Fabrication of Li4Ti5O12 (LTO) as Anode Material for Li-Ion Batteries
Abstract
:1. Introduction
2. Synthesis Methods
2.1. Starting Materials
2.1.1. Titanium Alkoxides
2.1.2. TiO2 as Ti Source
2.1.3. Intermediate Phase
2.1.4. Lithium Salts
2.2. Solid State Reaction
2.2.1. One-Step SSR
2.2.2. Two-Step SSR
2.2.3. SSR-Assisted Method
2.2.4. Sintering Process
2.3. Sol–Gel Process

2.4. Pechini Process
2.5. Hydrolysis (Wet-Chemical) Method
2.6. Mechanochemical Synthesis
2.7. Molten-Salt Synthesis
2.8. Solution-Combustion Method
2.9. Hydrothermal Method
2.10. Supercritical Synthesis
2.11. Solvothermal Method

2.12. Reflux Method
2.13. Templating Method
2.14. Spray Drying
2.15. Spray Pyrolysis Method
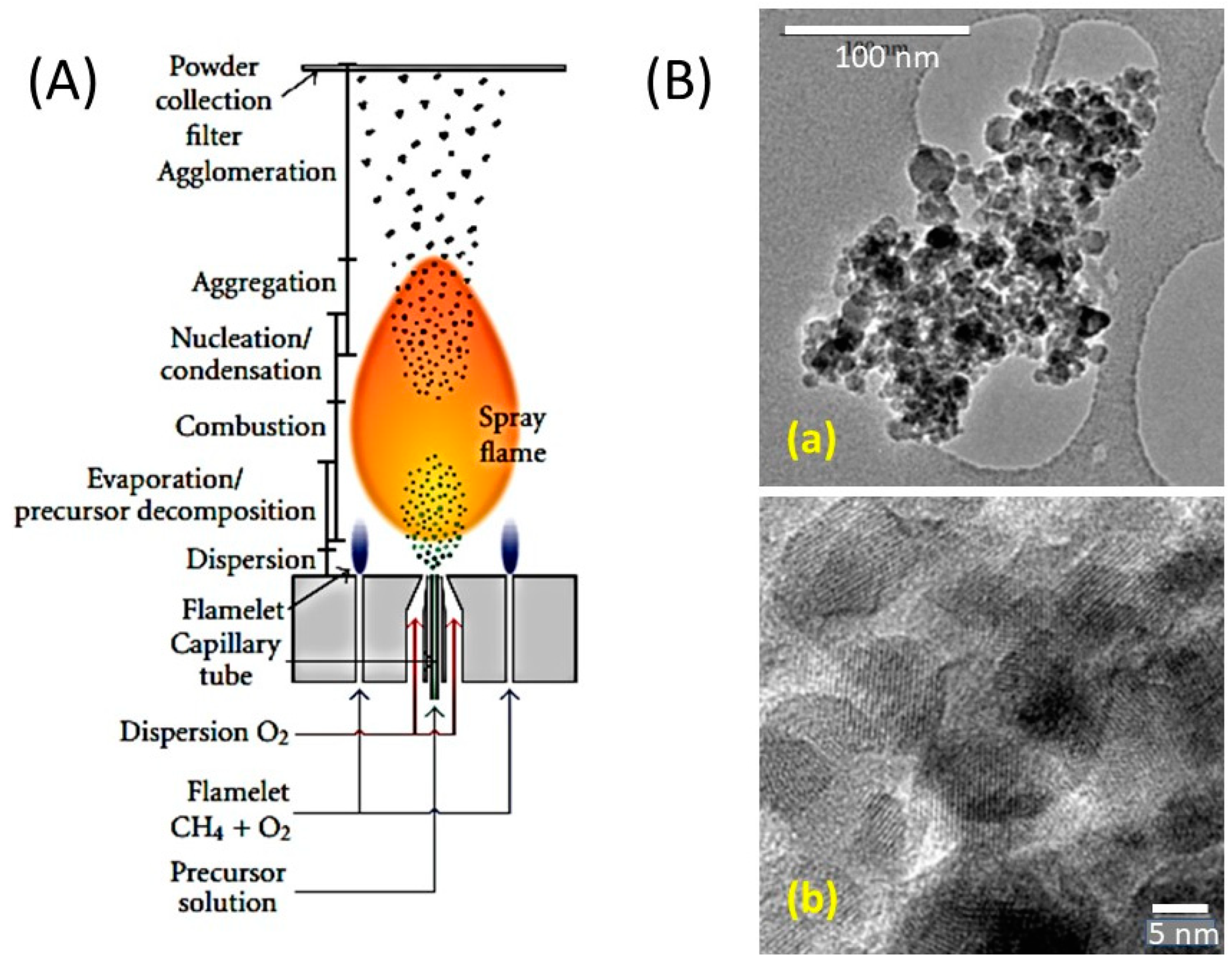
2.16. Sonochemical Process
2.17. Microwave Synthesis
2.18. Rheological Phase Reaction
2.19. Electrospinning Method
2.20. Ion Exchange
2.21. Thin-Film Techniques
2.22. 3D Ink Printing
2.23. Miscellaneous Treatments
3. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | acetic acid |
| AC | activated carbon |
| AHTO | amorphous hydrous titanium oxide |
| BET | Brunauer-Emmett-Teller |
| CA | citric acid |
| CNT | carbon nanotube |
| CTAB | cetyl trimethyl ammonium bromide |
| CTAC | cetyltrimethyl ammonium chloride |
| DI water | deionized water |
| DMEA | 2-dimethylaminoethanol |
| DTA | differential thermal analysis |
| EDTA | ethylene diamine tetraacetic acid |
| EtOH | ethanol |
| FSP | flame spray pyrolysis |
| HEBM | high-energy ball milling |
| HOP | hydroxypropyl |
| HTS | hydrothermal synthesis |
| LiAAc | lithium acetylacetonate |
| LIB | lithium-ion battery |
| LiOAc | lithium acetate dihydrate |
| LTH | lithium titanate oxide hydrate |
| MA | malic acid |
| MeOH | methanol |
| MWCNT | multiwalled carbon nanotube |
| MWHT | microwave-assisted hydrothermal |
| PAA | polyacrylate acid |
| PAN | polyacrylonitrile |
| PBI-b-PEO | poly(isobutylene)-b-polyethylene oxide |
| PEG | polyethylene glycol |
| PF | phenol-formaldehyde |
| PVA | polyvinyl alcohol |
| PVP | polyvinylpyrrolidone |
| rGO | reduced graphene oxide |
| SD | Spray drying |
| SDBS | sodium dodecyl benzene sulfonate |
| SEI | solid electrolyte interphase |
| SEM | scanning electron microscopy |
| SG | space group |
| SIB | sodium-ion battery |
| SOR | sodium–organic reduction reagent |
| SSR | solid-state reaction |
| TEA | triethanolamine |
| TBT | tetrabutyl titanate |
| TBOT | titanium tetrabutoxide |
| TEA | triethanolamine |
| TET | tetraethoxytitanium |
| TG | thermogravimetry analysis |
| TIP | titanium(IV) isopropoxide |
| TNBT | titanium n-butoxide |
| XRD | X-ray diffraction |
References
- Mauger, A.; Julien, C.M.; Goodenough, J.B.; Zaghib, K. Tribute to Michel Armand: From rocking chair—Li-ion to solid-state lithium batteries. J. Electrochem. Soc. 2020, 167, 070507. [Google Scholar] [CrossRef]
- Mauger, A.; Xie, H.; Julien, C.M. Composite anodes for lithium-ion batteries: Status and trends. AIMS Mater. Sci. 2016, 3, 1054–1106. [Google Scholar] [CrossRef]
- Jonker, G.H. Compounds in the system Li2O–TiO2 and their stability. In Proceedings of the Third International Symposium on the Reactivity of Solids, Madrid, Spain, 2–6 April 1956; pp. 413–421. [Google Scholar]
- Deschanvres, A.; Raveau, B.; Sekkal, Z. Mise en évidence et étude cristallographique d’une nouvelle solution solide de type spinelle Li1+xTi2−xO4, 0 ≤ x ≤ 0.33. Mater. Res. Bull. 1971, 6, 699–704. [Google Scholar] [CrossRef]
- Johnston, D.C.; Prakash, H.; Zachariasen, W.H.; Viswanathan, R. High temperature superconductivity in the Li-Ti-O ternary system. Mater. Res. Bull. 1973, 8, 777–784. [Google Scholar] [CrossRef]
- Murphy, D.W.; Cava, R.J.; Zahurak, S.M.; Santoro, A. Ternary LixTiO2 phases from insertion reaction. Solid State Ion. 1983, 9–10, 413–417. [Google Scholar] [CrossRef]
- Colbow, K.M.; Dahn, J.R.; Haering, R.R. Structure and electrochemistry of the spinel oxides LiTi2O4 and Li4/3Ti5/3O4. J. Power Sources 1989, 26, 397–402. [Google Scholar] [CrossRef]
- Ferg, E.; Gummow, R.J.; de Kock, A. Spinel anodes for lithium-ion batteries. J. Electrochem. Soc. 1995, 141, L147–L150. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Badway, F.; Du Pasquier, A.; Zheng, T. An asymmetric hybrid nonaqueous energy storage cell. J. Electrochem. Soc. 2001, 148, A930–A939. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Yamamoto, N. Zero-strain material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J. Electrochem. Soc. 1995, 142, 1431–1435. [Google Scholar] [CrossRef]
- Sun, X.; Radovanovic, P.V.; Cui, B. Advances in spinel Li4Ti5O12 anode materials for lithium-ion batteries. New J. Chem. 2015, 39, 38–63. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Huang, J.; He, X.; Chen, Y.; Zhang, R.; Lin, R.; Li, Y.; Yu, S.; Xing, X.; et al. Elucidating the limit of Li insertion into the spinel Li4Ti5O12. ACS Mater. Lett. 2019, 1, 96–102. [Google Scholar] [CrossRef]
- Sugiyama, J.; Umegaki, I.; Uyama, T.; McFadden, R.M.L.; Shiraki, S.; Hitosugi, T.; Salman, Z.; Saadanoui, H.; Morris, G.D.; MacFarlane, W.A.; et al. Lithium diffusion in spinel Li4Ti5O12 and LiTi2O4 films detected with 8Li β-NMR. Phys. Rev. B 2017, 96, 094402. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Z.; Mao, J. Mechanisms of the decrease in low-temperature electrochemical performance of Li4Ti5O12-based anode materials. Sci. Rep. 2017, 7, 15292. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.G.; Jang, M.W.; Hassoun, J.; Sun, Y.K.; Scrosati, B. A high-rate long-life Li4Ti5O12/Li[Ni0.45Co0.1Mn1.45]O4 lithium-ion battery. Nat. Commun. 2011, 2, 516. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Choi, D.; Yang, Z. Li-ion battery with LiFePO4 cathode and Li4Ti5O12 anode for stationary energy storage. Metall. Mater. Trans. A 2013, 44, 21–25. [Google Scholar] [CrossRef]
- Yang, C.; Hu, H.; Lin, S.J.; Chien, W. Electrochemical performance of V-doped spinel Li4Ti5O12/C composite anode in Li-half and Li4Ti5O12/LiFePO4–full cell. J. Power Sources 2014, 258, 424–433. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Charest, P.; Rodrigues, I.; Mauger, A.; Julien, C.M. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sources 2011, 196, 3949–3954. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Behi, M.; Jaguemont, J.; Ghanbarpour, M.; Behnia, M.; Berecibar, M.; Van Mierlo, J. Thermal management analysis using heat pipe in the high current discharging of lithium-ion battery in electric vehicles. J. Energy Storage 2020, 32, 101893. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Gandoman, F.H.; Akbarzadeh, M.; Khaleghi, S.; Kalogiannis, T.; Hosen, M.S.; Jaguemont, J.; Van Mierlo, J.; Berecibar, M. PCM assisted heat pipe cooling system for the thermal management of an LTO cell for high-current profiles. Case Stud. Therm. Eng. 2021, 25, 100920. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Kalogiannis, T.; He, J.; Patil, M.S.; Muller, J.-D.; Haider, A.; Mierlo, J.V.; Berecibar, M. Advanced hybrid thermal management system for LTO battery module under fast charging. Case Stud. Therm. Eng. 2022, 33, 101938. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, M.; Ma, S.; Luo, Y.; Zhang, B.; Wang, S.; Yan, L.; Tong, Z.; Lu, T.; Zhou, Y.-N.; et al. Li4Ti5O12-based battery energy storage system with dual-phase cathode. Energy Technol. 2023, 11, 2200899. [Google Scholar] [CrossRef]
- Piao, N.; Wang, P.-F.; Chen, L.; Deng, T.; Fan, X.; Wang, L.; He, X. Nonflammable all-fluorinated electrolytes enabling high-power and long-life LiNi0.5Mn1.5O4/Li4Ti5O12 lithium-ion batteries. Nano Energy 2023, 105, 108040. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Lee, U.; Allen, J.L. Electrical conductivity and rate-capability of Li4Ti5O12 as a function of heat-treatment atmosphere. J. Power Sources 2006, 154, 287–289. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J.L. Electrical conductivity and charge compensation in Ta doped Li4Ti5O12. J. Power Sources 2008, 180, 582–585. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Amine, K. Li4Ti5O12 spinel anodes. Nat. Energy 2021, 6, 683. [Google Scholar] [CrossRef]
- Vikram Babu, B.; Vijaya Babu, K.; Tewodros Aregai, G.; Seeta Devi, L.; Madhavi Latha, B.; Sushma Reddi, M.; Samatha, K.; Veeraiah, V. Structural and electrical properties of Li4Ti5O12 anode material for lithium-ion batteries. Results Phys. 2018, 9, 284–289. [Google Scholar] [CrossRef]
- Wagemaker, M.; Simon, D.R.; Kelder, E.M.; Schoonman, J.; Ringpfeil, C.; Haake, U.; Lützenkirchen-Hecht, D.; Frahm, R.; Mulder, F.M. A kinetic two-phase and equilibrium solid solution in spinel Li4+xTi5O12. Adv. Mater. 2006, 18, 3169–3173. [Google Scholar] [CrossRef]
- Borghols, W.J.H.; Wagemaker, M.; Lafont, U.; Kelder, E.M.; Mulder, F.M. Size effects in the Li4+xTi5O12 spinel. J. Am. Chem. Soc. 2009, 131, 17786–17792. [Google Scholar] [CrossRef]
- Kavan, L.; Prochazka, J.; Spitler, T.M.; Kalbac, M.; Zukalova, M.T.; Drezen, T.; Gratzel, M. Li insertion into Li4Ti5O12 (spinel): Charge capability vs. particle size in thin-film electrodes. J. Electrochem. Soc. 2003, 150, A1000–A1007. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 spinel anode: Fundamentals and advances in rechargeable batteries. InfoMat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Yi, T.-F.; Yang, S.-Y.; Xie, Y. Recent advances of Li4Ti5O12 as a promising next generation anode material for high power lithium-ion batteries. J. Mater. Chem. A 2015, 3, 5750–5777. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Liu, M.; Shao, Z. A comprehensive review of Li4Ti5O12-based electrodes for lithium-ion batteries: The latest advancements and future perspectives. Mater. Sci. Eng. R 2015, 98, 1–71. [Google Scholar] [CrossRef]
- Zhu, G.N.; Wang, Y.G.; Xia, Y.Y. Ti-Based compounds as anode materials for Li-ion Batteries. Energy Environ. Sci. 2012, 5, 6652–6667. [Google Scholar] [CrossRef]
- Yuan, T.; Tan, Z.; Ma, C.; Yang, J.; Ma, Z.-F.; Zheng, S. Challenges of spinel Li4Ti5O12 for lithium-ion battery industrial applications. Adv. Energy Mater. 2017, 7, 1601625. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.; Lu, Q.; Duo, X.; Sheng, X. A review of spinel lithium titanate (Li4Ti5O12) as electrode material for advanced energy storage devices. Ceram. Int. 2021, 47, 5870–5895. [Google Scholar] [CrossRef]
- Ezhyeh, Z.; Khodaei, M.; Torabi, F. Review on doping strategy in Li4Ti5O12 as an anode material for lithium-ion batteries. Ceram. Int. 2023, 49, 7105–7141. [Google Scholar] [CrossRef]
- Shen, Y.; Eltzholtz, J.; Iversen, B. Controlling size, crystallinity, and electrochemical performance of Li4Ti5O12 nanocrystals. Chem. Mater. 2013, 25, 5023–5030. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Tang, L.; He, Y.-B.; Gan, L.; Li, J.; Du, H.; Li, B.; Lin, Z.; Kang, F. A robust strategy for crafting monodisperse Li4Ti5O12 nanospheres as superior rate anode for lithium ion batteries. Nano Energy 2016, 21, 133–144. [Google Scholar] [CrossRef]
- Lin, C. Improvements of Li4Ti5O12 anode material for lithium-ion batteries. Mater. Res. Found. 2017, 12, 77–93. [Google Scholar]
- Meng, W.; Xu, Y.; Yan, B. In situ nano-sized spinel Li4Ti5O12 powder fabricated by a one-step roasting process in molten salts. J. Alloys Compd. 2018, 732, 784–791. [Google Scholar] [CrossRef]
- Chang-Jian, C.W.; Cho, E.C.; Huang, J.H.; Huang, J.H.; Chou, J.A.; Ho, B.C.; Lee, K.C.; Hsiao, Y.S. Spray-drying synthesis of Li4Ti5O12 microspheres in pilot scale using TiO2 nanosheets as starting materials and their application in high-rate lithium ion battery. J. Alloys Compd. 2019, 773, 376–386. [Google Scholar] [CrossRef]
- Shenouda, A.Y.; Murali, K.R. Electrochemical properties of doped lithium titanate compounds and their performance in lithium rechargeable batteries. J. Power Sources 2008, 176, 332–339. [Google Scholar] [CrossRef]
- Panero, S.; Reale, P.; Ronci, F.; Rossi Albertini, V.; Scrosati, B. Structural and electrochemical study on Li(Li1/3Ti5/3)O4 anode material for lithium ion batteries. Ionics 2000, 6, 461–465. [Google Scholar] [CrossRef]
- Ge, H.; Li, N.; Li, D.; Dai, C.; Wang, D. Electrochemical characteristics of spinel Li4Ti5O12 discharged to 0.01 V. Electrochem. Commun. 2008, 10, 719–722. [Google Scholar] [CrossRef]
- Lai, C.; Wu, Z.; Zhu, Y.; Wu, Q.; Liang Li, L.; Wang, C. Ball-milling assisted solid-state reaction synthesis of mesoporous Li4Ti5O12 for lithium-ion batteries anode. J. Power Sources 2013, 226, 71–74. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Dhananjaya, M.; Hussain, O.M.; Mauger, A.; Julien, C.M. Enhanced electrochemical performance of Li4Ti5O12 by niobium doping for pseudocapacitive applications. Micro 2021, 1, 28–42. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, A.; Dai, X.; Feng, L.; Li, J.; Li, J. Solid-state synthesis of submicron-sized Li4Ti5O12/Li2TiO3 composites with rich grain boundaries for lithium ion batteries. J. Power Sources 2014, 266, 114–120. [Google Scholar] [CrossRef]
- Sarantuya, L.; Sevjidsuren, G.; Zltantsog, P.; Tsogbadrakh, N. Synthesis, structure and electronic properties of Li4Ti5O12 anode material for lithium ion batteries. Solid State Phenom. 2018, 271, 9–17. [Google Scholar] [CrossRef]
- Zhang, E.; Zhang, H. Hydrothermal synthesis of Li4Ti5O12-TiO2 composites and Li4Ti5O12 and their applications in lithium-ion batteries. Ceram. Int. 2019, 45, 7419–7426. [Google Scholar] [CrossRef]
- Ge, H.; Chen, L.; Yuan, W.; Zhang, Y.; Fan, Q.; Osgood, H.; Matera, D.; Song, X.-M.; Wu, G. Unique mesoporous spinel Li4Ti5O12 nanosheets as anode materials for lithium-ion batteries. J. Power Sources 2015, 297, 436–441. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, L.; Fang, S.; Qiu, Z. Li4Ti5O12 hollow microspheres assembled by nanosheets as an anode material for high-rate lithium ion batteries. Electrochim. Acta 2009, 54, 6244–6249. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, Z.; Zhang, D.; Li, W.; Lu, Q. A new hydrothermal synthesis of spherical Li4Ti5O12 anode material for lithium-ion secondary batteries. J. Power Sources 2012, 219, 45–51. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, S.M.; Lee, J.W.; Lee, J.B.; Han, S.S.; Lee, H.C.; Kim, H.J. Spinel Li4Ti5O12 nanotubes for energy storage materials. J. Phys. Chem. C 2009, 113, 18420–18423. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, C.; Luo, H.; Zhang, X.; Xu, K.; Xia, Y. Facile synthesis of hierarchically porous microspheres for high rate lithium ion batteries. J. Mater. Chem. 2010, 20, 6998–7004. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, C.; Luo, H.; Zhang, X.; Yang, S.; Lu, X. In situ synthesis of high-loading Li4Ti5O12–graphene hybrid nanostructures for high rate lithium ion batteries. Nanoscale 2011, 3, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rong, H.; Li, B.; Xing, L.; Li, X.; Li, W. Microemulsion-assisted synthesis of ultrafine Li4Ti5O12/C nanocomposite with oleic acid as carbon precursor and particle size controller. J. Power Sources 2014, 246, 213–218. [Google Scholar] [CrossRef]
- Qian, K.; Tang, L.; Wagemaker, M.; He, Y.-B.; Liu, D.; Li, H.; Shi, R.; Li, B.; Kang, F. A facile surface reconstruction mechanism toward better electrochemical performance of Li4Ti5O12 in lithium-ion battery. Adv. Sci. 2017, 4, 1700205. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, H.; Tian, Z.; Qiu, W.; Li, X. Solvothermal synthesis and electrochemical characterization of amorphous lithium titanate materials. J. Alloys Compd. 2008, 455, 471–474. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, C.; Luo, H.; Zhang, X.; Chen, L.; Li, H. Novel template-free solvothermal synthesis of mesoporous Li4Ti5O12-C microspheres for high power lithium ion batteries. J. Mater. Chem. 2011, 21, 14414–14416. [Google Scholar] [CrossRef]
- Bach, S.; Pereira-Ramos, J.P.; Baffier, N. Electrochemical properties of sol–gel Li4/3Ti5/3O4. J. Power Sources 1999, 81–82, 273–276. [Google Scholar] [CrossRef]
- Shen, C.M.; Zhang, X.G.; Zhou, Y.K.; Li, H.L. Preparation and characterization of nanocrystalline Li4Ti5O12 by sol–gel method. Mater. Chem. Phys. 2002, 78, 437–441. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Lai, Q.-Y.; Lu, J.-Z.; Wang, H.-L.; Chen, Y.-D.; Ji, X.-Y. Synthesis and characterization of spinel Li4Ti5O12 anode material by oxalic acid-assisted sol gel method. J. Power Sources 2005, 158, 1358–1364. [Google Scholar] [CrossRef]
- Hao, Y.J.; Lai, Q.Y.; Xu, Z.; Liu, X.; Ji, X. Synthesis of TEA sol-gel method and electrochemical properties of Li4Ti5O12 anode material for lithium-ion battery. Solid State Ion. 2005, 176, 1201–1206. [Google Scholar] [CrossRef]
- Hao, Y.J.; Lai, Q.Y.; Lu, J.Z.; Liu, D.Q.; Ji, X.Y. Influence of various complex agents on electrochemical property of Li4Ti5O12 anode material. J. Alloys Compd. 2007, 439, 330–336. [Google Scholar] [CrossRef]
- Miao, X.; He, H.; Shi, L.; Zhao, X.; Fang, J. Sol-gel synthesis of nanocomposite/carbon nanotubes as anode materials for high-rate performance lithium-ion batteries. Adv. Mater. Res. 2014, 833, 45–49. [Google Scholar] [CrossRef]
- Mosa, J.; Aparicio, M. Sol-gel synthesis of nanocrystalline mesoporous Li4Ti5O12 thin-films as anodes for Li-ion microbatteries. Nanomaterials 2020, 10, 1369. [Google Scholar] [CrossRef]
- Priyono, S.; Sofyan, N.; Subhan, A.; Prihandoko, B.; Yuwono, A.H. Preparation of Al-doped Li4Ti5O12 anode material via sol-gel process with acidic catalyst for lithium-ion batteries. AIP Conf. Proc. 2023, 2538, 070003. [Google Scholar]
- Mani, J.; Katzke, H.; Habouti, S.; Moonoosawmy, K.R.; Dietze, M.; Es-Souni, M. A template-free synthesis and structural characterization of hierarchically nano-structured lithium-titanium-oxide films. J. Mater. Chem. 2012, 22, 6632–6638. [Google Scholar] [CrossRef]
- Jiang, C.H.; Ichihara, M.; Honma, I.; Zhou, H.S. Effect of particle dispersion on high rate performance of nano-sized Li4Ti5O12 anode. Electrochim. Acta 2007, 52, 6470–6475. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, R.; Bao, K.; Xie, H.; Zheng, S.; Guo, J.; Liu, G. Synthesis of nano- anode material for lithium ion batteries by a biphasic interfacial reaction route. Ceram. Int. 2016, 42, 11468–11472. [Google Scholar] [CrossRef]
