Aptasensor Integrated with Two-Dimensional Nanomaterial for Selective and Sensitive Electrochemical Detection of Ketamine Drug
Abstract
:1. Introduction
2. Material and Methodology
2.1. Chemicals, Reagents, and Apparatus
2.1.1. Apparatus/Instrument Used
2.1.2. Spiked Beverages: Alcoholic and Non-Alcoholic Drinks Were Taken for Spike Testing
2.2. Preparation of Standard Solutions
2.3. Synthesis of Nanomaterials
Preparation of Nanographite (NG) Sheets
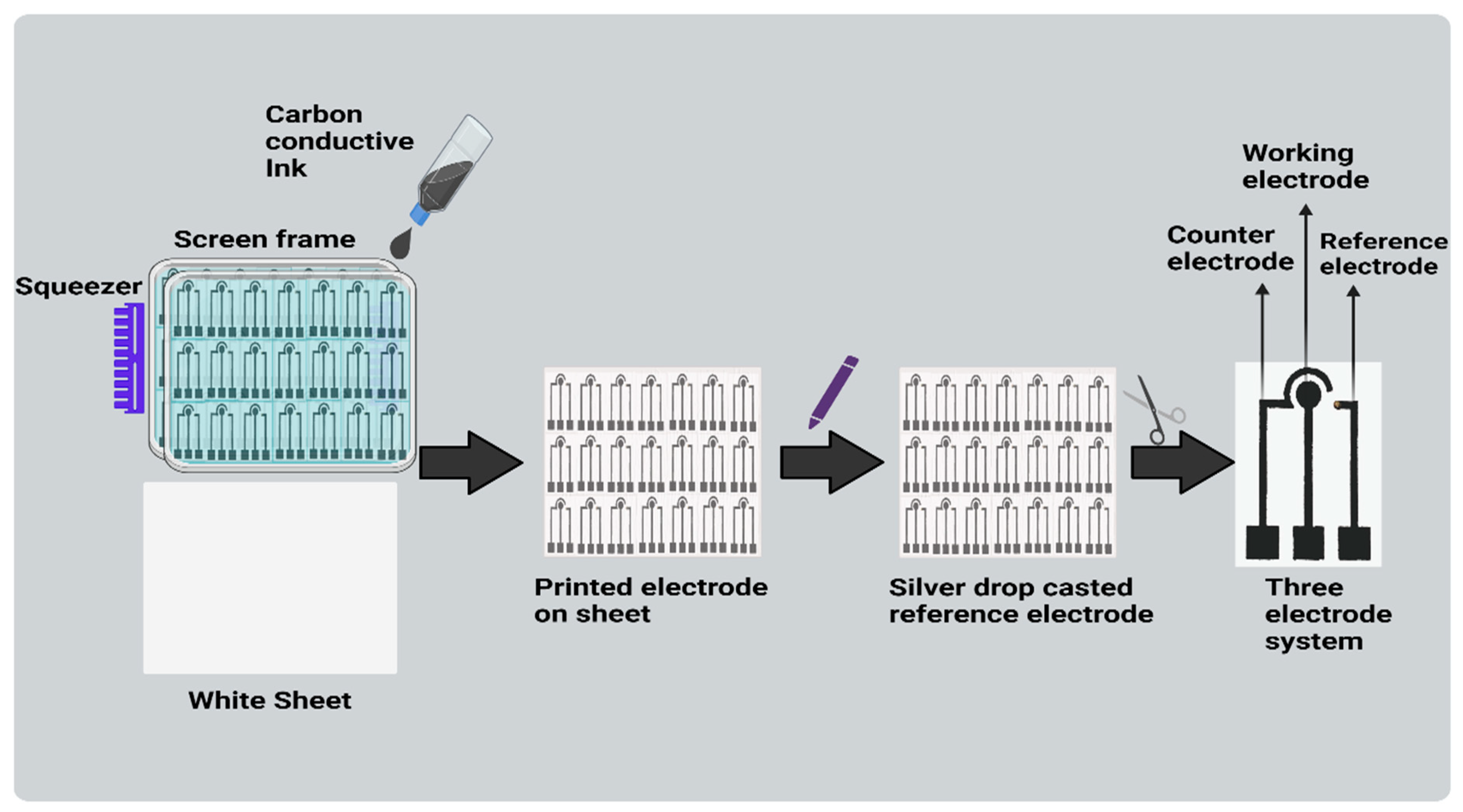
2.4. Fabrication of Paper-Based Three-Electrode System
2.5. Preparation of Spiked Beverages
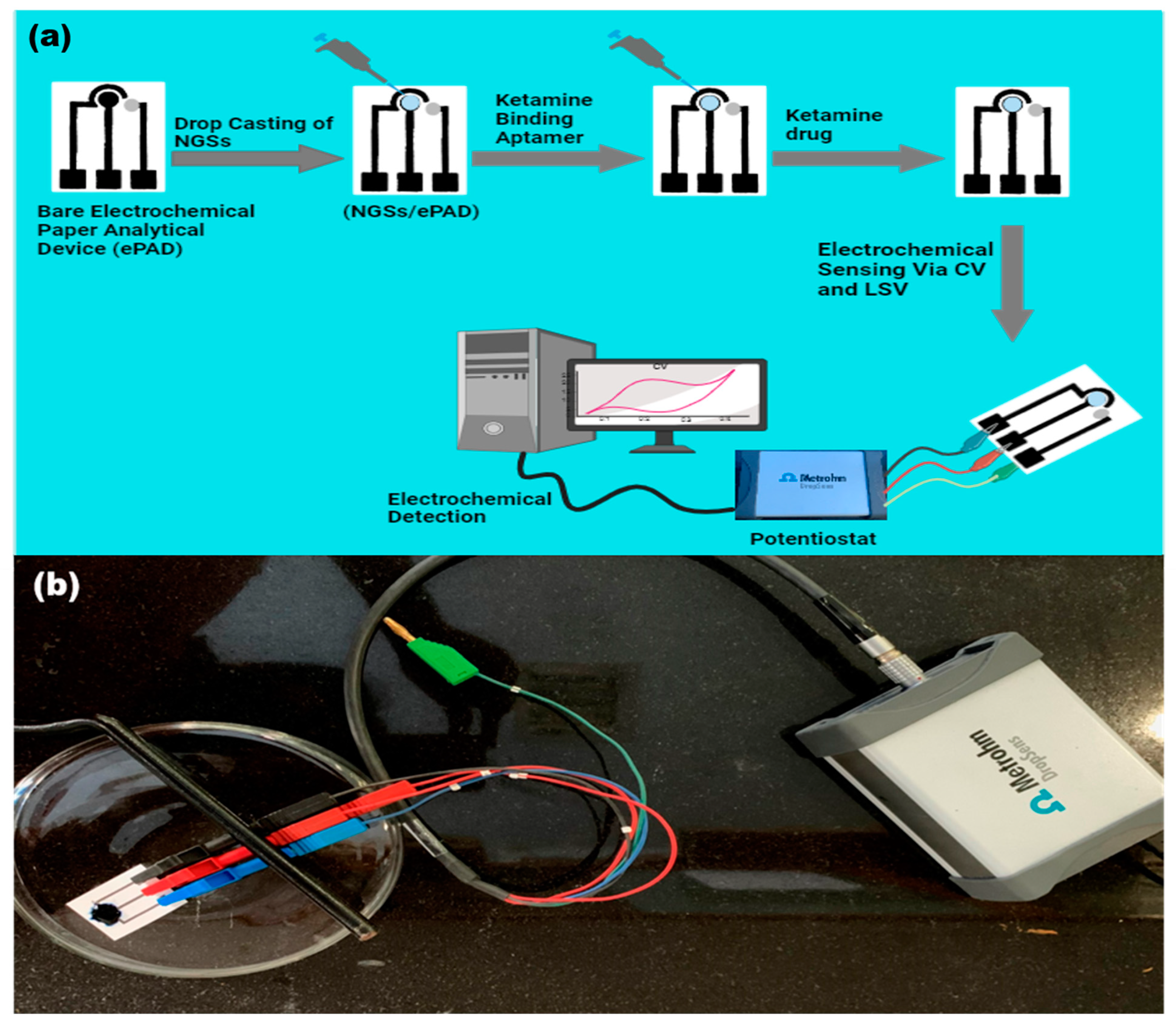
2.6. Immobilization and Deposition of Nanographite Sheets and Aptamer on a Paper-Based Sensor
2.7. Stages for Electrochemical Detection
2.8. Optimization of Different Parameters and Investigation of Repeatability and Stability and Methods for Real-Sample Analysis of Spiked Beverages
3. Results and Discussion
3.1. Sensing Strategy and Acquiring Signals
3.2. Characterization of Nanographite Sheets
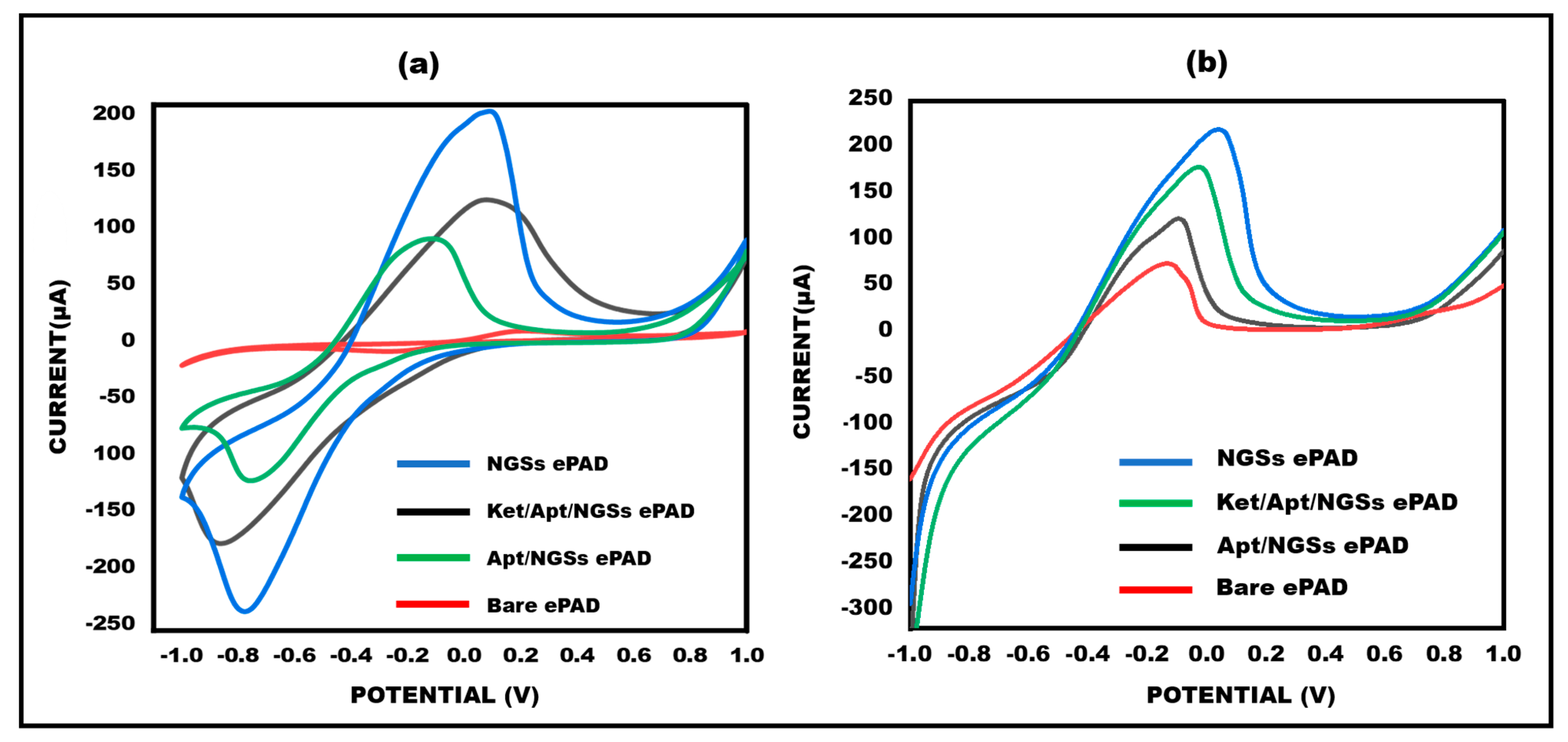
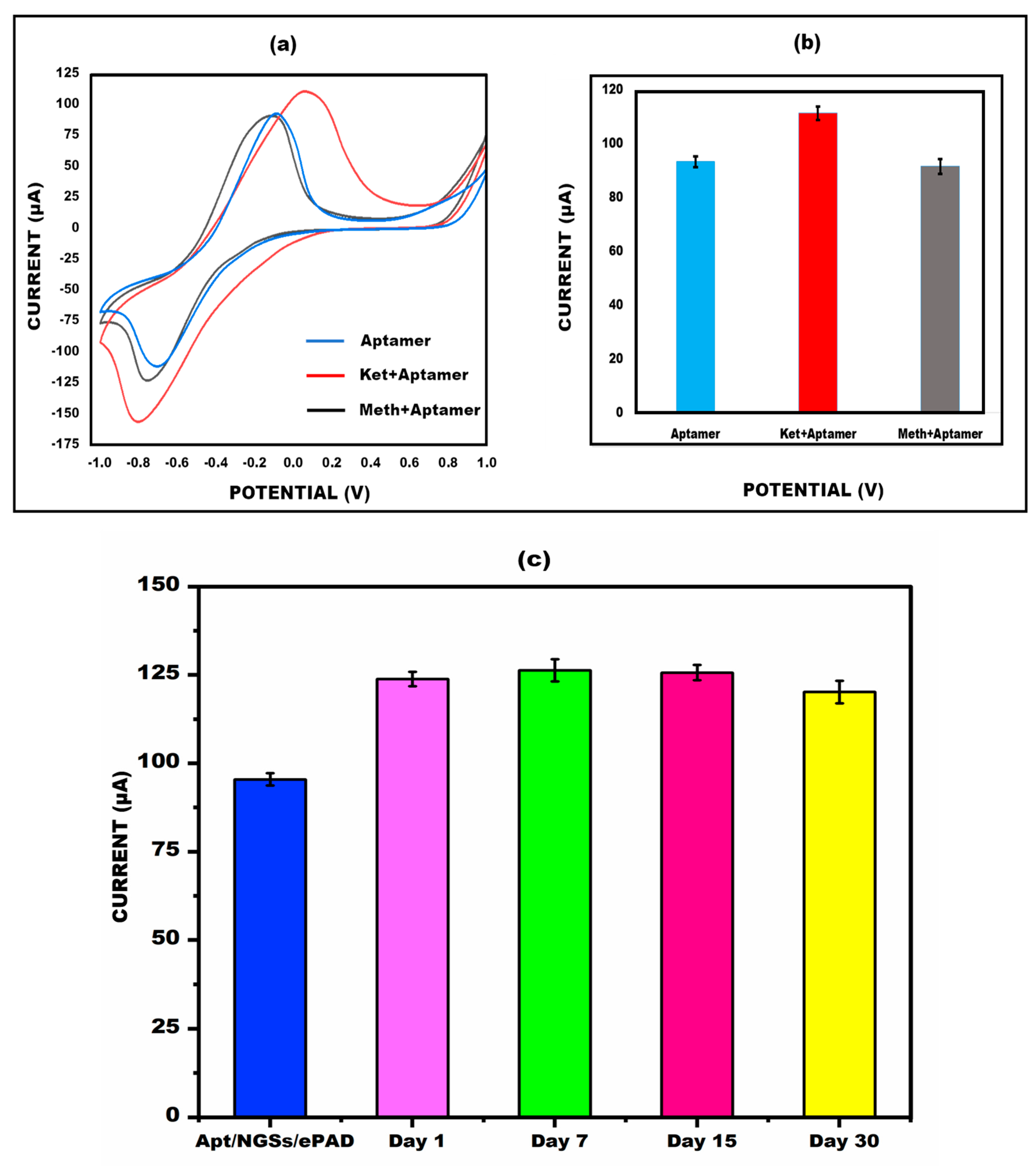
3.3. Electro-Chemical Properties of Ketamine/Aptamer/NGSs at Different Stages
3.4. Effects of Different Ketamine Concentrations on the Aptamer/NGSs Paper-Based Sensor
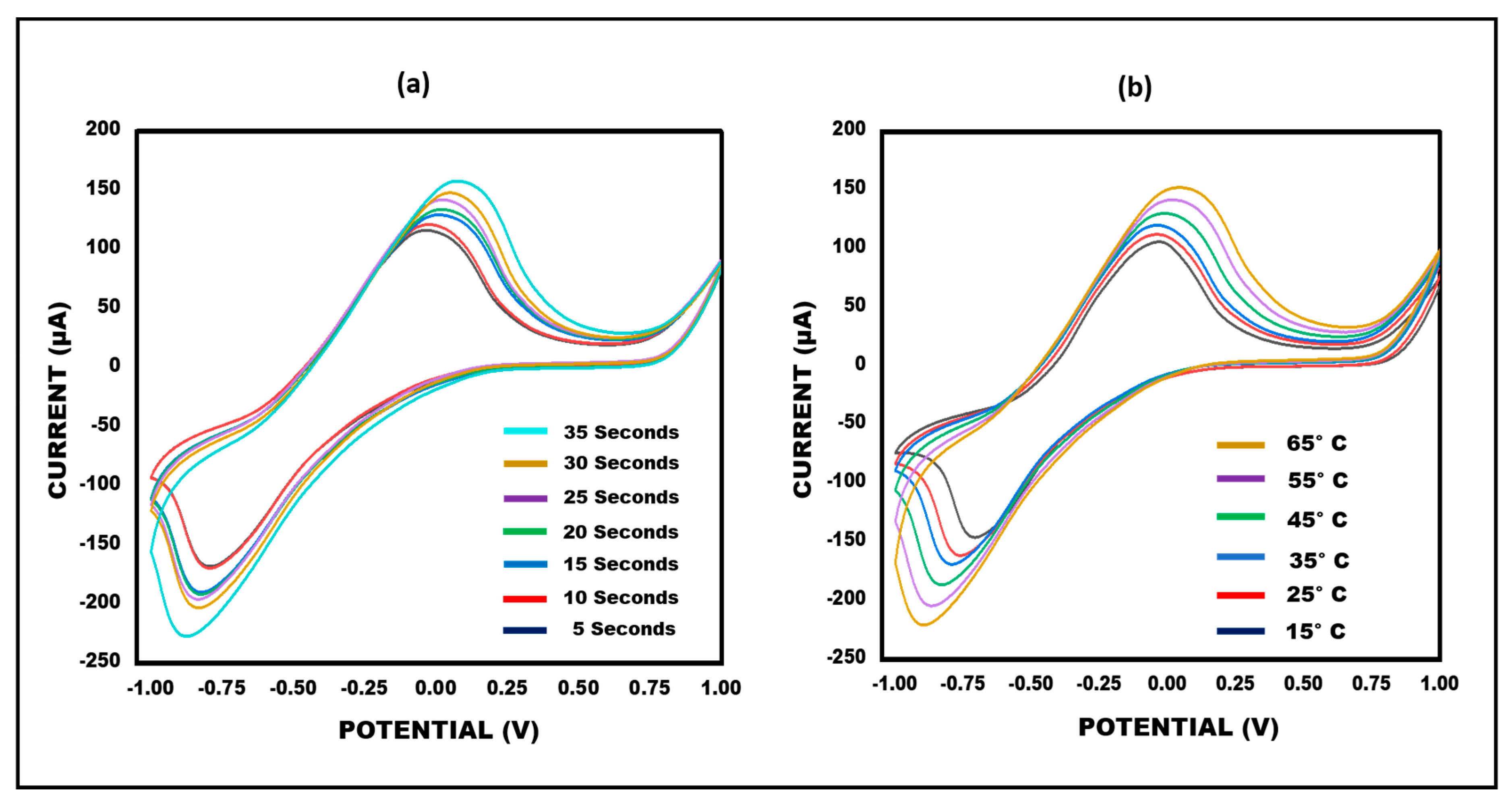
3.5. Optimization of Ketamine/Aptamer/NGSs Paper-Based Sensor in Terms of Temperature and Time
3.6. Limit of Detection and Accuracy (Recovery) Test
3.7. Examination of Cross-Reactivity (Specificity/Reliability) and Stability
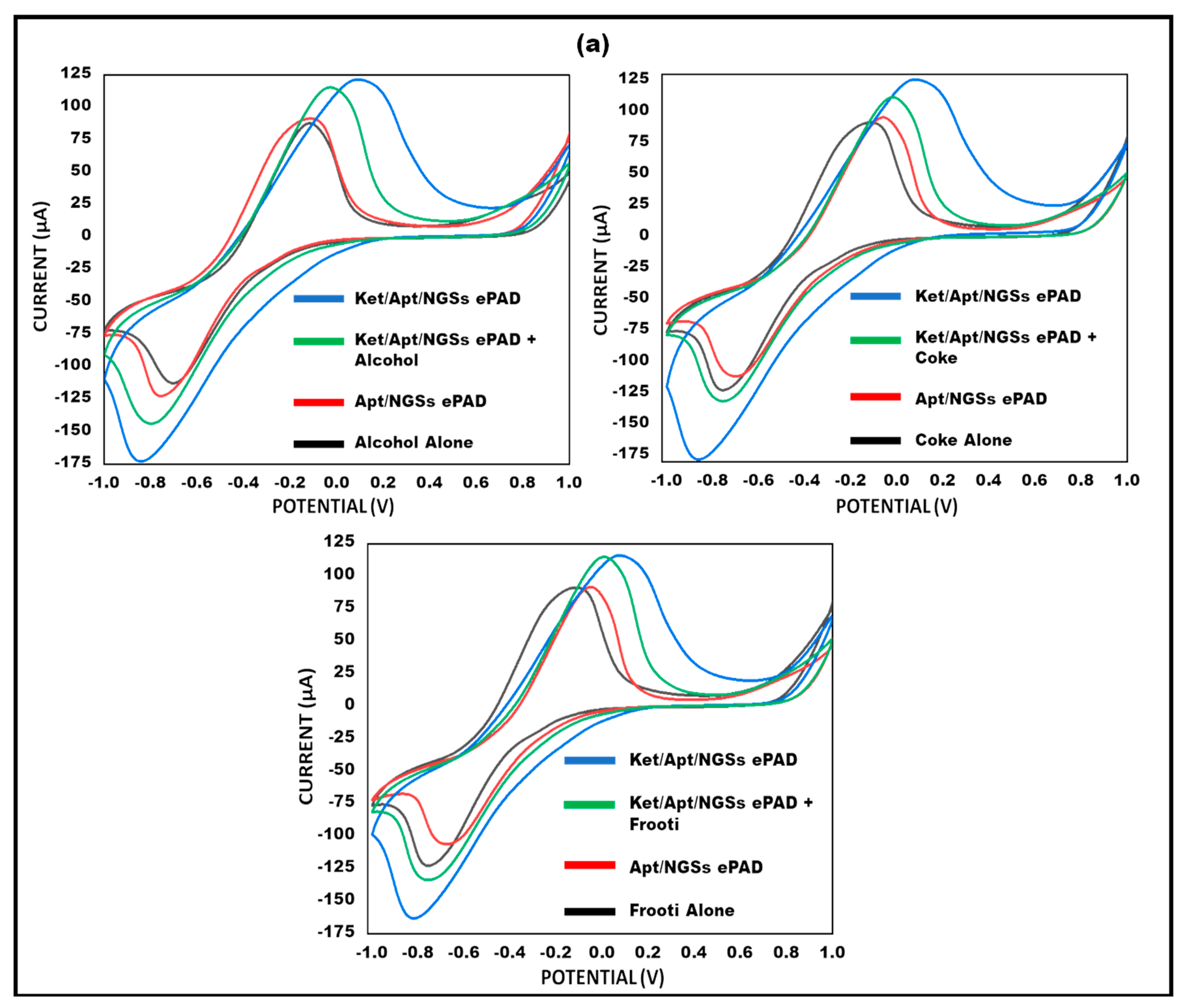
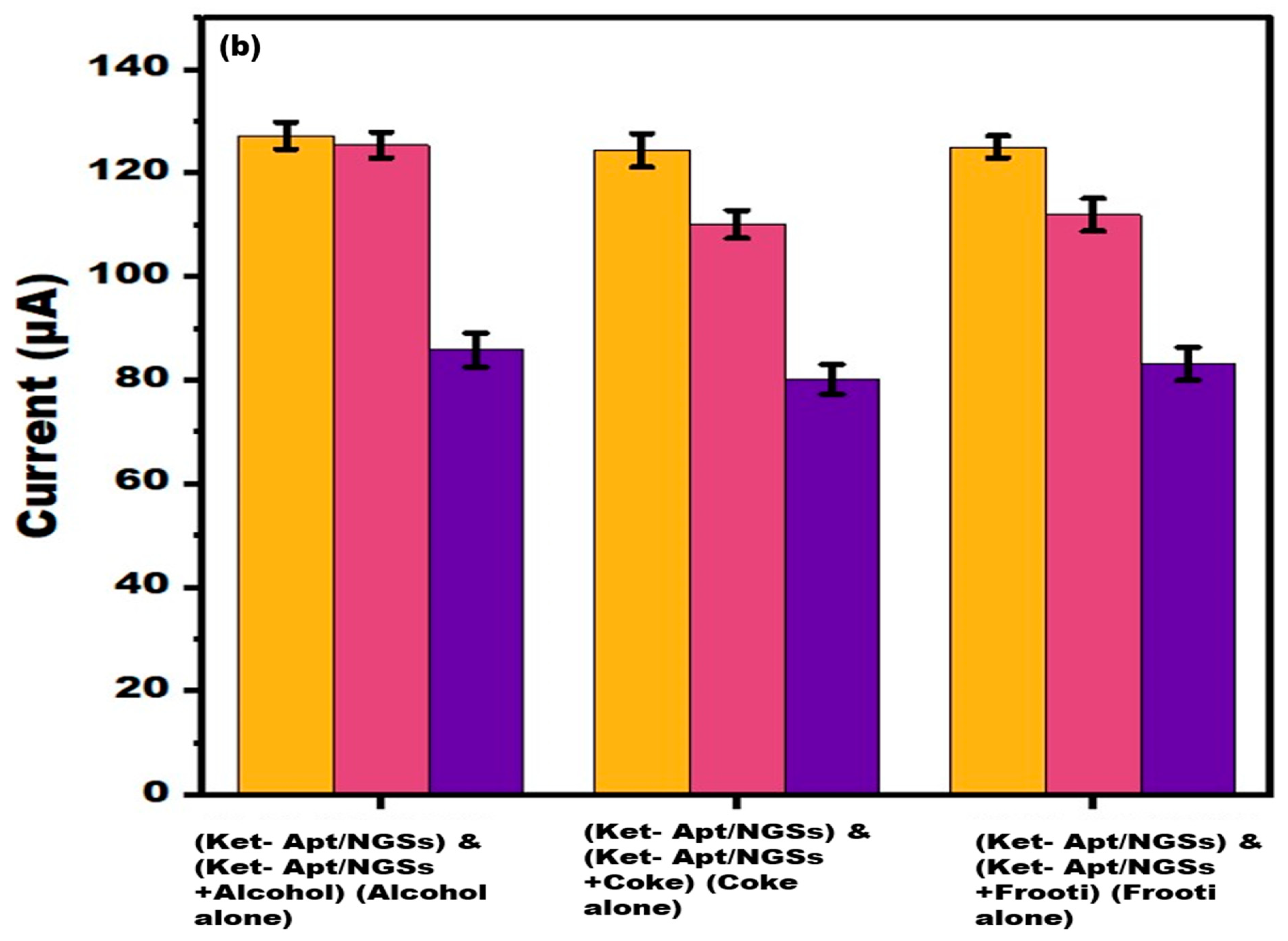
3.8. Analysis on Spiked Beverages
