A Current-Mode Analog Front-End for Capacitive Length Transducers in Pneumatic Muscle Actuators
Abstract
:1. Introduction
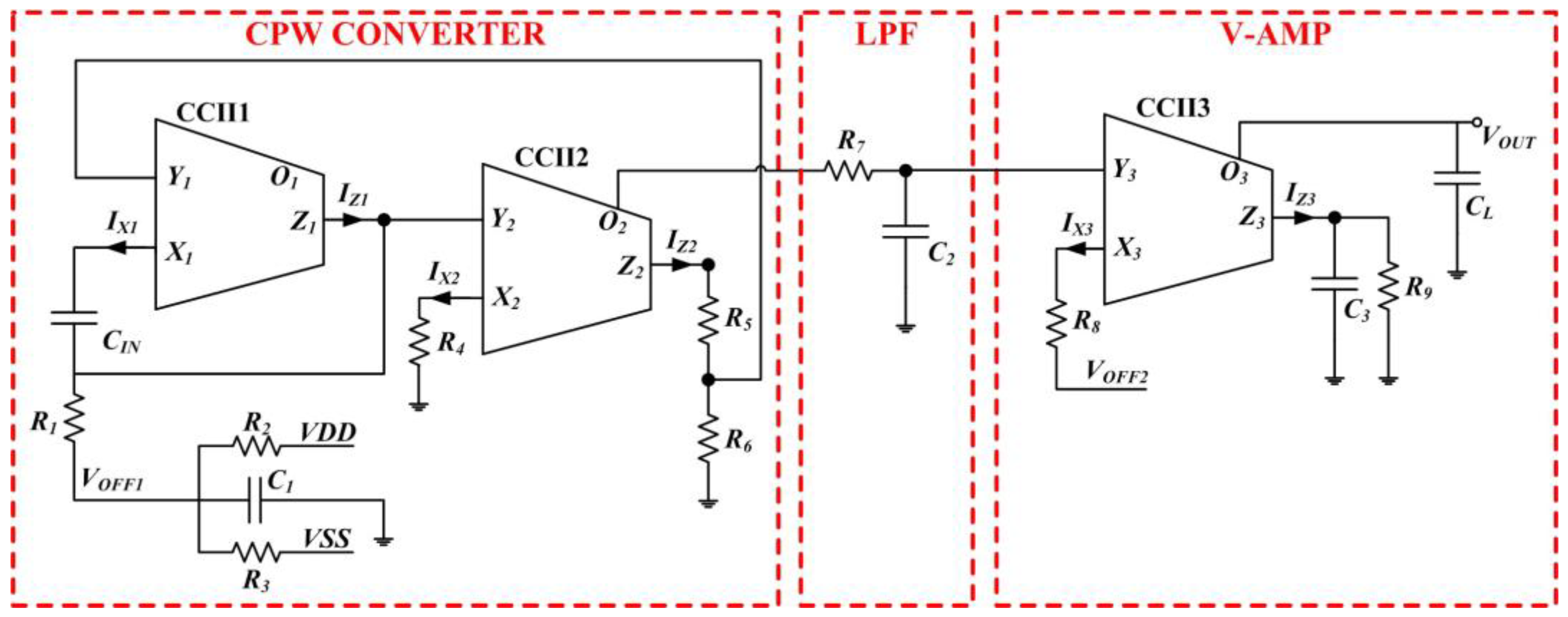
2. The CV-AFE Circuit for Capacitive Transducers: Main Parts and Operating Principles
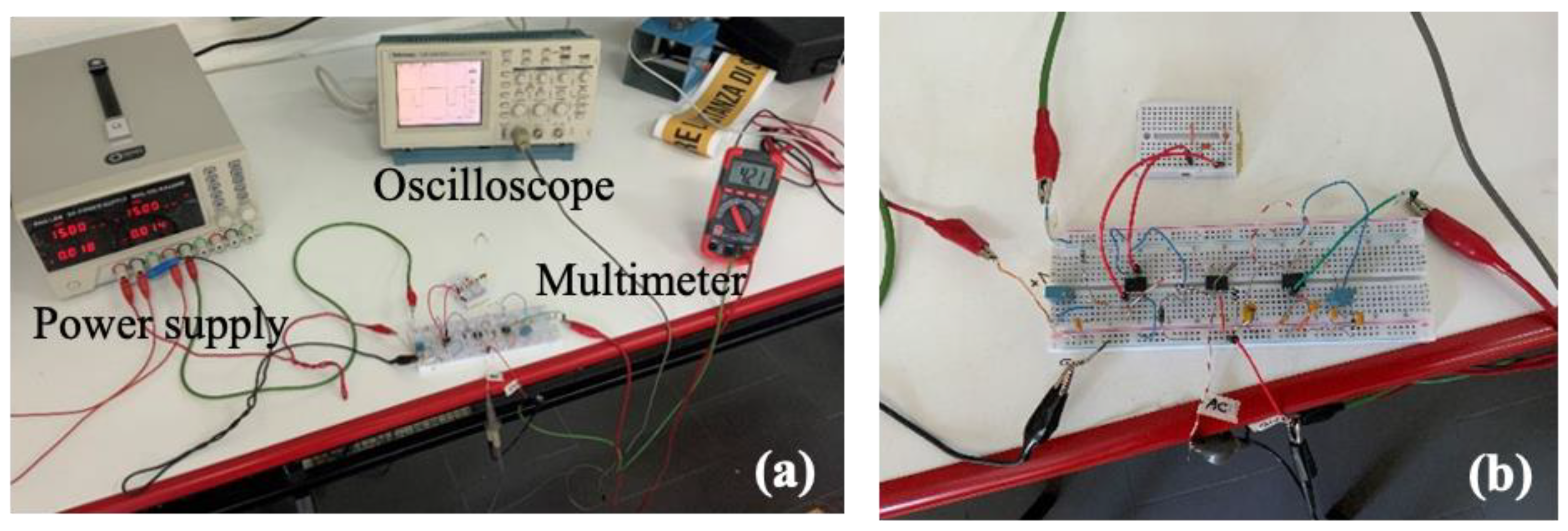
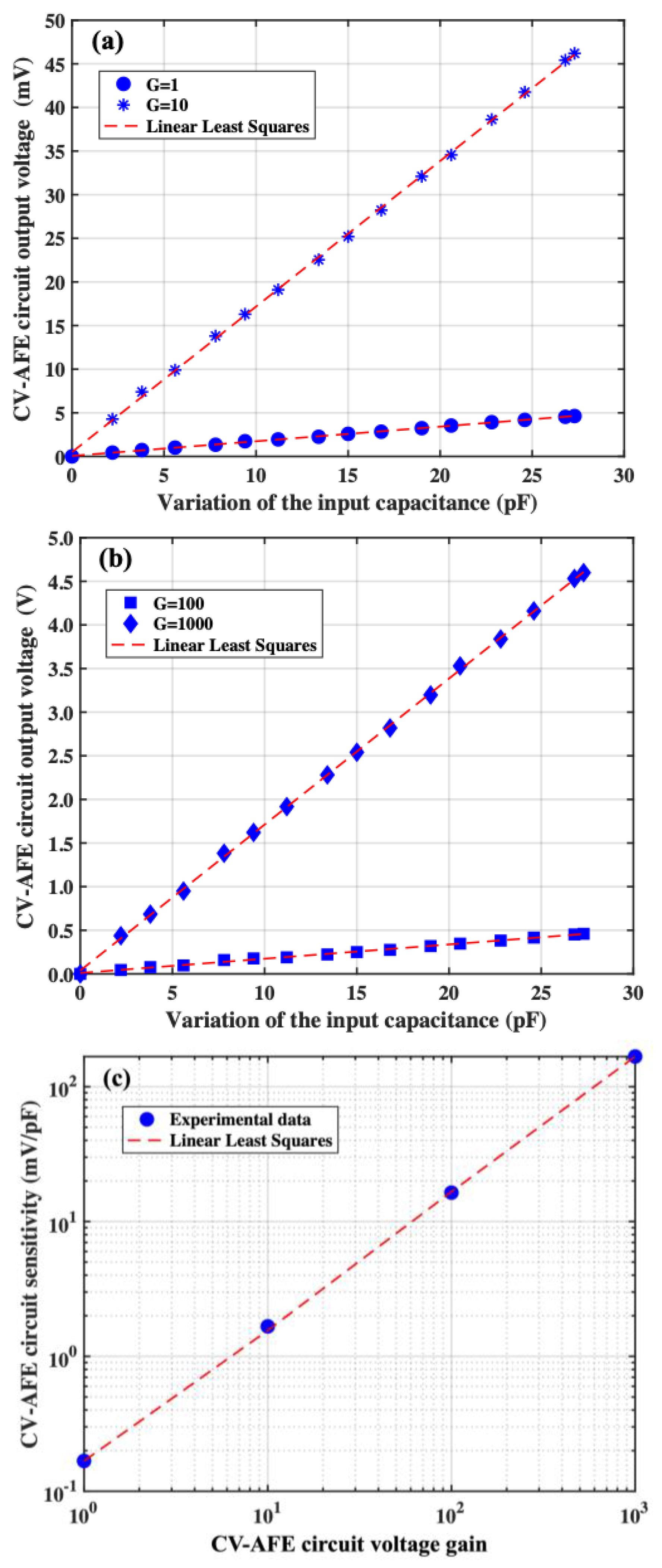
3. The CV-AFE Circuit Implementation: Experimental Validation and Electrical Characterization
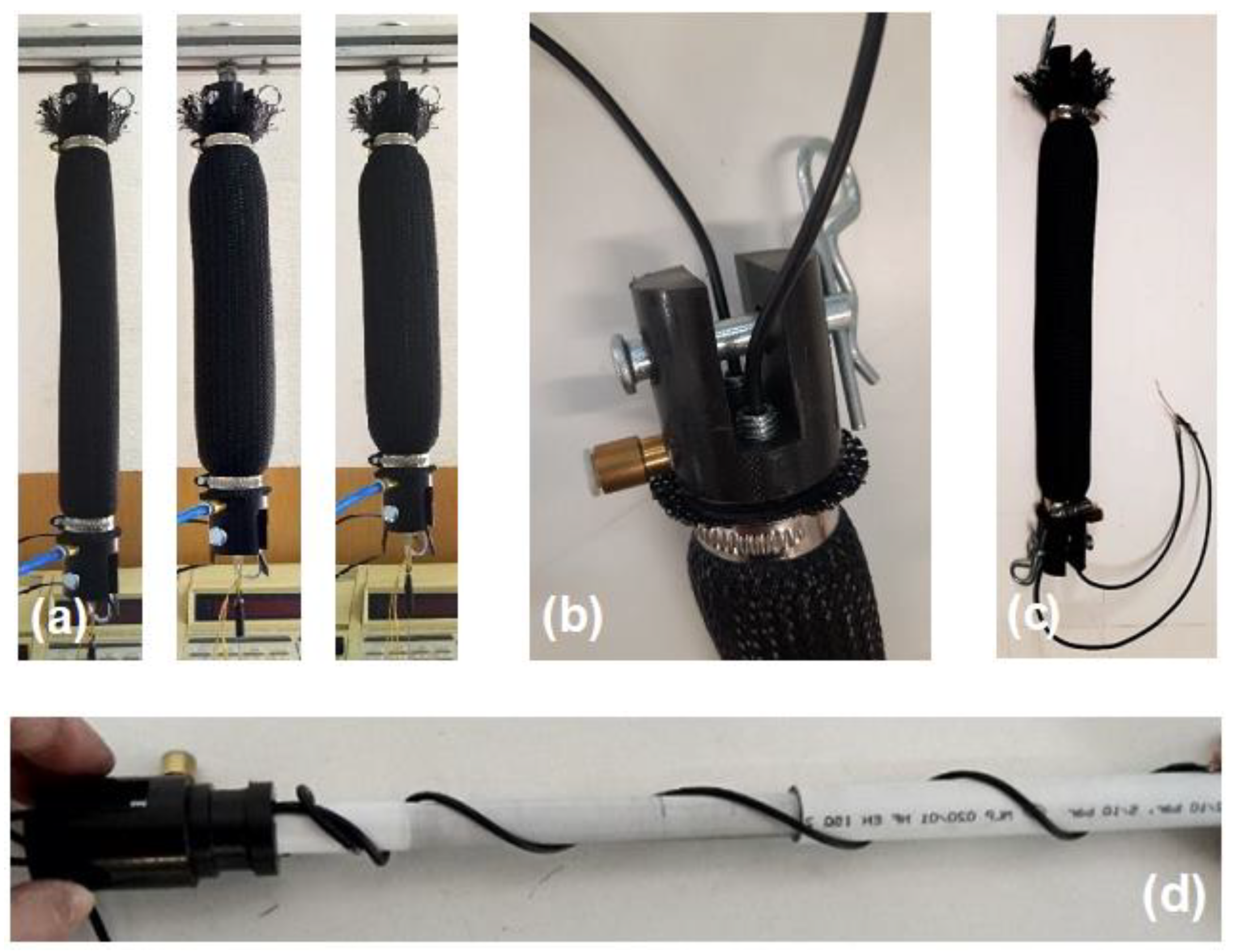
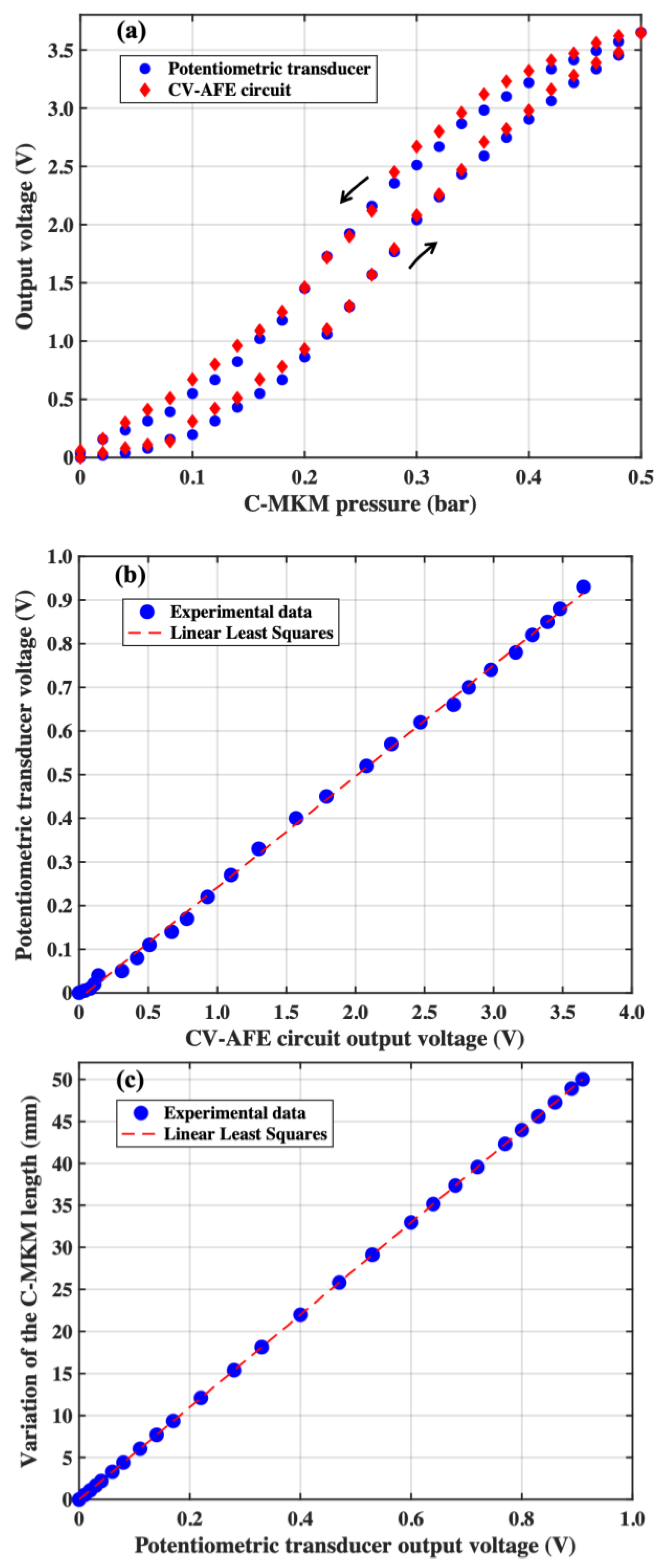
4. The CV-AFE Circuit Application as Length Transducer in Pneumatic Muscle Actuators
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Sun, N.; Liu, G.; Yang, T.; Fang, Y. Hysteresis Compensation-Based Intelligent Control for Pneumatic Artificial Muscle-Driven Humanoid Robot Manipulators with Experiments Verification. IEEE Trans. Autom. Sci. Eng. 2023, 1–14. [Google Scholar] [CrossRef]
- Ito, F.; Horiuchi, I.; Tsuru, K.; Nakamura, T. Development of an Earthworm-Type Electrical Wire Installation Assistance Robot Using Artificial Muscles. IEEE Robot. Autom. Lett. 2023, 8, 2999–3006. [Google Scholar] [CrossRef]
- Di Patrizio Stanchieri, G.; Saleh, M.; Sciulli, M.; De Marcellis, A.; Ibrahim, A.; Valle, M.; Faccio, M.; Palange, E. FPGA-Based Tactile Sensory Feedback System with Optical Fiber Data Communication Link for Prosthetic Applications. In Proceedings of the IEEE Conference on Electronics, Circuits and Systems (ICECS), Genoa, Italy, 27–29 November 2019; pp. 374–377. [Google Scholar]
