Intestinal Cells-on-Chip for Permeability Studies
Abstract
1. Introduction
2. Materials and Methods
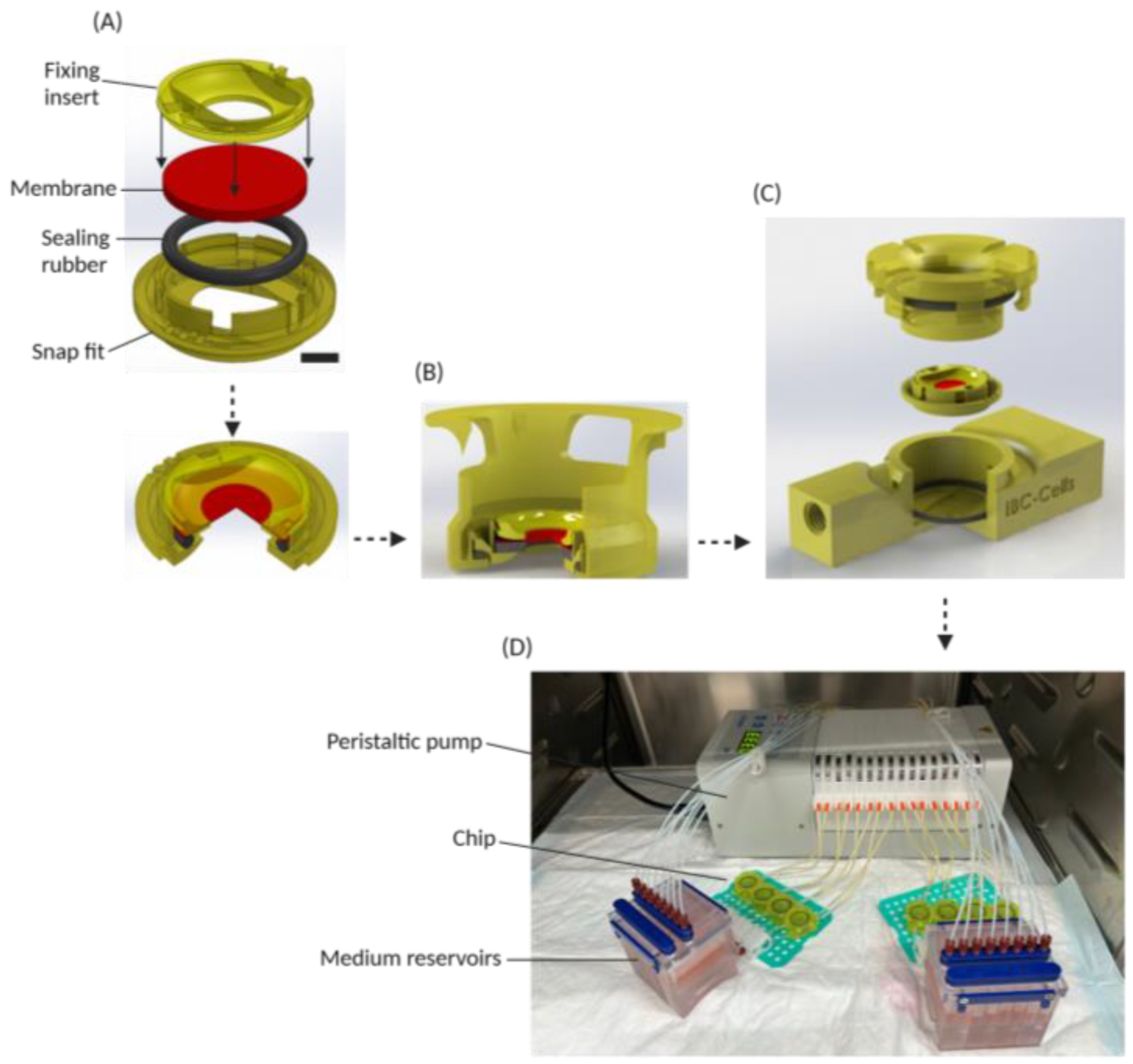
2.1. Design and Fabrication of the System
2.2. Caco-2 Cell Culture
2.3. Human Enteroid Culture
2.4. Cell Monolayer Culture
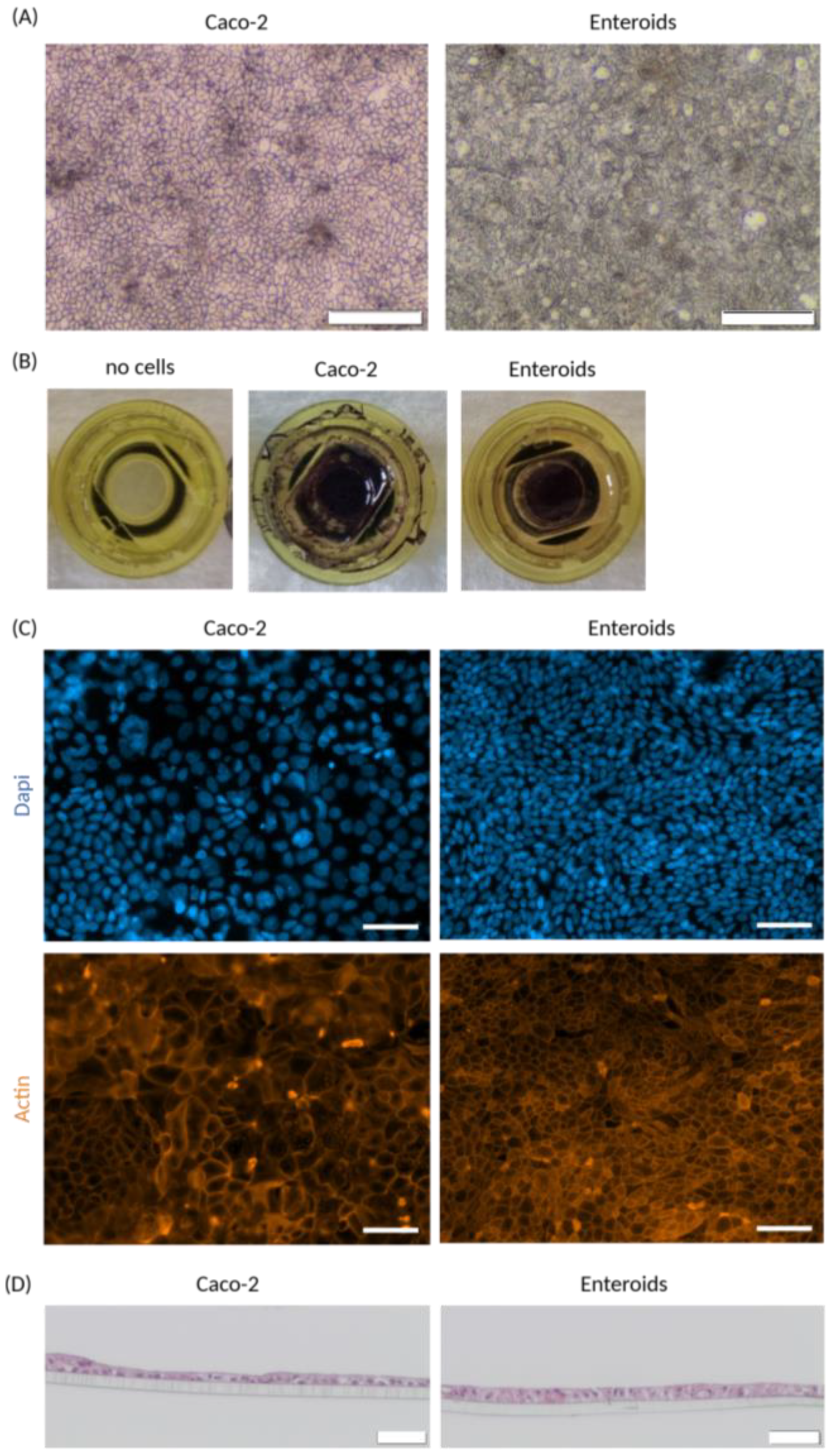
2.5. Cell Monolayer Morphology
2.6. Chip System Preparation
2.7. Epithelial Barrier Integrity Measurements
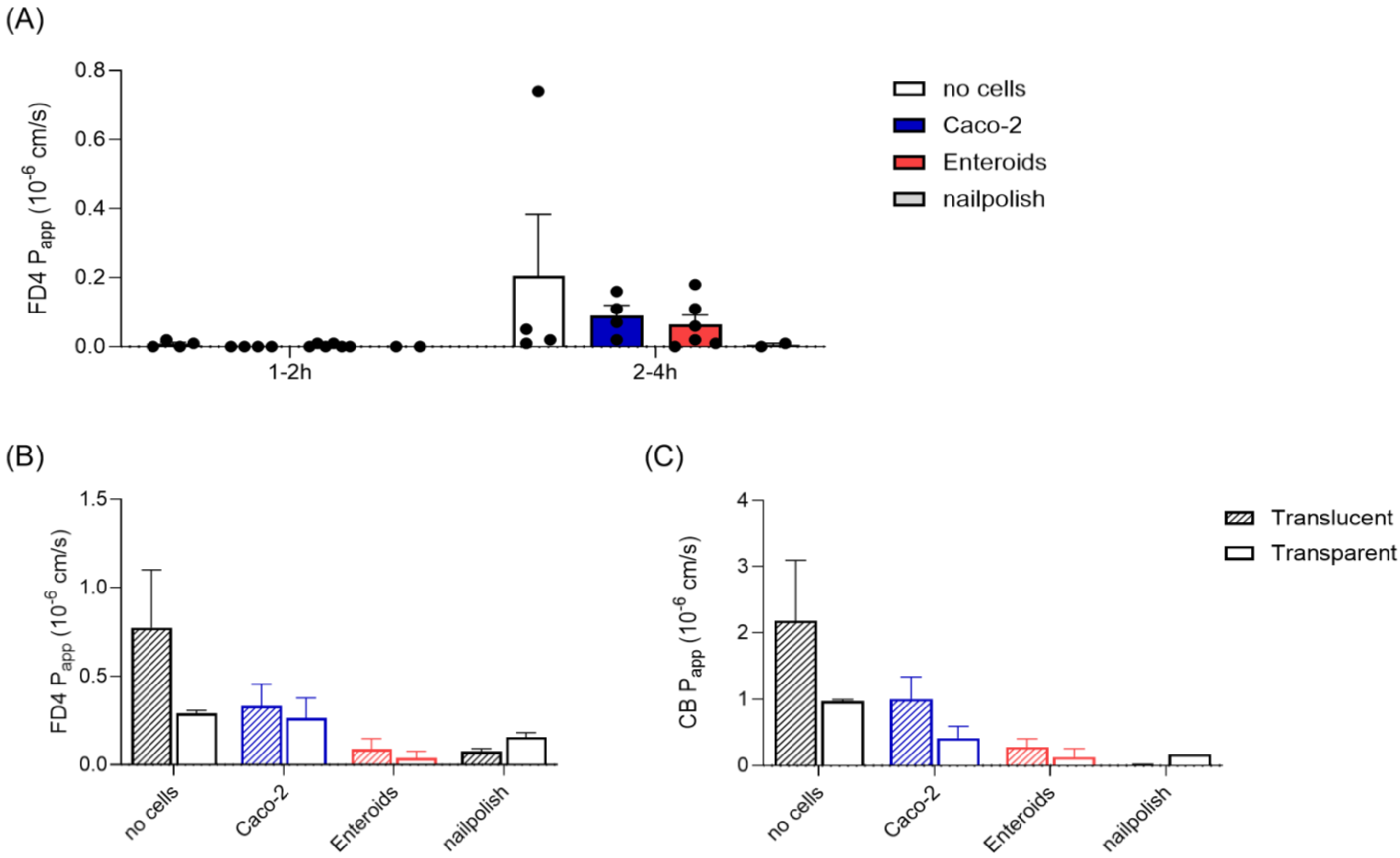
2.8. Epithelial Permeability Measurements
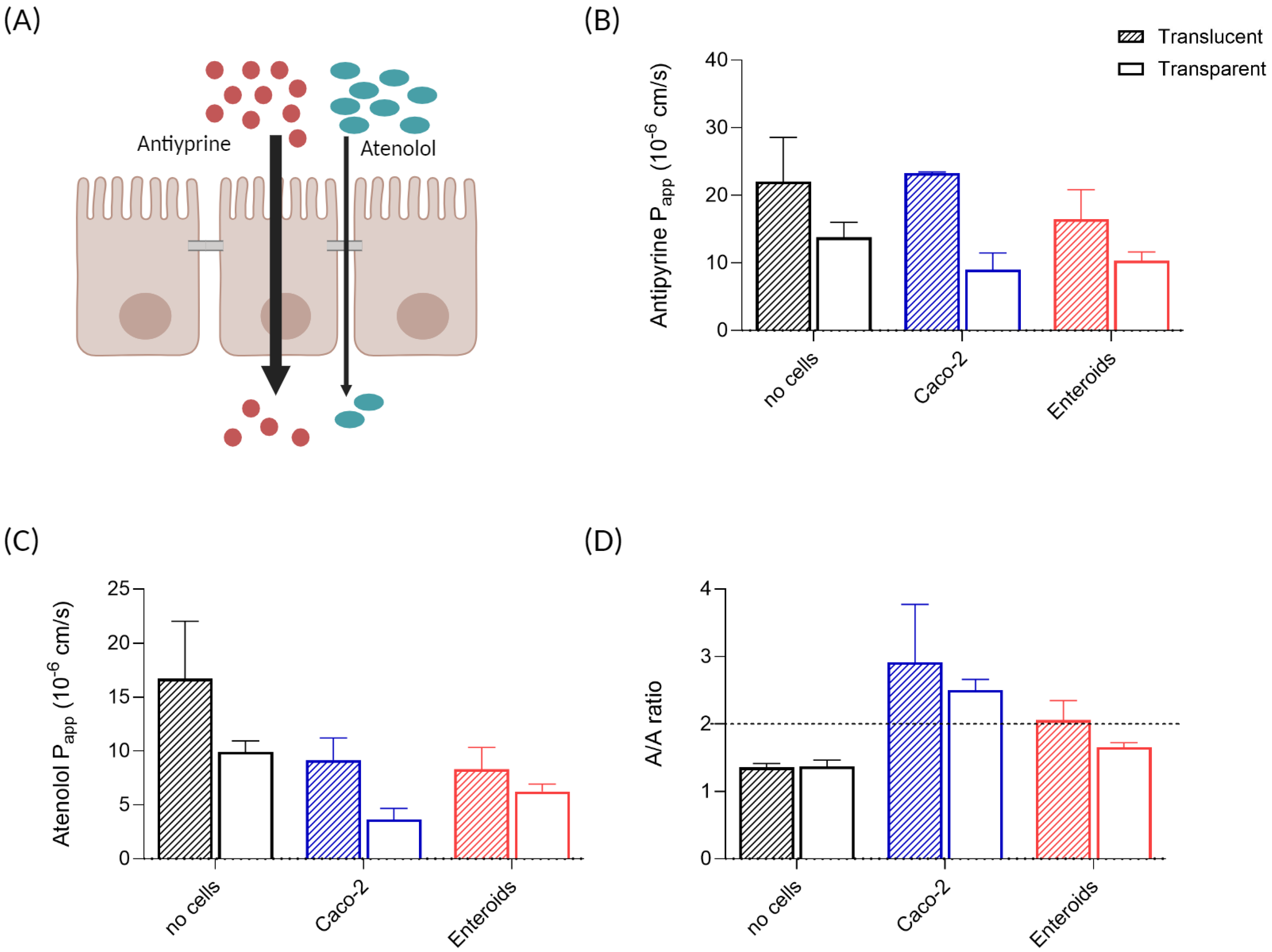
2.9. Drug Cocktail Experiment
2.10. Static Transwell Experiments
3. Results
3.1. Cell Disc and Chip Design
3.2. Cell Monolayer Culture (Static)
3.3. Epithelial Barrier Integrity
3.4. Epithelial Barrier Permeability
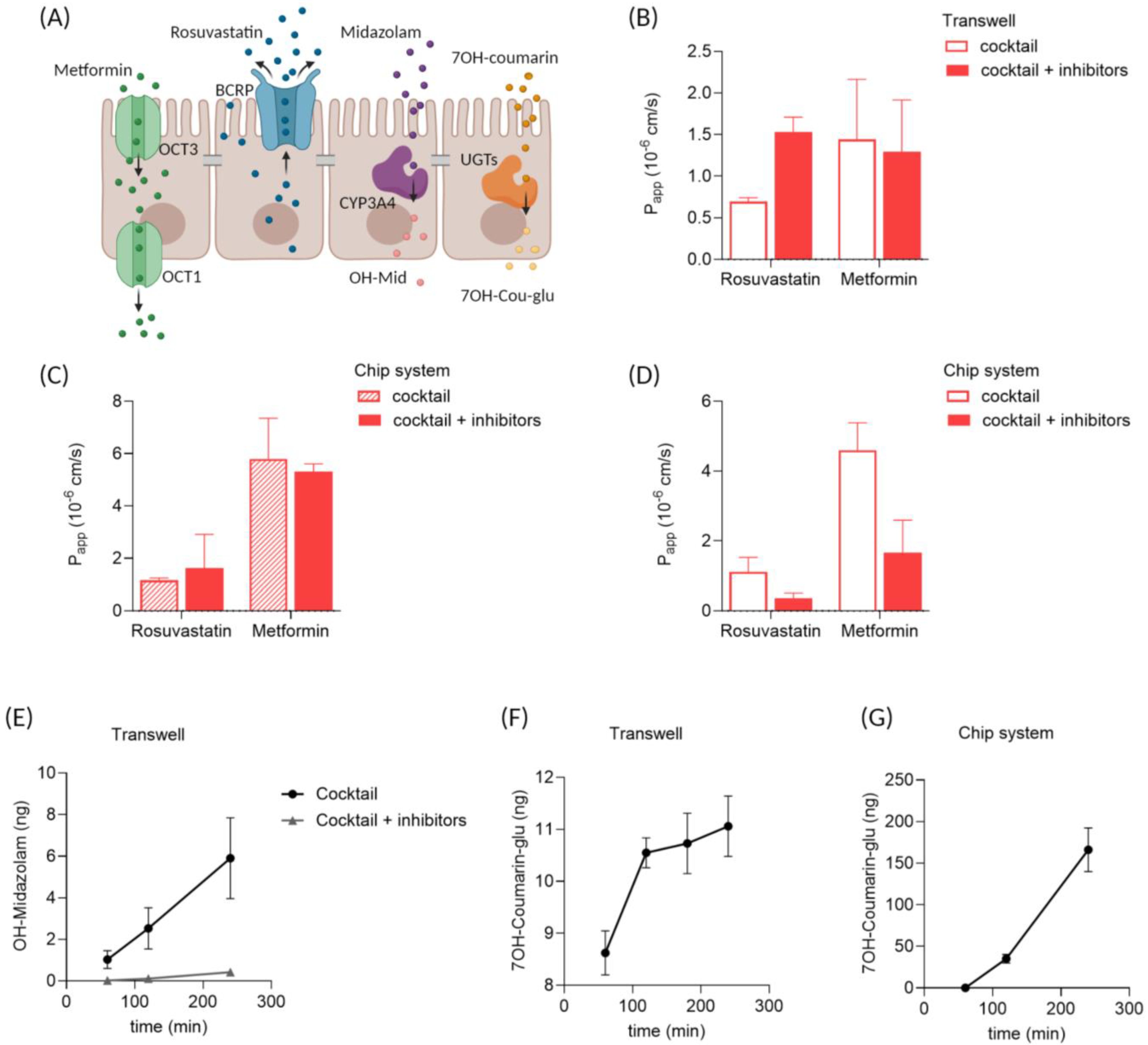
3.5. Drug Transport and Metabolism in Enteroid Monolayers
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- France, M.M.; Turner, J.R. The mucosal barrier at a glance. J. Cell Sci. 2017, 130, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Ghiboub, M.; Donkers, J.M.; van de Steeg, E.; van Tol, E.A.F.; Hakvoort, T.B.M.; de Jonge, W.J. The Progress of Intestinal Epithelial Models from Cell Lines to Gut-On-Chip. Int. J. Mol. Sci. 2021, 22, 13472. [Google Scholar] [CrossRef] [PubMed]
- Arian, C.M.; Imaoka, T.; Yang, J.; Kelly, E.J.; Thummel, K.E. Gutsy science: In vitro systems of the human intestine to model oral drug disposition. Pharmacol. Ther. 2022, 230, 107962. [Google Scholar] [CrossRef]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control. Release 2021, 335, 247–268. [Google Scholar] [CrossRef]