4. Conclusions and Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Bairros, A.V.; Lanaro, R.; de Almeida, R.M.; Yonamine, M. Determination of Ketamine, Norketamine and Dehydronorketamine in Urine by Hollow-Fiber Liquid-Phase Microextraction Using an Essential Oil as Supported Liquid Membrane. Forensic Sci. Int. 2014, 243, 47–54. [Google Scholar] [CrossRef]
- Kalmoe, M.C.; Janski, A.M.; Zorumski, C.F.; Nagele, P.; Palanca, B.J.; Conway, C.R. Ketamine and nitrous oxide: The evolution of NMDA receptor antagonists as antidepressant agents. J. Neurol. Sci. 2020, 412, 116778. [Google Scholar] [CrossRef]
- Narang, J.; Malhotra, N.; Singhal, C.; Mathur, A.; Chakraborty, D.; Ingle, A.; Pundir, C. Point of Care with Micro Fluidic Paper Based Device Incorporated with Nanocrys of Zeolite–GO for Electrochemical Sensing of Date Rape Drug. Procedia Technol. 2017, 27, 91–93. [Google Scholar] [CrossRef]
- Vidal Giné, C.; Ventura Vilamala, M.; Fornís Espinosa, I.; Gil Lladanosa, C.; Calzada Álvarez, N.; Fitó Fruitós, A.; Rodríguez Rodríguez, J.; Domíngo Salvany, A.; de La Torre Fornell, R. Crystals and Tablets in the Spanish Ecstasy Market 2000-2014: Are They the Same or Different in Terms of Purity and Adulteration? Forensic Sci. Int. 2016, 263, 164–168. [Google Scholar] [CrossRef]
- Gjerde, H.; Nordfjærn, T.; Bretteville-Jensen, A.L.; Edland-Gryt, M.; Furuhaugen, H.; Karinen, R.; Øiestad, E.L. Comparison of Drugs Used by Nightclub Patrons and Criminal Offenders in Oslo, Norway. Forensic Sci. Int. 2016, 265, 1–5. [Google Scholar] [CrossRef]
- Waddell-Smith, R.J.H. A Review of Recent Advances in Impurity Profiling of Illicit MDMA Samples. J. Forensic Sci. 2007, 52, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Lian, K.; Zhang, P.; Niu, L.; Bi, S.; Liu, S.; Jiang, L.; Kang, W. A Novel Derivatization Approach for Determination of Ketamine in Urine and Plasma by Gas Chromatography–Mass Spectrometry. J. Chromatogr. 2012, 1264, 104–109. [Google Scholar] [CrossRef]
