Strategies for Enhancing the Stability of Lithium Metal Anodes in Solid-State Electrolytes
Abstract
:1. Introduction
2. Sulfide SSEs
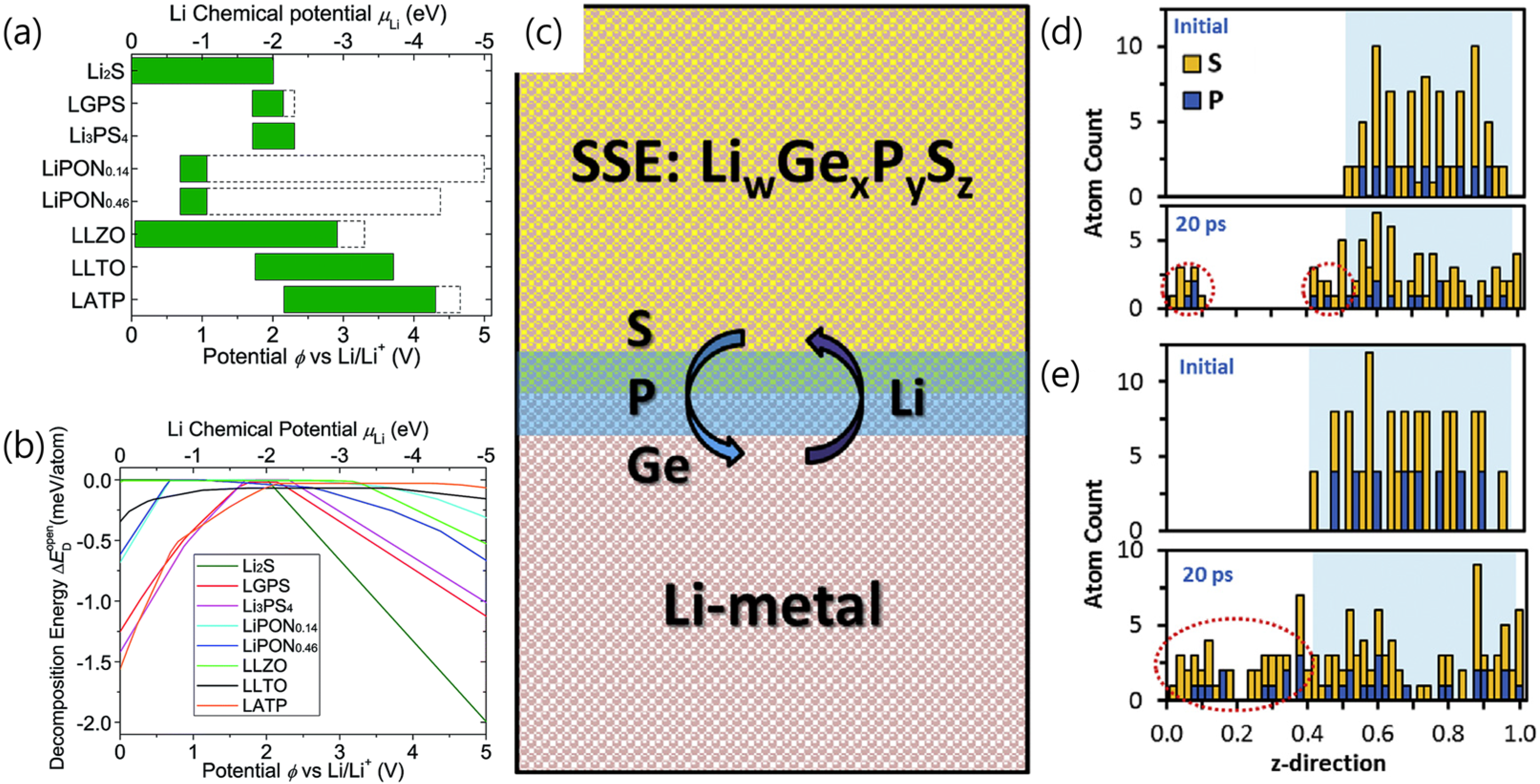
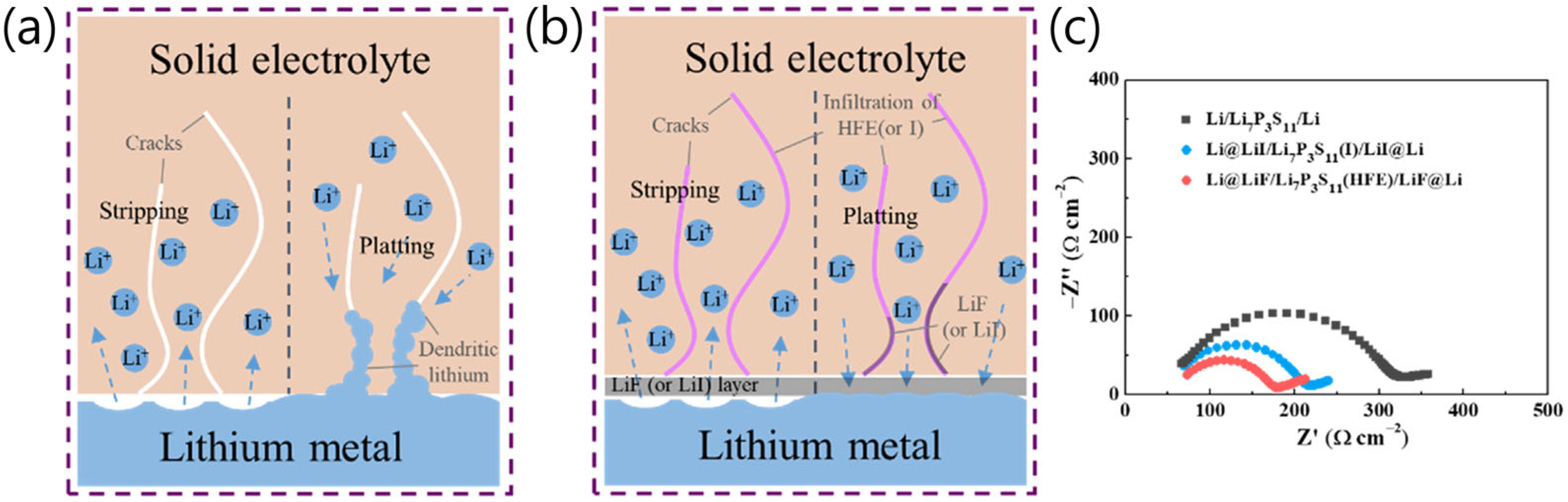
2.1. Challenges of Sulfide SSEs for Li Metal
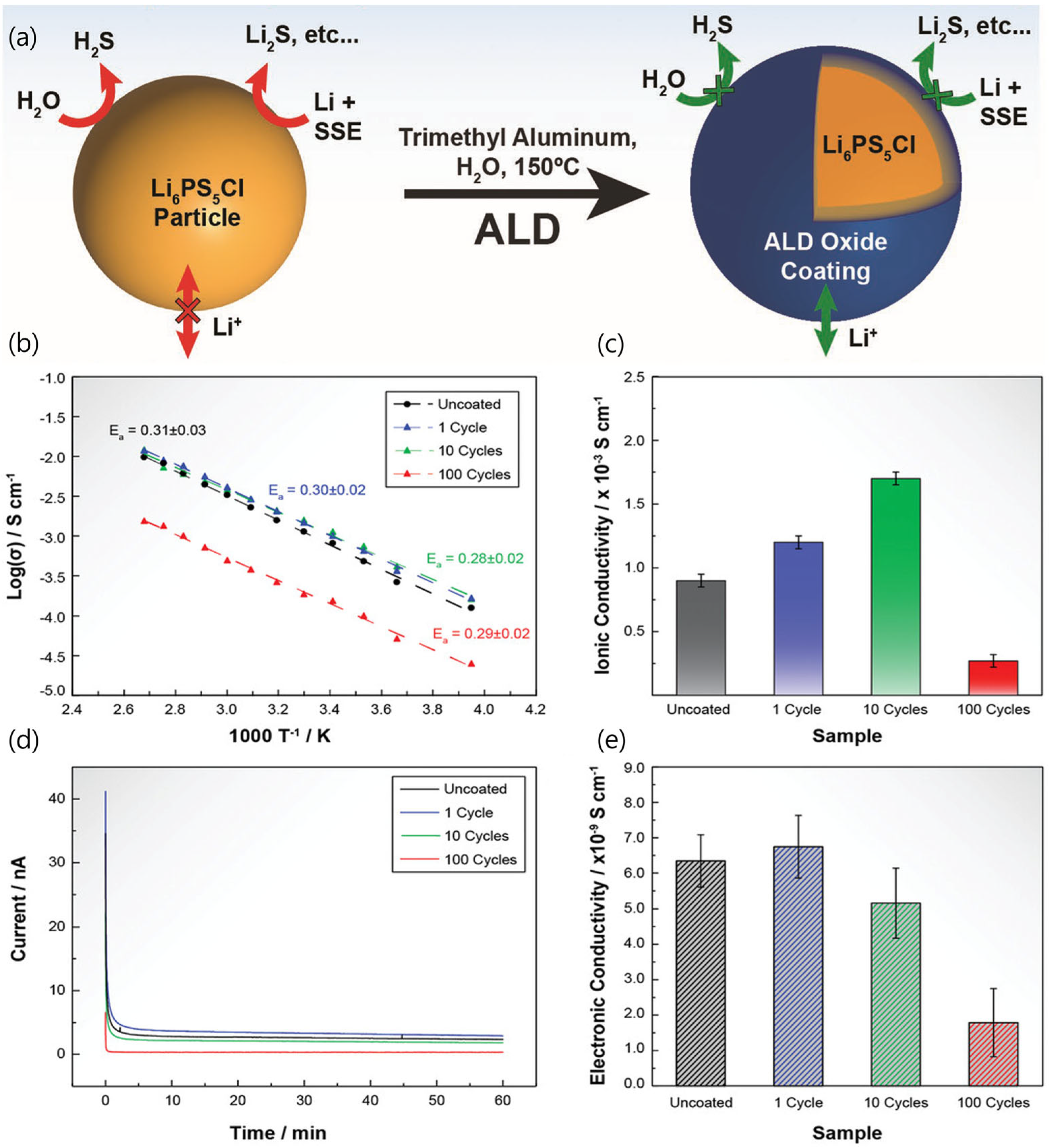
2.2. Strategies to Overcome Disadvantages of Sulfide SSEs for Li Metal
3. Oxide SSEs
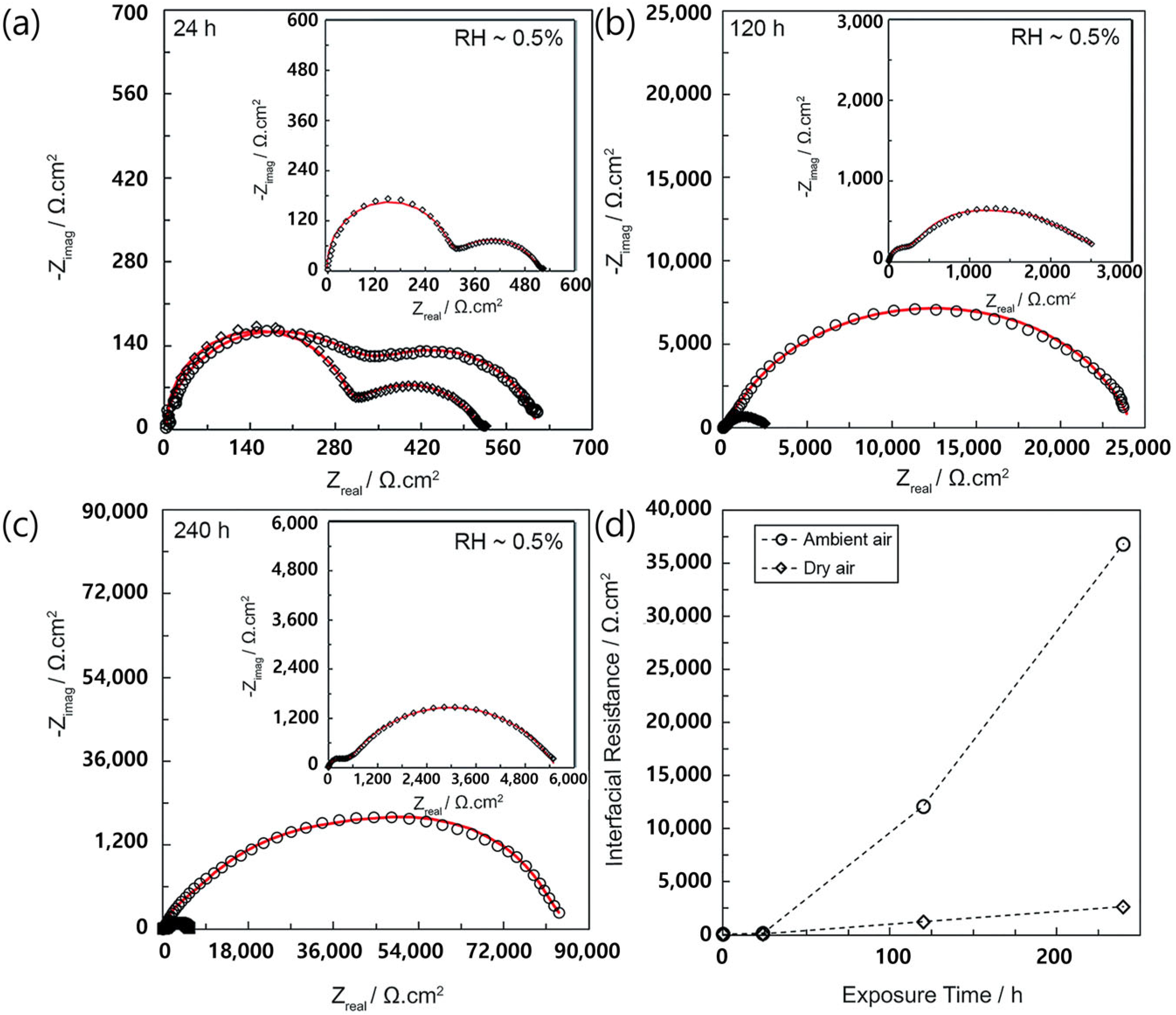
3.1. Challenges of Oxide SSEs for Li Metal
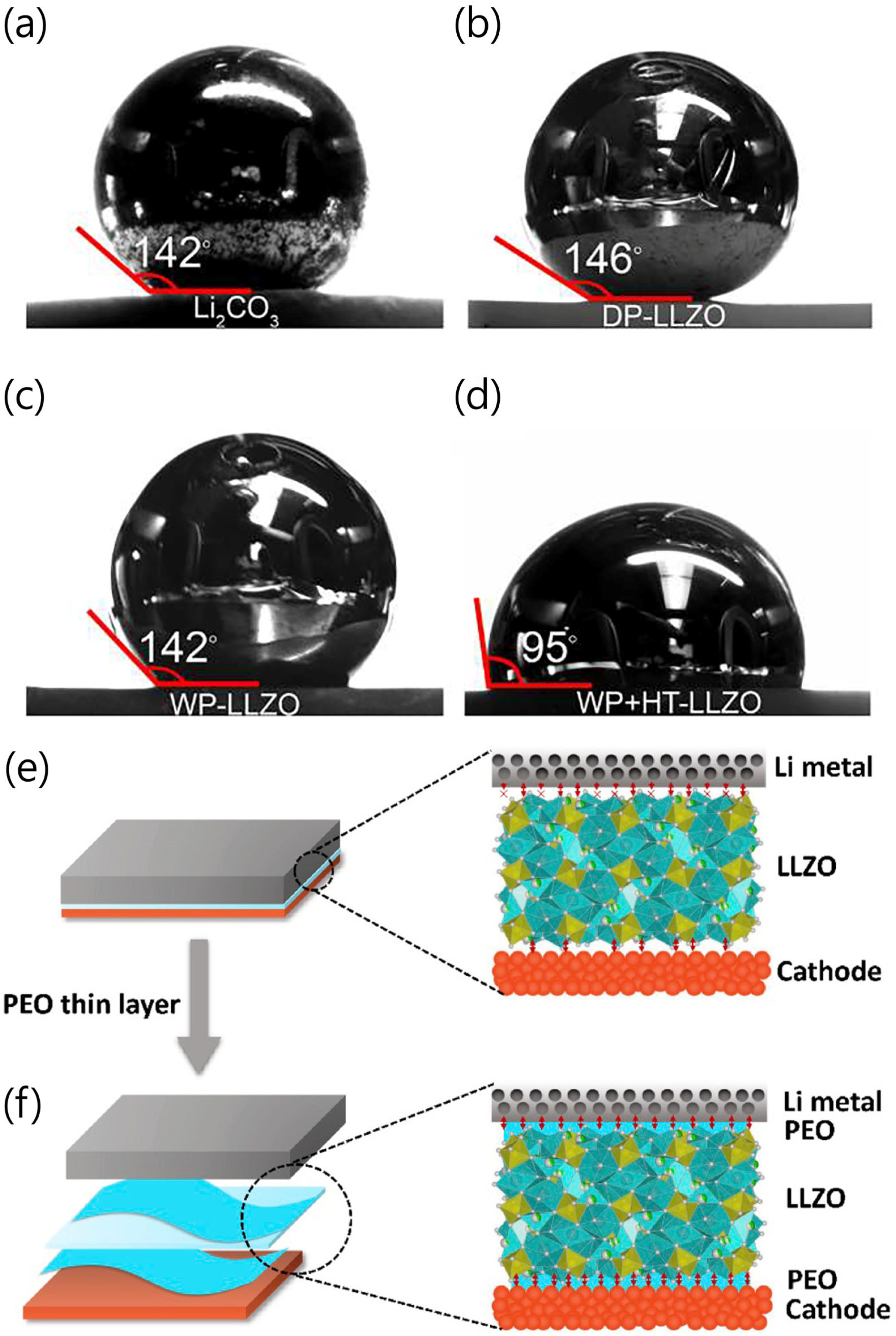
3.2. Strategies to Overcome Disadvantages of Oxide SSEs for Li Metal
4. Polymer SSEs
4.1. Challenges of Polymer SSEs for Li Metal
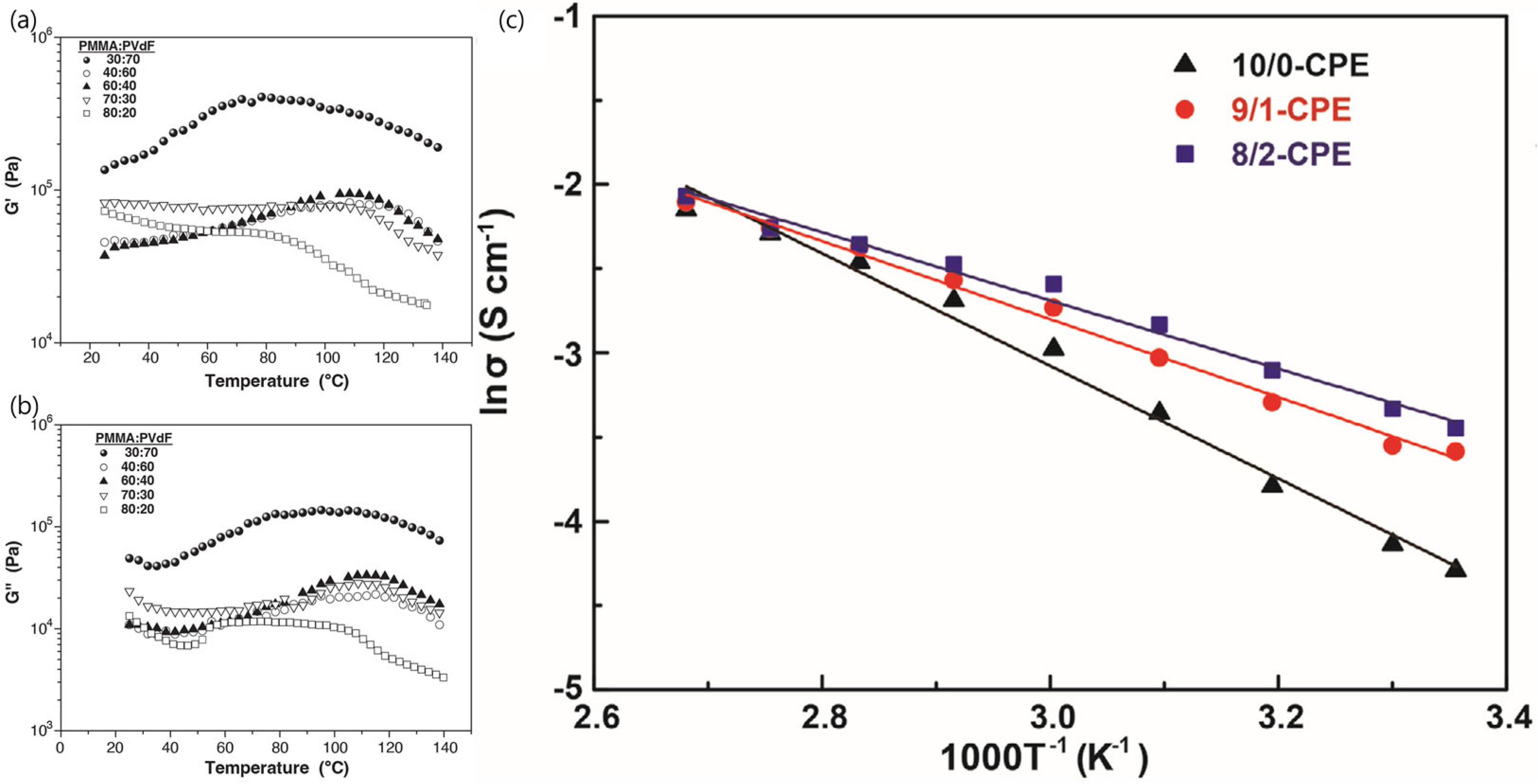
4.2. Strategies to Overcome Disadvantages of Polymer SSEs for Li Metal