- Ju, S.H.; Kang, Y.C. Effects of preparation conditions on the electrochemical and morphological characteristics of Li4Ti5O12 powders prepared by spray pyrolysis. J. Power Sources 2009, 189, 185–190. [Google Scholar] [CrossRef]
- Ju, S.H.; Kang, Y.C. Characteristics of spherical-shaped Li4Ti5O12 anode powders prepared by spray pyrolysis. J. Phys. Chem. Solids 2009, 70, 40–44. [Google Scholar] [CrossRef]
- Ju, S.H.; Kang, Y.C. Effects of types of drying control chemical additives on the morphologies and electrochemical properties of Li4Ti5O12 anode powders prepared by spray pyrolysis. J. Alloys Compd. 2010, 506, 913–916. [Google Scholar] [CrossRef]
- Ernst, F.O.; Kammler, H.K.; Roessler, A.; Pratsinis, S.E.; Stark, W.J.; Ufheil, J.; Novak, P. Electrochemically active flame-made nanosized spinels: LiMn2O4, Li4Ti5O12 and LiFe5O8. Mater. Chem. Phys. 2007, 101, 372–378. [Google Scholar] [CrossRef]
- Meierhofer, F.; Li, H.; Gockeln, M.; Kun, R.; Grieb, T.; Rosenauer, A.; Fritsching, U.; Kiefer, J.; Birkenstock, J.; Madler, L.; et al. Screening precursor−solvent combinations for Li4Ti5O12 energy storage material using flame spray pyrolysis. ACS Appl. Mater. Interfaces 2017, 9, 37760–37777. [Google Scholar] [CrossRef]
- Gockeln, M.; Pokhrel, S.; Meierhofer, F.; Glenneberg, J.; Schowalter, M.; Rosenauer, A.; Fritsching, U.; Busse, M.; Mädler, L.; Kun, R. Fabrication and performance of Li4Ti5O12/C Li-ion battery electrodes using combined double flame spray pyrolysis and pressure–based lamination technique. J. Power Sources 2018, 374, 97–106. [Google Scholar] [CrossRef]
- Terechshenko, A.; Sanbayeva, A.; Babaa, M.R.; Nurpeissova, A.; Bakenov, Z. Spray-pyrolysis preparation of Li4Ti5O12/Si composites for lithium-ion batteries. Eurasian Chem. Technol. J. 2019, 21, 69–73. [Google Scholar] [CrossRef]
- Xie, Z.; Song, Q.; Xie, H.; Yin, H.; Ning, Z. Chemically driven synthesis of Ti3+ self-doped Li4Ti5O12 spinel in molten salt. J. Am. Ceram. Soc. 2021, 104, 753–765. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, F.; Wu, F.; Wu, C.; Bao, L. Influence of composite LiCl–KCl molten salt on microstructure and electrochemical performance of spinel Li4Ti5O12. Electrochim. Acta 2008, 54, 322–327. [Google Scholar] [CrossRef]
- Sharmila, S.; Senthilkumar, B.; Kalai Selvan, R. Molten-salt synthesis and characterization of Li4Ti5O12. In AIP Conference Proceedings, Proceedings of the 55th DAE Solid State Physics; American Institute of Physics: College Park, MD, USA, 2011; Volume 1349, pp. 1325–1326. [Google Scholar]
- Guo, Q.; Li, S.; Wang, H.; Gao, Y.; Li, B. Molten salt synthesis of nano-sized Li4Ti5O12 doped with Fe2O3 for use as anode material in the lithium-ion battery. RSC Adv. 2014, 4, 60327–60333. [Google Scholar] [CrossRef]
- Xue, X.; Yan, H.; Fu, Y. Preparation of pure and metal-doped Li4Ti5O12 composites and their lithium-storage performances for lithium-ion batteries. Solid State Ion. 2019, 335, 1–6. [Google Scholar] [CrossRef]
- Kim, H.K.; Jegal, J.-P.; Kim, J.-Y.; Yoon, S.-B.; Roh, K.C.; Kim, K.-B. In situ fabrication of lithium titanium oxide by microwave-assisted alkalization for high-rate lithium-ion batteries. J. Mater. Chem. A 2013, 1, 14849–14852. [Google Scholar] [CrossRef]
- He, N.; Wang, B.; Huang, J. Preparation and electrochemical performance of monodisperse Li4Ti5O12 hollow spheres. J. Solid State Electrochem. 2010, 14, 1241–1246. [Google Scholar] [CrossRef]
- Yu, L.; Wu, H.B.; Lou, X.W. Mesoporous Li4Ti5O12 hollow spheres with enhanced lithium storage capability. Adv. Mater. 2013, 25, 2296–2300. [Google Scholar] [CrossRef] [PubMed]
- Kawade, U.V.; Jayswal, M.S.; Ambalkar, A.A.; Kadam, S.R.; Panmand, R.P.; Ambekar, J.D.; Kulkarni, M.V.; Kale, B.B. Surface modified Li4Ti5O12 by paper templated approach for enhanced interfacial Li+ charge transfer in Li-ion batteries. RSC Adv. 2018, 8, 38391–38399. [Google Scholar] [CrossRef]
- Hermawan, A.; Wibowo, A.; Asri, L.A.T.W.; Shu Yin, S.; Purwasasmita, B.S. Improved ionic conductivity of porous Li4Ti5O12 synthesized by sol-gel method using eggshell membrane as soft template. Mater. Res. Express 2019, 6, 075030. [Google Scholar] [CrossRef]
- Kanamura, K.; Chiba, T.; Dokko, K. Preparation of Li4Ti5O12 spherical particles for rechargeable lithium batteries. J. Eur. Ceram. Soc. 2006, 26, 577–581. [Google Scholar] [CrossRef]
- Kim, D.H.; Ahn, Y.S.; Kim, J. Polyol-mediated synthesis of Li4Ti5O12 nanoparticles and its electrochemical properties. J. Electrochem. Commun. 2005, 7, 1340–1344. [Google Scholar] [CrossRef]
- Kataoka, K.; Takahashi, Y.; Kijima, N.; Akimoto, J.; Ohshima, K. Single crystal growth and structure refinement of Li4Ti5O12. J. Phys. Chem. Solids 2008, 69, 1454–1456. [Google Scholar] [CrossRef]
- Wang, G.; Xu, J.; Wen, M.; Cai, R.; Ran, R.; Shao, Z. Influence of high-energy ball milling of precursor on the morphology and electrochemical performance of Li4Ti5O12–Ball-milling time. Solid State Ion. 2008, 179, 946–950. [Google Scholar] [CrossRef]
- Han, S.-W.; Jeong, J.; Yoon, D.-H. Effects of high-energy milling on the solid-state synthesis of pure nano-sized Li4Ti5O12 for high power lithium battery applications. Appl. Phys. A 2014, 114, 925–930. [Google Scholar] [CrossRef]
- Li, Y.; Xie, H.; Li, J.; Wang, J. Mechanochemical synthesis and electrochemical performances of Li4Ti5O12 anode materials for lithium-ion batteries. Adv. Mater. Res. 2012, 512–515, 1660–1663. [Google Scholar]
- Lu, H.W.; Zeng, W.; Li, Y.S.; Fu, Z.W. Fabrication and electrochemical properties of three-dimensional net architectures of anatase TiO2 and spinel Li4Ti5O12 nanofibers. J. Power Sources 2007, 164, 874–879. [Google Scholar] [CrossRef]
- Castano, N.; Cortes, M.A.; Garcia, E.; Martinez, H.V. Synthesis and morphological characterization of Li-Ti/PVP fibers as precursors for Li4Ti5O12 towards its future use as anode materials in Li-ion batteries by means of electrospinning. IOP Conf. Ser. Mater. Sci. Eng. 2018, 437, 012016. [Google Scholar] [CrossRef]
- Prakash, A.S.; Manikandan, P.; Ramesha, K.; Sathiya, M.; Tarascon, J.M.; Shukla, A.K. Solution–combustion synthesized nanocrystalline Li4Ti5O12 as high-rate performance Li-ion battery anode. Chem. Mater. 2010, 22, 2857–2863. [Google Scholar] [CrossRef]
- Cai, R.; Yu, X.; Liu, X.; Shao, Z. Li4Ti5O12/Sn composite anodes for lithium-ion batteries: Synthesis and electrochemical performance. J. Power Sources 2010, 195, 8244–8250. [Google Scholar] [CrossRef]
- Yuan, T.; Cai, R.; Wang, K.; Ran, R.; Liu, S.; Shao, Z. Combustion synthesis of high-performance Li4Ti5O12 for secondary Li-ion battery. Ceram. Int. 2009, 35, 1757–1768. [Google Scholar] [CrossRef]
- De Sloovere, D.; Marchal, W.; Ulu, F.; Vranken, T.; Verheijen, M.; Van Bael, M.K.; Hardy, A. Combustion synthesis as a low temperature route to Li4Ti5O12 based powders for lithium ion battery anodes. RSC Adv. 2017, 7, 18745–18754. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, K.; Cai, R.; Ran, R.; Shao, Z. Cellulose-assisted combustion synthesis of Li4Ti5O12 adopting anatase TiO2 solid as raw material with high electrochemical performance. J. Alloys Compd. 2009, 477, 665–672. [Google Scholar] [CrossRef]
- Yuan, T.; Cai, R.; Gu, P.; Shao, Z. Synthesis of lithium insertion material Li4Ti5O12 from rutile TiO2 via surface activation. J. Power Sources 2010, 195, 2883–2887. [Google Scholar] [CrossRef]
- Raja, M.W.; Mahanty, S.; Kundu, M.; Basu, R.N. Synthesis of nanocrystalline Li4Ti5O12 by a novel aqueous combustion technique. J. Alloys Compd. 2009, 468, 258–262. [Google Scholar] [CrossRef]
- Lee, S.S.; Byun, K.-T.; Park, J.P.; Kim, S.K.; Kwak, H.-Y.; Shim, I.-W. Preparation of nanoparticles by a simple sonochemical method. Dalton Trans. 2007, 37, 4182–4184. [Google Scholar] [CrossRef]
- Ghosh, S.; Mitra, S.; Barpanda, P. Sonochemical synthesis of nanostructured spinel Li4Ti5O12 negative insertion material for Li-ion and Na-ion batteries. Electrochim. Acta 2016, 222, 898–903. [Google Scholar] [CrossRef]
- Ni, H.; Song, W.-L.; Fan, L.-Z. A strategy for scalable synthesis of Li4Ti5O12/reduced graphene oxide toward high rate lithium-ion batteries. Electrochem. Commun. 2014, 40, 1–4. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Min, K.-M.; Shim, H.-W.; Seo, S.-D.; Hwang, I.-S.; Park, K.-S.; Kim, D.W. Facile synthesis of nano- for high-rate Li-ion battery anodes. Nanoscale Res. Lett. 2012, 7, 10. [Google Scholar] [CrossRef]
- Mao, S.; Huang, X.; Chang, J.; Cui, S.; Zhou, G.; Chen, J. One-step continuous synthesis of spherical Li4Ti5O12/graphene composite as an ultra-long cycle life lithium-ion battery anode. NPG Asia Mater. 2015, 7, e224. [Google Scholar] [CrossRef]
- Tang, B.; Li, A.; Tong, Y.; Song, H.; Chen, X.; Zhou, J.; Ma, Z. Carbon-coated Li4Ti5O12 tablets derived from metal-organic frameworks as anode material for lithium-ion batteries. J. Alloys Compd. 2017, 708, 6–13. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, L.; Qiu, Z.; Huang, J. Template-free synthesis of mesoporous spinel lithium titanate microspheres and their application in high-rate lithium ion batteries. J. Mater. Chem. 2009, 19, 5980–5984. [Google Scholar] [CrossRef]
- Wen, Z.; Gu, Z.; Huang, S.; Yang, J.; Lin, Z.; Yamamoto, O. Research on spray-dried lithium titanate as electrode materials for lithium ion batteries. J. Power Sources 2005, 146, 670–673. [Google Scholar] [CrossRef]
- Wu, X.; Xinghua Liang, X.; Zhang, X.; Lan, X.; Li, L.; Suo Gai, Q. Structural evolution of plasma sprayed amorphous Li4Ti5O12 electrode and ceramic/polymer composite electrolyte during electrochemical cycle of quasi-solid-state lithium battery. J. Adv. Ceram. 2021, 10, 347–354. [Google Scholar] [CrossRef]
- Yin, S.Y.; Song, L.; Wang, X.Y.; Zhang, M.F.; Zhang, K.L.; Zhang, Y.X. Synthesis of spinel Li4Ti5O12 anode material by a modified rheological phase reaction. Electrochim. Acta 2009, 54, 5629–5633. [Google Scholar] [CrossRef]
- Nugroho, A.; Yoon, D.; Joo, O.-S.; Chung, K.Y.; Kim, J. Continuous synthesis of Li4Ti5O12 nanoparticles in supercritical fluids and their electrochemical performance for anode in Li-ion batteries. Chem. Eng. J. 2014, 258, 357–366. [Google Scholar] [CrossRef]
- Doi, T.; Iriyama, Y.; Abe, T.; Ogumi, Z. Electrochemical insertion and extraction of lithium ion at uniform nanosized Li4/3Ti5/3O4 particles prepared by a spray pyrolysis method. Chem. Mater. 2005, 17, 1580–1582. [Google Scholar] [CrossRef]
- Naoi, K.; Ishimoto, S.; Isobe, Y.; Aoyagi, S. High-rate nano-crystalline Li4Ti5O12 attached on carbon nano-fibers for hybrid supercapacitors. J. Power Sources 2010, 195, 6250–6254. [Google Scholar] [CrossRef]
- Bresser, D.; Paillard, E.; Copley, M.; Bishop, P.; Winter, M.; Passerini, S. The importance of “going nano” for high power battery materials. J. Power Sources 2012, 219, 217–222. [Google Scholar] [CrossRef]
- Naoi, K. Nanohybrid capacitor: The next generation electrochemical capacitors. Fuel Cells 2010, 10, 825–833. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.Y.; Hou, M.Y.; Fan, L.; Wang, Y.; Xia, Y. Carbon-coated Li4Ti5O12 nanoparticles with high electrochemical performance as anode material in sodium-ion batteries. J. Mater. Chem. A 2017, 5, 10902–10908. [Google Scholar] [CrossRef]
- Jiang, C.H.; Hosono, E.; Ichihara, M.; Honma, I.; Zhou, H.S. Synthesis of nanocrystalline Li4Ti5O12 by chemical lithiation of anatase nanocrystals and postannealing. J. Electrochem. Soc. 2008, 155, A553–A556. [Google Scholar] [CrossRef]
- Lu, J.; Nan, C.; Peng, Q.; Li, Y. Single crystalline lithium titanate nanostructure with enhanced rate performance for lithium ion battery. J. Power Sources 2012, 202, 246–252. [Google Scholar] [CrossRef]
- Song, K.; Seo, D.-H.; Jo, M.R.; Kim, Y.-I.; Kang, K.; Kang, Y.-M. Tailored oxygen framework of Li4Ti5O12 nanorods for high-power Li ion battery. J. Phys. Chem. Lett. 2014, 5, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, G.L.; Liu, J.W.; Gao, X.P. Preparation of Li4Ti5O12 nanorods as anode materials for lithium-ion batteries. J. Electrochem. Soc. 2009, 156, 495–499. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, L.; Tan, J.L.; Tan, Z.; Huang, Z.; Wang, X. Synthesis of lithium titanate nanorods as anode materials for lithium and sodium ion batteries with superior electrochemical performance. J. Power Sources 2015, 283, 243–250. [Google Scholar] [CrossRef]
- Priyono, B.; Herwono, M.F.; Syahrial, A.Z.; Nugraha, M.R.; Faizah; Subhan, A. Enhancing lithium titanite (Li4Ti5O12) nanorods performance with graphite and nano tin as anode for lithium-ion batteries. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2020; Volume 2232, p. 030008. [Google Scholar]
- Luo, H.; Shen, L.; Rui, K.; Li, H.; Zhang, X. Carbon coated Li4Ti5O12 nanorods as superior anode material for high rate lithium ion batteries. J. Alloys Compd. 2013, 572, 37–42. [Google Scholar] [CrossRef]
- Wang, W.; Guo, Y.; Liu, L.; Wang, S.; Yang, X.; Guo, H. Gold coating for a high performance nanorod aggregates anode in lithium-ion batteries. J. Power Sources 2014, 245, 624–629. [Google Scholar] [CrossRef]
- Li, Y.; Song, J.; Tian, Q. Li4Ti5O12 nanowires intertwined with carbon nanotubes for ultra-long life and conductive additive-free anodes of lithium-ion batteries. Mater. Lett. 2023, 348, 134698. [Google Scholar] [CrossRef]
- Hu, G.; Wu, J.; Du, K.; Peng, Z.; Jia, M.; Yang, H.; Cao, Y. Surface-fluorinated Li4Ti5O12 nanowires/reduced graphene oxide composite as a high-rate anode material for lithium ion batteries. Appl. Surf. Sci. 2019, 479, 158–166. [Google Scholar] [CrossRef]
- Li, J.R.; Tang, Z.L.; Zhang, Z.T. Controllable formation and electrochemical properties of one-dimensional nanostructured spinel Li4Ti5O12. Electrochem. Commun. 2005, 7, 894–899. [Google Scholar] [CrossRef]
- Bachtiar, A.R.; Syahrial, A.Z.; Nugraha, M.R.; Faizah; Subhan, A.; Priyono, B. Enhancing performance of Li4Ti5O12 nanowire with addition of graphite and ZnO nanoparticle as anode for lithium-ion batteries. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2020; Volume 2232, p. 030003. [Google Scholar]
- Kim, T.-T.; Yu, C.-Y.; Yoon, C.S.; Kim, S.-J.; Sun, Y.-K.; Myung, S.-T. Carbon-coated Li4Ti5O12 nanowires showing high rate capability as an anode material for rechargeable sodium batteries. Nano Energy 2015, 12, 725–734. [Google Scholar] [CrossRef]
- Liu, H.; Tank, K.; Song, K.; van Aken, P.A.; Yu, Y.; Maier, J. Tiny Li4Ti5O12 nanoparticles embedded in carbon nanofibers as high-capacity and long-life anode materials for both Li-ion and Na-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 20813–20818. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Lu, Y.; Wei, F. Confined growth of Li4Ti5O12 nanoparticles in nitrogen-doped mesoporous graphene fibers for high-performance lithium-ion battery anodes. Nano Res. 2016, 9, 230–239. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Sun, Y.; Luo, W.; Chen, C.; Liu, Y.; Huang, Y. Highly porous Li4Ti5O12/C nanofibers for ultrafast electrochemical energy storage. Nano Energy 2014, 10, 163–171. [Google Scholar] [CrossRef]
- Bian, M.; Yang, Y.; Tian, L. Carbon-free Li4Ti5O12 porous nanofibers as high-rate and ultralong-life anode materials for lithium-ion batteries. J. Phys. Chem. Solids 2018, 113, 11–16. [Google Scholar] [CrossRef]
- Jo, M.R.; Jung, Y.S.; Kang, Y.-M. Tailored Li4Ti5O12 nanofibers with outstanding kinetics for lithium rechargeable batteries. Nanoscale 2012, 4, 6870–6875. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Li, Z.; Yang, K.; Gao, F. Pr-modified Li4Ti5O12 nanofibers as an anode material for lithium-ion batteries with outstanding cycling performance and rate performance. Ionics 2017, 23, 597–605. [Google Scholar] [CrossRef]
- Ji, X.; Lu, Q.; Guo, E.; Li, D.; Yao, L.; Liu, H.; Li, X. Bamboo-shaped Zn2+-doped Li4Ti5O12 nanofibers: One-step controllable synthesis and high-performance lithium-ion batteries. J. Electrochem. Soc. 2018, 165, A534–A541. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Luo, W.; Sun, Y.; Yang, Z.; Hu, C.; Huang, Y. Electrospun conformal Li4Ti5O12/C fibers for high-rate lithium-ion batteries. ChemElectroChem 2014, 1, 611–616. [Google Scholar] [CrossRef]
- Yagi, S.; Morinaga, T.; Togo, M.; Tsuda, H.; Shio, S.; Nakahira, A. Ion-exchange synthesis of Li4Ti5O12 nanotubes and nanoparticles for high-rate Li-ion batteries. Mater. Trans. 2016, 57, 42–45. [Google Scholar] [CrossRef]
- Jiang, Y.-M.; Wang, K.-X.; Wu, X.-Y.; Zhang, H.-J.; Bartlett, B.M.; Chen, J.-S. Li4Ti5O12/TiO2 hollow spheres composed nanoflakes with preferentially exposed Li4Ti5O12 (011) facets for high-rate lithium ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 19791–19796. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, W.; Li, X.; Zhong, X.; Liu, W.; Lin, Y.; Xia, R. Li4Ti5O12/Ti4O7/carbon nanotubes composite anode material for lithium-ion batteries. Micro Nano Lett. 2018, 13, 915–918. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Yang, Y.; Yu, W.; Ma, Q.; Dong, X.; Wang, J.; Liu, G. Electrochemical characteristics of Li4Ti5O12/Ag composite nanobelts prepared via electrospinning. Russ. J. Phys. Chem. A 2019, 93, 144–150. [Google Scholar] [CrossRef]
- Qin, W.; Chen, Y.; An, J.; Zhang, J.; Wen, X. High-loaded nanobelt-array/nanobelt-microsphere multilayer Li4Ti5O12 self-supported on Ti foils for high-performance lithium ion batteries. Electrochim. Acta 2022, 419, 140407. [Google Scholar] [CrossRef]
- Tang, Y.F.; Yang, L.; Qiu, Z.; Huang, J.S. Preparation and electrochemical lithium storage of flower-like spinel Li4Ti5O12 consisting of nanosheets. Electrochem. Commun. 2008, 10, 1513–1516. [Google Scholar] [CrossRef]
- Hong, Z.S.; Lan, T.B.; Xiao, F.Y.; Zhang, H.X.; Wei, M.D. Ultrafine Li4Ti5O12 nanosheets as a high performance anode for Li-ion battery. Funct. Mater. Lett. 2011, 4, 389–393. [Google Scholar] [CrossRef]