- Sergi, M.; Compagnone, D.; Curini, R.; D’Ascenzo, G.; Del Carlo, M.; Napoletano, S.; Risoluti, R. Micro-Solid Phase Extraction Coupled with High-Performance Liquid Chromatography-Tandem Mass Spectrometry for the Determination of Stimulants, Hallucinogens, Ketamine and Phencyclidine in Oral Fluids. Anal. Chim. Acta 2010, 675, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lee, M.R.; Cheng, F.C.; Wu, G.J. Determination of Ketamine and Metabolites in Urine by Liquid Chromatography-Mass Spectrometry. Talanta 2007, 72, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Tu, Y. An Electrochemical Impedimetric Immunosensor for Ultrasensitive Determination of Ketamine Hydrochloride. Sens. Actuators B Chem. 2013, 183, 150–156. [Google Scholar] [CrossRef]
- Suranshe, S.S.; Patil, A.; Deshmukh, T.; Chavhan, J. One Step Electrode Fabrication of Thin Film Graphene Oxide-Polypyrrole Composite by Electrodeposition Using Cyclic Voltammetry for Hybrid Type Supercapacitor Application. Electrochim. Acta 2023, 450, 142277. [Google Scholar] [CrossRef]
- Xia, Y.; Li, G.; Zhu, Y.; He, Q.; Hu, C. Facile Preparation of Metal-Free Graphitic-like Carbon Nitride/Graphene Oxide Composite for Simultaneous Determination of Uric Acid and Dopamine. Microchem. J. 2023, 190, 108726. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Politano, G.G.; Burza, S.; Versace, C. Cyclic Voltammetry and Impedance Measurements of Graphene Oxide Thin Films Dip-Coated on n-Type and p-Type Silicon. Crystals 2023, 13, 73. [Google Scholar] [CrossRef]
- Turan, H.E.; Medetalibeyoglu, H.; Polat, İ.; Yola, B.B.; Atar, N.; Yola, M.L. Graphene Quantum Dots Incorporated NiAl2O4 Nanocomposite Based Molecularly Imprinted Electrochemical Sensor for 5-Hydroxymethyl Furfural Detection in Coffee Samples. Anal. Methods 2023, 15, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Koppolu, R.; Blomquist, N.; Dahlström, C.; Toivakka, M. High-Throughput Processing of Nanographite-Nanocellulose-Based Electrodes for Flexible Energy Devices. Ind. Eng. Chem. Res. 2020, 59, 11232–11240. [Google Scholar] [CrossRef]