5. Conclusions
Funding
Conflicts of Interest
References
- Yoon, D.H.; Park, Y.J. Electrochemical Properties of Cathode According to the Type of Sulfide Electrolyte and the Application of Surface Coating. J. Electrochem. Sci. Technol. 2021, 12, 126–136. [Google Scholar] [CrossRef]
- Zhao, W.; Choi, W.; Yoon, W.-S. Nanostructured electrode materials for rechargeable lithium-ion batteries. J. Electrochem. Sci. Technol. 2020, 11, 195–219. [Google Scholar] [CrossRef]
- Adiraju, V.A.K.; Chae, O.B.; Robinson, J.R.; Lucht, B.L. Highly Soluble Lithium Nitrate-Containing Additive for Carbonate-Based Electrolyte in Lithium Metal Batteries. ACS Energy Lett. 2023, 8, 2440–2446. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, J.; Han, S.; Lee, J.; Lee, H.; Kwon, J.; Lee, J.; Seo, J.; Kim, P.J.; Song, T. Low-Resistance LiFePO4 Thick Film Electrode Processed with Dry Electrode Technology for High-Energy-Density Lithium-Ion Batteries. Small Sci. 2024, 2300302. [Google Scholar] [CrossRef]
- Lee, G.; Kim, I.T.; Hur, J. Highly conductive and robust telluride-carbon hybrid matrix for enhanced copper diphosphide anode in Li-ion batteries. J. Alloys Compd. 2023, 950, 169914. [Google Scholar] [CrossRef]
- Thieu, Q.Q.V.; Kidanu, W.G.; Nguyen, H.D.; Nguyen, T.L.T.; Le, M.L.P.; Nguyen, D.Q.; Tran, N.T.; Nguyen, X.V.; Kim, I.T.; Nguyen, T.L. Spinel Ni-ferrite advanced high-capacity anode for Li-ion batteries prepared via coprecipitation route. Ceram. Int. 2022, 48, 31470–31477. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Kim, I.T.; Hur, J. Core-shell Si@ c-PAN particles deposited on graphite as promising anode for lithium-ion batteries. Electrochim. Acta 2019, 297, 355–364. [Google Scholar] [CrossRef]
- Lim, Y.E.; Choi, W.S.; Kim, J.H.; Ahn, Y.N.; Kim, I.T. The Sn–red P–Fe–based alloy materials for efficient Li–ion battery anodes. J. Ind. Eng. Chem. 2023, 121, 299–311. [Google Scholar] [CrossRef]
- Tran, Q.N.; Park, C.H.; Le, T.H. Nanocrystalline Cellulose-Supported Iron Oxide Composite Materials for High-Performance Lithium-Ion Batteries. Polymers 2024, 16, 691. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Yang, S.; Kim, J.; Lee, S.; Seo, J.; Choi, J.; Kim, P.J. Uniform Li Deposition through the Graphene-Based Ion-Flux Regulator for High-Rate Li Metal Batteries. ACS Appl. Mater. Interfaces 2024, 16, 3416–3426. [Google Scholar] [CrossRef]
- Paul, S. Materials and electrochemistry: Present and future battery. J. Electrochem. Sci. Technol. 2016, 7, 115–131. [Google Scholar] [CrossRef]
- Chae, O.B.; Lucht, B.L. Interfacial Issues and Modification of Solid Electrolyte Interphase for Li Metal Anode in Liquid and Solid Electrolytes. Adv. Energy Mater. 2023, 13, 2203791. [Google Scholar] [CrossRef]
- Chae, O.B.; Kim, J.; Lucht, B.L. Modification of lithium electrodeposition behavior by variation of electrode distance. J. Power Sources 2022, 532, 231338. [Google Scholar] [CrossRef]