- Wu, L.; Leng, X.; Liu, Y.; Wei, S.; Li, C.; Wang, G.; Lian, J.; Jiang, Q.; Nie, A.; Zhang, T.-Y. A strategy for synthesis of nanosheets consisting of alternating spinel Li4Ti5O12 and rutile TiO2 lamellas for high-rate anodes of lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 4649–4657. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.B.; Tian, Y.; Wei, X.L. Free-standing electrodes composed of carbon-coated Li4Ti5O12 nanosheets and reduced graphene oxide for advanced sodium ion batteries. J. Power Sources 2017, 337, 180–188. [Google Scholar] [CrossRef]
- Lai, C.; Dou, Y.Y.; Li, X.; Gao, X.P. Improvement of the high rate capability of hierarchical structured Li4Ti5O12 induced by the pseudocapacitive effect. J. Power Sources 2010, 195, 3676–3679. [Google Scholar] [CrossRef]
- Sha, Y.; Zhao, B.; Ran, R.; Cai, R.; Shao, Z. Synthesis of well-crystalized Li4Ti5O12 nanoplates for lithium-ion batteries with outstanding rate capability and cycling stability. J. Mater. Chem. A 2013, 1, 13233–13243. [Google Scholar] [CrossRef]
- Liu, J.; Wei, X.; Liu, X.-W. Two-dimensional wavelike spinel lithium titanate for fast lithium storage. Sci. Rep. 2015, 5, 9782. [Google Scholar] [CrossRef]
- Salvatore, K.L.; Vila, M.N.; Renderos, G.; Li, W.; Housel, L.M.; Tong, X.; McGuire, S.C.; Gan, J.; Paltis, A.; Lee, K.; et al. Probing the physicochemical behavior of variously doped Li4Ti5O12 nanoflowers. ACS Phys. Chem. Au 2022, 2, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, H.; Cao, G.P.; Wang, B.Y.; Wang, X.D. Phenol-formaldehyde resin-assisted synthesis of pure porous Li4Ti5O12 for rate capability improvement. Mater. Res. Bull. 2011, 46, 2312–2316. [Google Scholar] [CrossRef]
- Kang, E.; Jung, Y.S.; Kim, G.-H.; Chun, J.; Wiesner, U.; Dillon, A.C.; Kim, J.K.; Lee, J. Highly improved rate capability for a lithium-ion battery nano-Li4Ti5O12 negative electrode via carbon-coated mesoporous uniform pores with a simple self-assembly method. Adv. Funct. Mater. 2011, 21, 4349–4357. [Google Scholar] [CrossRef]
- Saxena, S.; Sil, A. Nanoporous Li4Ti5O12 material for the electrode of lithium ion battery. IETE Techn. Rev. 2016, 33, 60–63. [Google Scholar] [CrossRef]
- Shao, D.; He, J.; Luo, Y.; Liu, W.; Yu, X.; Fang, Y. Synthesis and electrochemical performance of nanoporous Li4Ti5O12 anode material for the lithium-ion batteries. J. Solid State Electrochem. 2012, 16, 2047–2053. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Lin, J.; Yang, J.; Feng Wu, F.; Li, L.; Chen, R. Structural and electrochemical characteristics of hierarchical Li4Ti5O12 as high-rate anode material for lithium-ion batteries. Electrochim. Acta 2021, 368, 137470. [Google Scholar] [CrossRef]
- Liu, J.; Song, K.; van Aken, P.A.; Maier, J.; Yu, Y. Self-supported Li4Ti5O12-C nanotube arrays as high-rate and long-life anode materials for flexible Li-ion batteries. Nano Lett. 2014, 14, 2597–2603. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chen, Y.; An, J.; Wen, X. Self-supported Li4Ti5O12 nanobelt array anode with long-life and improved low-temperature performance for flexible lithium-ion batteries. Ceram. Int. 2022, 48, 22194–22203. [Google Scholar] [CrossRef]
- Liu, J.; Wei, A.; Pan, G.; Shen, S.; Xiao, Z.; Zhao, Y.; Xia, X. Self-supported hierarchical porous Li4Ti5O12/carbon arrays for boosted lithium ion storage. J. Energy Chem. 2021, 54, 754–760. [Google Scholar] [CrossRef]
- Pawlitzek, F.; Althues, H.; Schumm, B.; Kaskel, S. Nanostructued networks for energy stotage: Vertically aligned carbon nanotubes (VACNT) as current collectors for high-power Li4Ti5O12(LTO)//LiMn2O4(LMO) lithium ion batteries. Batteries 2017, 3, 37. [Google Scholar] [CrossRef]
- Shen, L.; Uchaker, E.; Zhang, X.; Cao, G. Hydrogenated Li4Ti5O12 nanowire arrays for high rate lithium ion batteries. Adv. Mater. 2012, 24, 6502–6506. [Google Scholar] [CrossRef]
- Xia, Q.; Jabeen, N.; Savilov, S.V.; Aldoshind, S.M.; Xia, H. Black mesoporous Li4Ti5O12–δ nanowall arrays with improved rate performance as advanced 3D anodes for microbatteries. J. Mater. Chem. A 2016, 4, 17543–17551. [Google Scholar] [CrossRef]
- Jiang, C.; Ding, W.; Wu, H.; Yu, Z.; Ma, L.; Zou, Z. Hierarchical Li4Ti5O12 nanosheet arrays anchoring on carbon fiber cloth as ultra-stable free-standing anode of Li-ion battery. Ceram. Int. 2018, 44, 3040–3047. [Google Scholar] [CrossRef]
- Kim, S.D.; Rana, K.; Ahn, J.-H. Additive-free synthesis of Li4Ti5O12 nanowire arrays on freestanding ultrathin graphite as a hybrid anode for flexible lithium ion batteries. J. Mater. Chem. A 2016, 4, 19197–19206. [Google Scholar] [CrossRef]
- Sorensen, E.M.; Barry, S.J.; Jung, H.K.; Rondinelli, J.R.; Vaughey, J.T.; Poeppelmeier, K.R. Three-dimensionally ordered macroporous Li4Ti5O12: Effect of wall structure on electrochemical properties. Chem. Mater. 2006, 18, 482–489. [Google Scholar] [CrossRef]
- Li, N.; Zhou, G.; Li, F.; Wen, L.; Cheng, H.-M. A self-standing and flexible electrode of Li4Ti5O12 nanosheets with a N-doped carbon coating for high rate lithium ion batteries. Adv. Funct. Mater. 2013, 23, 5429–5435. [Google Scholar] [CrossRef]
- Chen, S.; Xin, Y.; Zhou, Y.; Ma, Y.; Zhou, H.; Qi, L. Self-supported Li4Ti5O12 nanosheet arrays for lithium ion batteries with excellent rate capability and ultralong cycle life. Energy Environ. Sci. 2014, 7, 1924–1930. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M.; Zaghib, K. Structural studies of Li4/3Me5/3O4 (Me = Ti, Mn) electrode materials: Local structure and electrochemical aspects. J. Power Sources 2004, 136, 72–79. [Google Scholar] [CrossRef]
- Leonidov, I.A.; Leonidova, O.N.; Perelyaeva, A.L.; Samigullina, R.F.; Kovyazina, S.A.; Patrakeev, M.V. Structure, ionic conduction, and phase transformations in lithium titanate Li4Ti5O12. Phys. Solid State 2003, 45, 2183–2188. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M. Lattice vibrations of materials for lithium rechargeable batteries. I. Lithium manganese oxide spinel. Mater. Sci. Eng. B 2003, 97, 217–230. [Google Scholar] [CrossRef]
- Pelegov, D.V.; Slautin, B.N.; Gorshkov, V.S.; Zelenovskiy, P.S.; Kiselev, E.A.; Kholkin, A.L.; Shur, V.Y. Raman spectroscopy “big data” and local heterogeneity of solid state synthesized lithium titanate. J. Power Sources 2017, 346, 143–150. [Google Scholar] [CrossRef]
- Mosa, J.; Aparicio, M.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Li4Ti5O12 thin-film electrodes by in-situ synthesis of lithium alkoxide for Li-ion microbatteries. Electrochim. Acta 2014, 149, 293–299. [Google Scholar] [CrossRef]
- Tran, M.V.; Huynh, N.L.T.; Nguyen, T.T.; Ha, D.T.C.; Le, P.M.L. Facile solution route to synthesize nanostructure Li4Ti5O12 for high rate Li-ion battery. J. Nanomater. 2016, 2016, 4261069. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Zhong, H.; Yan, X.; Ouyang, C.; Zhang, L. W6+ and Br− codoped Li4Ti5O12 anode with super rate performance for Li-ion batteries. J. Mater. Chem. A 2015, 3, 13706–13716. [Google Scholar] [CrossRef]
- Pelegov, D.V.; Nasara, R.N.; Tu, C.; Li, S. Defects in Li4Ti5O12 induced by carbon deposition: An analysis of unidentified bands in Raman spectra. Phys. Chem. Chem. Phys. 2019, 21, 20757–20763. [Google Scholar] [CrossRef]
- Kang, C.-Y.; Krajewski, M.; Lin, J.-Y. Impact of titanium precursors on formation and electrochemical properties of Li4Ti5O12 anode materials for lithium-ion batteries. J. Solid State Electrochem. 2021, 25, 575–582. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Huang, S.; Yu, G.; Gao, L.; Liu, H.; Yu, Y. Nano-sized Li4Ti5O12 anode material with excellent performance prepared by solid state reaction: The effect of precursor size and morphology. Electrochim. Acta 2013, 112, 356–363. [Google Scholar] [CrossRef]
- Lin, C.; Fan, X.; Xin, Y.; Cheng, F.; Lai, M.O.; Zhou, H.; Lu, L. Monodispersed mesoporous Li4Ti5O12 submicrospheres as anode materials for lithium-ion batteries: Morphology and electrochemical performances. Nanoscale 2014, 6, 6651–6660. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Duh, J.-G.; Tsai, M.-C.; Lee, C.-Y. Self-assembled synthesis of monodispersed mesoporous Li4Ti5O12 beads and their applications in secondary lithium-ion batteries. Electrochim. Acta 2012, 83, 47–52. [Google Scholar] [CrossRef]
- Pawlitzek, F.; Pampel, J.; Schmuck, M.; Althues, H.; Schumm, B.; Kaskel, S. High-power lithium ion batteries based on preorganized necklace type Li4Ti5O12/VACNT nano-composites. J. Power Sources 2016, 325, 1–6. [Google Scholar] [CrossRef]
- Tang, Y.; Tan, X.; Hou, G.; Zheng, G. Nanocrystalline Li4Ti5O12-coated TiO2 nanotube arrays as three-dimensional anode for lithium-ion batteries. Electrochim. Acta 2014, 117, 172–178. [Google Scholar]
- Wang, G.; Wang, H.; Ma, G.; Du, X.; Du, L.; Jing, P.; Wang, Y.; Wu, K.; Wu, H.; Wang, Q.; et al. Investigation on process mechanism of a novel energy-saving synthesis for Li4Ti5O12 anode material. J. Energy Chem. 2022, 70, 266–275. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Y.; Liu, Y.; Huang, Z.-D.; He, Y.; Kim, J.-K. Percolation threshold of graphene nanosheets as conductive additives in Li4Ti5O12 anodes of Li-ion batteries. Nanoscale 2013, 5, 2100–2106. [Google Scholar] [CrossRef]
- Li, Z.; Ding, F.; Zhao, Y.; Wang, Y.; Li, J.; Yang, K. synthesis and electrochemical performance of Li4Ti5O12 submirospheres coated with TiN as anode materials for lithium-ion batteries. Ceram. Int. 2016, 42, 15464–15470. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Duh, J.-G. Porous Li4Ti5O12 anode material synthesized by one-step solid-state method for electrochemical properties enhancement. J. Alloys Compd. 2011, 509, 3682–3685. [Google Scholar] [CrossRef]
- Haetge, J.; Hartman, P.; Brezenski, K.; Janek, J.; Brezenski, T. Ordered large-pore mesoporous Li4Ti5O12 spinel thin film electrodes with nanocrystalline framework for high rate rechargeable lithium batteries: Relationships among charge storage, electrical conductivity, and nanoscale structure. Chem. Mater. 2011, 23, 4384–4393. [Google Scholar] [CrossRef]
- Nowack, L.V.; Waser, O.; Yarema, O.; Wood, V. Rapid, microwave-assisted synthesis of battery-grade lithium titanate (LTO). RSC Adv. 2013, 3, 15618–15621. [Google Scholar] [CrossRef]
- Nguyen Huynh, L.T.; Duy Ha, C.T.; Nguyen, V.D.; Nguyen, D.Q.; Phung Le, M.L.; Tran, V.M. Structure and electrochemical properties of Li4Ti5O12 prepared via low-temperature precipitation. J. Chem. 2019, 2019, 1727859. [Google Scholar]
- Mahmoud, A.; Saadoune, I.; Lippens, P.; Chamas, M. The design and study of new Li-ion full cells of LiCo2/3Ni1/6Mn1/6O2 positive electrode paired with MnSn2 and Li4Ti5O12 negative electrodes. Solid State Ion. 2017, 300, 175–181. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.M.; Scofield, M.E.; Yue, S.Y.; McBean, C.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S.; Wong, S.S. Enhanced performance of “flower-like” Li4Ti5O12 motifs as anode materials for high-rate lithium-ion batteries. ChemSusChem 2015, 8, 3304–3313. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Li, W.; Zhong, X.; Gu, L.; Yu, Y. Nitridation Br-doped Li4Ti5O12 anode for high rate lithium ion batteries. J. Power Sources 2014, 266, 323–331. [Google Scholar] [CrossRef]
- Hong, H.-J.; Ban, G.; Lee, S.-M.; Park, I.-S.; Lee, Y.-J. Synthesis of 3D-structured Li4Ti5O12 from titanium(IV) oxysulfate (TiOSO4) solution as a highly sustainable anode material for lithium-ion batteries. J. Alloys Compd. 2020, 844, 156203. [Google Scholar] [CrossRef]
- Spitler, T.; Prochazka, J.; Kavan, L.; Graetzel, M.; Sugnaux, F. High Performance Lithium Titanium Spinel Li4Ti5O12 for Electrode Material. U.S. Patent 7,547,490 B2, 16 June 2009. [Google Scholar]
- Han, S.Y.; Kim, I.Y.; Jo, K.Y.; Hwang, S.J. Solvothermal-assisted hybridization between reduced graphene oxide and lithium metal oxides: A facile route to graphene-based composite materials. J. Phys. Chem. C 2012, 116, 7269–7279. [Google Scholar] [CrossRef]
- Jung, H.G.; Myung, S.T.; Yoon, C.S.; Son, S.B.; Oh, K.H.; Amine, K.; Scrosati, B.; Sun, Y.K. Microscale spherical carbon-coated Li4Ti5O12 as ultra high power anode material for lithium batteries. Energy Environ. Sci. 2011, 4, 1345–1351. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Li, X.; Wu, L.; Wang, X.; Zhang, X.; Wang, Z.; Xiong, X.; Guo, H. Preparation and characterization of spinel Li4Ti5O12 anode material from industrial titanyl sulfate solution. J. Alloys Compd. 2011, 509, 596–601. [Google Scholar] [CrossRef]
- Tsai, M.C.; Tsai, T.L.; Lin, C.T.; Chung, R.J.; Sheu, H.S.; Chiu, H.T.; Lee, C.Y. Tailor made Mie scattering color filters made by size-tunable titanium dioxide particles. J. Phys. Chem. C 2008, 112, 2697–2702. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Ning, F.; Lu, X.; Liu, Y.; Nie, L.; Ouyang, C.; Zhang, L. Calcium doping of lithium titanium oxide nanospheres: A combined first-principles and experimental study. Energy Technol. 2017, 5, 539–543. [Google Scholar] [CrossRef]
- Zhang, F.; Yi, F.; Gao, A.; Shu, D.; Sun, Z.; Mao, J.; Zhou, X.; Zhu, Z.; Sun, Y. Interfacial electrostatic self-assembly in water-in-oil microemulsion assisted synthesis of Li4Ti5O12/graphene for lithium-ion-batteries. J. Alloys Compd. 2020, 819, 153018. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Xiao, X.; Higgins, D.; Lee, D.; Hassan, F.; Chen, Z. Hierarchical Li4Ti5O12-TiO2 composite microsphere consisting of nanocrystals for high power Li-ion batteries. Electrochim. Acta 2013, 108, 104–111. [Google Scholar] [CrossRef]
- Yu, S.-H.; Pucci, A.; Herntrich, T.; Willinger, M.-G.; Baek, S.-H.; Sungac, Y.-E.; Pinna, N. Surfactant-free nonaqueous synthesis of lithium titanium oxide (LTO) nanostructures for lithium ion battery applications. J. Mater. Chem. 2011, 21, 806–810. [Google Scholar] [CrossRef]
- Wu, L.; Kan, S.R.; Lu, S.G.; Zhang, X.J.; Jin, W.H. Effect of particle size and agglomeration of TiO2 on synthesis and electrochemical properties of Li4Ti5O12. Trans. Nonferrous Met. Soc. China 2007, 17, s117–s212. [Google Scholar]
- Yuan, T.; Cai, R.; Shao, Z. Different effect of the atmospheres on the phase formation and performance of Li4Ti5O12 prepared from ball-milling-assisted solid-phase reaction with pristine and carbon precoated TiO2 as starting materials. J. Phys. Chem. C 2011, 115, 4943–4952. [Google Scholar] [CrossRef]
- Xu, R.; Li, J.; Tang, Z.; Zhang, Z. Li4Ti5O12 heat treated under nitrogen ambient with outstanding rate capabilities. J. Nanomater. 2011, 2011, 635416. [Google Scholar] [CrossRef]
- Mahmoud, A.; Amarilla, J.M.; Lasri, K.; Saadoune, I. Influence of the synthesis method on the electrochemical properties of the Li4Ti5O12 spinel in Li-half and Li-ion full-cells. A systematic comparison. Electrochim. Acta 2013, 93, 163–172. [Google Scholar] [CrossRef]
- Chen, X.; Guan, X.; Li, L.; Li, G. Defective mesoporous Li4Ti5O12−y: An advanced anode with anomalous capacity and cycling stability at a high rate of 20C. J. Power Sources 2012, 210, 297–302. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Gu, L.; Guo, Y.G.; Li, H.; He, X.Q.; Tsukimoto, S.; Ikuhara, Y.; Wan, L.J. Rutile-TiO2 nanocoating for a high-rate Li4Ti5O12 anode of a lithium-ion battery. J. Am. Chem. Soc. 2012, 134, 7874–7879. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.; Demopoulos, G.P. A novel green approach to synthesis of nanostructured Li4Ti5O12 anode material. ECS Trans. 2013, 50, 119–128. [Google Scholar] [CrossRef]
- Demopoulos, G.; Chiu, H.; Zaghib, K.; Guerfi, A. Layered and Spinel Lithium Titanates and Processes for Preparing the Same. World Patent WO 2014/056111 A1, 17 April 2014. [Google Scholar]
- Feng, X.; Zou, H.; Xiang, H.; Guo, X.; Zhou, T.; Wu, Y.; Xu, W.; Yan, P.; Wang, C.; Zhang, J.-G.; et al. Ultrathin Li4Ti5O12 nanosheets as anode materials for lithium and sodium storage. ACS Appl. Mater. Interfaces 2016, 8, 16718–16726. [Google Scholar] [CrossRef]
- Suzuki, S.; Kozawa, T.; Murakami, T.; Naito, M. Mechanochemical-hydrothermal synthesis of layered lithium titanate hydrate nanotubes at room temperature and their conversion to Li4Ti5O12. Mater. Res. Bull. 2017, 90, 218–223. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Geng, D.; Li, Y.; Wang, D.; Li, R.; Sun, X.; Cai, M.; Verbrugge, M.W. Microwave-assisted hydrothermal synthesis of nanostructured spinel Li4Ti5O12 as anode materials for lithium ion batteries. Electrochim. Acta 2012, 63, 100–104. [Google Scholar] [CrossRef]
- Xu, G.B.; Li, W.; Yang, L.W.; Wei, X.L.; Ding, J.W.; Zhong, J.X.; Chu, P.K. Highly-crystalline ultrathin Li4Ti5O12 nanosheets decorated with silver nanocrystals as a high-performance anode material for lithium ion batteries. J. Power Sources 2015, 276, 247–254. [Google Scholar] [CrossRef]
- Feng, X.-Y.; Li, X.; Tang, M.; Gan, A.; Hu, Y.-Y. Enhanced rate performance of Li4Ti5O12 anodes with bridged grain boundaries. J. Power Sources 2017, 354, 172–178. [Google Scholar] [CrossRef]
- Yuan, T.; Cai, R.; Ran, R.; Zhou, Y.; Shao, Z. A mechanism study of synthesis of Li4Ti5O12 from TiO2 anatase. J. Alloys Compd. 2010, 505, 367–373. [Google Scholar] [CrossRef]
- Lin, C.; Lai, M.O.; Li, L.; Zhou, H.; Xin, Y. Structure and high performance of Ni2+ doped Li4Ti5O12 for lithium ion battery. J. Power Sources 2013, 244, 272–279. [Google Scholar] [CrossRef]
- Hu, X.; Li, Z.; Yang, K.; Huai, Y.; Deng, Z. Effects of carbon source and carbon content on electrochemical performances of Li4Ti5O12/C prepared by one-step solid-state reaction. Electrochim. Acta 2011, 56, 5046–5053. [Google Scholar] [CrossRef]
- Shen, Y.; Søndergaard, M.; Christensen, M.; Birgisson, S.; Iversen, B.B. Solid state formation mechanism of Li4Ti5O12 from an anatase TiO2 source. Chem. Mater. 2014, 26, 3679–3686. [Google Scholar] [CrossRef]
- Harrison, M.R.; Edwards, P.P.; Goodenough, J.B. The superconductor-semiconductor transition in the Li1+xTi2–xO4 spinel system. Philos. Mag. B 1985, 52, 679–699. [Google Scholar] [CrossRef]
- Jhan, Y.R.; Duh, J.G. Synthesis of entanglement structure in nanosized Li4Ti5O12/multi-walled carbon nanotubes composite anode material for Li-ion batteries by ball-milling-assisted solid-state reaction. J. Power Sources 2012, 198, 294–297. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, W.; Dai, X.; Feng, L.; Zhang, H.; Zhou, A.; Li, J. Solid-state synthesis of graphite carbon-coated Li4Ti5O12 anode for lithium ion batteries. Ionics 2014, 20, 1377–1383. [Google Scholar] [CrossRef]