- Andres, B.; Forsberg, S.; Dahlström, C.; Blomquist, N.; Olin, H. Enhanced Electrical and Mechanical Properties of Nanographite Electrodes for Supercapacitors by Addition of Nanofibrillated Cellulose. Phys. Status Solidi B Basic. Res. 2014, 251, 2581–2586. [Google Scholar] [CrossRef]
- Speranza, G. The Role of Functionalization in the Applications of Carbon Materials: An Overview. C—J. Carbon. Res. 2019, 5, 84. [Google Scholar] [CrossRef]
- Kausar, A. Avant-Garde Polymer and Nano-Graphite-Derived Nanocomposites—Versatility and Implications. C—J. Carbon. Res. 2023, 9, 13. [Google Scholar] [CrossRef]
- Chen, S.; Lin, C.; Lin, M. Electronic properties of nanographite ribbons in a spatially modulated electric field. Diam. Relat. Mater. 2008, 17, 1545–1549. [Google Scholar] [CrossRef]
- Zen, J.-M.; Kumar, A.S.; Tsai, D.-M. Recent Updates of Chemically Modified Electrodes in Analytical Chemistry. Electroanalysis 2003, 15, 1073–1087. [Google Scholar] [CrossRef]
- Downs, A.M.; Gerson, J.; Leung, K.K.; Honeywell, K.M.; Kippin, T.; Plaxco, K.W. Improved Calibration of Electrochemical Aptamer-Based Sensors. Sci. Rep. 2022, 12, 5535. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Wu, H.; Wang, Y.; Niu, L.; Li, F. Integrated Aptasensor Array for Sweat Drug Analysis. Anal. Chem. 2022, 94, 7936–7943. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Slosse, A.; Van Echelpoel, R.; Montiel, N.F.; Van Durme, F.; De Wael, K. Portable Electrochemical Detection of Illicit Drugs in Smuggled Samples: Towards More Secure Borders. Chem. Proc. 2021, 5, 44. [Google Scholar]
