Puster, U.; Richtering, W.; Stevens, M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Nin, L.; Betancourt, N.; Schreyer, D.; Chen, X. Characterization of Cell Damage and Proliferative Ability during and after Bioprinting. ACS Biomater. Sci. Eng. 2018, 4, 3906–3918. [Google Scholar]
- Huang, J.; Cai, R.; Zhang, K. Experiments and Analysis of Drop on Demand Cell Printing. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 93–96. [Google Scholar]
- Ringeisen, B.; Kim, H.; Barron, J.A.; Krizman, D.B.; Chrisey, D.B.; Jackman, S.; Auyeung, R.Y.C.; Spargo, B.J. Laser Printing of Pluripotent Embryonal Carcinoma Cells. Tissue Eng. 2004, 10, 483–491. [Google Scholar] [CrossRef]
- Ning, L.; Yang, B.; Mohabatpour, F.; Betancourt, N.; Sarker, M.; Papagerakis, P.; Chen, X. Process-induced cell damage: Pneumatic versus screw-driven bioprinting. Biofabrication 2020, 12, 025011. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, C.; Jin, S.; Kim, T. Study of the process-induced cell damage in forced extrusion bioprinting. Biofabrication 2021, 13, 035048. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, G.; Huang, Y. Modeling of Bubble ExpansionInduced Cell Mechanical Profile in Laser-Assisted Cell Direct Writing. J. Manuf. Sci. Eng. 2009, 131, 0510131–05101310. [Google Scholar] [CrossRef]
- Cai, R.; Huang, J. Modeling and Analysis of the Droplet Landing Process in Cell Direct-Writing. Int. J. Autom. Technol. 2013, 7, 353–358. [Google Scholar]
- Orkwis, J.A.; Wolf, A.K.; Mularczyk, Z.J.; Bryan, A.E.; Smith, C.S.; Brown, R.; Krutko, M.; McCann, A.; Collar, R.M.; Esfandiari, L.; et al. Mechanical stimulation of a bioactive, functionalized PVDF-TrFE scaffold provides electrical signaling for nerve repair applications. Biomater. Adv. 2022, 140, 213081. [Google Scholar] [CrossRef]
- Goldblatt, Z.E.; Choshali, H.A.; Cirka, H.A.; Liang, V.; Wen, Q.; McCollum, D.; Rahbar, N.; Billiar, K.L. Heterogeneity Profoundly Alters Emergent Stress Fields in Constrained Multicellular Systems. Biophys. J. 2020, 118, 15–25. [Google Scholar] [CrossRef]
- Marion, M.; Temam, R. Navier-stokes equations: Theory and approximation. In Handbook of Numerical Analysis; Elsevier: Amsterdam, The Netherlands, 1998; Volume 6, pp. 503–689. [Google Scholar]
- Carpenter, N.J.; Taylor, R.L.; Katona, M.G. Lagrange constraints for transient finite element surface contact. Int. J. Numer. Methods Eng. 1991, 32, 103–128. [Google Scholar] [CrossRef]
- Ştefancu, A.; Melenciuc, S.; Budescu, M. Penalty Based Algorithms for Frictional Contact Problems. Bull. Polytech. Inst. Jassy Constr. Archit. Sect. 2011, 3, 119–129. [Google Scholar]