- Yi, T.F.; Yang, S.Y.; Li, X.Y.; Yao, J.H.; Zhu, Y.R.; Zhu, R.S. Sub-micrometric Li4−xNaxTi5O12 (0 ≤ x ≤ 0.2) spinel as anode material exhibiting high-rate capability. J. Power Sources 2014, 246, 505–511. [Google Scholar] [CrossRef]
- Yang, L.X.; Gao, L.J. Li4Ti5O12/C composite electrode material synthesized involving conductive carbon precursor for Li-ion battery. J. Alloys Compd. 2009, 485, 93–97. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J. Spinel Li4Ti5O12 nanowires for high-rate Li-ion intercalation electrode. Electrochem. Solid-State Lett. 2007, 10, A81–A83. [Google Scholar] [CrossRef]
- Han, S.-W.; Ryu, J.H.; Jeong, J.; Yoon, D.-H. Solid-state synthesis of Li4Ti5O12 for high power lithium-ion battery applications. J. Alloys Compd. 2013, 570, 144–149. [Google Scholar] [CrossRef]
- Yao, W.; Zhuang, W.; Ji, X.; Chen, J.; Lu, X.; Wang, C. Solid-state synthesis of Li4Ti5O12 whiskers from TiO2-B. Mater. Res. Bull. 2016, 75, 204–210. [Google Scholar] [CrossRef]
- Cheng, L.; Yan, J.; Zhu, G.-N.; Luo, J.-Y.; Wang, C.-X.; Xia, Y.-Y. General synthesis of carbon-coated nanostructure Li4Ti5O12 as a high rate electrode material for Li-ion intercalation. J. Mater. Chem. 2010, 20, 595–602. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Zhang, Y.; Wang, J.; Yan, P.; Zhang, C.; He, D. The influence of the TiO2 particle size on the properties of Li4Ti5O12 anode material for lithium-ion battery. Ceram. Int. 2014, 40, 3799–3804. [Google Scholar] [CrossRef]
- Ohtake, T.; Iijima, K. Li4Ti5O12 synthesis with high specific surface area and single phase. J. Mater. Sci. Chem. Eng. 2015, 3, 68–73. [Google Scholar]
- Ma, G.; Cheng, M. Preparation and properties of Li4Ti5O12/C composites. Integr. Ferroelectr. 2019, 548, 42–49. [Google Scholar] [CrossRef]
- Purwanto, A.; Muzayanha, S.U.; Yudha, C.S.; Widiyandari, H.; Jumari, A.; Dyartanti, E.R.; Nizam, M.; Putra, M.I. High performance of salt-modified–LTO anode in LiFePO4 battery. Appl. Sci. 2020, 10, 7135. [Google Scholar] [CrossRef]
- Bai, X.; Li, T.; Bai, Y.-J. Capacity degradation of Li4Ti5O12 during long-term cycling in terms of composition and structure. Dalton Trans. 2020, 49, 10003–10010. [Google Scholar] [CrossRef]
- Allen, J.L.; Jow, T.R.; Wolfenstine, J. Low temperature performance of nanophase Li4Ti5O12. J. Power Sources 2006, 159, 1340–1345. [Google Scholar] [CrossRef]
- Yao, X.L.; Xie, S.; Nian, H.Q.; Chen, C.H. Spinel Li4Ti5O12 as a reversible anode material down to 0 V. J. Alloys Compd. 2008, 465, 375–379. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Peng, W.; Guo, H.; Li, X. An improved solid-state reaction to synthesize Zr-doped Li4Ti5O12 anode material and its application in LiMn2O4/Li4Ti5O12 full-cell. Ceram. Int. 2014, 40, 10053–10059. [Google Scholar] [CrossRef]
- Zaghib, K.; Simoneau, M.; Armand, M.; Gauthier, M. Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries. J. Power Sources 1999, 81–82, 300–305. [Google Scholar] [CrossRef]
- Berbenni, V.; Milanese, C.; Bruni, G.; Marini, A. Mechano-thermally activated solid-state synthesis of Li4Ti5O12 spinel from Li2CO3-TiO2 mixtures. Z. Naturforsch. B Chem. Sci. 2010, 65, 23–26. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wang, Q.; Xie, X.; Lou, Y.; Xia, B. The effects of Li2CO3 particle size on the properties of lithium titanate as anode material for lithium-ion batteries. Ionics 2014, 20, 1553–1560. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Dai, X.; Yan, X.; Huang, B.; Li, J. Electrochemical performance of ZnO-coated Li4Ti5O12 composite electrodes for lithium-ion batteries with the voltage ranging from 3 to 0.01 V. R. Soc. Open Sci. 2018, 5, 180762. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wen, Z.Y.; Zhu, X.J.; Gu, Z.H. Preparation and electrochemical performance of Ag doped Li4Ti5O12. Electrochem. Commun. 2004, 6, 1093–1097. [Google Scholar] [CrossRef]
- Zhou, T.P.; Feng, X.Y.; Guo, X.; Wu, W.W.; Cheng, S.; Xiang, H.F. Solid-state synthesis and electrochemical performance of Ce-doped Li4Ti5O12 anode materials for lithium-ion batteries. Electrochim. Acta 2015, 174, 369–375. [Google Scholar] [CrossRef]
- Becker, D.; Haberkorn, R.; Kickelbick, G. Mechanochemical induced structure transformations in lithium titanates: A detailed PXRD and 6Li MAS NMR study. Inorganics 2018, 6, 117. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, D.; Lin, Y.; Wu, X.; Yan, P.; Zhang, C.; He, D. Enhancing the high-rate performance of Li4Ti5O12 anode material for lithium-ion battery by a wet ball milling assisted solid-state reaction and ultra-high speed nano-pulverization. J. Power Sources 2014, 266, 60–65. [Google Scholar] [CrossRef]
- Natalia, V.; Gustami, A.P.; Rahmawati, F.; Lestari, W.W.; Purwanto, A. Lithium titanate (LTO) synthesis through solid state reaction and its performance for LiFePO4/LTO battery. J. Math. Fund. Sci. 2018, 50, 290–302. [Google Scholar] [CrossRef]
- Xiao, H.; Huang, X.; Ren, Y.; Ding, X.; Zhou, S. Fabrication of Li4Ti5O12@CN composite with enhanced rate properties. Front. Chem. 2019, 7, 432. [Google Scholar] [CrossRef]
- Krajewski, M.; Michalska, M.; Hamankiewicz, B.; Ziolkowska, D.; Korona, K.P.; Jasinski, J.B.; Maria Kaminska, M.; Lipinska, L.; Czerwinski, A. Li4Ti5O12 modified with Ag nanoparticles as an advanced anode material in lithium-ion batteries. J. Power Sources 2014, 245, 764–771. [Google Scholar] [CrossRef]
- Kim, S.; Alauzun, J.G.; Louvain, N.; Brun, N.; Stievano, L.; Boury, B.; Monconduit, L.; Mutin, P.H. Alginic acid aquagel as a template and carbon source in the synthesis of Li4Ti5O12/C nanocomposites for application as anodes in Li-ion batteries. RSC Adv. 2018, 8, 32558–32564. [Google Scholar] [CrossRef]
- Yi, T.-F.; Xie, Y.; Zhu, Y.-R.; Zhu, R.-S.; Shen, H. Structural and thermodynamical stability of Li4Ti5O12 anode material for lithium-ion battery. J. Power Sources 2013, 222, 448–454. [Google Scholar] [CrossRef]
- Qiao, Y.; Hu, X.; Liu, Y.; Huang, Y. Li4Ti5O12 nanocrystallites for high-rate lithium-ion batteries synthesized by a rapid microwave-assisted solid-state process. Electrochim. Acta 2012, 63, 118–123. [Google Scholar] [CrossRef]
- Shi, L.; Hu, X.; Huang, Y. Fast microwave-assisted synthesis of Nb-doped Li4Ti5O12for high-rate lithium-ion batteries. J. Nanopart. Res. 2014, 16, 2332. [Google Scholar] [CrossRef]
- Ohtake, T. Single phase Li4Ti5O12 synthesis for nanoparticles by two steps sintering. J. Mater. Sci. Chem. Eng. 2015, 3, 5–10. [Google Scholar]
- Bach, S.; Pereira-Ramos, J.P.; Baffier, N. Electrochemical behaviour of a lithium titanium spinel compound synthesized via a sol-gel process. J. Mater. Chem. 1998, 8, 251–253. [Google Scholar] [CrossRef]
- Venkateswarlu, M.; Chen, C.H.; Do, J.S.; Lin, C.W.; Chou, T.C.; Hwang, B.J. Electrochemical properties of nano-sized Li4Ti5O12 powders synthesized by a sol–gel process and characterized by X-ray absorption spectroscopy. J. Power Sources 2005, 146, 204–208. [Google Scholar] [CrossRef]
- Rho, Y.H.; Kanamura, K. Li+ ion diffusion in Li4Ti5O12 thin film electrode prepared by PVP sol-gel method. J. Solis State Chem. 2004, 177, 2094–2100. [Google Scholar] [CrossRef]
- Rho, Y.H.; Kanamura, K. Preparation of Li4/3Ti5/3O4 thin film electrodes by a PVP sol-gel coating method and their electrochemical properties. J. Electrochem. Soc. 2004, 151, A106–A110. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, C.; Wan, C. Synthesis and characterization of spherical La-doped nanocrystalline Li4/3Ti5/3O4/C compound for Lithium-ion batteries. J. Electrochem. Soc. 2010, 157, K39–K42. [Google Scholar] [CrossRef]
- Wang, G.J.; Gao, J.; Fu, L.J.; Zhao, N.H.; Wu, Y.P.; Takamura, T. Preparation and characteristic of carbon-coated Li4/3Ti5/3O4 anode material. J. Power Sources 2007, 174, 1109–1112. [Google Scholar] [CrossRef]
- Liu, D.Q.; Lai, Q.Y.; Hao, Y.J. Study on synthesis and mechanism of Li4/3Ti5/3O4 by sol-gel method. Chin. J. Inorg. Chem. 2004, 20, 829–832. [Google Scholar]
- Xiang, H.F.; Tian, B.B.; Lian, P.C.; Li, Z.; Wang, H. Sol-gel synthesis and electrochemical performance of Li4Ti5O12/graphene composite anode for lithium-ion batteries. J. Alloys Compd. 2011, 509, 7205–7209. [Google Scholar] [CrossRef]
- Fawwaz, T.A.; Retna, D.P.; Riesma, T.; Ade, U.H.; Sri, R.; Damish; Hanif, Y.; Oka, P.A.; Nendar, H.; Yelvia, D.; et al. Synthesis of lithium titanium oxide (Li4Ti5O12) through sol-gel- method and the effect of graphene addition in lithium-ion battery anodes. Defect Diff. Forum 2022, 417, 227–240. [Google Scholar]
- Zhang, C.; Zhang, Y.; Wang, J.; Wang, D.; He, D.; Xia, Y. Li4Ti5O12 prepared by a modified citric acid sol-gel method for lithium-ion battery. J. Power Sources 2013, 236, 118–125. [Google Scholar] [CrossRef]
- Supriadi, C.P.; Syahrial, A.Z.; Subhan, A. The effect of sol-gel derived TiO2 crystallite size to Li4Ti5O12 anode performance in lithium-ion battery. Ionics 2020, 26, 5907–5914. [Google Scholar] [CrossRef]
- Livage, J. Vanadium pentoxide gels. Chem. Mater. 1991, 3, 578–593. [Google Scholar] [CrossRef]
- Julien, C.; El-Farh, L.; Rangan, S.; Massot, M. Studies of LiNi1–yCoyO2 cathode materials prepared by a citric acid-assisted sol-gel method for lithium batteries. J. Sol-Gel Sci. Technol. 1999, 15, 63–72. [Google Scholar] [CrossRef]
- Luo, G.; He, J.; Song, X.; Huang, X.; Yu, X.; Fang, Y.; Chen, D. Bamboo carbon assisted sol-gel synthesis of Li4Ti5O12 anode material with enhanced electrochemical activity for lithium ion battery. J. Alloys Compd. 2015, 621, 268–768. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Lai, Q.-Y.; Liu, D.-Q.; Xu, Z.-U.; Ji, X.-Y. Synthesis by citric acid sol-gel method and electrochemical properties of Li4Ti5O12 anode material for lithium-ion battery. Mater. Chem. Phys. 2005, 94, 382–387. [Google Scholar] [CrossRef]
- Yang, G.; Su, Z.; Fang, H.; Yao, Y.; Li, Y.; Yang, B.; Ma, W. Synthesis and performance of Li4Ti5O12/C with little inert carbon. Electrochim. Acta 2013, 93, 158–162. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lin, J.-Y. One-pot sol-gel synthesis of Li4Ti5O12/C anode materials for high-performance Li-ion batteries. Electrochim. Acta 2014, 142, 43–50. [Google Scholar] [CrossRef]
- Kurajica, S. A brief review on the use of chelation agents in sol-gel Synthesis with emphasis on β-diketones and β-ketoesters. Chem. Biochem. Eng. Q. 2019, 33, 295–301. [Google Scholar] [CrossRef]
- Kirillov, S.A.; Romanova, I.V.; Lisnycha, T.V.; Potapenko, A.V. High-rate electrochemical performance of Li4Ti5O12 obtained from TiCl4 by means of a citric acid aided route. Electrochim. Acta 2018, 286, 163–171. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.-M.; Yang, H.; Shen, X. Characterization and electrochemical properties of carbon-coated Li4Ti5O12 prepared by a citric acid sol–gel method. J. Alloys Compd. 2011, 509, 712–718. [Google Scholar] [CrossRef]
- Purwamargapratala, Y.; Sujatno, A.; Sabayu, Y.L.; Kartini, E. Synthesis of Li4Ti5O12 (LTO) by sol-gel method for lithium ion battery anode. IOP Conf. Ser. Mater. Sci. Eng. 2019, 553, 012062. [Google Scholar] [CrossRef]
- Stenina, I.A.; Il’in, A.B.; Yaroslavtsev, A.B. Synthesis and ionic conductivity of Li4Ti5O12. Inorg. Mater. 2015, 51, 62–67. [Google Scholar] [CrossRef]
- Mahmoud, A.; Amarilla, J.M.; Saadoune, I. Effect of thermal treatment used in the sol-gel synthesis method of Li4Ti5O12 spinel on its electrochemical properties as anode for lithium ion batteries. Electrochim. Acta 2015, 163, 213–222. [Google Scholar] [CrossRef]
- Zhou, W.; Shao, Z.; Jin, W. Synthesis of nanocrystalline conducting composite oxides based on a non-ion selective combined complexing process for functional applications. J. Alloys Compd. 2006, 426, 368–374. [Google Scholar] [CrossRef]
- Rho, Y.H.; Kanamura, K. Preparation of Li4Ti5O12 thin film electrode with PVP sol-gel for a rechargeable lithium microbattery. J. Surf. Sci. Soc. Jpn. 2003, 24, 423–428. [Google Scholar] [CrossRef]
- Fang, W.; Cheng, X.; Zuo, P.; Ma, Y.; Liao, L.; Yin, G. Hydrothermal-assisted sol-gel synthesis of Li4Ti5O12/C nano-composite for high-energy lithium-ion batteries. Solid State Ion. 2013, 244, 52–56. [Google Scholar] [CrossRef]
- Chang, L.-J.; Luo, D.-H.; Zhang, H.-L.; Qi, X.-W.; Wang, Z.-Y.; Liu, Y.-G.; Zhai, Y.-C. Synthesis and performance of Li4Ti5O12 anode materials using the PVP-assisted combustion method. Chin. Chem. Lett. 2014, 25, 1569–1572. [Google Scholar] [CrossRef]
- Lee, J.-M.; Jun, Y.-D.; Kim, D.-W.; Lee, Y.-H.; Oh, S.-G. Effects of PVP on the formation of silver–polystyrene heterogeneous nanocomposite particles in novel preparation route involving polyol process: Molecular weight and concentration of PVP. Mater. Chem. Phys. 2009, 114, 549–555. [Google Scholar] [CrossRef]
- Ma, G.; Cheng, M. Preparation and performance of Li4Ti5O12 by sol-gel method. Integr. Ferroelectr. 2020, 209, 119–124. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, B.; Wei, Y.; Tao, B.; Zhao, Y. Simple sol-gel method synthesis of 3-dimension Li4Ti5O12-TiO2 nanostructures using butterfly wings as biotemplates for high-rate performance lithium-ion batteries. J. Alloys Compd. 2017, 705, 58–63. [Google Scholar] [CrossRef]
- Qiu, C.; Yuan, Z.; Liu, L.; Ye, N.; Liu, J. Sol-gel preparation and electrochemical properties of La-doped anode material for lithium-ion batteries. J. Solid State Electrochem. 2013, 17, 841–847. [Google Scholar] [CrossRef]
- Kavan, L.; Grätzel, M. Facile synthesis of nanocrystalline Li4Ti5O12 (spinel) exhibiting fast Li insertion. Electrochem. Solid State Lett. 2002, 5, A39–A42. [Google Scholar] [CrossRef]
- Pershina, S.V.; Antonov, B.D.; Farlenkov, A.S. Optimization of technology for synthesis of Li4Ti5O12 anode materials for lithium-ion batteries. Russ. J. Appl. Chem. 2021, 94, 30–37. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Yang, H. Synthesis and electrochemical properties of highly dispersed Li4Ti5O12 nanocrystalline for lithium secondary batteries. Trans. Nonferrous Met. Soc. China 2012, 22, 613–620. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Z.; Yang, T.; Liao, C.; Wang, Z.; Sun, K. Facile preparation of nanocrystalline Li4Ti5O12 and its high electrochemical performance as anode material for lithium-ion batteries. Electrochem. Commun. 2011, 13, 654–656. [Google Scholar] [CrossRef]
- Morsy, S.M.I. Role of surfactants in nanotechnology and their applications. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 237–260. [Google Scholar]
- Dokan, F.K.; Sahan, H.; Ozdemir, B.; Ozdemir, N.; Patat, S. Synthesis and characterization of spinel Li4Ti5O12 anode material by CTAB assisted sol-gel method. Acta Phys. Pol. A 2014, 125, 648–649. [Google Scholar] [CrossRef]
- Khomane, R.B.; Prakash, A.S.; Ramesha, K.; Sathiya, M. CTAB-assisted sol-gel synthesis of Li4Ti5O12 and its performance as anode material for Li- ion batteries. Mater. Res. Bull. 2011, 46, 1139–1142. [Google Scholar] [CrossRef]
- Chen, J.; Yang, S.; Fang, S.; Hirani, S.; Tachibana, K. Synthesis of hierarchical mesoporous nest-like Li4Ti5O12 for high-rate lithium ion batteries. J. Power Sources 2012, 200, 59–66. [Google Scholar] [CrossRef]
- Li, D.; Zhao, W.; Cao, L.; Gao, Y.; Liu, Y.; Wang, W.; Qi, T. Mixed-surfactant-assisted synthesis of dual-phase Li4Ti5O12-TiO2 hierarchical microspheres as high-performance anode materials for Li-ion batteries. ChemSusChem 2019, 12, 4412–4420. [Google Scholar] [CrossRef] [PubMed]
- Akintola, T.; Shellikeri, A.; Akintola, T.; Zheng, J.P. The influence of Li4Ti5O12 preparation method on lithium-ion capacitor performance. Batteries 2021, 7, 33. [Google Scholar] [CrossRef]
- Meyer, F.; Hempelmann, R.; Mathur, S.; Veith, M. Microemulsion mediated sol-gel synthesis of nano-scaled MAl2O4 (M=Co, Ni, Cu) spinels from single-source heterobimetallic alkoxide precursors. J. Mater. Chem. 1999, 9, 1755–1763. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kanamori, K.; Kiyomura, T.; Kurata, H.; Nakanishi, K.; Abe, T. Hierarchically porous Li4Ti5O12 anode materials for Li- and Na-ion batteries: Effects of nanoarchitectural design and temperature dependence of the rate capability. Adv. Energy Mater. 2015, 5, 1400730. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Li, M.; Wang, X.; Bai, S.; Shi, Y.; Tian, J.; Shan, Z.; Meng, M.; Liu, P.; et al. Nanosheet-assembled hierarchical Li4Ti5O12 microspheres for high-volumetric-density and high-rate Li-ion battery anode. Energy Storage Mater. 2019, 21, 361–371. [Google Scholar] [CrossRef]
- Erdas, A.; Ozcan, S.; Guler, M.O.; Akbulut, H. Sol-gel synthesis of nanocomposite Cu–Li4Ti5O12 structures for ultrahigh rate Li-ion batteries. Acta Phys. Pol. A 2015, 127, 1026–1028. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Wang, X.; Zhang, Y.; Ding, J.; Guo, Y. Synthesis of Li4Ti5O12/V2O5 nanocomposites for lithium-ion batteries by one-pot co-precipitation method. J. Mater. Sci. Mater. Electron. 2021, 32, 12134–12138. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Lin, Y.; Xiong, D.-B.; Wang, D.; Wu, X.; He, D. Influence of Sc3+ doping in B-site on electrochemical performance of Li4Ti5O12 anode materials for lithium-ion battery. J. Power Sources 2014, 250, 50–57. [Google Scholar] [CrossRef]
- Wei, G.; Rambo, C.R.; Guo, Y.; Ning, Z.; Guo, S.; Zhao, M.; Huang, Z.; Zhang, C.; He, D. Graphene coated La3+/Sc3+ co-doped Li4Ti5O12 anodes for enhanced Li-ion battery performance. Mater. Lett. 2017, 193, 179–182. [Google Scholar] [CrossRef]
- Alias, N.; Kufian, M.; Teo, L.; Majid, S.; Arof, A. Synthesis and characterization of Li4Ti5O12. J. Alloys Compd. 2009, 486, 645–648. [Google Scholar] [CrossRef]
- Llaín-Jiménez, H.A.; Buchberger, D.A.; Winkowska-Struzik, M.; Ratyński, M.; Krajewski, M.; Boczar, M.; Hamankiewicz, B.; Czerwiński, A. Correlation between lithium titanium oxide powder morphology and high rate performance in lithium-ion batteries. Batteries 2022, 8, 168. [Google Scholar] [CrossRef]
- Rajoba, S.J.; Shirsat, A.N.; Tyagi, D.; Jadhav, L.D.; Kalubarme, R.S.; Wani, B.N.; Varma, S. Sol-gel assisted spinel Li4Ti5O12 and its performance and stability as anode for long life Li-ion battery. Mater. Lett. 2021, 285, 129134. [Google Scholar] [CrossRef]