- Asghary, M.; Raoof, J.B.; Ojani, R.; Hamidi-Asl, E. A genosensor based on CPE for study the interaction between ketamine as an anesthesia drug with DNA. Int. J. Biol. Macromol. 2015, 80, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Slosse, A.; Van Echelpoel, R.; Montiel, N.F.; Langley, A.R.; Van Durme, F.; De Wael, K. Rapid On-Site Detection of Illicit Drugs in Smuggled Samples with a Portable Electrochemical Device. Chemosensors 2022, 10, 108. [Google Scholar] [CrossRef]
- Chen, C.A.; Wang, P.W.; Yen, Y.C.; Lin, H.L.; Fan, Y.C.; Wu, S.M.; Chen, C.F. Fast Analysis of Ketamine Using a Colorimetric Immunosorbent Assay on a Paper-Based Analytical Device. Sens. Actuators B Chem. 2019, 282, 251–258. [Google Scholar] [CrossRef]
- Rui, H.; Ting, Y.; Yan, M.Y. Advances in the Application of Novel Carbon Nanomaterials in Illicit Drug Detection. New J. Chem. 2022, 47, 2161–2172. [Google Scholar] [CrossRef]
- Yang, Y.; Zhai, S.; Liu, C.; Wang, X.; Tu, Y. Disposable Immunosensor Based on Electrochemiluminescence for Ultrasensitive Detection of Ketamine in Human Hair. ACS Omega 2019, 4, 801–809. [Google Scholar] [CrossRef]
- Radi, A.-E. Electrochemical Aptamer-Based Biosensors: Recent Advances and Perspectives. Int. J. Electrochem. 2011, 2011, 863196. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Jiang, X.; Cao, F.; Liu, W. An Enzyme-Free FRET Nanoprobe for Ultrasensitive Ketamine Detection Based on ATP-Fueled Target Recycling. RSC Adv. 2019, 9, 36884–36889. [Google Scholar] [CrossRef]
- Muniyalakshmi, M.; Sethuraman, K.; Silambarasan, D. Synthesis and Characterization of Graphene Oxide Nanosheets. Mater. Today Proc. 2020, 21, 408–410. [Google Scholar] [CrossRef]
- Schram, J.; Parrilla, M.; Sleegers, N.; Samyn, N.; Bijvoets, S.M.; Heerschop, M.W.J.; Van Nuijs, A.L.N.; De Wael, K. Identifying Electrochemical Fingerprints of Ketamine with Voltammetry and Liquid Chromatography-Mass Spectrometry for Its Detection in Seized Samples. Anal. Chem. 2020, 92, 13485–13492. [Google Scholar] [CrossRef] [PubMed]