- Chen, C.T.; Lien, W.Y.; Chen, C.T.; Twu, M.J.; Wu, Y.C. Dynamic Modeling and Motion Control of a Cable-Driven Robotic Exoskeleton with Pneumatic Artificial Muscle Actuators. IEEE Access 2020, 8, 149796–149807. [Google Scholar] [CrossRef]
- Khan, M.A.; Shaik, S.; Tariq, M.H.; Kamal, T. McKibben Pneumatic Artificial Muscle Robot Actuators—A Review. In Proceedings of the 2023 International Conference on Robotics and Automation in Industry (ICRAI), Peshawar, Pakistan, 3–5 March 2023; pp. 1–6. [Google Scholar]
- Dzedzickis, A.; Subačiuté-Žemaitié, J.; Šutinys, E.; Samukaité-Bubniené, U.; Bučinskas, V. Advanced Applications of Industrial Robotics: New Trends and Possibilities. Appl. Sci. 2022, 12, 135. [Google Scholar] [CrossRef]
- Do Rosario Carvalho, A.D.; Karanth, N.P.; Desai, V. Design and characterization of a pneumatic muscle actuator with novel end-fittings for medical assistive applications. Sens. Actuators A Phys. 2021, 331, 112877. [Google Scholar] [CrossRef]
- Durante, F.; Antonelli, M.G.; Beomonte Zobel, P. Development of an active exoskeleton for assisting back movements in lifting weights. Int. J. Mech. Eng. Robot. Res. 2018, 7, 353–360. [Google Scholar] [CrossRef]
- Laksanacharoen, S. Artificial muscle construction using natural rubber latex in Thailand. In Proceedings of the 3rd Thailand and Material Science and Technology Conference, Bangkok, Thailand, 10–11 August 2004; pp. 10–11. [Google Scholar]
- Nguyen, H.T.; Trinh, V.C.; Le, T.D. An adaptive fast terminal sliding mode controller of exercise-assisted robotic arm for elbow joint rehabilitation featuring pneumatic artificial muscle actuator. Actuators 2020, 9, 118. [Google Scholar] [CrossRef]