- Najihah, A.I.; Priyono, S.; Imam Supardi, Z.A.; Subhan, A.; Prihandoko, B. Synthesized of Li4Ti5O12 materials via sol-gel method to lithium ion battery anodes. IOP Conf. Ser. J. Phys. 2020, 1491, 012038. [Google Scholar] [CrossRef]
- Priyono, S.; Nuroniah, I.; Subhan, A.; Sanjaya, E.; Prihandoko, B. Synthesis and characterization of Li4Ti5O12 with sol gel method as a lithium-ion battery anode material. J. Sains Mater. Indones. 2019, 20, 67–74. [Google Scholar] [CrossRef]
- Chang, C.-M.; Chen, Y.-C.; Ma, W.-L.; Wang, P.-H.; Lee, C.-F.; Chen, H.-S.; Chen-Yang, Y.W. Sol-gel synthesis of low carbon content and low surface area Li4Ti5O12/carbon black composite as high-rate anode materials for lithium ion battery. RSC Adv. 2015, 5, 74381–74390. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, C.; Luo, H.; Zhang, X.; Xu, K.; Zhang, F. In situ growth of Li4Ti5O12 on multi-walled carbon nanotubes: Novel coaxial nanocables for high rate lithium ion batteries. J. Mater. Chem. 2011, 21, 761–767. [Google Scholar] [CrossRef]
- Zhong, Z. Synthesis of Mo4+ substituted spinel Li4Ti5–xMoxO12. Electrochem. Solid-State Lett. 2007, 10, A267–A269. [Google Scholar] [CrossRef]
- Zhang, A.; Zheng, Z.-M.; Cheng, F.-Y.; Liang, Z.; Chen, J. Preparation of Li4Ti5O12 submicrospheres and their application as anode materials of rechargeable lithium-ion batteries. Sci. China Chem. 2011, 54, 936–940. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Li, Y.-J.; Xu, C.; Kong, L.; Li, L. Synthesis and characterization of Li4Ti5O12 via a hydrolysis process from TiCl4 aqueous solution. Rare Met. 2014, 33, 459–465. [Google Scholar] [CrossRef]
- Li, D.; Shen, G.; Zhao, W.; Gao, Y.; Hui, Z.; Liu, Y.; Yi, L.; Wang, W.; Cao, L.; Liu, Y.; et al. Synthesis of Li4Ti5O12 with theoretical capacity in Li2CO3-ammonia ball milling system. Mater. Res. Bull. 2019, 114, 177–183. [Google Scholar] [CrossRef]
- Wang, R.; Cao, X.; Zhao, D.; Zhu, L.; Xie, L.; Liu, J.; Liu, Y. Wet-chemistry synthesis of Li4Ti5O12 as anode materials rendering high-rate Li-ion storage. Int. J. Energy Res. 2020, 44, 4211–4223. [Google Scholar] [CrossRef]
- Xu, C.; Xue, L.; Zhang, W.; Fan, X.; Yan, Y.; Li, Q.; Huang, Y.; Zhang, W. Hydrothermal synthesis of Li4Ti5O12/TiO2 nanocomposite as high performance anode material for Li-ion batteries. Electrochim. Acta 2014, 147, 506–512. [Google Scholar] [CrossRef]
- Xie, L.-L.; Xu, Y.-D.; Zhang, J.J.; Cao, X.-Y.; Wang, B.; Yan, X.-Y.; Qu, L.-B. Facile Synthesis and characterization of Li4Ti5O12 as anode material for lithium ion batteries. Int. J. Electrochem. Sci. 2013, 8, 1701–1712. [Google Scholar] [CrossRef]
- Shin, J.W.; Hong, C.H.; Yoon, D.H. Effects of TiO2 starting materials on the solid-state formation of Li4Ti5O12. J. Am. Ceram. Soc. 2012, 95, 1894–1900. [Google Scholar] [CrossRef]
- Hong, C.H.; Noviyanto, A.; Ryu, J.H.; Kim, J.; Yoon, D.H. Effects of the starting materials and mechanochemical activation on the properties of solid-state reacted Li4Ti5O12 for lithium ion batteries. Ceram. Int. 2012, 38, 301–310. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Zhang, X.; Wang, J.; Nie, P.; Che, Q.; Ding, B. Nitrogen-doped carbon coated Li4Ti5O12 nanocomposite: Superior anode materials for rechargeable lithium ion batteries. J. Power Sources 2013, 221, 122–127. [Google Scholar] [CrossRef]
- Veljković, I.; Poleti, D.; Karanović, L.; Zdujić, M.; Branković, G. Solid state synthesis of extra phase-pure Li4Ti5O12 spinel. Sci. Sinter. 2011, 4, 343–351. [Google Scholar] [CrossRef]
- Guerfi, A.; Yuichi, A.; Charest, P.; Mamoru, S.; Zaghib, K. Li4Ti5O12 material prepared by mechanochemical activation: Structure and electrochemical performance. ECS Meet. Abstr. 2006, MA2006-02, 206. [Google Scholar] [CrossRef]
- Priyono, B.; Winowatan, P.W.; Syahrial, A.Z.; Faizah; Subhan, A. Optimizing the performance of Li4Ti5O12/LTO by addition of silicon nanoparticles in half cell lithium_ion battery anode. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012121. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wang, Q.; Xie, X.; Lou, Y.; Han, X.; Xia, B. Microsized TiO2 activated by high-energy ball milling as starting material for the preparation of Li4Ti5O12 anode material. Powder Technol. 2013, 247, 204–210. [Google Scholar] [CrossRef]
- Jia, P.; Shao, Z.; Liu, K. Synthesis and electrochemical performance of Li4Ti5O12 by high temperature ball milling method. Mater. Lett. 2014, 125, 218–220. [Google Scholar] [CrossRef]
- Jia, P.; Shao, Z.; Liu, K. Pretreatments-assisted high temperature ball milling route to Li4Ti5O12 and its electrochemical performance. Mater. Lett. 2014, 130, 71–74. [Google Scholar] [CrossRef]
- Michalska, M.; Krajewski, M.; Ziolkowska, D.; Hamankiewicz, B.; Andrzejczuk, M.; Lipinska, L.; Korona, K.P.; Czerwinski, A. Influence of milling time in solid-state synthesis on structure, morphology and electrochemical properties of Li4Ti5O12 of spinel structure. Powder Technol. 2014, 266, 372–377. [Google Scholar] [CrossRef]
- Yan, G.; Fang, H.; Zhao, H.; Li, G.; Yang, Y.; Li, L. Ball milling-assisted sol–gel route to Li4Ti5O12 and its electrochemical properties. J. Alloys Compd. 2009, 470, 544–547. [Google Scholar] [CrossRef]
- HKim, H.J.; Oh, M.H.; Son, W.K.; Kim, T.I.; Park, S.G. Novel synthesis method and electrochemical characteristics of lithium titanium oxide as anode material for high power device. In Proceedings of the 2006 IEEE 8th International Conference on Properties & Applications of Dielectric Materials, Bali, Indonesia, 26–30 June 2006; pp. 464–467. [Google Scholar]
- Wang, D.; Zhang, C.; Zhang, Y.; Wang, J.; He, D. Synthesis and electrochemical properties of La-doped Li4Ti5O12 as anode material for Li-ion battery. Ceram. Int. 2013, 39, 5145–5149. [Google Scholar] [CrossRef]
- Jang, I.-S.; Kang, S.-H.; Kang, Y.C.; Roh, K.C.; Chun, J. Facile synthesis of surface fluorinated-Li4Ti5O12/carbon nanotube nanocomposites for a high-rate capability anode of lithium-ion batteries. Appl. Surf. Sci. 2022, 605, 154710. [Google Scholar] [CrossRef]
- Ye, Z.; Zhong, F.; Chen, Y.; Zou, Z.; Jiang, C. Unique CNTs-chained Li4Ti5O12 nanoparticles as excellent high rate anode materials for Li-ion capacitors. Ceram. Int. 2022, 48, 20237–20244. [Google Scholar] [CrossRef]
- Chauque, S.; Oliva, F.Y.; Visintin, A.; Barraco, D.; Leiva, E.P.M.; Cámara, O.R. Lithium titanate as anode material for lithium ion batteries: Synthesis, post-treatment and its electrochemical response. J. Electroanal. Chem. 2017, 799, 142–155. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, D.; Yu, J.; Zhang, L.; Huang, X.; Xu, D.; Yu, X. Synthesis and electrochemical performance of cubic Co-doped Li4Ti5O12 anode material for high-performance lithium-ion batteries. J. Electroanal. Chem. 2016, 776, 188–192. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, H.J.; Zhang, J.J.; Xiong, H.M.; Xia, Y.Y. Nanosized Li4Ti5O12 prepared by molten salt method as an electrode material for hybrid electrochemical supercapacitors. J. Electrochem. Soc. 2006, 153, A1472–A1477. [Google Scholar] [CrossRef]
- Nithya, V.D.; Sharmila, S.; Vediappan, K.; Lee, C.W.; Vasylechko, L.; Kalai Selvan, R. Electrical and electrochemical properties of molten-saltsynthesized 0.05 mol Zr- and Si-doped Li4Ti5O12 microcrystals. J. Appl. Electrochem. 2014, 44, 647–654. [Google Scholar] [CrossRef]
- Nithya, V.D.; Kalai Selvan, R.; Kumaran, V.; Sharmila, S.; Lee, C.W. Molten salt synthesis and characterization of Li4Ti5−xMnxO12 (x = 0.0, 0.05 and 0.1) as anodes for Li-ion batteries. Appl. Surf. Sci. 2012, 261, 515–519. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Q.; Chen, G.; Shen, M.; Li, B. Molten salt synthesis of different ionic radii metallic compounds doped lithium titanate used in Li-ion battery anodes. Mater. Trans. 2017, 58, 383–389. [Google Scholar] [CrossRef]
- Rahman, M.; Wang, J.Z.; Hassan, M.F.; Wexler, D.; Liu, H.K. Amorphous carbon coated high grain boundary density dual phase Li4Ti5O12-TiO2: A nanocomposite anode material for Li-ion batteries. Adv. Energy. Mater. 2011, 1, 212–220. [Google Scholar] [CrossRef]
- Sharmila, S.; Senthilkumar, B.; Nithya, V.D.; Vediappan, K.; Lee, C.W.; Kalai Selvan, R. Electrical and electrochemical properties of molten salt-synthesized Li4Ti5–xO12SnxO12 (x = 0.0, 0.05 and 0.1) as anodes for Li-ion batteries. J. Phys. Chem. Solids 2013, 74, 1515–1521. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wang, J.-Z.; Hassan, M.F.; Chou, S.; Wexler, D.; Liu, H.-K. Basic molten salt process—A new route for synthesis of nanocrystalline Li4Ti5O12–TiO2 anode material for Li-ion batteries using eutectic mixture of LiNO3–LiOH–Li2O2. J. Power Sources 2010, 195, 4297–4303. [Google Scholar] [CrossRef]
- Julien, C.; Camacho-Lopez, M.A.; Lemal, M.; Mohan, T.; Chitra, S.; Kalyani, P.; Gopukumar, S. Combustion synthesis and properties of substituted lithium cobalt oxides in lithium batteries. Solid State Ion. 2000, 135, 241–248. [Google Scholar] [CrossRef]
- Sha, Y.; Yuan, T.; Zhao, B.; Cai, R.; Wang, H.; Shao, Z. Solid lithium electrolyte-Li4Ti5O12 composites as anodes of lithium-ion batteries showing high-rate performance. J. Power Sources 2013, 231, 177–185. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Cui, W.; Xiao, Q.; Zhao, J. Fast solution-combustion synthesis of nitrogen-modified Li4Ti5O12 nanomaterials with Improved electrochemical performance. ACS Appl. Mater. Interfaces 2014, 6, 7895–7901. [Google Scholar] [CrossRef]
- Shi, W.D.; Song, S.Y.; Zhang, H.J. Hydrothermal synthesis strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef]
- Li, B.; Han, C.; He, Y.-B.; Yang, C.; Du, H.; Yang, Q.-H.; Kang, F. Facile synthesis of Li4Ti5O12/C composite with super rate performance. Energy Environ. Sci. 2012, 5, 9595–9602. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Hon, M.-H.; Kuan, C.-Y.; Leu, I.-C. Hydrothermal synthesis of nanosheets as anode materials for lithium ion batteries. RSC Adv. 2015, 5, 35224–35229. [Google Scholar] [CrossRef]
- Li, N.; Mei, T.; Zhu, Y.C.; Wang, L.; Liang, J.; Zhang, X.; Qian, Y.; Tang, K. Hydrothermal synthesis of layered Li1.81H0.19Ti2O5·xH2O nanosheets and their transformation to single-crystalline Li4Ti5O12 nanosheets as the anode materials for Li-ion batteries. CrystEngComm 2012, 14, 6435–6440. [Google Scholar] [CrossRef]
- Fattakhova, D.; Krtil, P. Electrochemical activity of hydrothermally synthesized Li-Ti-O cubic oxides toward Li insertion. J. Electrochem. Soc. 2002, 149, A1224–A1229. [Google Scholar] [CrossRef]
- Wu, S.C.; Guo, Y.X.; Zhou, J.H.; Zhao, J.; Ding, X.; Hu, J. Effect of heat-treatment temperature on the structure and properties of Li4Ti5O12 nanorods prepared by the hydrothermal ion exchange method. J. Inorg. Mater. 2011, 26, 123–128. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, L.; Huang, J.; Zhou, S.; Huang, Y.; Cai, Y. Hydrothermal synthesis of Zn-doped Li4Ti5O12 with improved high rate properties for lithium ion batteries. Ceram. Int. 2013, 39, 6139–6143. [Google Scholar] [CrossRef]
- Kim, K.-T.; Kim, S.-J.; Myung, S.-T. Fast Li+ transfer achieved by carbon coating on Li4Ti5O12 nanobelts. ECS Meet. Abstr. 2013, MA2013-02, 393. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, J.; Abruna, H.D.; Liu, H.-K.; Li, H. Rapid synthesis of Li4Ti5O12/graphene composite with superior rate capability by a microwave-assisted hydrothermal method. Nano Energy 2014, 8, 297–304. [Google Scholar] [CrossRef]
- Ding, M.; Liu, H.; Zhu, J.; Zhao, X.; Pang, L.; Qin, Y.; Deng, L. Constructing of hierarchical yolk-shell structure Li4Ti5O12-SnO2 composites for high rate lithium ion batteries. Appl. Surf. Sci. 2018, 448, 389–399. [Google Scholar] [CrossRef]
- He, Y.; Muhetaer, A.; Li, J.; Wang, F.; Liu, C.; Li, Q.; Xu, D. Ultrathin Li4Ti5O12 nanosheet based hierarchical microspheres for high-rate and long-life Li-ion batteries. Adv. Energy Mater. 2017, 7, 1700950. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, L.; Huang, J.; Wang, D.; Wu, J.; Cai, Y. Hydrothermal synthesis of Li4Ti5O12 microsphere with high capacity as anode material for lithium ion batteries. Ceram. Int. 2013, 39, 2695–2698. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Li, J.; Yang, Q. Hydrothermal synthesis and electrochemical performances of Ag-coated nanoflake-like Li4Ti5O12 as an anode material for lithium-ion batteries. Int. J. Electrochem. Sci. 2021, 16, 21035. [Google Scholar] [CrossRef]
- Prasad, G.V.; Reddy, T.M.; Narayana, A.L.; Hussain, O.M.; Gopal, T.V.; Shaikshavali, P. Construction of the embedded Li4Ti5O12-MWCNTs nanocomposite electrode for diverse applications in electrochemical sensing and rechargeable battery. J. Inorg. Organomet. Polymers Mater. 2023, 33, 1261–1279. [Google Scholar] [CrossRef]
- Wang, M.; He, Y.; Hong, W.; Zhang, S.Y.; Yang, C.X.; Yan, C. Three-dimensional network microstructure design of the Li4Ti5O12/rGO nanocomposite as an anode material for high-performance lithium-ion batteries. J. Phys. Chem. C 2023, 127, 10025–10037. [Google Scholar] [CrossRef]
- Fang, W.; Dong, E.; Zhang, Y.; Yang, L.; Zhang, L.; Zhang, H.; Wang, Y.; Che, G.; Yin, G. Self-assembled Li4Ti5O12/rGO nanocomposite anode for high power lithium-ion batteries. Inorg. Chem. Commun. 2022, 144, 109753. [Google Scholar] [CrossRef]
- Sha, Y.; Xu, X.; Li, L.; Cai, R.; Shao, Z. Hierarchical carbon-coated acanthosphere-like Li4Ti5O12 microspheres for high-power lithium-ion batteries. J. Power Sources 2016, 314, 18–27. [Google Scholar] [CrossRef]
- Wang, M.; Fang, P.G.; Chen, Y.; Leng, X.Y.; Yan, Y.; Yang, S.B.; Xu, P.; Yan, C. Synthesis of highly stable LTO/rGO/SnO2 nanocomposite via in situ electrostatic self-assembly for high-performance lithium-ion batteries. Adv. Func. Mater. 2023, 33, 2213902. [Google Scholar] [CrossRef]
- Zhu, M.; Deng, X.; Li, W.; He, M.; Xiong, D.; Feng, Z. Porous layered SnO2-LTO@C as a high-performance anode material for lithium-ion batteries. Ionics 2022, 28, 3699–3708. [Google Scholar] [CrossRef]
- Fang, W.; Ma, Y.; Zuo, P.; Cheng, X.; Yin, G. Nano-Li4Ti5O12 pore microspheres: A high power electrode material for lithium ion batteries. Int. J. Electrochem. Sci. 2013, 8, 1949–1956. [Google Scholar] [CrossRef]
- Kim, S.; Fang, S.H.; Zhang, Z.X.; Chen, J.Z.; Yang, L.; Penner-Hahn, J.E.; Deb, A. The electrochemical and local structural analysis of the mesoporous Li4Ti5O12 anode. J. Power Sources 2014, 268, 294–300. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Wang, Z.; Guo, H. Petal-like Li4Ti5O12–TiO2 nanosheets as high-performance anode materials for Li-ion batteries. Nanoscale 2013, 5, 6936–6943. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, Y.; Zhang, H.; Wang, X.; Li, G.; Wang, Y.; Jiao, L.; Yuan, H. Small amount of reduced graphene oxide modified Li4Ti5O12 nanoparticles for ultrafast high-power lithium ion battery. J. Power Sources 2015, 278, 693–702. [Google Scholar] [CrossRef]
- Yi, T.-F.; Yang, S.-Y.; Zhu, Y.-R.; Ye, M.-F.; Xie, Y.; Zhu, R.-S. Enhanced rate performance of Li4Ti5O12 anode material by ethanol-assisted hydrothermal synthesis for lithium-ion battery. Ceram. Int. 2014, 40, 9853–9858. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Guan, Y.; Jin, Y.; Zhu, W.; Guo, X.; Qiu, X. Nano-Li4Ti5O12 with high rate performance synthesized by a glycerol assisted hydrothermal method. J. Power Sources 2013, 243, 661–667. [Google Scholar] [CrossRef]
- Li, X.-P.; Mao, J. Sol-hydrothermal synthesis of Li4Ti5O12/rutile-TiO2 composite as high rate anode material for lithium ion batteries. Ceram. Int. 2014, 40, 13553–13558. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Huang, L.; Zhou, Z.; Wang, J.; Liu, H.; Wu, H. Hierarchical carambola-like Li4Ti5O12-TiO2 composites as advanced anode materials for lithium-ion batteries. Electrochim. Acta 2016, 195, 124–133. [Google Scholar] [CrossRef]
- Chou, S.-L.; Wang, J.-Z.; Liu, H.-K.; Dou, S.-X. Rapid synthesis of Li4Ti5O12 microspheres as anode materials and its binder effect for lithium-ion battery. J. Phys. Chem. C 2011, 115, 16220–16227. [Google Scholar] [CrossRef]
- Hui, Y.; Cao, L.; Xu, Z.; Huang, J.; Ouyang, H.; Li, J. Mesoporous Li4Ti5O12 nanoparticles synthesized by a microwave-assisted hydrothermal method for high rate lithium-ion batteries. J. Electroanal. Chem. 2016, 763, 45–50. [Google Scholar] [CrossRef]
- Cheng, J.; Che, R.; Liang, C.; Liu, J.; Wang, M.; Xu, J. Hierarchical hollow Li4Ti5O12 urchin-like microspheres with ultra-high specific surface area for high rate lithium ion batteries. Nano Res. 2014, 7, 1043–1053. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.; Xu, S.; Lan, L.; Lu, L.; Li, S. Synthesis of spherical silver-coated Li4Ti5O12 anode material by a sol-gel-assisted hydrothermal method. Nanoscale Res. Lett. 2017, 12, 576. [Google Scholar] [CrossRef]
- Pryono, B.; Murti, P.B.; Syahrial, A.Z.; Subhan, A. Optimizing the performance of Li4Ti5O12 anode synthesized from TiO2 xerogel and LiOH with hydrothermal-ball mill method by using acetylene black. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2017; Volume 1826, p. 020005. [Google Scholar]
- Gao, L.; Wang, L.; Dai, S.; Cao, M.; Zhong, Z.; Shen, Y.; Wang, M. Li4Ti5O12-TiO2 nanowire arrays constructed with stacked nanocrystals for high-rate lithium and sodium ion batteries. J. Power Sources 2017, 344, 223–232. [Google Scholar] [CrossRef]
- Ma, G.; Cheng, M. Hydrothermal method preparing lithium ion battery anode material Li4Ti5O12 using metatitanic acid. Ferroelectrics 2018, 536, 181–186. [Google Scholar] [CrossRef]
- Nugroho, A.; Kim, S.J.; Chung, K.Y.; Cho, B.-W.; Lee, Y.-W.; Kim, J. Facile synthesis of nanosized Li4Ti5O12 in supercritical water. Electrochem. Commun. 2011, 13, 650–653. [Google Scholar] [CrossRef]
- Nugroho, A.; Kim, S.J.; Chung, K.Y.; Kim, J. Synthesis of Li4Ti5O12 in supercritical water for Li-ion batteries: Reaction mechanism and high-rate performance. Electrochim. Acta 2012, 78, 623–632. [Google Scholar] [CrossRef]