- Leung, C.M.; De Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Y.C.; Lu, H.; Jin, D. Advances in Spheroids and Organoids on a Chip. Adv. Funct. Mater. 2023, 33, 2215043. [Google Scholar] [CrossRef]
- Pimenta, J.; Ribeiro, R.; Almeida, R.; Costa, P.F.; da Silva, M.A.; Pereira, B. Organ-on-Chip Approaches for Intestinal 3D In Vitro Modeling. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 351–367. [Google Scholar] [CrossRef]
- Eslami Amirabadi, H.; Donkers, J.M.; Wierenga, E.; Ingenhut, B.; Pieters, L.; Stevens, L.; Donkers, T.; Westerhout, J.; Masereeuw, R.; Bobeldijk-Pastorova, I.; et al. Intestinal explant barrier chip: Long-term intestinal absorption screening in a novel microphysiological system using tissue explants. Lab Chip 2022, 22, 326–342. [Google Scholar] [CrossRef]
- Donkers, J.M.; Wiese, M.; van den Broek, T.J.; Wierenga, E.; Agamennone, V.; Schuren, F.; van de Steeg, E. A host-microbial metabolite interaction gut-on-a-chip model of the adult human intestine demonstrates beneficial effects upon inulin treatment of gut microbiome. Microbiome Res. Rep. 2024, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Michiba, K.; Watanabe, K.; Imaoka, T.; Nakai, D. Recent Advances in the Gastrointestinal Complex in Vitro Model for ADME Studies. Pharmaceutics 2023, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Ong, W.S.Y.; Subelzu, N.; Gleeson, J.P. Validation of a Caco-2 microfluidic Chip model for predicting intestinal absorption of BCS Class I-IV drugs. Int. J. Pharm. 2024, 656, 124089. [Google Scholar] [CrossRef] [PubMed]
- Streekstra, E.J.; Keuper-Navis, M.; van den Heuvel, J.; van den Broek, P.; Stommel, M.W.J.; Bervoets, S.; O’Gorman, L.; Greupink, R.; Russel, F.G.M.; van de Steeg, E.; et al. Human enteroid monolayers as a potential alternative for Ussing chamber and Caco-2 monolayers to study passive permeability and drug efflux. Eur. J. Pharm. Sci. 2024, 201, 106877. [Google Scholar] [CrossRef]