- Van Echelpoel, R.; Schram, J.; Parrilla, M.; Daems, D.; Slosse, A.; Van Durme, F.; De Wael, K. Electrochemical Methods for On-Site Multidrug Detection at Festivals. Sens. Diagn. 2022, 1, 793–802. [Google Scholar] [CrossRef]
- Asghary, M.; Raoof, J.B.; Rahimnejad, M.; Ojani, R. Microbial Fuel Cell-Based Self-Powered Biosensing Platform for Determination of Ketamine as an Anesthesia Drug in Clinical Serum Samples. J. Iran. Chem. Soc. 2018, 15, 445–453. [Google Scholar] [CrossRef]
- Ioni, Y.V.; Tkachev, S.V.; Bulychev, N.A.; Gubin, S.P. Preparation of Finely Dispersed Nanographite. Inorg. Mater. 2011, 47, 597–602. [Google Scholar] [CrossRef]
- Pretschuh, C.; Schwarzinger, C.; Abdala, A.A.; Vukusic, S. Characterization of Conductive Nanographite Melamine Composites. Open J. Compos. Mater. 2014, 4, 61–71. [Google Scholar] [CrossRef]
- Radon, A.; Lukowiec, D. Structure of Nanographite Synthesised by Electrochemical Oxidation and Exfoliation of Polycrystalline Graphite. Micro Nano Lett. 2017, 12, 955–959. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile Synthesis and Characterization of Graphene Nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Kala, D.; Sharma, T.K.; Gupta, S.; Verma, V.; Thakur, A.; Kaushal, A.; Trukhanov, A.V.; Trukhanov, S.V. Graphene Oxide Nanoparticles Modified Paper Electrode as a Biosensing Platform for Detection of the htra Gene of O. tsutsugamushi. Sensors 2021, 21, 4366. [Google Scholar] [CrossRef] [PubMed]