- Martens, M.; Seel, T.; Zawatzki, J.; Boblan, I. A novel framework for a systematic integration of pneumatic-muscle-actuator-driven joints into robotic systems via a torque control interface. Actuators 2018, 7, 82. [Google Scholar] [CrossRef]
- Tsai, T.C.; Chiang, M.H. Design and control of a 1-DOF robotic lower-limb system driven by novel single pneumatic artificial muscle. Appl. Sci. 2019, 10, 43. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Komatsu, R.; Machii, K.; Yokota, M.; Tobe, M.; Ibrahim, A.N.; Fukui, T.; Habu, Y. Pace Running of a Quadruped Robot Driven by Pneumatic Muscle Actuators: An Experimental Study. Appl. Sci. 2022, 12, 4146. [Google Scholar] [CrossRef]
- Zhong, S.; Gai, Z.; Yang, Y.; Zhao, Y.; Qi, Y.; Yang, Y.; Peng, Y. A contraction length feedback method for the McKibben pneumatic artificial muscle. Sens. Actuators A Phys. 2022, 334, 113321. [Google Scholar] [CrossRef]
- Felt, W.; Chin, K.Y.; Remy, C.D. Contraction Sensing with Smart Braid McKibben Muscles. IEEE/ASME Trans. Mechatron. 2016, 21, 1201–1209. [Google Scholar] [CrossRef]
- Ho, V.A.; Hirai, S. Measuring McKibben Actuator Shrinkage using Fiber Sensor. In Proceedings of the 24th IEEE International Symposium on Robot and Human Interactive Communication, Kobe, Japan, 31 August–4 September 2015; pp. 628–633. [Google Scholar]
- Legrand, J.; Loenders, B.; Vos, A.; Schoevaerdts, L.; Poorten, E.V. Integrated Capacitance Sensing for Miniature Artificial Muscle Actuators. IEEE Sens. J. 2020, 20, 1363–1372. [Google Scholar] [CrossRef]