- Streekstra, E.J.; Keuper-Navis, M.; van den Heuvel, J.; van den Broek, P.; Greupink, R.; Stommel, M.W.J.; de Boode, W.P.; Botden, S.; Russel, F.G.M.; van de Steeg, E.; et al. The potential of enteroids derived from children and adults to study age-dependent differences in intestinal CYP3A4/5 metabolism. Eur. J. Pharm. Sci. 2024, 201, 106868. [Google Scholar] [CrossRef]
- Maalderink, H.H.; Bruning, F.B.; De Schipper, M.M.; Van Der Werff, J.J.; Germs, W.W.; Remmers, J.J.; Meinders, E.R. 3D Printed structural electronics: Embedding and connecting electronic components into freeform electronic devices. Plast. Rubber Compos. 2018, 47, 35–41. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6. [Google Scholar] [CrossRef]
- Delon, L.C.; Guo, Z.; Oszmiana, A.; Chien, C.C.; Gibson, R.; Prestidge, C.; Thierry, B. A systematic investigation of the effect of the fluid shear stress on Caco-2 cells towards the optimization of epithelial organ-on-chip models. Biomaterials 2019, 225, 119521. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Drug Development and Drug Interactions | Table of Substrates, Inhibitors and Inducers; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2023. [Google Scholar]
- Gong, L.; Goswami, S.; Giacomini, K.M.; Altman, R.B.; Klein, T.E. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2012, 22, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Donkers, J.M.; van der Vaart, J.I.; van de Steeg, E. Gut-on-a-Chip Research for Drug Development: Implications of Chip Design on Preclinical Oral Bioavailability or Intestinal Disease Studies. Biomimetics 2023, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Trier, S.; Rahbek, U.L.; Dufva, M.; Kutter, J.P.; Andresen, T.L. A multi-chamber microfluidic intestinal barrier model using Caco-2 cells for drug transport studies. PLoS ONE 2018, 13, e0197101. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, J.P.; Zhang, S.Y.; Subelzu, N.; Ling, J.; Nissley, B.; Ong, W.; Nofsinger, R.; Kesisoglou, F. Head-to-Head Comparison of Caco-2 Transwell and Gut-on-a-Chip Models for Assessing Oral Peptide Formulations. Mol. Pharm. 2024, 21, 3880–3888. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Tatsuoka, H.; Tsuda, M.; Sumi, T.; Eguchi, Y.; So, K.; Higuchi, Y.; Takayama, K.; Torisawa, Y.; Yamashita, F. Intestinal Permeability of Drugs in Caco-2 Cells Cultured in Microfluidic Devices. Biol. Pharm. Bull. 2022, 45, 1246–1253. [Google Scholar] [CrossRef]
- Santbergen, M.J.C.; van der Zande, M.; Gerssen, A.; Bouwmeester, H.; Nielen, M.W.F. Dynamic in vitro intestinal barrier model coupled to chip-based liquid chromatography mass spectrometry for oral bioavailability studies. Anal. Bioanal. Chem. 2020, 412, 1111–1122. [Google Scholar] [CrossRef]
- Marrella, A.; Buratti, P.; Markus, J.; Firpo, G.; Pesenti, M.; Landry, T.; Ayehunie, S.; Scaglione, S.; Kandarova, H.; Aiello, M. In vitro demonstration of intestinal absorption mechanisms of different sugars using 3D organotypic tissues in a fluidic device. Altex 2020, 37, 255–264. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Ayehunie, S.; Landry, T.; Stevens, Z.; Armento, A.; Hayden, P.; Klausner, M. Human Primary Cell-Based Organotypic Microtissues for Modeling Small Intestinal Drug Absorption. Pharm. Res. 2018, 35, 72. [Google Scholar] [CrossRef]