- Pimpang, P.; Wongrerkdee, S.; Choopun, S. Charge Transfer Improvement of ZnO-Based Dye-Sensitized Solar Cells Modified with Graphite Nanosheets and Bilayer Photoelectrode Structures. Ferroelectrics 2019, 552, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Dong, Y.; Yang, J.; Li, Y.; Huang, C. Modified Nano-Graphite/Fe3O4 Composite as Efficient Adsorbent for the Removal of Methyl Violet from Aqueous Solution. J. Mol. Liq. 2014, 196, 348–356. [Google Scholar] [CrossRef]
- Hadi, A.; Zahirifar, J.; Karimi-Sabet, J.; Dastbaz, A. Graphene Nanosheets Preparation Using Magnetic Nanoparticle Assisted Liquid Phase Exfoliation of Graphite: The Coupled Effect of Ultrasound and Wedging Nanoparticles. Ultrason. Sonochem. 2018, 44, 204–214. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, J.H.; Chen, L.Y.; Feng, L.; Chen, Z.M.; Zheng, J.X.; Qin, S.N.; Li, G.W.; Salminen, K.; Sun, J.J. Rapid Nanomolar Detection of Ketamine in Biofluids Based on Electrochemical Aptamer-Based Sensor for Drugged Driving Screening within 30 s. Sens. Actuators B Chem. 2023, 390, 133903. [Google Scholar] [CrossRef]
- Yehia, A.M.; Farag, M.A.; Tantawy, M.A. A Novel Trimodal System on a Paper-Based Microfluidic Device for on-Site Detection of the Date Rape Drug “Ketamine”. Anal. Chim. Acta 2020, 1104, 95–104. [Google Scholar] [CrossRef]
- Musile, G.; Wang, L.; Bottoms, J.; Tagliaro, F.; McCord, B. The Development of Paper Microfluidic Devices for Presumptive Drug Detection. Anal. Methods 2015, 7, 8025–8033. [Google Scholar] [CrossRef]
- Abu Shawish, H.M.; Saadeh, S.M.; Tamos, H.; Abed-Almonem, K.I.; Al Khalili, O. A New Potentiometric Sensor for the Determination of Ketamine Hydrochloride in Ampoules and Urine. Anal. Methods 2015, 7, 301–308. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Farag, M.A.; Yehia, A.M. A Gold-Carbon Dots Nanoprobe for Dual Mode Detection of Ketamine HCl in Soda Drinks. New J. Chem. 2020, 44, 7058–7064. [Google Scholar] [CrossRef]


| Initial Concentration μg/mL | Concentration Added (µg/mL) | Current Measured (µA) | Expected Current Measured (μA) | Recovery (%) |