- Hayashi, H.; Nakamura, T.; Ebina, T. Hydrothermal synthesis of Li4Ti5O12 nanoparticles using a supercritical flow reaction system. J. Ceram. Soc. Jpn 2014, 122, 78–82. [Google Scholar] [CrossRef]
- Nugroho, A.; Kim, S.J.; Chang, W.; Chung, K.Y. Facile synthesis of hierarchical mesoporous Li4Ti5O12 microspheres in supercritical methanol. J. Power Sources 2013, 244, 164–169. [Google Scholar] [CrossRef]
- Laumann, A.; Bremholm, M.; Hald, P.; Holzapfel, M.; Fehr, K.T.; Iversen, B.B. Rapid green continuous flow supercritical synthesis of high performance Li4Ti5O12 nanocrystals for Li ion battery applications. J. Electrochem. Soc. 2012, 159, A166–A171. [Google Scholar] [CrossRef]
- Mathew, V.; Lim, J.; Gim, J.; Kim, D.; Moon, J.; Kang, J.; Kim, J. Optimized Li4Ti5O12 nanoparticles by solvothermal route for Li-ion batteries. J. Nanosci. Nanotechnol. 2011, 11, 7294–7298. [Google Scholar] [CrossRef]
- Li, N.; Liang, J.; Wei, D.; Zhu, Y.; Qian, Y. Solvothermal synthesis of micro-/nanoscale Cu/Li4Ti5O12 composites for high-rate Li-ion batteries. Electrochim. Acta 2014, 123, 346–352. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Zeng, M.; Wu, X.; Bai, Y.; Li, J. Solvothermal synthesis for dual-phase Li4Ti5O12/TiO2 composites for high-stability lithium storage. Mater. Res. Express 2020, 7, 015515. [Google Scholar] [CrossRef]
- Chen, J.Z.; Yang, L.; Fang, S.H.; Tang, Y.F. Synthesis of sawtooth-like Li4Ti5O12 nanosheets as anode materials for Li-ion batteries. Electrochim. Acta 2010, 55, 6596–6600. [Google Scholar] [CrossRef]
- Kageyama, H.; Oaki, Y.; Imai, H. Basicity-controlled synthesis of Li4Ti5O12 nanocrystals by a solvothermal method. RSC Adv. 2014, 4, 44124–44129. [Google Scholar] [CrossRef]
- Feckl, J.M.; Fominykh, K.; Döblinger, M.; Fattakhova-Rohlfing, D.; Bein, T. Nanoscale porous framework of lithium titanate for ultrafast lithium insertion. Angew. Chem. Int. Ed. 2012, 51, 7459–7463. [Google Scholar] [CrossRef]
- Kim, H.-K.; Roh, K.C.; Kang, K.; Kim, K.-B. Synthesis of nano-Li4Ti5O12 decorated on non-oxidized carbon nanotubes with enhanced rate capability for lithium-ion batteries. RSC Adv. 2013, 3, 14267–14272. [Google Scholar] [CrossRef]
- Gangaja, B.; Nair, S.; Santhanagopalan, D. Surface-engineered Li4Ti5O12 nanostructures for high-power Li-ion batteries. Nano-Micro Lett. 2020, 30, 12. [Google Scholar] [CrossRef]
- Santhoshkumar, P.; Shaji, N.; Sim, G.S.; Nanthagopal, M.; Lee, C.W. Towards environment-friendly and versatile energy storage devices: Design and preparation of mesoporous Li4Ti5O12-TiO2 nano-hybrid electrode materials. Appl. Surf. Sci. 2022, 583, 152490. [Google Scholar] [CrossRef]
- Ho, C.K.; Li, C.Y.V.; Chan, K.Y.; Yung, H.; Tay, Y.Y. Interfacing TiO2(B) nanofibers with Li4Ti5O12 towards highly reversible and durable TiO2-based anode for Li−ion batteries. Energy Technol. 2019, 7, 107–112. [Google Scholar] [CrossRef]
- Lu, S.; Shang, Y.; Zheng, W.; Huang, Y.; Wang, R.; Zeng, W.; Zhan, H.; Yang, Y.; Mei, J. TiO2(B) nanosheets modified Li4Ti5O12 microsphere anode for high-rate lithium-ion batteries. Nanotechnology 2022, 33, 245404. [Google Scholar] [CrossRef]
- Singhai, A.; Skandan, G. Nanostructured Li4Ti5O12 Powders and Method of Making the Same. US Patent 6,827,921, 7 December 2004. [Google Scholar]
- Meng, T.; Zeng, R.; Sun, Z.; Yi, F.; Shu, D.; Li, K.; Li, S.; Zhang, F.; Cheng, H.; He, C. Chitosan-confined synthesis of N-doped and carbon-coated Li4Ti5O12 nanoparticles with enhanced lithium storage for lithium-ion batteries. J. Electrochem. Soc. 2018, 165, A1046–A1053. [Google Scholar] [CrossRef]
- Yin, Y.; Luo, X.; Xu, B. In-situ self-assembly synthesis of low-cost, long-life, shape-controllable spherical Li4Ti5O12 anode material for Li-ion batteries. J. Alloys Compd. 2022, 904, 164026. [Google Scholar] [CrossRef]
- Dong, H.; Yin, Y.; Zhang, Z.; Yang, S. Synthesis and properties of Li4Ti5O12/C composite by a microwave-assisted method using PAM as both the template and the carbon source. Phys. Scr. 2012, 86, 055802. [Google Scholar] [CrossRef]
- Hao, X.; Bartlett, B.M. Li4Ti5O12 nanocrystals synthesized by carbon templating from solution precursors yield high performance thin film Li-ion battery electrodes. Adv. Energy Mater. 2013, 3, 753–761. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, K.H.; Park, B.G.; Kim, H.G.; Park, Y.J. Synthesis of Li4Ti5O12 thin film with inverse hemispheric structure. Bull. Korean Chem. Soc. 2010, 31, 360–364. [Google Scholar] [CrossRef]
- Liu, J.; Wei, A.X.; Chen, M.; Xia, X. Rational synthesis of Li4Ti5O12/N-C nanotube arrays as advanced high-rate electrodes for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 3857–3863. [Google Scholar] [CrossRef]
- Singh, D.P.; Mulder, F.M.; Wagemaker, M. Templated spinel Li4Ti5O12 Li-ion battery electrodes combining high rates with high energy density. Electrochem. Commun. 2013, 35, 124–127. [Google Scholar] [CrossRef]
- Vertruyen, B.; Eshraghi, N.; Piffet, C.; Bodart, J.; Mahmound, A.; Boschini, F. Spray-drying of electrode materials for lithium- and sodium-ion batteries. Materials 2018, 11, 1076. [Google Scholar] [CrossRef]
- Nowack, L.V.; Bunjaku, T.; Wegner, K.; Pratsinis, S.E.; Luisier, M.; Wood, V. Design and fabrication of microspheres with hierarchical internal structure for tuning performance. Adv. Sci. 2015, 2, 1500078. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Li, X.; Guo, H.; Yue, P.; Xiong, X.; He, Z.; Zhang, Q. Characterization of spherical-shaped Li4Ti5O12 prepared by spray-drying. Electrochim. Acta 2012, 78, 331–339. [Google Scholar] [CrossRef]
- Alaboina, P.K.; Ge, Y.; Uddin, M.-J.; Liu, Y.; Lee, D.; Park, S.; Zhang, X.; Cho, S.-J. Nanoscale porous lithium titanate anode for superior high temperature performance. ACS Appl. Mater. Interfaces 2016, 8, 12127–12133. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Ye, J.; Zhao, S.; He, P.; Zhou, H. Fabrication of high-energy Li-ion cells with Li4Ti5O12 microspheres as anode and 0.5 Li2MnO3·0.5 LiNi0.4Co0.2Mn0.4O2 microspheres as cathode. Chem. Asian J. 2016, 11, 1273–1280. [Google Scholar] [CrossRef]
- Deng, L.; Yang, W.-H.; Zhou, S.-X.; Chen, J.-T. Effect of carbon nanotubes addition on electrochemical performance and thermal stability of Li4Ti5O12 anode in commercial LiMn2O4/Li4Ti5O12 full-cell. Chin. Chem. Lett. 2015, 26, 1529–1534. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, C.; Wan, C. Influence of carbon additive on the properties of spherical Li4Ti5O12 and LiFePO4 materials for lithium-ion batteries. Ionics 2010, 16, 417–424. [Google Scholar] [CrossRef]
- He, Z.; Wang, Z.; Wu, F.; Guo, H.; Li, X.; Xiong, X. Spherical Li4Ti5O12 synthesized by spray-drying from a different kind of solution. J. Alloys Compd. 2012, 540, 39–45. [Google Scholar] [CrossRef]
- Hsiao, K.-C.; Liao, S.-C.; Chen, J.-M. Microstructure effect on the electrochemical property of Li4Ti5O12 as an anode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 7242–7247. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Chen, I.-L.; Jiang, Y.-R.; Lin, J.-Y. Synthesis of spinel lithium titanate anodes incorporated with rutile titania nanocrystallites by spray-drying followed by calcination. Solid State Ion. 2011, 201, 60–67. [Google Scholar] [CrossRef]
- Jung, H.-G.; Kim, J.; Scrosati, B.; Sun, Y.-K. Micron-sized, carbon-coated Li4Ti5O12 as high power anode material for advanced lithium batteries. J. Power Sources 2011, 196, 7763–7766. [Google Scholar] [CrossRef]
- Kadoma, Y.; Chiba, Y.; Yoshikawa, D.; Mitobe, Y.; Kumagai, N.; Ui, K. Influence of the carbon source on the surface and electrochemical characteristics of lithium excess Li4.3Ti5O12 carbon composite. Electrochemistry 2012, 80, 759–761. [Google Scholar] [CrossRef]
- Lee, B.; Yoon, J.R. Synthesis of high-performance Li4Ti5O12 and its application to the asymmetric hybrid capacitor. Electron. Mater. Lett. 2013, 9, 871–873. [Google Scholar] [CrossRef]
- Li, C.; Li, G.; Wen, S.; Ren, R. Spray-drying synthesis and characterization of Li4Ti5O12 anode material for lithium ion batteries. J. Adv. Oxid. Technol. 2017, 20, 183. [Google Scholar] [CrossRef]
- Lu, X.; Gu, L.; Hu, Y.-S.; Chiu, H.-C.; Li, H.; Demopoulos, G.P.; Chen, L. New insight into the atomic-scale bulk and surface structure evolution of Li4Ti5O12 anode. J. Am. Chem. Soc. 2015, 137, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ming, H.; Jia, Z.; Zhang, Y.; Ming, J.; Zhou, Q.; Zheng, J. High tap density Li4Ti5O12 microspheres: Synthetic conditions and advanced electrochemical performance. Energy Technol. 2017, 5, 1680–1686. [Google Scholar] [CrossRef]
- Ruan, D.; Kim, M.-S.; Yang, B.; Qin, J.; Kim, K.-B.; Lee, S.-H.; Liu, Q.; Tan, L.; Qiao, Z. 700 F hybrid capacitors cells composed of activated carbon and Li4Ti5O12 microspheres with ultra-long cycle life. J. Power Sources 2017, 366, 200–206. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Wang, Z.; Guo, H.; He, Z.; Zhang, Q.; Xiong, X.; Yue, P. Low-temperature synthesis of nano-micron Li4Ti5O12 by an aqueous mixing technique and its excellent electrochemical performance. J. Power Sources 2012, 202, 374–379. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Suzuki, N.; Kadoma, Y.; Ui, K.; Kumagai, N. Li excess Li4+xTi5–xO12–δ/C composite using spray-drying method and its electrode properties. Funct. Mater. Lett. 2012, 5, 1250001. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, W.; Wang, Z.; Li, X.; Xiong, X.; Guo, H.; Wang, Z.; Wu, F. Li4Ti5O12/reduced graphene oxide composite as a high rate capability material for lithium ion batteries. Solid State Ion. 2013, 236, 30–36. [Google Scholar] [CrossRef]
- Zheng, X.; Dong, L.; Dong, C. Easy synthesis of Li4Ti5O12/C microspheres containing nanoparticles as anode material for high-rate lithium batteries. Surf. Rev. Lett. 2014, 21, 1450023. [Google Scholar] [CrossRef]
- Zhu, G.-N.; Liu, H.-J.; Zhuang, J.-H.; Wang, C.-X.; Wang, Y.-G.; Xia, Y.-Y. Carbon-coated nano-sized Li4Ti5O12 nanoporous micro-sphere as anode material for high-rate lithium-ion batteries. Energy Environ. Sci. 2011, 4, 4016. [Google Scholar] [CrossRef]
- Zhu, W.; Zhuang, Z.; Yang, Y.; Zhang, R.; Lin, Z.; Lin, Y.; Huang, Z. Synthesis and electrochemical performance of hole-rich Li4Ti5O12 anode material for lithium-ion secondary batteries. J. Phys. Chem. Solids 2016, 93, 52–58. [Google Scholar] [CrossRef]
- Wen, S.; Li, G.; Ren, R.; Li, C. Preparation of spherical Li4Ti5O12 anode materials by spray drying. Mater. Lett. 2015, 148, 130–133. [Google Scholar] [CrossRef]
- Xu, G.; Quan, X.; Gao, H.; Li, J.; Cai, Y.; Cheng, X.; Guo, L. Facile spray drying route for large scale nitrogen-doped carbon-coated Li4Ti5O12 anode material in lithium-ion batteries. Solid State Ion. 2017, 304, 40–45. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Kadoma, Y.; Kima, J.-M.; Ui, K.; Kumagai, N.; Kitamura, N.; Idemoto, Y. Spray-drying synthesized lithium-excess Li4+xTi5–xO1–δ and its electrochemical property as negative electrode material for Li-ion batteries. Electrochim. Acta 2010, 55, 1872–1879. [Google Scholar] [CrossRef]
- Lee, B.-G.; Lee, S.-H. Application of hybrid supercapacitor using granule Li4Ti5O12/activated carbon with variation of current density. J. Power Sources 2017, 343, 545–549. [Google Scholar] [CrossRef]
- Kumagai, N.; Yoshikawa, D.; Kadoma, Y.; Ui, K. Spray-drying synthesized lithium-excess Li4+xTi4.95−xNb0.05O12−δ and its electrochemical property as negative electrode material for Li-ion batteries. Electrochemistry 2010, 78, 754–756. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Cao, C.; Han, X.; Zhang, J.; Xie, X.; Xia, B. Spray drying of spherical Li4Ti5O12/C powders using polyvinyl pyrrolidone as binder and carbon source. J. Alloys Compd. 2015, 621, 162–169. [Google Scholar] [CrossRef]
- Piffet, C.; Vertruyen, B.; Caes, S.; Thomassin, J.-M.; Broze, G.; Malherbe, C.; Boschini, F.; Cloots, R.; Mahmoud, A. Aqueous processing of flexible, free-standing Li4Ti5O12 electrodes for Li-ion batteries. Chem. Eng. J. 2020, 397, 125508. [Google Scholar] [CrossRef]
- Yuan, T.; Li, W.-T.; Zhang, W.; He, Y.-S.; Zhang, C.; Liao, X.-Z.; Ma, Z.-F. One-pot spray dried graphene sheets encapsulated nano-Li4Ti5O12 microspheres for a hybrid BatCap system. Ind. Eng. Chem. Res. 2014, 53, 10849–10857. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Wang, Z.; Guo, H. Synthesis of chromium-doped lithium titanate microspheres as high-performance anode material for lithium ion batteries. Ceram. Int. 2014, 40, 13195–13204. [Google Scholar] [CrossRef]
- Jia, X.L.; Kan, Y.F.; Zhu, X.; Ning, G.Q.; Lu, Y.F.; Wei, F. Building flexible Li4Ti5O12/CNT lithium-ion battery anodes with superior rate performance and ultralong cycling stability. Nano Energy 2014, 10, 344–352. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, J.; Yuan, C.; Kuai, L.; Geng, B. Mesoporous spherical Li4Ti5O12/TiO2 composites as an excellent anode material for lithium ion batteries. Electrochim. Acta 2016, 212, 41–46. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, S.-W.; Kwon, T.-S.; Park, H.S. Spray-drying assisted synthesis of a Li4Ti5O12/C composite for high rate performance lithium ion batteries. Ceram. Int. 2018, 44, 2683–2690. [Google Scholar] [CrossRef]
- Lee, G.-W.; Kim, M.-S.; Jeong, J.H.; Roh, H.-K.; Roh, K.C.; Kim, K.-B. Comparative study of Li4Ti5O12 composites prepared with pristine, oxidized, and surfactant-treated multiwalled carbon nanotubes for high-power hybrid supercapacitors. ChemElectroChem 2018, 5, 2357–2366. [Google Scholar] [CrossRef]
- Chien, W.-C.; Wu, Z.-H.; Hsieh, Y.-C.; Wu, Y.-S.; Wu, S.-H.; Yang, C.-C. Electrochemical performance of Li4Ti5O12 anode materials synthesized using a spray-drying method. Ceram. Int. 2020, 46, 26923–26935. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Lin, J.-Y. Influence of Li addition on charge-discharge behavior of spinel lithium titanate. J. Alloys Compd. 2010, 506, 231–236. [Google Scholar] [CrossRef]
- Jamin, C.; Jungers, T.; Piffet, C.; Mahmoud, A.; Cloots, R.; Vertruyen, B.; Boschini, F. Li4Ti5O12 powders by spray-drying: Influence of the solution concentration and particle size on the electrochemical properties. IOP Conf. Ser. J. Phys. 2018, 1081, 012001. [Google Scholar] [CrossRef]
- Lenggoro, I.W.; Hata, T.; Iskandar, F.; Lunden, M.M.; Okuyama, K. An experimental and modeling investigation of particle production by spray pyrolysis using a laminar flow aerosol reactor. J. Mater. Res. 2000, 15, 733–743. [Google Scholar] [CrossRef]
- Zhu, Y.; Choi, S.H.; Fan, X.; Shin, J.; Ma, Z.; Zachariah, M.R.; Choi, J.W.; Wang, C. Recent progress on spray pyrolysis for gigh performance electrode materials in lithium and sodium rechargeable batteries. Adv. Energy Mater. 2017, 7, 1601578. [Google Scholar] [CrossRef]
- Laine, R.M.; Baranwal, R.; Hinklin, T.; Treadwell, D.; Sutorik, A.; Bickmore, C.; Waldner, K.; Neo, S.S. Making nanosized oxide powders from precursors by flame spray pyrolysis. Key Eng. Mater. 1999, 159, 17–24. [Google Scholar] [CrossRef]
- Ogihara, T.; Yamada, M.; Fujita, A.; Akao, S.; Myoujin, K. Effect of organic acid on the electrochemical properties of Li4Ti5O12/C composite powders synthesized by spray pyrolysis. Mater. Res. Bull. 2011, 46, 796–800. [Google Scholar] [CrossRef]
- Karhunen, T.; Lähde, A.; Leskinen, J.; Büchel, R.; Waser, O.; Tapper, U.; Jokiniemi, J. Transition metal-doped lithium titanium oxide nanoparticles made using flame spray pyrolysis. ISRN Nanotechnol. 2011, 2011, 180821. [Google Scholar] [CrossRef]
- Waser, O.; Brenner, O.; Groehn, A.J.; Pratsinis, S.E. Process design for size-controlled flame spray synthesis of Li4Ti5O12 and electrochemical performance. Chem. Proc. Eng. 2017, 38, 51–66. [Google Scholar] [CrossRef]
- Ju, S.H.; Kang, Y.C. Effects of drying control chemical additive on properties of Li4Ti5O12 negative powders prepared by spray pyrolysis. J. Power Sources 2010, 195, 4327–4331. [Google Scholar] [CrossRef]
- Takahashi, M.; Tani, J.; Kido, H.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. Thin film electrode materials Li4Ti5O12 and LiCoO2 prepared by spray pyrolysis method. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 122004. [Google Scholar] [CrossRef]
- Yamada, M.; Kodera, T.; Ogihara, T. Synthesis and electrochemical properties of C/Li4Ti5O12 powders by spray pyrolysis using aqueous solution of organic acid. Electrochemistry 2010, 78, 463–466. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, Y.C. Electrochemical properties of nanosized Li4Ti5O12 powders prepared by flame spray pyrolysis. Int. J. Electrochem. Sci. 2013, 8, 3379–3389. [Google Scholar] [CrossRef]
- Du, G.; Winton, B.R.; Hashim, I.M.; Sharma, N.; Konstantinov, K.; Reddy, M.V.; Guo, Z. Mass production of Li4Ti5O12 with a conductive network via in-situ spray pyrolysis as a long cycle life, high rate anode material for lithium ion batteries. RSC Adv. 2014, 4, 38568–38574. [Google Scholar] [CrossRef]
- Birrozzi, A.; Copley, M.; von Zamory, J.; Pasqualini, M.; Calcaterra, S.; Nobili, F.; Di Cicco, A.; Rajantie, H.; Briceno, M.; Bilbe, E.; et al. Scaling up “nano” Li4Ti5O12 for high-power lithium-ion anodes using large scale flame spray pyrolysis. J. Electrochem. Soc. 2015, 162, A2331–A2338. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Zhao, Y.; Zhu, G.; Yu, A. A facile one-step spray pyrolysis method to synthesize spherical Li4Ti5O12 for lithium-ion battery. Mater. Lett. 2014, 129, 101–103. [Google Scholar] [CrossRef]
- Yang, K.M.; Ko, Y.N.; Yun, J.-Y.; Kang, Y.C. Preparation of Li4Ti5O12 yolk–shell powders by spray pyrolysis and their electrochemical properties. Chem. Asian J. 2014, 9, 443–446. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, J.R. Preparation and characterization of Li4Ti5O12 synthesized using hydrogen titanate nanowire for hybrid super capacitor. J. Adv. Ceram. 2013, 2, 285–290. [Google Scholar] [CrossRef]
- Bhat, M.H.; Miura, A.; Vinatier, P.; Levasseur, A.; Rao, K.J. Microwave synthesis of lithium lanthanum titanate. Solid State Commun. 2003, 125, 557–562. [Google Scholar] [CrossRef]
- Li, J.; Jin, Y.-L.; Zhang, X.-G.; Yang, H. Microwave solid-state synthesis of spinel Li4Ti5O12 nanocrystallites as anode material for lithium-ion batteries. Solid State Ion. 2007, 178, 1590–1594. [Google Scholar] [CrossRef]