- Kasendra, M.; Luc, R.; Yin, J.; Manatakis, D.V.; Kulkarni, G.; Lucchesi, C.; Sliz, J.; Apostolou, A.; Sunuwar, L.; Obrigewitch, J.; et al. Duodenum Intestine-Chip for preclinical drug assessment in a human relevant model. Elife 2020, 9, e50135. [Google Scholar] [CrossRef]
- Takenaka, T.; Harada, N.; Kuze, J.; Chiba, M.; Iwao, T.; Matsunaga, T. Application of a Human Intestinal Epithelial Cell Monolayer to the Prediction of Oral Drug Absorption in Humans as a Superior Alternative to the Caco-2 Cell Monolayer. J. Pharm. Sci. 2016, 105, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lennernas, H.; Welage, L.S.; Barnett, J.L.; Landowski, C.P.; Foster, D.; Fleisher, D.; Lee, K.D.; Amidon, G.L. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm. Res. 2002, 19, 1400–1416. [Google Scholar] [CrossRef] [PubMed]
- Brück, S.; Strohmeier, J.; Busch, D.; Drozdzik, M.; Oswald, S. Caco-2 cells—Expression, regulation and function of drug transporters compared with human jejunal tissue. Biopharm. Drug Dispos. 2017, 38, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef]
- Kwon, O.; Jung, K.B.; Lee, K.R.; Son, Y.S.; Lee, H.; Kim, J.J.; Kim, K.; Lee, S.; Song, Y.K.; Jung, J.; et al. The development of a functional human small intestinal epithelium model for drug absorption. Sci. Adv. 2021, 7, eabh1586. [Google Scholar] [CrossRef]
- Kourula, S.; Derksen, M.; Jardi, F.; Jonkers, S.; van Heerden, M.; Verboven, P.; Theuns, V.; Van Asten, S.; Huybrechts, T.; Kunze, A.; et al. Intestinal organoids as an in vitro platform to characterize disposition, metabolism, and safety profile of small molecules. Eur. J. Pharm. Sci. 2023, 188, 106481. [Google Scholar] [CrossRef]