|---|---|---|---|---|
| 0.01 | 0.1 | 112.651 | 115.608 | 97% |
| 0.01 | 1 | 116.314 | 121.541 | 95% |
| Methods | Linear Range (µM) | LOD (μM) | References |
|---|---|---|---|
| Electrochemical aptamer-based (EAB) sensor | 0.01–3.0 μM | 0.01 µM | [45] |
| Fluorimetry | 20–1000 µM | 200 µM | [46] |
| Colorimetry | 105–315 µM | 4.1 µg | [47] |
| Potentiometric sensor | 9–10,000 µM | 7.3 µM | [48] |
| Fluorescent carbon dots (CDs) | 0.5–650 µM | 230 nm | [49] |
| ePAD sensor | 0.01–5.0 μM | 0.01 µM | Proposed sensor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleman, S.; Anzar, N.; Patil, S.; Shadan; Parvez, S.; Khanuja, M.; Pilloton, R.; Narang, J. Aptasensor Integrated with Two-Dimensional Nanomaterial for Selective and Sensitive Electrochemical Detection of Ketamine Drug. Micromachines 2024, 15, 312. https://doi.org/10.3390/mi15030312
Suleman S, Anzar N, Patil S, Shadan, Parvez S, Khanuja M, Pilloton R, Narang J. Aptasensor Integrated with Two-Dimensional Nanomaterial for Selective and Sensitive Electrochemical Detection of Ketamine Drug. Micromachines. 2024; 15(3):312. https://doi.org/10.3390/mi15030312
Chicago/Turabian StyleSuleman, Shariq, Nigar Anzar, Shikha Patil, Shadan, Suhel Parvez, Manika Khanuja, Roberto Pilloton, and Jagriti Narang. 2024. "Aptasensor Integrated with Two-Dimensional Nanomaterial for Selective and Sensitive Electrochemical Detection of Ketamine Drug" Micromachines 15, no. 3: 312. https://doi.org/10.3390/mi15030312
APA StyleSuleman, S., Anzar, N., Patil, S., Shadan, Parvez, S., Khanuja, M., Pilloton, R., & Narang, J. (2024). Aptasensor Integrated with Two-Dimensional Nanomaterial for Selective and Sensitive Electrochemical Detection of Ketamine Drug. Micromachines, 15(3), 312. https://doi.org/10.3390/mi15030312