- Balaji, S.; Mutharasu, D.; Sankara Subramanian, N.; Ramanathan, K. A review on microwave synthesis of electrode materials for lithium-ion batteries. Ionics 2009, 15, 765–777. [Google Scholar] [CrossRef]
- Yang, L.H.; Dong, C.; Guo, J. Hybrid microwave synthesis and characterization of the compounds in the Li-Ti-O system. J. Power Sources 2008, 175, 575–580. [Google Scholar] [CrossRef]
- Liu, H.W.; Feng, C.Q.; Tang, H.; Song, L.; Zhang, K.L. Synthesis and electrochemical properties of indium doped spinel LiMn2O4. J. Mater. Sci. 2004, 15, 495–497. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Y.; Wang, K.; Xie, J. Synthesis and electrochemical properties of Li4Ti5O12/C composite by the PVB rheological phase method. J. Phys. Chem. Solids 2008, 69, 2037–2040. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, G.; Gao, L. Electrochemical characterization of Li4Ti5O12/C anode material prepared by starch-sol-assisted rheological phase method for Li-ion battery. J. Nanomater. 2012, 2012, 876197. [Google Scholar] [CrossRef]
- Abureden, S.; Hassan, F.M.; Lui, G.; Ahn, W.; Sy, S.; Yu, A.P.; Chen, Z.W. Multigrain electrospun nickel doped lithium titanate nanofibers with high power lithium ion storage. J. Mater. Chem. A 2016, 4, 12638–12647. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, W.; Xue, M.; Xie, Z.; Zhao, D.; Zhang, M.; Chen, J.; Cao, T. Graphene as a conductive additive to enhance the high-rate capabilities of electrospun Li4Ti5O12 for lithium-ion batteries. Electrochim. Acta 2010, 55, 5813–5818. [Google Scholar] [CrossRef]
- Guo, B.; Li, Y.; Yao, Y.; Lin, Z.; Ji, L.; Xu, G.; Liang, Y.; Shi, Q.; Zhang, X. Electrospun Li4Ti5O12/C composites for lithium-ion batteries with high rate performance. Solid State Ion. 2011, 204–205, 61–65. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, Q.; Li, Z.; Lei, G.; Zhang, P.; Wu, L. Synthesis of Li4Ti5O12 fibers as a high-rate electrode material for lithium-ion batteries. J. Solid State Electrochem. 2012, 16, 3307–3313. [Google Scholar] [CrossRef]
- Ji, X.; Li, D.; Lu, Q.; Guo, E.; Yao, L. Electrospinning preparation of one-dimensional Ce3+-doped Li4Ti5O12 sub-microbelts for high-performance lithium-ion batteries. J. Nanopart. Res. 2017, 19, 393. [Google Scholar] [CrossRef]
- Li, X.; Yang, K.; Gao, F.; Hu, C.; Liu, H.; Li, D.; Wang, L. Electrospinning synthesis of spinel Li4Ti5O12 and its characterization. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012098. [Google Scholar] [CrossRef]
- Park, H.; Song, T.; Han, H.; Paik, U. Electrospun Li4Ti5O12 nanofibers sheathed with conductive TiN/TiOxNy layer as an anode material for high power Li-ion batteries. J. Power Sources 2013, 244, 726–730. [Google Scholar] [CrossRef]
- Chen, C.H.; Feng, J.J.; Shao, W.Q.; Chen, S.O.; He, L.Z.; Li, Y.Q.; Ban, Y.Q.; Hei, F.; Guo, X.J. Electrochemical performance of Li4Ti5O12/TiO2/Li0.4TiO2/C composite prepared by the electrospinning method using as lithium battery anode material. Mater. Sci. Forum 2016, 852, 959–962. [Google Scholar] [CrossRef]
- Zou, H.L.; Xiang, H.F.; Liang, X.; Feng, X.Y.; Cheng, S.; Jin, Y.; Chen, C.H. Electrospun Li3.9Cr0.3Ti4.8O12 nanofibers as anode material for high-rate and low-temperature lithium-ion batteries. J. Alloys Compd. 2017, 701, 99–106. [Google Scholar] [CrossRef]
- Kim, J.-G.; Shi, D.; Park, M.-S.; Jeong, G.; Heo, Y.-U.; Seo, M.; Kim, Y.-J.; Kim, J.H.; Dou, S.X. Controlled Ag-driven superior rate-capability of Li4Ti5O12 anodes for lithium rechargeable batteries. Nano Res. 2013, 6, 365–372. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, X.; Sunarso, J.; Cai, R.; Chu, S.; Miao, J.; Zhou, W.; Shao, Z. Two-step fabrication of Li4Ti5O12-coated carbon nanofibers as a flexible film electrode for high-power lithium-ion batteries. ChemElectroChem 2017, 4, 2286–2292. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, S.; Jiang, J.; Wu, R.; Niu, X.; Chen, J.S. In-situ construction of Li4Ti5O12/rutile TiO2 heterostructured nanorods for robust and high-power lithium storage. Nano Res. 2023, 16, 1513–1521. [Google Scholar] [CrossRef]
- Wang, J.; Shen, L.; Li, H.; Ding, B.; Nie, P.; Dou, H.; Zhang, X. Mesoporous Li4Ti5O12/carbon nanofibers for high-rate lithium-ion batteries. J. Alloys Compd. 2014, 587, 171–176. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Zhang, C.; Fu, H.; Nan, X.; Yang, Y. High power high safety battery with electrospun Li3V2(PO4)3 cathode and Li4Ti5O12 anode with 95% energy efficiency. Energy Storage Mater. 2016, 5, 93–102. [Google Scholar] [CrossRef]
- Wu, Y.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishna, S. Electrochemical studies on electrospun Li(Li1/3Ti5/3)O4 grains as an anode for Li-ion batteries. Electrochim. Acta 2012, 67, 33–40. [Google Scholar] [CrossRef]
- Kim, E.-K.; Choi, B.-H.; Jee, M.-J.; Kwon, Y.-J.; Seo, H.; Kim, Y.-J.; Kim, K.-B. Synthesis and electrochemical characteristics of Li4Ti5O12 nanofibers by hydrothermal method. J. Korean Ceram. Soc. 2010, 47, 627–632. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagai, H.; Sotokawa, T.; Kumashiro, Y.; Ishibai, Y.; Akimoto, J. Ion-exchange synthesis and improved Li insertion property of lithiated H2Ti12O25 as a negative electrode material for lithium-ion batteries. J. Asian Ceram. Soc. 2016, 4, 75–80. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Li, J.; Cai, W.; Zhang, J.; Yu, L.; Jin, Z.; Zhang, Z. Preparation of Li4Ti5O12 by solution ion-exchange of sodium titanate nanotube and evaluation of electrochemical performance. J. Nanopart. Res. 2013, 15, 2005. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Chabot, V.; Gu, M.; Wang, C.; Xiao, X.; Chen, Z. Dual phase Li4Ti5O12-TiO2 nanowire arrays as integrated anodes for high-rate lithium-ion batteries. Nano Energy 2014, 9, 383–391. [Google Scholar] [CrossRef]
- Li, N.; Katase, T.; Zhu, Y.; Matsumoto, T.; Umemura, T.; Ikuhara, Y.; Ohta, H. Solid-liquid phase epitaxial growth of Li4Ti5O12 thin film. Appl. Phys. Express 2016, 9, 125501. [Google Scholar] [CrossRef]
- Wang, C.-L.; Liao, Y.C.; Hsu, F.C.; Tai, N.H.; Wu, M.K. Preparation and characterization of thin film Li4Ti5O12 electrodes by magnetron sputtering. J. Electrochem. Soc. 2005, 152, A653–A657. [Google Scholar] [CrossRef]
- Kumatani, A.; Shiraki, S.; Takagi, Y.; Suzuki, T.; Ohsawa, T.; Gao, X.; Ikuhara, Y.; Hitosugi, T. Epitaxial growth of Li4Ti5O12 thin films using RF magnetron sputtering. Jpn. J. Appl. Phys. 2014, 53, 058001. [Google Scholar] [CrossRef]
- Pagani, F.; Döbeli, M.; Battaglia, C. Lithium-ion transport in Li4Ti5O12 epitaxial thin films vs state of charge. Batter. Supercaps 2021, 4, 316–321. [Google Scholar] [CrossRef]
- Deng, J.Q.; Lu, Z.G.; Belharouak, I.; Amine, K.; Chung, C.Y. Preparation and electrochemical properties of Li4Ti5O12 thin film electrodes by pulsed laser deposition. J. Power Sources 2009, 193, 816–821. [Google Scholar] [CrossRef]
- Kumatani, A.; Ohsawa, T.; Shimizu, R.; Takagi, Y.; Shiraki, S.; Hitosugi, T. Growth processes of lithium titanate thin films deposited by using pulsed laser deposition. Appl. Phys. Lett. 2012, 101, 123103. [Google Scholar] [CrossRef]
- Yu, P.; Li, C.; Guo, X. Sodium storage and pseudocapacitance charge in textured Li4Ti5O12 thin films. J. Phys. Chem. C 2014, 118, 10616–10624. [Google Scholar] [CrossRef]
- Yu, X.; Wang, R.; He, Y.; Hu, Y.; Li, H.; Huang, X. Electrochromic behavior of transparent Li4Ti5O12/FTO electrode. Electrochem. Solid-State Lett. 2010, 13, J99–J101. [Google Scholar] [CrossRef]
- Deng, J.; Lu, Z.; Chung, C.Y.; Hanc, X.; Wang, Z.; Zhou, H. Electrochemical performance and kinetic behavior of lithium ion in Li4Ti5O12 thin film electrodes. Appl. Surf. Sci. 2014, 314, 936–941. [Google Scholar] [CrossRef]
- Zhao, M.; Lian, J.; Jia, Y.; Jin, K.; Xu, L.; Hu, Z.; Yang, X.; Kang, S. Investigation of the optical properties of LiTi2O4 and Li4Ti5O12 spinel films by spectroscopic ellipsometry. Opt. Mater. Express 2016, 6, 3366–3374. [Google Scholar] [CrossRef]
- Schichtel, P.; Geiß, M.; Leichtweiß, T.; Sann, J.; Weber, D.A.; Janek, J. On the impedance and phase transition of thin film all-solid-state batteries based on the Li4Ti5O12 system. J. Power Sources 2017, 360, 593–604. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Pulsed laser deposited films for energy storage and conversion: A review. Coatings 2018, 9, 386. [Google Scholar] [CrossRef]
- Rho, Y.H.; Kanamura, K.; Umegaki, T. Preparation of Li4/3Ti5/3O4 thin film anode with high electrochemical response for rechargeable lithium batteries by sol–gel method. Chem. Lett. 2001, 30, 1322–1323. [Google Scholar] [CrossRef]
- Rho, Y.H.; Kanamura, K.; Fujisaki, M.; Hamagami, J.; Suda, S.; Umegaki, T. Preparation of Li4Ti5O12 and LiCoO2 thin film electrodes from precursors obtained by sol-gel method. Solid State Ion. 2002, 151, 151–157. [Google Scholar] [CrossRef]
- Wude, F.; Berkemeier, F.; Schmitz, G. Lithium diffusion in sputter-deposited Li4Ti5O12 thin films. J. Power Sources 2012, 215, 109–115. [Google Scholar]
- Hirayama, M.; Kim, K.; Toujigamori, T.; Cho, W.; Kanno, R. Epitaxial growth and electrochemical properties of Li4Ti5O12 thin-film lithium battery anodes. Dalton Trans. 2011, 40, 2882–2887. [Google Scholar] [CrossRef]
- Kim, K.; Toujigamori, T.; Suzuki, K.; Taminato, S.; Tamura, K.; Mizuki, J.; Hirayama, M.; Kanno, R. Characterization of nano-sized epitaxial Li4Ti5O12 (110) film electrode for lithium batteries. Electrochemistry 2012, 80, 800–803. [Google Scholar] [CrossRef]
- Pfenninger, R.; Afyon, S.; Garbayo, I.; Struzik, M.; Rupp, J.L.M. Lithium titanate anode thin films for Li-ion solid state battery based on garnets. Adv. Funct. Mater. 2018, 28, 1800879. [Google Scholar] [CrossRef]
- Mesoraca, S.; Kleibeuker, J.E.; Prasad, B.; MacManus-Driscoll, J.L.; Blamire, M.G. Lithium outdiffusion in LiTi2O4 thin films grown by pulsed laser deposition. J. Cryst. Growth 2016, 454, 134–138. [Google Scholar] [CrossRef]
- Chopdekar, R.V.; Wong, F.J.; Takamura, Y.; Arenholz, E.; Suzuki, Y. Growth and characterization of superconducting spinel oxide LiTi2O4 thin films. Phys. C Supercond. 2009, 469, 1885–1891. [Google Scholar] [CrossRef]
- Pagani, F.; Stilp, E.; Pfenninger, R.; Reyes, E.R.; Remhof, A.; Balogh-Michels, Z.; Neels, A.; Sastre-Pellicer, J.; Stiefel, M.; Döbeli, M.; et al. Epitaxial thin films as a model system for Li-ion conductivity in Li4Ti5O12. ACS Appl. Mater. Interfaces 2018, 10, 44494–44500. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.M.; Hendriks, T.A.; Vasileiadis, A.; Vos, C.M.; Verhallen, T.; Singh, D.P.; Wagemaker, M.; Huijben, M. Doubling reversible capacities in epitaxial Li4Ti5O12 thin film anodes for microbatteries. ACS Appl. Energy Mater. 2019, 2, 3410–3418. [Google Scholar] [CrossRef]
- Daminabo, S.; Goel, S.; Grammatikos, S.; Yazdani Nezhad, H.; Thakur, V.K. Fused deposition modeling-based additive manufacturing (3D printing): Techniques for polymer material systems. Mater. Today Chem. 2020, 16, 100248. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D Printing of interdigitated Li-ion microbattery architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, G.; Liu, L.; Jiang, Z. High-performance thin-film Li4Ti5O12 electrodes fabricated by using ink-jet printing technique and their electrochemical properties. J. Solid State Electrochem. 2009, 13, 705–711. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, R.; He, Z.; Zhao, K.; Pan, L. Printing 3D gel polymer electrolyte in lithium-ion microbattery using stereolithography. J. Electrochem. Soc. 2017, 164, A1852–A1857. [Google Scholar] [CrossRef]
- Delannoy, P.E.; Riou, B.; Lestriez, B.; Guyomard, D.; Brousse, T.; Le Bideau, J. Toward fast and cost-effective ink-jet printing of solid electrolyte for lithium microbatteries. J. Power Sources 2015, 274, 1085–1090. [Google Scholar] [CrossRef]
- Zhou, L.; Ning, W.; Wu, C.; Zhang, D.; Wei, W.; Ma, J.; Li, C.; Chen, L. 3D-printed microelectrodes with a developed conductive network and hierarchical pores toward high areal capacity for microbatteries. Adv. Mater. Technol. 2018, 4, 1800402. [Google Scholar] [CrossRef]
- Wei, T.S.; Ahn, B.Y.; Grotto, J.; Lewis, J.A. 3D printing of customized Li-ion batteries with thick electrodes. Adv. Mater. 2018, 30, 1703027. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K.; et al. 3D-printed all-fiber Li-ion battery toward wearable energy storage. Adv. Funct. Mater. 2017, 27, 1703140. [Google Scholar] [CrossRef]
- Kohlmeyer, R.R.; Blake, A.J.; Hardin, J.O.; Carmona, E.A.; Carpena-Núñez, J.; Maruyama, B.; Daniel Berrigan, J.; Huang, H.; Durstock, M.F. Composite batteries: A simple yet universal approach to 3D printable lithium-ion battery electrodes. J. Mater. Chem. A 2016, 4, 16856–16884. [Google Scholar] [CrossRef]
- Ragones, H.; Menkin, S.; Kamir, Y.; Gladkikh, A.; Mukra, T.; Kosa, G.; Golodnitsky, D. Towards smart free form-factor 3D printable batteries. Sustain. Energy Fuels 2018, 2, 1542–1549. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.-M.; Kwon, M.-S.; Song, M.S.; Park, Y.; Doo, S.-G. Inkjet printed electrode for rechargeable lithium thin film battery. ECS Meet. Abstr. 2009, MA2009-02, 691. [Google Scholar] [CrossRef]
- Lawes, S.; Sun, Q.; Lushington, A.; Xiao, B.; Liu, Y.; Sun, X. Inkjet-printed silicon as high performance anodes for Li-ion batteries. Nano Energy 2017, 36, 313–321. [Google Scholar] [CrossRef]
- Viviani, P.; Gibertini, E.; Iervolino, F.; Levi, M.; Magagnin, L. Carbon additive effect on the electrochemical performances of inkjet printed thin-film Li4Ti5O12 electrodes. J. Manufact. Proc. 2021, 72, 411–418. [Google Scholar] [CrossRef]
- Zhao, E.; Qin, C.; Jung, H.-R.; Berdichevsky, G.; Nese, A.; Marder, S.; Yushin, G. Lithium titanate confined in carbon nanopores for asymmetric supercapacitors. ACS Nano 2016, 10, 3977–3984. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, J.; Xu, H.; Sun, S.; Xu, Y.; Zhou, M.; Gao, X.; Sun, Z. Plasma-introduced oxygen defects confined in Li4Ti5O12 nanosheets for boosting lithium-ion diffusion. ACS Appl. Mater. Interfaces 2019, 11, 17384–17392. [Google Scholar] [CrossRef]
- Kitta, M.; Kojima, T.; Kataoka, R.; Yazawa, K.; Tada, K. Realizing the single-phase spinel-type sodium titanium oxide with the Li4Ti5O12-like structure for building stable sodium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 9322–9331. [Google Scholar] [CrossRef]
- Ritala, M.; Leskela, M.; Rauhala, E.; Haussalo, P. Atomic layer epitaxy growth of TiN thin films. J. Electrochem. Soc. 1995, 142, 2731–2737. [Google Scholar] [CrossRef]
- Richard, M.N.; Fuller, E.W.; Dahn, J.R. the effect of ammonia reduction on the spinel electrode materials, LiMn2O4 and Li(Li1/3Mn5/3)O4. Solid State Ion. 1994, 73, 81–91. [Google Scholar] [CrossRef]
- Santucci, S.; Lozzi, L.; Passacantando, M.; Picozzi, P.; Alfonsetti, R.; Diamanti, R.; Moccia, G. Study by X-ray photoelectron spectroscopy and X-ray diffraction of the growth of TiN thin films obtained by nitridation of Ti layers. Thin Solid Films 1996, 290–291, 376–380. [Google Scholar] [CrossRef]
- Wan, Z.N.; Cai, R.; Jiang, S.M.; Shao, Z.P. Nitrogen- and TiN-modified Li4Ti5O12: One-step synthesis and electrochemical performance optimization. J. Mater. Chem. 2012, 22, 17773–17781. [Google Scholar] [CrossRef]
- Kahrisi, M.; Kashani, H.; Ghaffarinejad, A. Improving the cyclability and rate capability of Li4Ti5O12 anode material by Cu2+ and F− co-doping. ChemistrySelect 2023, 8, e202204198. [Google Scholar] [CrossRef]
- Ali, B.; Muhammad, R.; Islam, M.; Anang, D.A.; Han, D.-S.; Moeez, I.; Chung, K.Y.; Cho, M.; Kim, J.Y.; Kim, M.G.; et al. Cd-doped Li4–xCdxTi5O12 (x = 0.20) as a high rate capable and stable anode material for lithium-ion batteries. ACS Appl. Energy Mater. 2023, 6, 4198–4210. [Google Scholar] [CrossRef]
- Noreochim, L.; Prabowo, R.S.; Widyastuti, W.; Susanti, D.; Subhan, A.; Idris, N.H. Enhanced high-rate capability of iodide-doped Li4Ti5O12 as an anode for lithium-ion batteries. Batteries 2023, 9, 38. [Google Scholar] [CrossRef]
- Qasim, K.F.; Mousa, M.A. Physicochemical properties of oriented crystalline assembled polyaniline/metal doped Li4Ti5O12 composites for Li-ion storage. J. Inorg. Organomet. Polym. 2023, 33, 2601–2617. [Google Scholar] [CrossRef]
- Yeo, S.; Raj, M.R.; Lee, G. Oxygen vacancy-modulated zeolitic Li4Ti5O12 microsphere anode for superior lithium-ion battery. Electrochim. Acta 2023, 441, 141809. [Google Scholar] [CrossRef]
- Tian, K.; Hui, X.; Wang, H.; Zhang, Z.; Zhang, L.; Wang, C.; Yin, L. In situ growth of Li4Ti5O12 nanoparticles on Ti3C2Tx MXene for efficient electron transfer as high-rate anode of Li ion battery. Electrochim. Acta 2022, 415, 140242. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Lai, C.; Yang, T.; Chang, X.; Zhang, M.; Sheng, L.; Yang, Z.; Ye, D.; Huang, K.; et al. Ti3C2 MXene-derived Li4Ti5O12 nanoplates with in-situ formed carbon quantum dots for metal-ion battery anodes. J. Colloid Interface Sci. 2023, 629, 263–269. [Google Scholar] [CrossRef]
- Meng, Q.; Hao, Q.; Chen, F.; Wang, L.; Li, N.; Sun, X. Li4Ti5O12 hollow macrospheres combine high tap density and excellent low-temperature performance as anode materials for Li-ion batteries. Mater. Character. 2023, 203, 113089. [Google Scholar] [CrossRef]
- Liang, K.; Ren, Y. Research progress on lithium titanate as anode material for sodium-ion batteries. Mater. Rep. 2020, 34, 9041–9047. [Google Scholar]
- Doyle, M.; Newman, J. The use of mathematical modeling in the design of lithium/polymer battery systems. Electrochim. Acta 1995, 40, 2191–2196. [Google Scholar] [CrossRef]
- Christensen, J.; Srinivasan, V.; Newman, J. Optimization of lithium titanate electrodes for high-power cells. J. Electrochem. Soc. 2006, 153, A560–A565. [Google Scholar] [CrossRef]
- Kashkooli, A.G.; Lui, G.; Farhad, S.; Lee, D.U.; Feng, K.; Yu, A.; Chen, Z. Nano-particle size effect on the performance of Li4Ti5O12 spinel. Electrochim. Acta 2016, 196, 33–40. [Google Scholar] [CrossRef]