- Macedo, M.H.; Barros, A.S.; Martínez, E.; Barrias, C.C.; Sarmento, B. All layers matter: Innovative three-dimensional epithelium-stroma-endothelium intestinal model for reliable permeability outcomes. J. Control Release 2022, 341, 414–430. [Google Scholar] [CrossRef]
- Asal, M.; Rep, M.; Bontkes, H.J.; van Vliet, S.J.; Mebius, R.E.; Gibbs, S. Towards Full Thickness Small Intestinal Models: Incorporation of Stromal Cells. Tissue Eng. Regen. Med. 2024, 21, 369–377. [Google Scholar] [CrossRef]
- Kemas, A.M.; Zandi Shafagh, R.; Taebnia, N.; Michel, M.; Preiss, L.; Hofmann, U.; Lauschke, V.M. Compound Absorption in Polymer Devices Impairs the Translatability of Preclinical Safety Assessments. Adv. Healthc. Mater. 2024, 13, e2303561. [Google Scholar] [CrossRef]
- Grant, J.; Özkan, A.; Oh, C.; Mahajan, G.; Prantil-Baun, R.; Ingber, D.E. Simulating drug concentrations in PDMS microfluidic organ chips. Lab Chip 2021, 21, 3509–3519. [Google Scholar] [CrossRef]
- Frost, T.S.; Jiang, L.; Lynch, R.M.; Zohar, Y. Permeability of Epithelial/Endothelial Barriers in Transwells and Microfluidic Bilayer Devices. Micromachines 2019, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Kulthong, K.; Duivenvoorde, L.; Sun, H.; Confederat, S.; Wu, J.; Spenkelink, B.; de Haan, L.; Marin, V.; van der Zande, M.; Bouwmeester, H. Microfluidic chip for culturing intestinal epithelial cell layers: Characterization and comparison of drug transport between dynamic and static models. Toxicol. In Vitro 2020, 65, 104815. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Z.; Su, W.; Wang, L.; Zhu, Y.; Qin, J. A Biomimetic Human Gut-on-a-Chip for Modeling Drug Metabolism in Intestine. Artif. Organs 2018, 42, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Corral-Nájera, K.; Chauhan, G.; Serna-Saldívar, S.O.; Martínez-Chapa, S.O.; Aeinehvand, M.M. Polymeric and biological membranes for organ-on-a-chip devices. Microsyst. Nanoeng. 2023, 9, 107. [Google Scholar] [CrossRef]
- Arık, Y.B.; de Sa Vivas, A.; Laarveld, D.; van Laar, N.; Gemser, J.; Visscher, T.; van den Berg, A.; Passier, R.; van der Meer, A.D. Collagen I Based Enzymatically Degradable Membranes for Organ-on-a-Chip Barrier Models. ACS Biomater. Sci. Eng. 2021, 7, 2998–3005. [Google Scholar] [CrossRef]
- Mondrinos, M.J.; Yi, Y.S.; Wu, N.K.; Ding, X.; Huh, D. Native extracellular matrix-derived semipermeable, optically transparent, and inexpensive membrane inserts for microfluidic cell culture. Lab Chip 2017, 17, 3146–3158. [Google Scholar] [CrossRef]
| Company | Product nr. | Material | Pore Size Ø | Pore Density | Porosity | Pore Alignment | Membrane Thickness | |
|---|---|---|---|---|---|---|---|---|
| TranswellTM transparent | Corning® | #3470-Clear | Polyester (PET) | 0.4 µm | 4·10⁶ pores per cm2 | 0.5% | parallel | 10 µm |
| ipCELLCULTURE™ transparent | It4ip ion track technology | 2000M12/ 640N403/10 | Polyester (PET) | 0.4 µm | 4·10⁶ pores per cm2 | 0.5% | parallel | 12 µm |
| ipCELLCULTURE™ translucent | It4ip ion track technology | 2000M12/ 811N403/10 | Polyester (PET) | 0.4 µm | 1·108 pores per cm2 | 12.6% | crossed | 12 µm |
| Cell Type | Compound | Papp A to B (×10−6 cm/s) | ||
|---|---|---|---|---|
| Flow | Static | |||
| This study * | Enteroids | FD4 | 0 | 0.01 |
| Atenolol | 3.08 | 0.69 | ||
| Antipyrine | 5.40 | 21.55 | ||