- Doumit, M.D.; Pardoel, S. Dynamic contraction behaviour of pneumatic artificial muscle. Mech. Syst. Signal Process. 2017, 91, 93–110. [Google Scholar] [CrossRef]
- Kuriyama, S.; Ding, M.; Kurita, Y.; Ueda, J.; Ogasawara, T. Flexible Sensor for McKibben Pneumatic Artificial Muscle Actuator. Int. J. Autom. Technol. 2009, 3, 731–740. [Google Scholar] [CrossRef]
- Yano, T.; Fujimoto, S.; Akagi, T.; Kobayashi, W. Development of Outer Diameter Sensor for Position Control of McKibben Artificial Actuator Using Hall-effect Sensor. Int. J. Mech. Eng. Robot. Res. 2020, 9, 190–196. [Google Scholar] [CrossRef]
- Erin, O.; Pol, N.; Valle, L.; Park, Y.L. Design of A Bio-Inspired Pneumatic Artificial Muscle with Self-Contained Sensing. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2115–2119. [Google Scholar]
- Antonelli, M.G.; Beomonte Zobel, P.; De Marcellis, A.; Palange, E. Design and characterization of a Mckibben pneumatic muscle prototype with an embedded capacitive length transducer. Machines 2022, 10, 1156. [Google Scholar] [CrossRef]
- Antonelli, M.G.; Beomonte Zobel, P.; Durante, F.; Zeer, M. Modeling-Based EMG Signal (MBES) Classifier for Robotic Remote-Control Purposes. Actuators 2022, 11, 65. [Google Scholar] [CrossRef]
- Abdellatif, S.O.; Moustafa, A.; Khalid, A.; Ghannam, R. Integration of Capacitive Pressure Sensor-on-Chip with Lead-Free Perovskite Solar Cells for Continuous Health Monitoring. Micromachines 2023, 14, 1676. [Google Scholar] [CrossRef]