- Lim, J.; Choi, E.; Mathew, V.; Kim, D.; Ahn, D.; Gim, J.; Kang, S.-H.; Kim, J. Enhanced high-rate performance of Li4Ti5O12 nanoparticles for rechargeable Li-ion batteries. J. Electrochem. Soc. 2011, 158, A275–A280. [Google Scholar] [CrossRef]

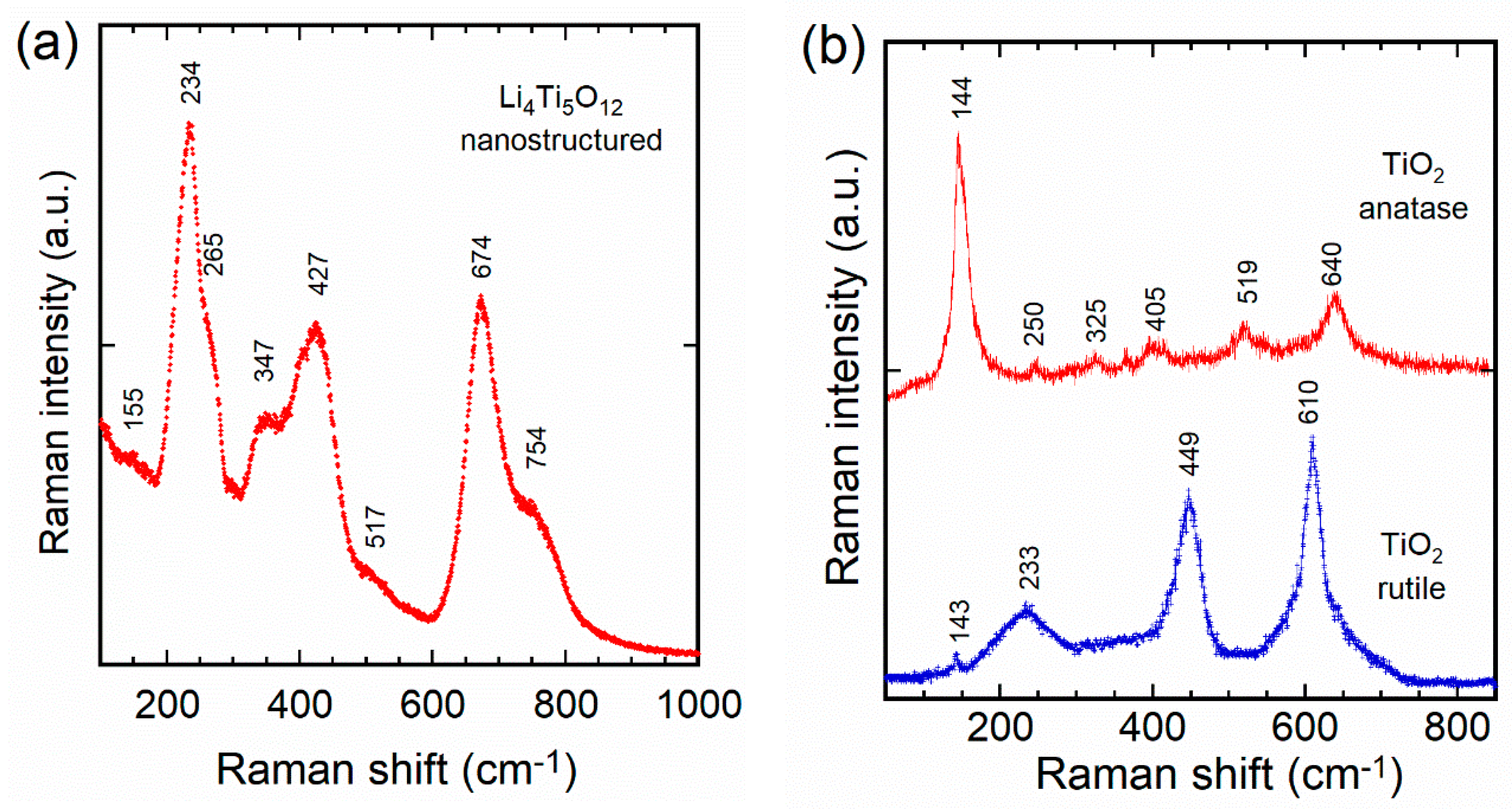


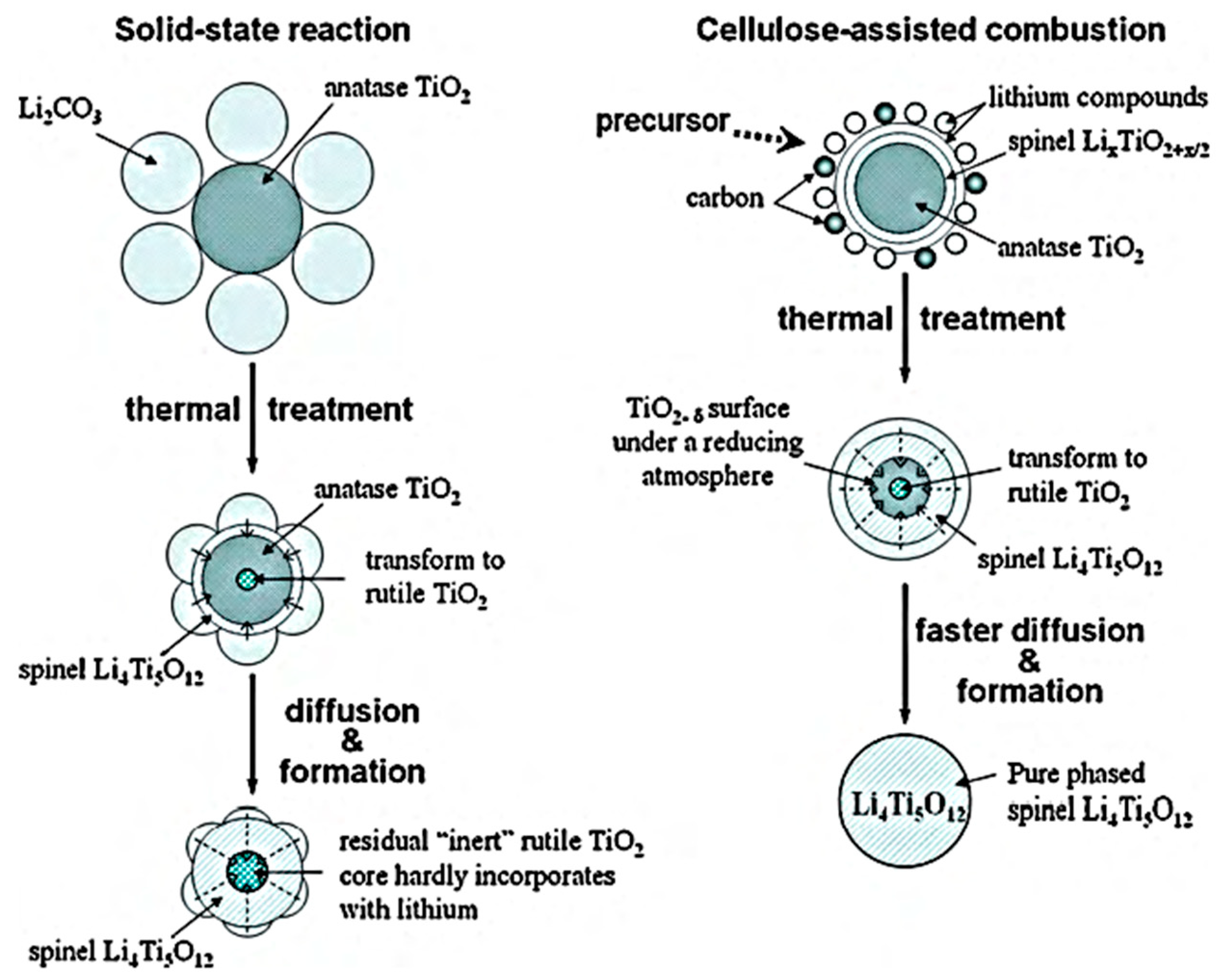

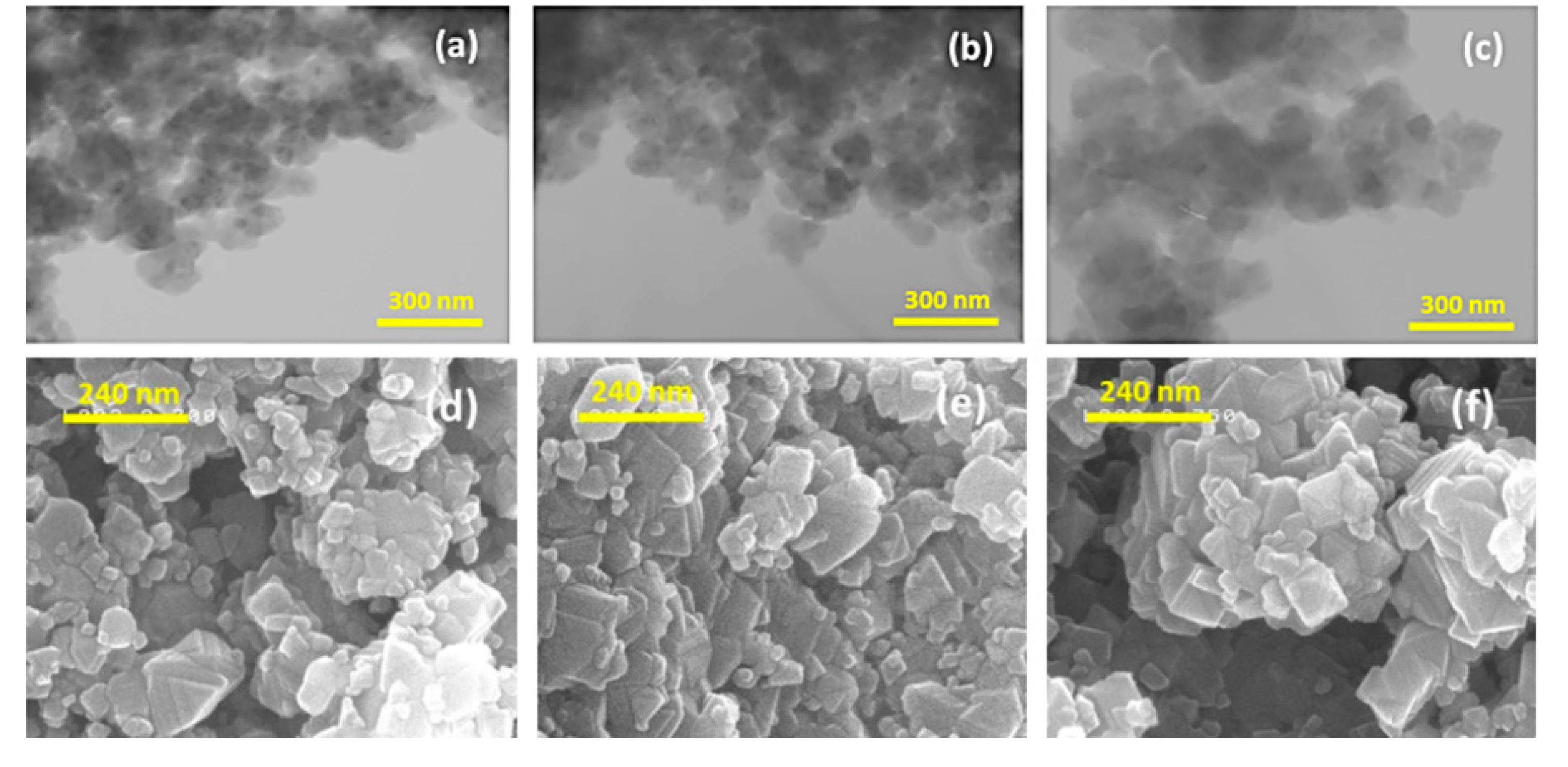
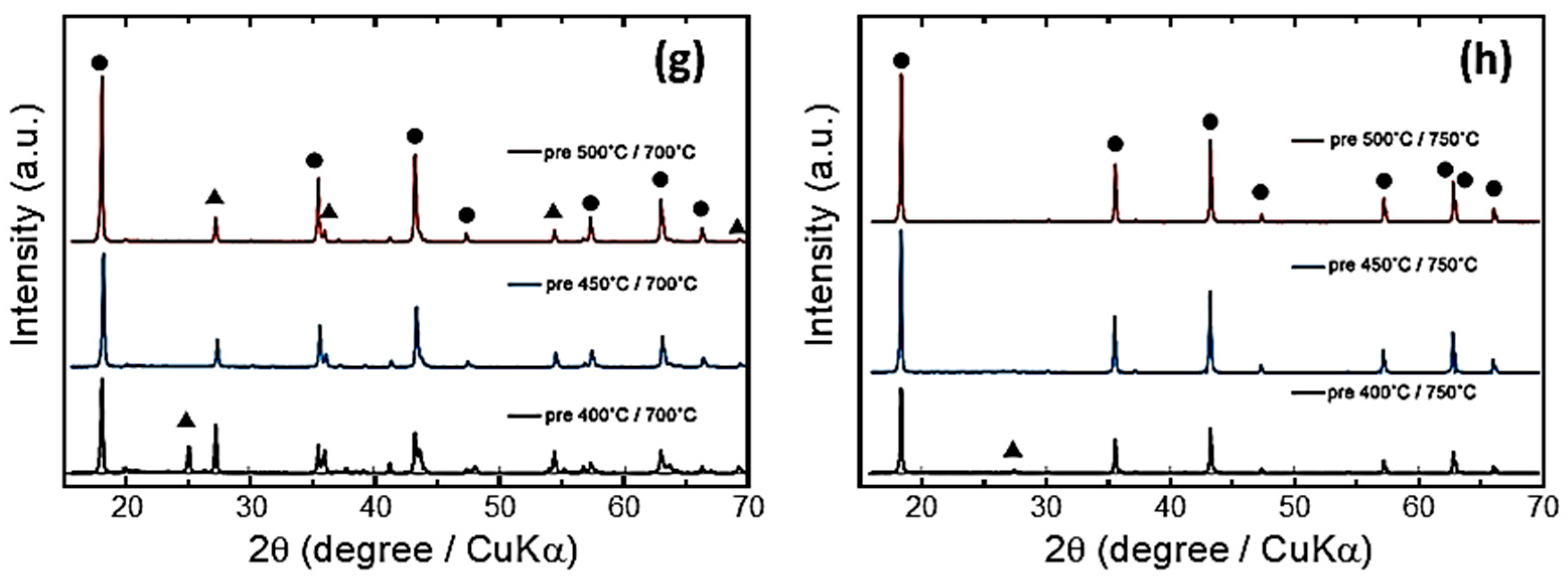
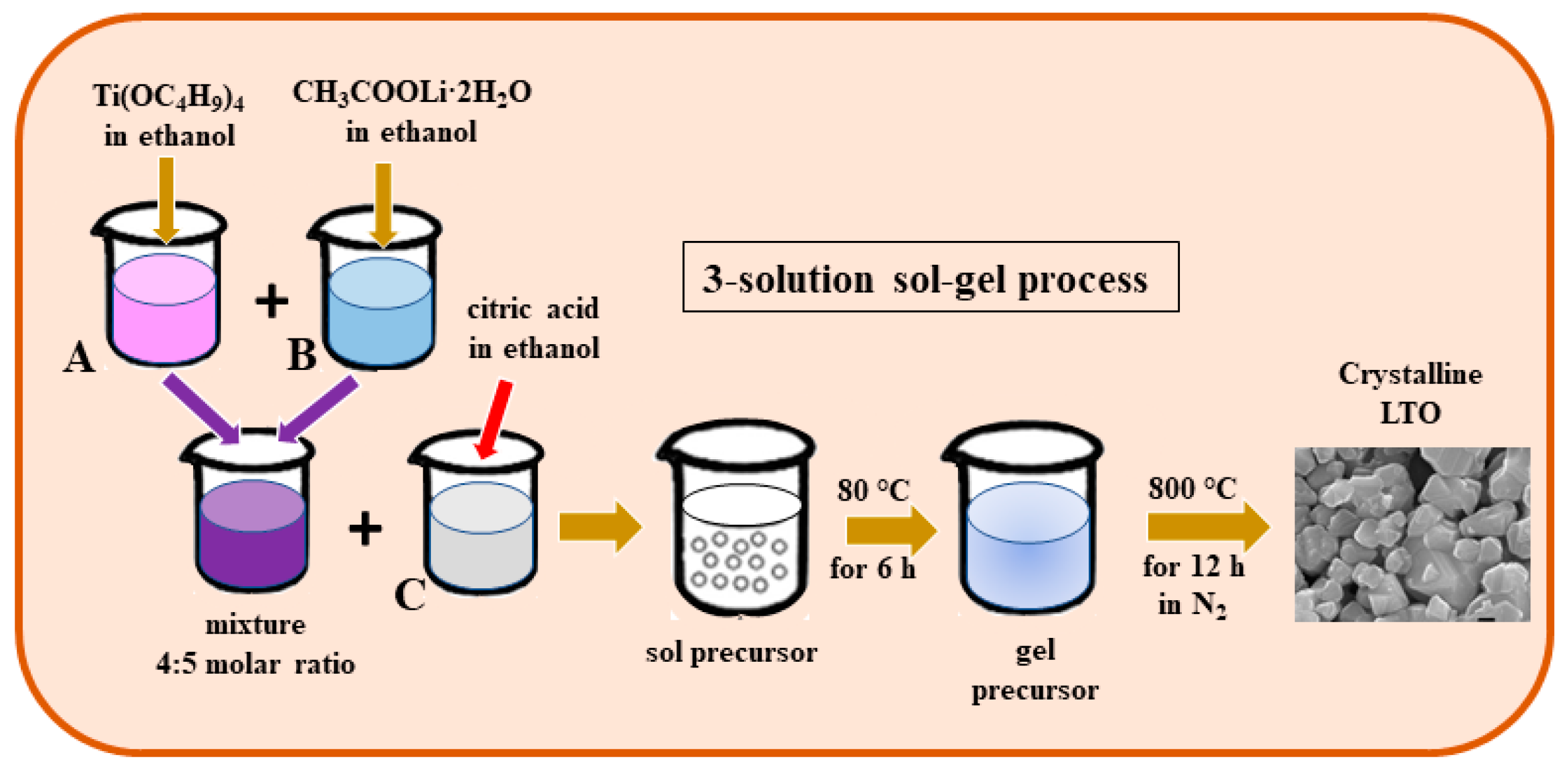
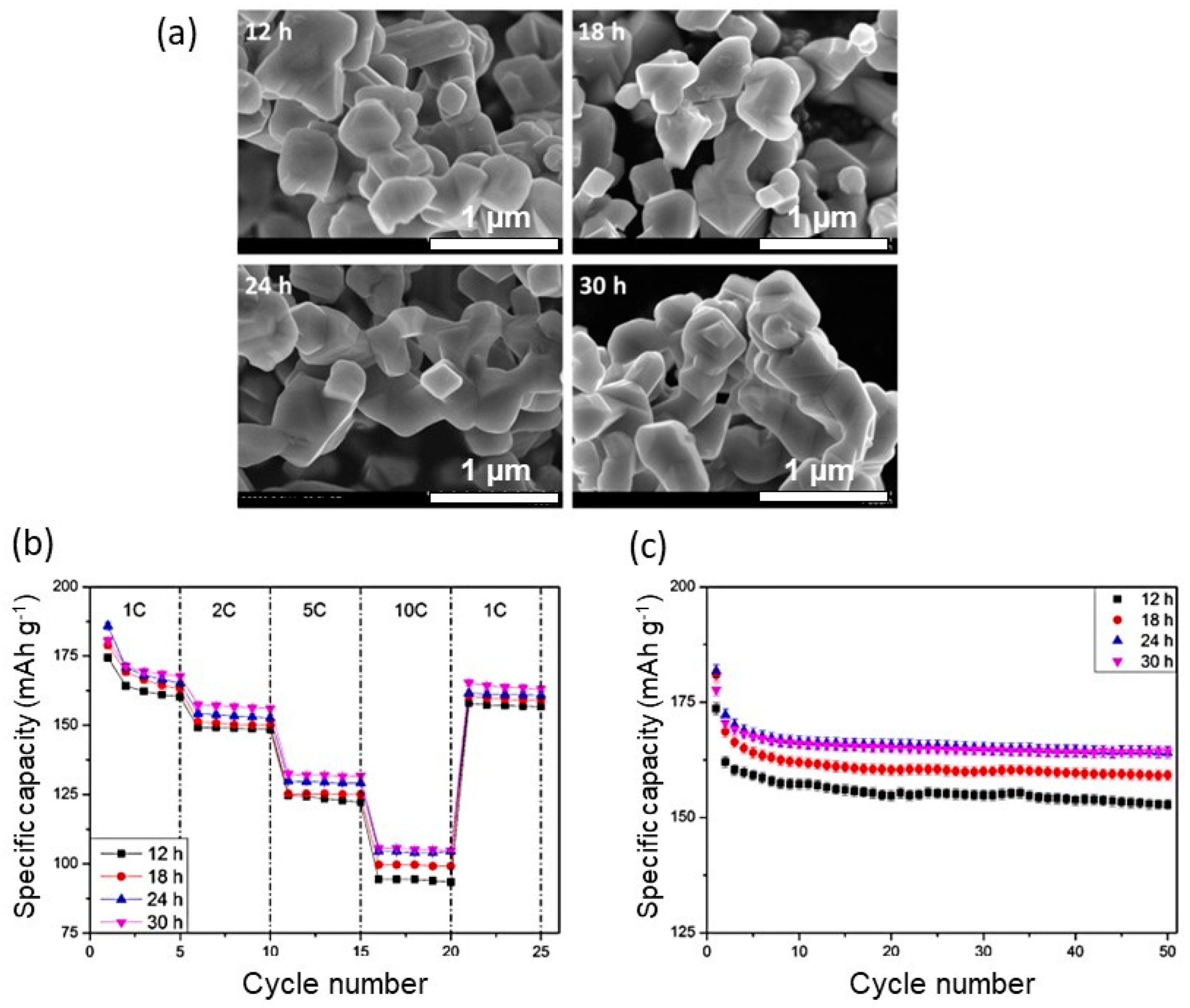
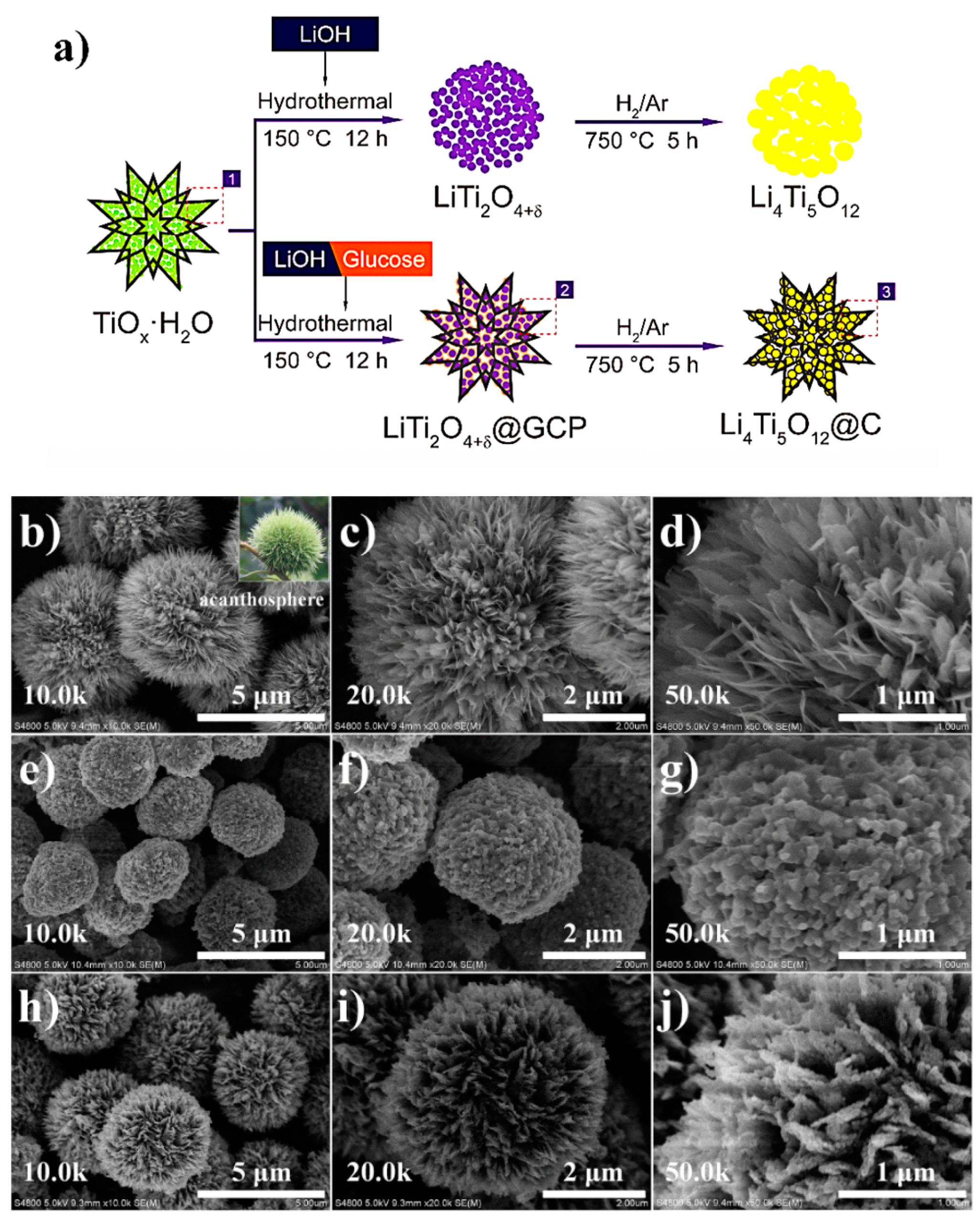
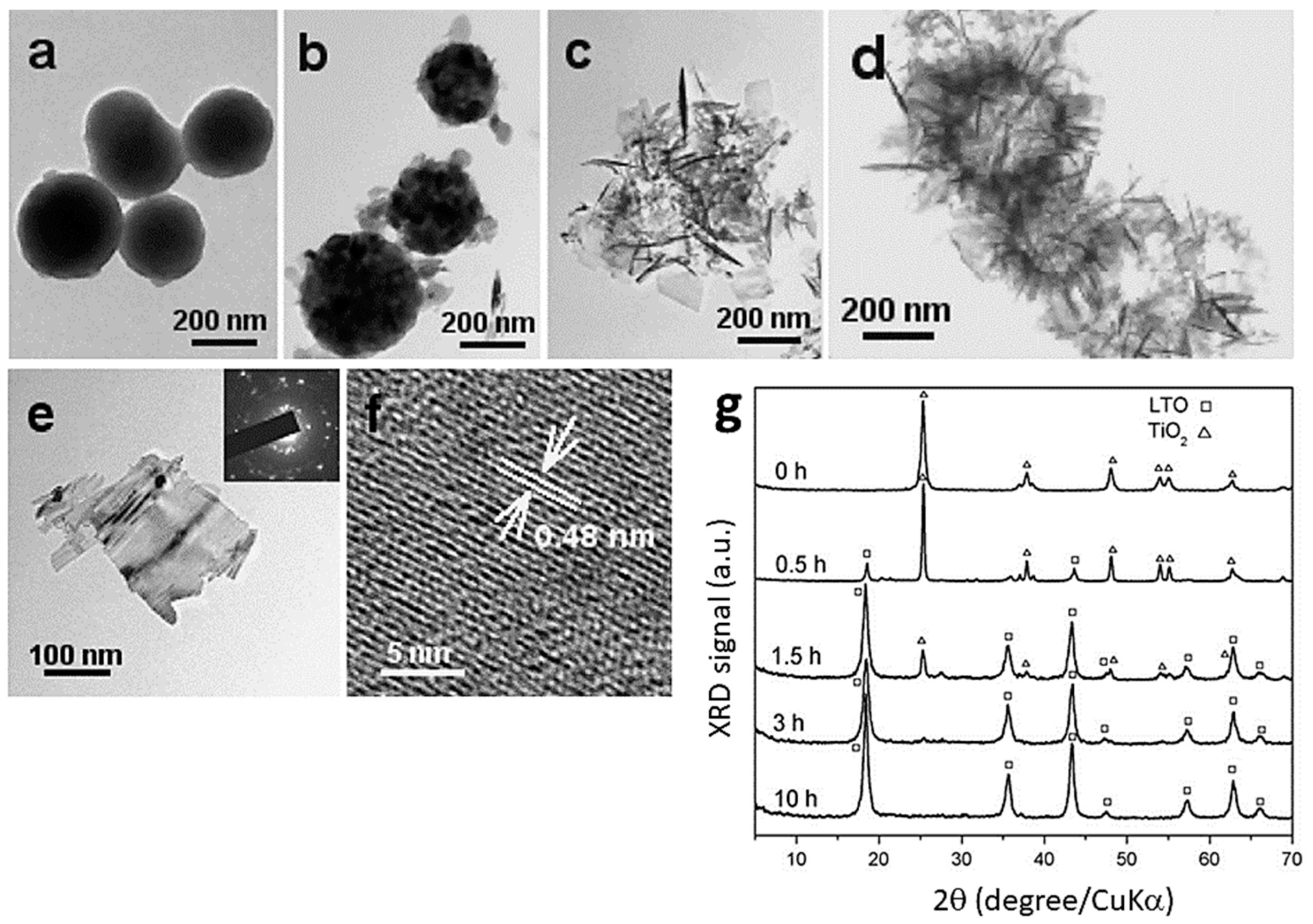
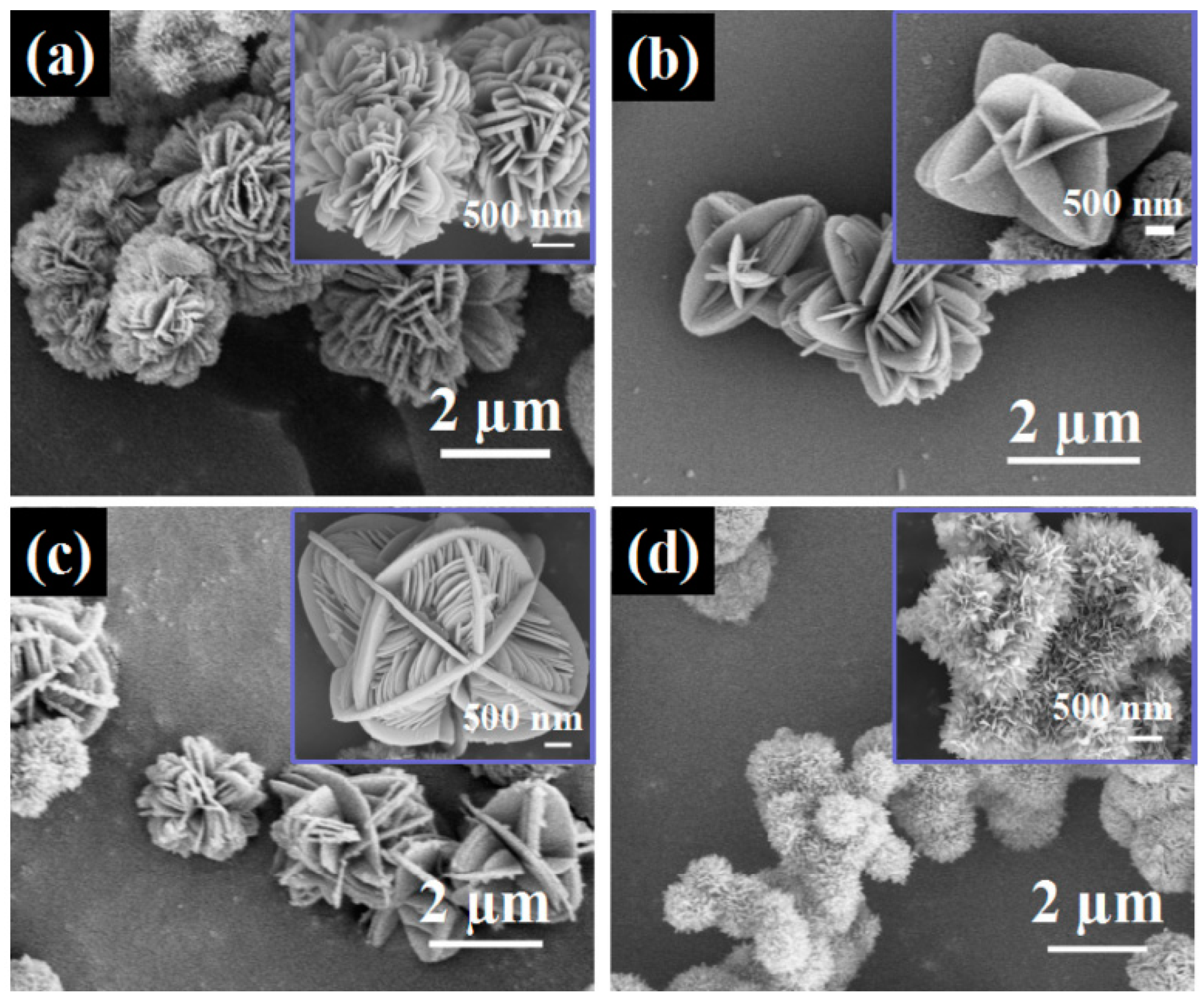
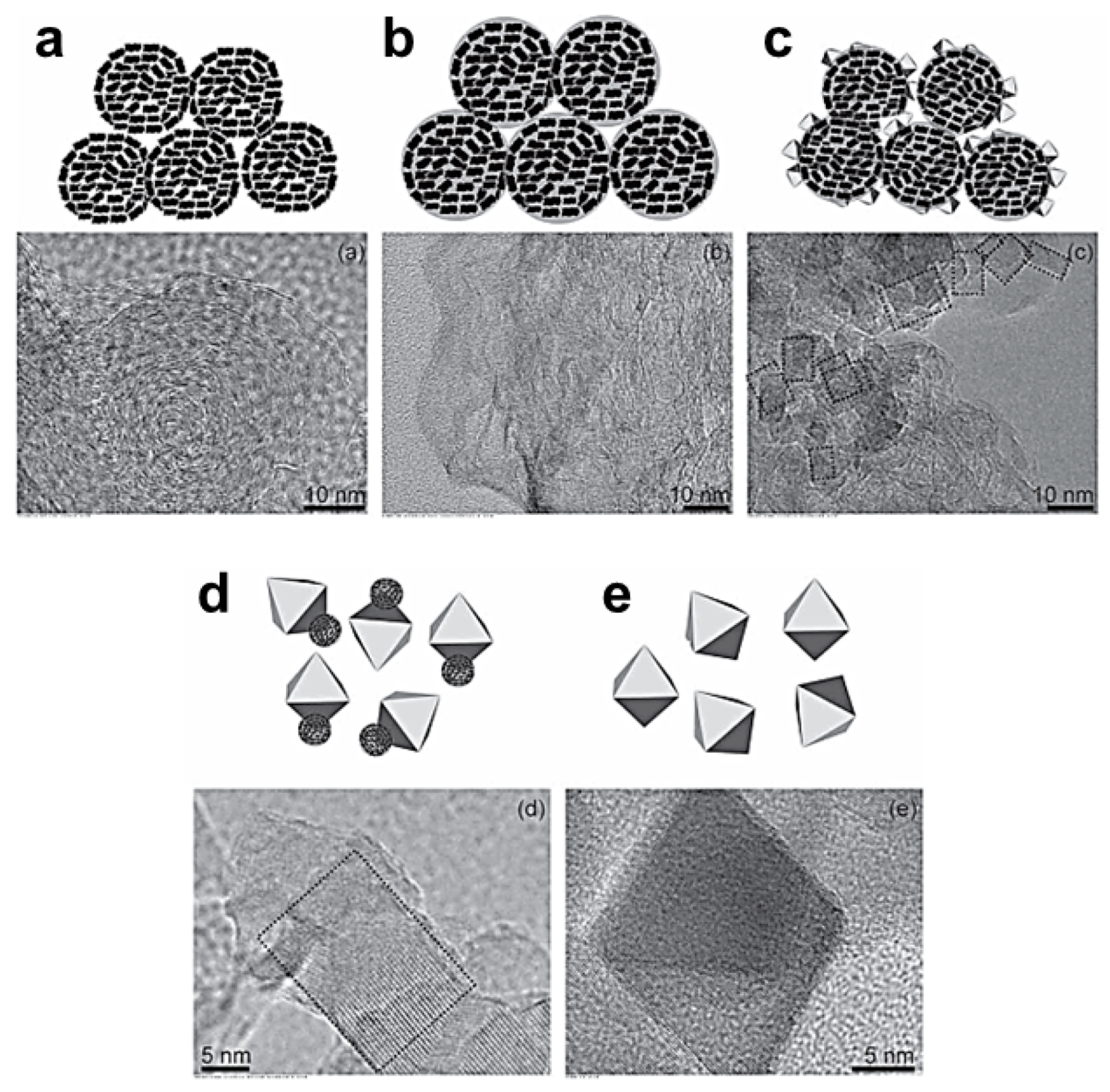
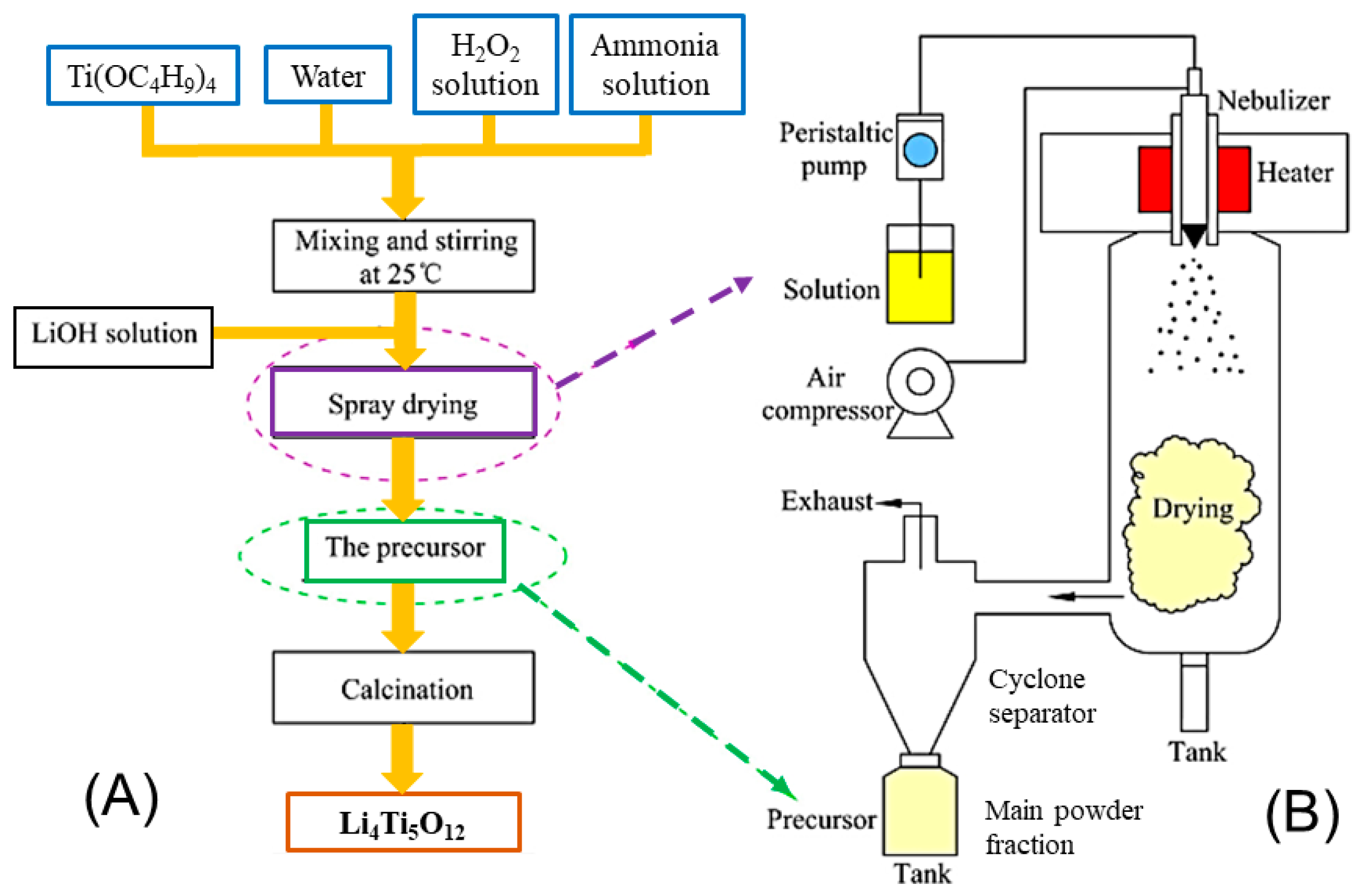

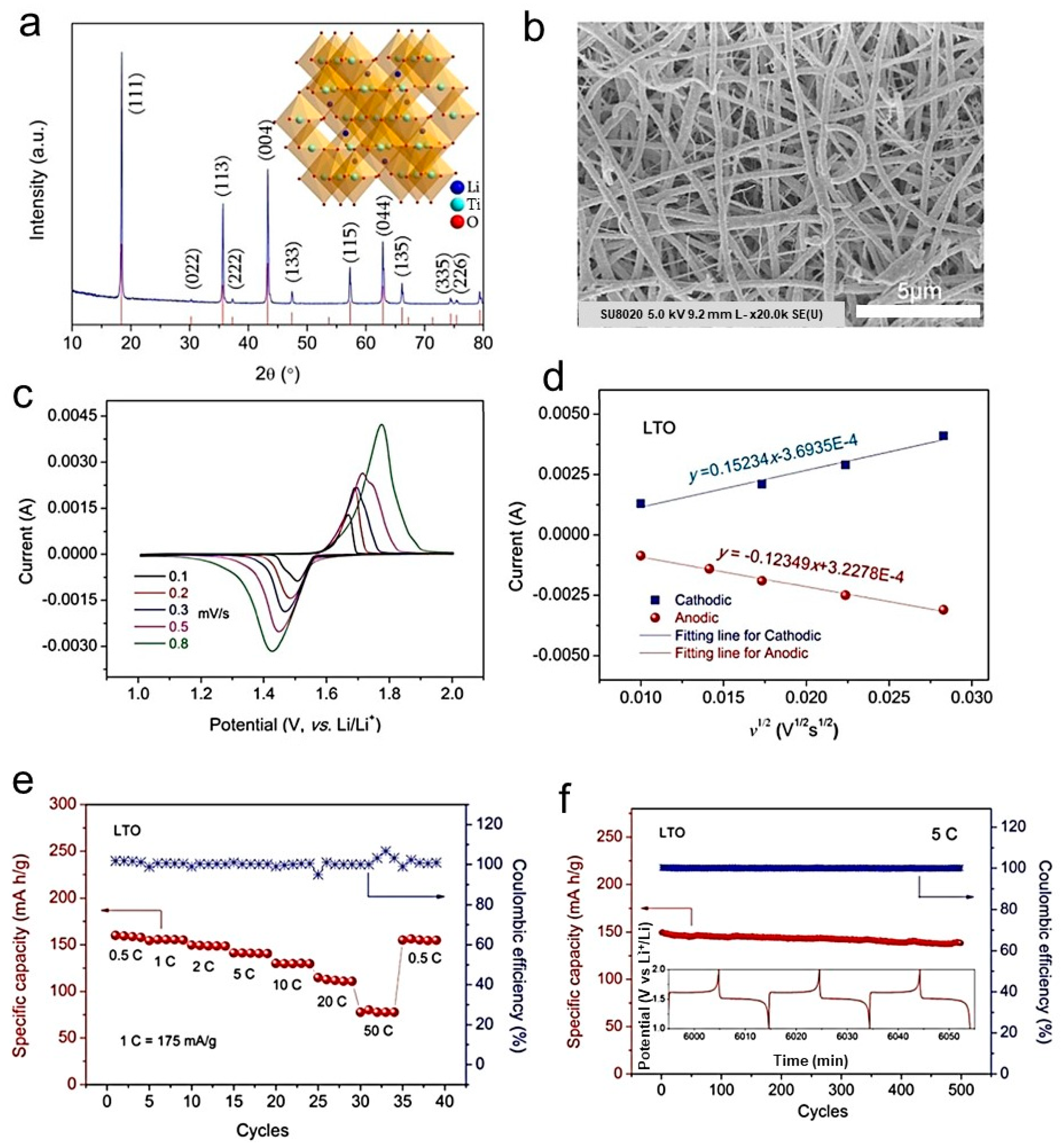
| Type | Particle | Method (a) | Reagents (b) | Particule Size (nm) | Ref. |
|---|---|---|---|---|---|
| 0D | nanoparticle | CL | A-TiO2+LiOH | 30 | [119] |
| nanosphere | HL | TiN+LiOH+PVP | dia. ~120 | [39] | |
| 1D | nanorod | HT | A-TiO2+LiOH | dia. ~200 | [122] |
| nanowire | HT+IE | TiO2+NaOH+LiOH | dia. 50–100 | [129] | |
| nanofiber | ES | LAA+TIP+PVP | dia. ~500 | [135] | |
| nanotube | HR | TNBT+CTACl+LiOH | outer 11/inner 6 | [54] | |
| nanoflake | HT | TBuT+EG+LiOH | 200–400 | [142] | |
| nanobelt | ES | TIP+LiNO3+DMF+PVP | dia. 100 | [144] | |
| 2D | nanoplate | HT | TBuT+LiOH+BA | 300 × 8 | [152] |
| nanosheet | EF+CL | H0.68Ti1.83O4+LiOH | 400 × 2.5 | [168] | |
| wave-like | HL | Li+DMEA+TBuT | 400 × 10 | [152] | |
| 3D | nanoflowers | HT | LiOH+H2O2+TNBT | - | [153] |
| nanoporous | HT | A-TiO2+LiOH | pore dia. 2.6 | [55] | |
| mesoporous | SG | TIP+Li+AC+PBI-b-PEO | pore = 20 | [67] | |
| hierarchical | US+HT | TBuT+LiOH+CTAB | - | [158] | |
| nanotube arrays | TP | TiO2+LiOH | - | [159] | |
| nanobelt arrays | IE+HT | Ti foil+HCL+LIOH | - | [160] | |
| nanoflake arrays | TP+HT | TIP+LiOH | 10–50 | [161] | |
| nanosheet arrays | HT+TD | Ti foil+LiOH | ~14 | [169] | |
| nanowire arrays | HT+IE | Ti foil+LiOH | 90 | [163] | |
| nanowall arrays | HT | Ti foil+NaOH+LiOH | 10–20 | [164] |
| Raman Shift (cm−1) | Assignment | |||||
|---|---|---|---|---|---|---|
| Observed | From Ref. [174] | from Ref. [175] | From Ref. [176] | From Ref. [177] | ||
| 234 | 225 | 232 | 233 | 232 | νb(O−Li−O) | F2g1 |
| 265 | 272 | 271 | - | 271 | νb(O−Ti−O) | F2g2 |
| 347 | 340 | 347 | 348 | 355 | νs(Li16dO6) | F2g3 |
| 427 | 420 | 430 | 419 | 428 | νs(Li8aO4) | Eg |
| 517 | 514 | 520 | - | 6 | overtone | - |
| 674 | 675 | 671 | 670 | 672 | νs(TiO6) | A1g |
| 754 | - | 749 | 748 | 730 | νs(TiO6) | A1g shoulder |
| Synthesis | Ti Source (a) | Li Source | Solution/Additive (b) | Particle Size (nm) | Ref. |
|---|---|---|---|---|---|
| Solid-state reaction | TiO2 | Li2CO3 | acetone | >1000 | [185] |
| Solid-state reaction | TiO2 | Li2CO3 | urea | 400 | [179] |
| Hydrolysis | TiN | LiOH∙H2O | H2O2+NH3 | 120 | [39] |
| Solvothermal | TBT | LiOH∙H2O | water-ethanol (2:3) | 800–1300 | [186] |
| Spray pyrolysis | TIP | Li(CH3COO) | 2-propanol (IPA) | 120 | [115] |
| Ball milling | A-TiO2 | Li2CO3 | ammonium salt | 230 | [93] |
| Spray drying | R-TiO2 | Li2CO3 | polyvinyl butyral | 1000 | [111] |
| Hydrothermal | TIP | LiOH∙H2O | ethanol-water | 200–400 | [110] |
| Wet chemistry | TiOCl2+NH4OH | Li(CH3COO)∙2H2O | water+oxalic acid | 5000 | [167] |
| Combustion | TiO2 | LiOH∙H2O | methyl alcohol | 30–40 | [104] |
| Combustion | Ti(OH)4 | LiNO3 | HNO3 | 40–80 | [103] |
| Combustion | R-TiO2 | Li2CO3 | cellulose/HNO3 | - | [102] |
| Combustion | A-TiO2 | LiNO3 | cellulose/glycine | 200–800 | [101] |
| Ball milling | TIP | LiNO3 | lactic acid/NH4NO3 | 200–300 | [100] |
| Solid-state reaction | TiCl4 | LiCl | 70 wt.% oxalic acid | 41 (c) | [187] |
| Sol–gel | TIP | Li ethoxide | PBI-b-PEO | 20–30 | [67] |
| Soft-templating | TBT | Li(CH3COO)∙2H2O | 2-methoxyethanol | 18 (d) | [188] |
| Microwave | TIP | dissolved Li metal | benzyl alcohol | 150 (d) | [189] |
| Precipitation | TBT | LiOH | DI water | 10 (e) | [190] |
| Sol–gel | TiCl4 | LiOH∙H2O | ethanol+TEA | 360 | [191] |
| Hydrothermal | Ti foil | LiOH∙H2O | H2O2+DI water | ~12 | [192] |
| Solid-phase | H2TiO3 | Li2CO3 | - | 800 | [194] |
| Ti Source | Li Source (a) | Synthesis Procedure | Particle Size (µm) | Ref. |
|---|---|---|---|---|
| anatase TiO2 | Li2CO3 | mixed in acetone; 900 °C/10 h in air | ~1.5 | [43] |
| anatase TiO2 | Li2CO3 | ball milled; 800 °C/5 h in air, N2, H2/Ar | 0.3–0.4 | [205] |
| anatase TiO2 | LiOAc | intermediate phase Li2TiO3 | 0.1 | [231] |
| TiO(OH)2 | Li2TiO3 | Li:Ti = 0.85; 750–800 °C/6 h in air | 1–5 | [232] |
| TiO2+6% NaCl | LiOH | Li:Ti = 0.8; 800 °C/12 h in O2 | 1.8–2.3 | [233] |
| Ti(C4H9O)4 | LiOH·H2O | dissolved in ethyl alcohol 750 °C/5 h in air | - | [234] |
| rutile TiO2 | Li2CO3 | milling methanol; 800 °C/12 h in air | - | [45] |
| rutile TiO2 | Li2CO3 | ground with methanol; 800 °C/12 h in air | 0.35 | [235] |
| Ti foil | LiOH | 30% (w/w) H2O2 aqueous solution; 130 °C/5 h | 1/0.012 | [192] |
| anatase TiO2 | Li2CO3 | ground with ethanol/3 h; 900 °C/5 h in air | 0.4–0.5 | [47] |
| anatase TiO2 | Li2CO3 | ball mill 400 rpm/4 h; 750 °C/12 h | ≈0.7 | [48] |
| TiO2 | Li2CO3 | ball mil/48 h with hexamethylene; 900 °C/24 h | - | [236] |
| TiO2 | Li2CO3 | phenolic resin+ethyl alcool slurry solution | 0.2–0.5 | [237] |
| H2TiO3 | Li2CO3 | direct mixing + sintering at 700 °C/6 h in Ar | 0.8 | [184] |
| Type (a) | Reagents (b) | Sintering (°C:h) | Particle Size (nm) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| NPs | A-TiO2+Li2CO3 | 800:2 | 40 | [email protected] A g−1 (500) | [119] |
| NPs | A-TiO2+LiOH+PAA | 800:8 | ~300 | 133@10C (50) | [219] |
| MPs | A-TiO2+LiOH | 800:12 | ~2000 | 114@10C (1065) | [233] |
| AGs | LiCl+TiCl4+OA | 800:10 | - | 70@10C (200) | [187] |
| SpGs | TiO2+Li2CO3 | 800:5 | ~300 | 80@40C (5) | [205] |
| Sub-µ | R-TiO2+Li2CO3 | 800:36 | 700 | 35@30C (5) | [235] |
| Sub-µ | TiO2+Li2CO3 | 850:24 | ~600 | 93@5C (200) | [224] |
| SpGs | A-TiO2+Li2CO3 | 750:12 | ~700 | 60@10C (500) | [48] |
| NPs | A-TiO2+Li2CO3 | 800:3 | 162 | 139@10C (3) | [227] |
| NPs | TiCl4+H2C2O4+LiCl | 800:10 | 200 | 70@10C (100) | [222] |
| NPs | TiO2+Li2CO3 | 800:20 | 200–500 | 170@1C (50) | [248] |
| AGs | A-TiO2+LiOAc | 800:3 | - | 130@10C (10) | [249] |
| NPs | A-TiO2+Li2CO3 | 850:24 | ~500 | 212@1C (150) | [250] |
| Ti Source | Li Source | Complexing Agent | Solvent | Particle Size (nm) | Ref. |
|---|---|---|---|---|---|
| TBT | LiOAc | Triethanolamine | ethanol | 223–264 | [64] |
| TIP | LiOEt | PEG | ethanol | 4–5 | [285] |
| TBT | LiOAc | acetic acid | isopropyl alcohol | 100 | [62] |
| TIP | LiOH | n-octanol+HOP | acetonitrile | 450 | [89] |
| TET | Li2CO3 | citric acid | TiH4O4+HNO3 | 300–650 | [286] |
| TBT | LiOAc | lauric acid | alcohol | 65 | [287] |
| TBT | Li2CO3 | oxalic acid | alcohol | 200 | [63] |
| TBT | LiOAc | glacial acetic acid | ethanol+water | 400–800 | [267] |
| TBT | LiOAc | propionic acid | alcohol | 150 × 60 | [283] |
| TIP | LiOAc | P123 copolymer | ethanol | 90–110 | [288] |
| TBT | LiOAc | PVP | isopropyl alcohol | 20–75 | [279] |
| Type (a) | Reagents (b) | Sintering (°C:h) | Particle Size (nm) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| NCs | TBT+ LiOAc | 800:10 | 65 | 163@1C (50) | [287] |
| NCs | TBT+LiOAc | 700:12 | 136 | 157@10C (1) | [303] |
| TF | TBT+LiOAc | 650 | 18 (pores) | 148@64C (3000) | [188] |
| NCs | TIP+LiOAc | 700:5 | 100 | 108@40C (250) | [288] |
| NPs | TIP+LiOH | 800:10 | 450 | [email protected] (80) | [89] |
| NSs | TBT+LiOAc | 800:1 | 150 × 60 | 56@20C (80) | [283] |
| NPs | TBT+Li2CO3 | 750:5 | 200–500 | 160@1C (1000) | [263] |
| NPs | TBT+LiOAc | 800:12 | 300 | 60@10C (50) | [178] |
| NPs | TIP+LiOAc | 800:12 | 200 | [email protected] (30) | [291] |
| NPs | TBT+Li2CO3 | 750:5 | ~100 | 145@5C (400) | [301] |
| NPs | TBT+Li2CO3 | 800:20 | 200 | [email protected] mA cm−2 (35) | [63] |
| NPs | TIP+LiOAc | 800:2 | 50–200 | [email protected] (200) | [304] |
| NPs | TBT+LiOAc+HCl | 900:2 | 240 | 302@1C (3) | [305] |
| NPs | TIP+LiOH+CA | 800:2 | - | [email protected] (3) | [306] |
| NPs | TIP+LiOAc | 800:12 | 5–14 | 214@1C (150) | [307] |
| NCs | TBT+LiOH+EtOH | 600:2 | 50–200 | 90@40C (100) | [308] |
| Ti Source | Li Source | Solution | Process | Ref. |
|---|---|---|---|---|
| TBT | LiOH | DI water/ethanol | stirring for 24 h | [106] |
| TBT+ammonia | LiOH | glycerol/water | formation of clear solution | [314] |
| TBT | LiOAc | DI water | drying at 90 °C for 5 h | [313] |
| TBT | LiOH | DI water | slurry dried at 100 °C + grinding | [315] |
| TBT | Li metal | DMEA | H2 byproduct is used as fuel | [152] |
| TiN | LiOH | DI water/H2O2 | stirring with ammonia | [39] |
| Type (a) | Reagents (b) | Sintering (°C:h) | Particle Size (nm) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| NPs | A-TiO2+LiOAc+AG | 800:10 | 50–400 | 153@1C (1000) | [316] |
| Nc1 | A-TiO2+Li2CO3+EtOH | 750:12 | <1 µm | 108@10C (500) | [48] |
| Nc1 | A-TiO2+Li2CO3+EtOH | 750:12 | 100–200 | 260@88 A g−1 (160) | [241] |
| NPs | TiO2+Li2CO3+EA | 800:20 | 200–500 | 171@1C (50) | [325] |
| C-Ncs | TiO2+Li2CO3+EA | 800:10 | 50–400 | 142@1C (1000) | [318] |
| NPs | R-TiO2+Li2CO3 | 800:3 | ~400 | 150@4C (3) | [328] |
| HEBM | A-TiO2+LiOH+GL | 800:12 | 150 | 140@1C (1) | [92] |
| HEBM | A-TiO2+Li2CO3+PAA | 800:3 | 146 | 120@20C (3) | [93] |
| NPs | TOS+LiOH | 500:2.5 | ~1 µm | [email protected] A g−1 (50) | [46] |
| NPs | TBT+Li2CO3+NH3 | 800:7 | 200–500 | [email protected] (300) | [312] |
| NPs | TiCl4+LiOAc+CA | 800:10 | 500 | 141@30 mA g−1 (30) | [326] |
| NPs | A-TiO2+Li2CO3 | 800:8 | 620 | 135@10C (10) | [329] |
| MPs | a-TiO2+Li2CO3+Et | 800:12 | 1 µm | [email protected] (100) | [330] |
| Reagents (a) | Chelate (b) | Sintering (°C:h) | Particle Size (nm) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| TiO2+LiOAc | PVP | 800:8 | 200–500 | 142@5C (20) | [280] |
| TiO2+LiNO3 | glycine | 750:2 | 40–80 | 175@1C (1) | [217] |
| TIP+LiNO3 | lactic acid | 300 | 200–300 | 132@1C (100) | [100] |
| TBT+LiNO3 | glycine | 850:17 | 50–100 | 187@1C (50) | [99] |
| R-TiO2+Li2CO3 | cotton fiber | 800:5 | - | 130@2C (50) | [102] |
| TBT+LiNO3 | glycine | 800:5 | 50–350 | 111@10C (150) | [341] |
| TiNt+LiNO3 | glycine | 800:½ | 50–100 | 159@1C (200) | [342] |
| TiNt+LiNO3 | glycine | 800 | <150 | [email protected] (100) | [97] |
| Reagents (a) | HT Reaction (°C:h) | Sintering (°C:h) | Particle Size (nm) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| TIP+LiOH+EG | 170:36 | 500:2 | 200 | 152@8C (100) | [146] |
| TIP+LiOH+H2O2 | 150:¼ | 500:2 | ~100 | [email protected] (30) | [351] |
| TBT+LiOH | 180:12 | 600:3 | 200–300 | [email protected] (50) | [50] |
| TBT+LiOH | 180:12 | 550:6 | 300–600 | 150@20C (5) | [345] |
| TBT+LiOH | 120 | 500:6 | 6–11 (b) | [email protected] (25) | [54] |
| TBT/LiOH | 180:24 | 500:10 | nanosheets | 115@5C (50) | [354] |
| Ti(SO4)2+LiOH | 100:20 | 800:2 | 500 | [email protected] (70) | [53] |
| TBT+LiOH | 180:36 | 66:6 | nanoflakes | [email protected] (100) | [355] |
| H2TiO3+LiOH | 170:24 | - | - | [email protected] (30) | [376] |
| TBT+LiOEt | 150:10 | 400–500 | <100 | 140@2C (1) | [195] |
| TBT+LiOH | 180:36 | 700:6 | nanosheets | 144@ 2C (600) | [149] |
| Na2Ti3O7+LiOH | 180:24 | 450:1 | 500 × 40 | 0.55@3 mA cm−2 (1000) (c) | [145] |
| H2Ti3O7+LiOH | 150:12 | 500:4 | 50 × 10 | 95@5C (300) | [129] |
| TBT+LiOAc | 170:72 | 1050:1 | 30–40 | 80@60C (5) | [362] |
| TBT+LiOAc+PVP | 160:24 | 800:10 | 20–75 | 125@20C (50) | [279] |
| TiO2(P25)+LiOH | 120:24 | 400:4 | nanosheets | 126@20C (3000) | [353] |
| Reagents (a) | HT Reaction (°C:h) | Sintering (°C:h) | Particle Size (nm) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| TBT+LTB+P123 | 170:20 | 400:10 | 35–55 | 140@100C (1000) | [387] |
| TIP+LiOH+EtOH | 180:10 | 600:3 | 20 | 125@1C (100) | [383] |
| TBT+LIOH+EG | 180:16 | 600:8 | 100–200 | 160@10C (1000) | [200] |
| TBT+LiOAc+EtOH | - | 550:2 | 0.5–1 µm | 92@20C (100) | [60] |
| TIP+Li+BA | 250:48 | 750:1 | 1–2 µm | 148@1C (200) | [203] |
| TBT+LiOH+EtOH | 200:24 | 600:3 | 200 | [email protected] (100) | [384] |
| TiO2+LiOH+EtOH | 180:16 | 700:4 | ~660 | 106@10C (100) | [180] |
| TBL+LiOH+EtOH | 200:12 | 500:3 | 13 | 105@10C (10) | [386] |
| Reagents (a) | Template | Particle Size (nm) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| TIP+Li+EtOH+Tol | carbon black | ~70 | 160@1C (100) | [397] |
| TIP+Li+EtOH+OA | PI-b-PEO | mesoporous | 115@10C (500) | [155] |
| TiCl4+LiOAc | PMMA | microporous | [email protected] mA cm−2 | [167] |
| TiCl4+LiOH | ZnO nanorods | nanotube arrays | 110@40C (200) | [399] |
| R-TiO2+Li2CO3 | PFR | ~800 | 60@20C (5) | [154] |
| TiO2+LiOH | ZnO nanorods | nanotubes (b) | 144@10C (500) | [159] |
| TBT+LiOAc | NPGF | fibers | 146@1C (100) | [134] |
| TOT+LiOAc+EtOH | whatman paper | 250–300 | 88@5C (1100) | [87] |
| Reagents (a) | Sintering (°C:h) | Particle Size (µm) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| TIP+LiOH+H2O2 | 675:16 | 1–5 | 75@20C (100) | [432] |
| TiO2 (P25)+LiOH | 750:10 | ~5 | 140@1C (200) | [437] |
| TiO2+LiOH+PEG | 850:2 | 0.6 | 120@4C (10) | [430] |
| TBT+LiOH | 650:16 | 1–1.5 | 100@20C (100) | [403] |
| TIP+Li2CO3+OA | 800:12 | 0.2–0.4 | 120@2C (200) | [407] |
| A-TiO2+LiOH | 850:12 | 0.3–0.5 | 40@20C (2) | [410] |
| A-TiO2+Li2CO3+APC | 850:5 | 0.2 | 110@5C (100) | [404] |
| A-TiO2+Li2CO3 | 800:12 | 10–20 | 145@1C (250) | [405] |
| n-TiO2+Li2CO3 | 850:12 | 1.18 | [email protected] (1) | [406] |
| TIP+H2O2+LiOH+NH3 | 700:16 | 1–2 | 135@5C (100) | [408] |
| A-TiO2+Li2CO3 | 850:8 | 10 | 173@1C (400) | [416] |
| TBT+LiOH+AA | 750:4 | 0.2 | 192@2C (20) | [424] |
| TIP+LiNO3+IsoP | 800:2 | 3.5–5.5 | 50@10C (10) | [440] |
| Reagents (a) | Sintering (°C:h) | Particle Size (nm) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| TBT+LiOAc+EtOH | 800:1 | 800 | 146@10C (500) | [450] |
| LNT-EtOH+TIP-EHA | - | ~10 | 146@1C (450) | [77] |
| Li-acac+TIP+EHA+xylene | - | ~14 | 100@25C (10) | [445] |
| LTN+TIP in water | 800:12 | 700 | [email protected] (70) | [72] |
| Li-acac+TIP+EHA | none | ~10 | 87@1C (200) | [77] |
| LiOH+TIP+LA | 800 | ~1000 | 162@1C (100) | [443] |
| THE+LiOAc+MeOH | none | 20 | 140@ 1C (75) | [451] |
| TBT+LNT+EG | none | 600 | [email protected] (50) | [452] |
| TIP+LNT | 800:3 | 700 | 175@1C (100) | [453] |
| TIP+LNT | 700:3 | 300 | [email protected] (50) | [449] |
| Ti Source (a) | Li Source (b) | Solvent (c) | Polymer (d) | Performance (e) (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| TIP | LiOAc | DMF+AA | PVAc | 75@10C (1) | [476] |
| TIP | LiOAc | EtOH+AA | PVP+Pluronic | 123@5C (100) | [474] |
| TIP | LiAAc | isoproxyl alcohol | F127 | [email protected] (30) | [95] |
| TiOAc | LiOAc | EtOH | PVP | 165@1C (100) | [133] |
| nano-LTO | - | DMF | PVP | 115@2C (1) | [464] |
| TBT | LiOAc | ethanol+AAc+AA | PAN | 139@5C (500) | [474] |
| TIP | LiAAc | isoproxyl alcohol | PVP | 120@10C (100) | [140] |
| TIP | LiOAc | EtOH+AA | PVP | 120@1C (200) | [471] |
| TBT | LiAAc | EtOH+AA | PVP | 60@25C (1) | [468] |
| TIP | LiOAc | EtOH+AA | PVP | 140@10C (30) | [137] |
| TIP | LAAc | EtOH+AA | PVP | 132@20C (300 | [135] |
| First Step | Second Step | Particle (a) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| TiO2/NaOH/HNO3 | H-TiOx/LiOH | NTs 7 nm | 110@1C (30) | [130] |
| TiO2/Na2CO3/HCl | H2Ti3O7/LiNO3 | 1–2 µm | 190@5C (3) | [478] |
| TiO2/NaOH/HCl | H2Ti2O5/LiOH | NFs | 118@5C (4) | [477] |
| Ti/NaOH+NaCl/HCl | H2Ti2O5/LiOH | NW arrays | 125@30C (5000) | [375] |
| - | TiO2/NaOH/LiOH | 10–50 nm | 60@50C (1) | [141] |
| TiO2/NaOH/HCl | H2Ti3O7/LiOH | NWs | [email protected] (50) | [132] |
| Ti/NaOH/HCl | H-TiOx/LiOH | arrays 7 µm | 0.2@1 mA cm−2 (1000) (b) | [165] |
| - | TiO2/NaOH/LiOH | 20–30 nm | 148@10C (200) | [479] |
| TiO2/NaOH/HCl | H2Ti2O5/LiOH | NWs 80 nm | 125@10C (100) | [480] |
| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Solid state reaction | Simple method; use of starting materials at low cost; large-scale production | Need for high-temperature treatment; formation of unwanted byproducts; difficult to adjust particle size, particle size distribution, and lack of stoichiometric control |
| Hydrothermal/solvothermal | Production of nanomaterials; well controlled through liquid-phase reaction | Expensive method; time-consuming; not feasible for mass production; organic residues appear with organic solvents |
| Sol–gel | Low-cost; offer products with a homogeneous distribution of uniform, submicron-size particles with good stoichiometric control | Use of chelating agent for the control of nanostructure and morphology; difficult to use for mass production |
| Solution-combustion | Needs additional post-annealing treatment | |
| Microwave | Fast synthesis route; low-temperature process; rapid volumetric heating, high reaction rate; small particle size | Purity and the crystallinity of the products are dependent upon the irradiation power and time; difficult to use for mass production |
| Spray pyrolysis | Direct synthesis of powder from precursor solutions; products with high purity and homogeneity; industrially scalable | Difficult to control the morphology and chemical composition |
| Electrospinning | Simple, versatile, and low-cost technology to produce well-defined fibers at the nanoscale | Two-step procedure; expensive |
| Templating | Formation of mesoporous structures; monodispersed products | Removal of the templates after synthesis may damage the desired configurations; not suitable for large-scale production |
| Molten salt | Low temperature and short time; suitable for small particle size | Not suitable for large-scale production |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julien, C.M.; Mauger, A. Fabrication of Li4Ti5O12 (LTO) as Anode Material for Li-Ion Batteries. Micromachines 2024, 15, 310. https://doi.org/10.3390/mi15030310
Julien CM, Mauger A. Fabrication of Li4Ti5O12 (LTO) as Anode Material for Li-Ion Batteries. Micromachines. 2024; 15(3):310. https://doi.org/10.3390/mi15030310
Chicago/Turabian StyleJulien, Christian M., and Alain Mauger. 2024. "Fabrication of Li4Ti5O12 (LTO) as Anode Material for Li-Ion Batteries" Micromachines 15, no. 3: 310. https://doi.org/10.3390/mi15030310