- Lim, C.; Zhou, E.; Quek, S. Mechanical Models for Living Cells—A Review. J. Biomech. 2006, 39, 195–216. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Y.; Grujicic, M.; Chrisey, D. Study of Impact-induced Mechanical Efects in Cell Direct Writing Using Smooth Particle Hydrodynamic Method. J. Manuf. Sci. Eng. 2008, 130, 021012-1–021012-10. [Google Scholar] [CrossRef]
- Zhang, B. Engineering Mechanics; China Machine Press: Beijing, China, 2011; pp. 185–186. [Google Scholar]
- Bao, G.; Jiang, T.; Ravanbakhsh, H.; Reyes, A.; Ma, Z.; Strong, M.; Wang, H.; Kinsella, J.M.; Li, J.; Mongeau, L. Triggered micropore-forming bioprinting of porous viscoelastic hydrogels. Mater. Horiz. 2020, 7, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Lanero, T.; Cavalleri, O.; Krol, S.; Rolandi, R.; Gliozzi, R. Mechanical properties of single living cells encapsulated in polyelectrolyte matrixes. J. Biotechnol. 2006, 124, 723–731. [Google Scholar] [CrossRef] [PubMed]
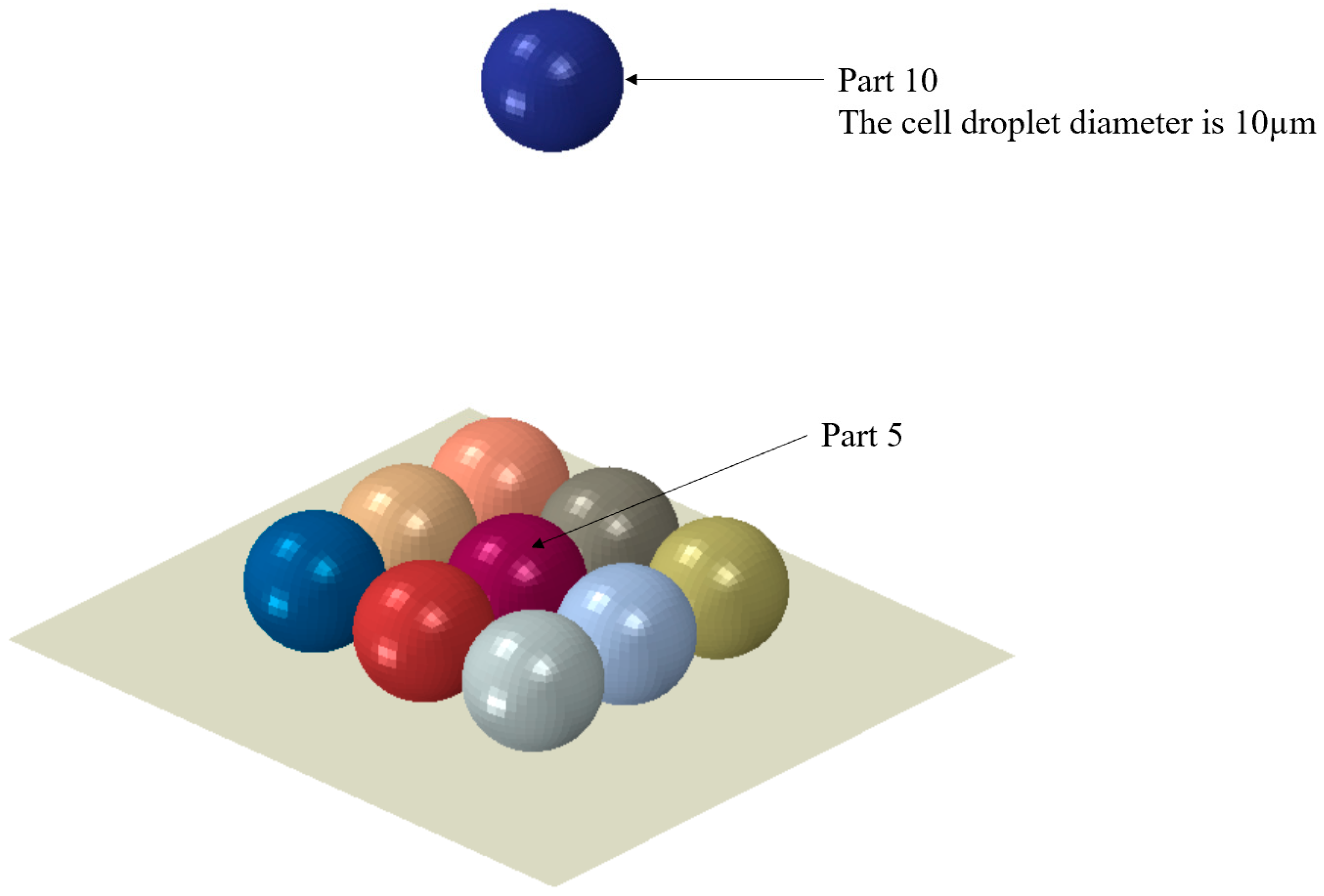






| Cell Droplet at the Center of the Substrate (Part 5) | Descending Cell Droplet (Part 10) | |||||||
|---|---|---|---|---|---|---|---|---|
| Velocity (m/s) | 10 | 20 | 30 | 40 | 10 | 20 | 30 | 40 |
| Stress maximum (MPa) | 3.32 | 5.58 | 8.74 | 20.61 | 2.41 | 3.67 | 5.11 | 16.7 |
| Maximum strain | 0.093 | 0.219 | 0.415 | 1.309 | 0.093 | 0.143 | 0.207 | 1.296 |
| growth rate of maximum stress | − | 68.07% | 56.63% | 135.8% | − | 52.28% | 39.23% | 226.8% |
| growth rate of maximum strain | − | 134.7% | 89.50% | 215.4% | − | 53.26% | 44.76% | 520.6% |
| Velocity (m/s) | 10 | 20 | 30 | 40 | |
|---|---|---|---|---|---|
| Maximum Stress(MPa) | Part 10 | 2.31 | 3.58 | 4.65 | 6.02 |
| Part 5_2 | 2.43 | 4.11 | 6.11 | 6.70 | |
| Part 5_1 | 3.12 | 5.09 | 7.75 | 13.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Pang, F.; Lai, S.; Cai, R.; Lai, C.; Yu, Z.; Zhu, Y.; Wu, M.; Zhang, H.; Kong, C. Numerical Analysis of the Cell Droplet Loading Process in Cell Printing. Micromachines 2024, 15, 1335. https://doi.org/10.3390/mi15111335
Wang Y, Pang F, Lai S, Cai R, Lai C, Yu Z, Zhu Y, Wu M, Zhang H, Kong C. Numerical Analysis of the Cell Droplet Loading Process in Cell Printing. Micromachines. 2024; 15(11):1335. https://doi.org/10.3390/mi15111335
Chicago/Turabian StyleWang, Yankun, Fagui Pang, Shushan Lai, Renye Cai, Chenxiang Lai, Zexin Yu, Yiwei Zhu, Min Wu, Heng Zhang, and Chunyu Kong. 2024. "Numerical Analysis of the Cell Droplet Loading Process in Cell Printing" Micromachines 15, no. 11: 1335. https://doi.org/10.3390/mi15111335
APA StyleWang, Y., Pang, F., Lai, S., Cai, R., Lai, C., Yu, Z., Zhu, Y., Wu, M., Zhang, H., & Kong, C. (2024). Numerical Analysis of the Cell Droplet Loading Process in Cell Printing. Micromachines, 15(11), 1335. https://doi.org/10.3390/mi15111335