- Zhang, C.; Gallichan, R.; Budgett, D.M.; McCormick, D. A Capacitive Pressure Sensor Interface IC with Wireless Power and Data Transfer. Micromachines 2020, 11, 897. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasan, E.; Duraisamy, G.N.R.; Elangovan, M.K.; Sarasam, A.S.T. A 0.8 V, 14.76 nVrms, Multiplexer-Based AFE for Wearable Devices Using 45 nm CMOS Techniques. Micromachines 2023, 14, 1816. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, B.; Mun, Y.; Kim, J.; Han, K.; Roh, Y.; Song, D.; Huh, S.; Ko, H. Reconfigurable Sensor Analog Front-End Using Low-Noise Chopper-Stabilized Delta-Sigma Capacitance-to-Digital Converter. Micromachines 2018, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Ko, S. A Mutual Capacitance Touch Readout IC with Synchronization in Touch and Mobile Display Driving for High Refresh Rate AMOLED Panels. Micromachines 2021, 12, 922. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Ren, M.; Xu, H. A Readout Circuit for MEMS Gas Sensor. Micromachines 2023, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Barile, G.; Centurelli, F.; Ferri, G.; Monsurrò, P.; Pantoli, L.; Stornelli, V.; Tommasino, P.; Trifiletti, A. A New Fully Closed-Loop, High-Precision, Class-AB CCII for Differential Capacitive Sensor Interfaces. Electronics 2022, 11, 903. [Google Scholar] [CrossRef]
- De Marcellis, A.; Reig, C.; Cubells-Beltrán, M.-D. A Capacitance-to-Time Converter-Based Electronic Interface for Differential Capacitive Sensors. Electronics 2019, 8, 80. [Google Scholar] [CrossRef]
- Demori, M.; Baù, M.; Ferrari, M.; Ferrari, V. Interrogation Techniques and Interface Circuits for Coil-Coupled Passive Sensors. Micromachines 2018, 9, 449. [Google Scholar] [CrossRef]