| Rosuvastatin | 1.11 | 0.70 | ||
| Metformin | 4.60 | 1.44 | ||
| Zhang 2024 [13] | Caco-2 | FD3 | 0.24 | 0.08 |
| Atenolol | 0.72 | 0.26 | ||
| Antipyrine | 32.9 | 38.5 | ||
| Tan 2018 [24] | Caco-2 | FD4 | 0.25 | <0.1 |
| Gleeson 2024 [25] | Caco-2 | FD3 | 0.32 | 0.053 |
| Sasaki 2022 [26] | Caco-2 | Atenolol | 0.10 | 0.43 |
| Kulthong 2020 [43] | Caco-2 | Antipyrine | 5.4 | 22.7 |
| Santbergen 2020 [27] | Caco-2/HT-29 | Verapamil | 19.5 | 20.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keuper-Navis, M.; Eslami Amirabadi, H.; Donkers, J.; Walles, M.; Poller, B.; Heming, B.; Pieters, L.; de Wagenaar, B.; Myszczyszyn, A.; Sinnige, T.; et al. Intestinal Cells-on-Chip for Permeability Studies. Micromachines 2024, 15, 1464. https://doi.org/10.3390/mi15121464
Keuper-Navis M, Eslami Amirabadi H, Donkers J, Walles M, Poller B, Heming B, Pieters L, de Wagenaar B, Myszczyszyn A, Sinnige T, et al. Intestinal Cells-on-Chip for Permeability Studies. Micromachines. 2024; 15(12):1464. https://doi.org/10.3390/mi15121464
Chicago/Turabian StyleKeuper-Navis, Marit, Hossein Eslami Amirabadi, Joanne Donkers, Markus Walles, Birk Poller, Bo Heming, Lisanne Pieters, Bjorn de Wagenaar, Adam Myszczyszyn, Theo Sinnige, and et al. 2024. "Intestinal Cells-on-Chip for Permeability Studies" Micromachines 15, no. 12: 1464. https://doi.org/10.3390/mi15121464
APA StyleKeuper-Navis, M., Eslami Amirabadi, H., Donkers, J., Walles, M., Poller, B., Heming, B., Pieters, L., de Wagenaar, B., Myszczyszyn, A., Sinnige, T., Spee, B., Masereeuw, R., & van de Steeg, E. (2024). Intestinal Cells-on-Chip for Permeability Studies. Micromachines, 15(12), 1464. https://doi.org/10.3390/mi15121464