- Ahmadpour Bijargah, A.; Heidary, A.; Torkzadeh, P.; Nihtianov, S. Design trade-offs of a capacitance-to-voltage converter with a zoom-in technique for grounded capacitive sensors. Int. J. Circ. Theor. Appl. 2018, 46, 2231–2247. [Google Scholar] [CrossRef]
- Devaraj, S.V.; Baghini, M.S. A Parasitic Insensitive High-Frequency Capacitance-to-Voltage Converter ASIC for Dielectric Measurements. IEEE Sens. J. 2023, 23, 2393–2402. [Google Scholar] [CrossRef]
- Li, L.; Lai, X.; Wang, Y.; Niu, Z. High-Power-Efficiency Readout Circuit Employing Average Capacitance-to-Voltage Converter for Micro-Electro-Mechanical System Capacitive Accelerometers. Sensors 2023, 23, 8547. [Google Scholar] [CrossRef]
- Utz, A.; Walk, C.; Haas, N.; Fedtschenko, T.; Stanitzki, A.; Mir, M.; Görtz, M.; Kraft, M.; Kokozinski, R. An ultra-low noise capacitance to voltage converter for sensor applications in 0.35 µm CMOS. J. Sens. Sens. Syst. 2017, 6, 285–301. [Google Scholar] [CrossRef]
- Arefin, M.S.; Redouté, J.-M.; Yuce, M.R. A MEMS Interface IC with Low-Power and Wide-Range Frequency-to-Voltage Converter for Biomedical Applications. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 455–466. [Google Scholar] [CrossRef]
- Ferri, G.; Parente, F.R.; Stornelli, V. Current-mode differential capacitance to voltage converter for position sensing. In Proceedings of the 2017 European Conference on Circuit Theory and Design (ECCTD), Catania, Italy, 4–6 September 2017; pp. 1–4. [Google Scholar]
- Arshad, A.; Tasnim, R.; Alam, A.H.M.Z.; Khan, S. Capacitance-to-voltage converter design to measure small change in capacitance produced by human body movement. In Proceedings of the 2015 IEEE International WIE Conference on Electrical and Computer Engineering (WIECON-ECE), Dhaka, Bangladesh, 19–20 December 2015; pp. 114–117. [Google Scholar]
- Pulugu, P.; Ghosh, S.; Rokade, S.; Choudhury, K.; Arya, N.; Kumar, P. A perspective on implantable biomedical materials and devices for diagnostic applications. Curr. Opin. Biomed. Eng. 2021, 18, 100287. [Google Scholar] [CrossRef]
- Di Patrizio Stanchieri, G.; De Marcellis, A.; Faccio, M.; Palange, E.; Guler, U. A Fully-Analogue Light-to-Frequency Converter Circuit for Optical Sensing Applications. IEEE Sens. J. 2022, 22, 16120–16130. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Kim, Y.S.; Mahmood, M.; Kwon, S.; Zavanelli, N.; Kim, H.S.; Rim, Y.S.; Epps, F.; Yeo, W.H. Fully integrated, stretchable, wireless skin-conformal bioelectronics for continuous stress monitoring in daily life. Adv. Sci. 2020, 7, 2000810. [Google Scholar] [CrossRef]
- Tchamako, A.T.; Ottaviani, L.; Rahajandraibe, W.; Vervisch, W.; Vervisch, V.; Walder, J.P. Front end electronics for radiation detectors based on SiC: Application to high dose per pulse charged particle beam current measurement. IEEE Sens. J. 2022, 22, 2326–2337. [Google Scholar] [CrossRef]
- Di Patrizio Stanchieri, G.; De Marcellis, A.; Battisti, G.; Faccio, M.; Palange, E.; Guler, U. A 1.8 V Low-Power Low-Noise High Tunable Gain TIA for CMOS Integrated Optoelectronic Biomedical Applications. Electronics 2022, 11, 1271. [Google Scholar] [CrossRef]
- AD844, Current Feedback Op-Amp Datasheet; Analog Devices Inc.: Norwood, MA, USA, 1990.
- Toumazou, C.; Payne, A.; Haigh, D. Analogue IC Design: The Current Mode Approach; Peter Peregrinus: London, UK, 1990. [Google Scholar]
- Di Patrizio Stanchieri, G.; De Marcellis, A.; Faccio, M.; Palange, E.; Guler, U. A Novel Light-to-Frequency Converter Based Analog Front-End for Optical Sensing Applications. In Proceedings of the IEEE Sensors, Sydney, Australia, 31 October–4 November 2021; pp. 1–4. [Google Scholar]
- Antonelli, M.G.; Beomonte Zobel, P.; Durante, F.; Raparelli, T. Numerical modelling and experimental validation of a McKibben pneumatic muscle actuator. J. Intell. Mater. Syst. Struct. 2017, 28, 2737–2748. [Google Scholar] [CrossRef]



| Parameters | [39] | [37] | [38] | [34] | This Work |
|---|---|---|---|---|---|
| Year | 2015 | 2016 | 2017 | 2023 | 2024 |
| Implementation type | Discrete components | Integrated | Discrete components | Integrated | Discrete components |
| COTS components | 2 (LM555 + LM2917) | n/a | 4 CCII (AD844) | n/a | 3 CCII (AD844) |
| Sensitivity (mV/pF) | 10 | 54 | 37.5 | 95 | 167.3 |
| Gain/sensitivity tunability | no | yes | no | no | yes |
| Resolution (fF) | N/A | 10 | N/A | 10.6 | 5 |
| Capacitance variation range (pF) | 0.01–800 | 0.01–27 | 140–260 | 0.01–24 | 57–84 |
| Capacitance variation range tunability | no | no | yes | yes | yes |
| External signals | not required | not required | not required | required | not required |
| External reference capacitors | not required | not required | required | required | not required |
| Offset compensation | No | no | no | no | yes |
| Capacitive sensing applications | Body movement measurements | pH measurements | Position measurements | Dielectric measurements | Pneumatic muscle length measurements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Patrizio Stanchieri, G.; De Marcellis, A.; Faccio, M.; Palange, E.; Antonelli, M.G.; Beomonte Zobel, P. A Current-Mode Analog Front-End for Capacitive Length Transducers in Pneumatic Muscle Actuators. Micromachines 2024, 15, 377. https://doi.org/10.3390/mi15030377
Di Patrizio Stanchieri G, De Marcellis A, Faccio M, Palange E, Antonelli MG, Beomonte Zobel P. A Current-Mode Analog Front-End for Capacitive Length Transducers in Pneumatic Muscle Actuators. Micromachines. 2024; 15(3):377. https://doi.org/10.3390/mi15030377
Chicago/Turabian StyleDi Patrizio Stanchieri, Guido, Andrea De Marcellis, Marco Faccio, Elia Palange, Michele Gabrio Antonelli, and Pierluigi Beomonte Zobel. 2024. "A Current-Mode Analog Front-End for Capacitive Length Transducers in Pneumatic Muscle Actuators" Micromachines 15, no. 3: 377. https://doi.org/10.3390/mi15030377