- Kim, M.; Park, H.G.; Park, K. A strategy of enhancing the ionic conductivity of Li7La3Zr2O12 under accurate sintering conditions. Phys. Chem. Chem. Phys. 2022, 24, 29159–29164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Cheng, X.-B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Fan, L.; Zhuang, H.L.; Gao, L.; Lu, Y.; Archer, L.A. Regulating Li deposition at artificial solid electrolyte interphases. J. Mater. Chem. A 2017, 5, 3483–3492. [Google Scholar] [CrossRef]
- Jurng, S.; Brown, Z.L.; Kim, J.; Lucht, B.L. Effect of electrolyte on the nanostructure of the solid electrolyte interphase (SEI) and performance of lithium metal anodes. Energy Environ. Sci. 2018, 11, 2600–2608. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Xu, R.; Han, F.; Ji, X.; Fan, X.; Tu, J.; Wang, C. Interface engineering of sulfide electrolytes for all-solid-state lithium batteries. Nano Energy 2018, 53, 958–966. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Li, Y.; Song, S.; Kim, H.; Nomoto, K.; Kim, H.; Sun, X.; Hori, S.; Suzuki, K.; Matsui, N.; Hirayama, M. A lithium superionic conductor for millimeter-thick battery electrode. Science 2023, 381, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Vasylenko, A.; Daniels, L.M.; Collins, C.M.; Corti, L.; Chen, R.; Niu, H.; Manning, T.D.; Antypov, D.; Dyer, M.S. Superionic lithium transport via multiple coordination environments defined by two-anion packing. Science 2024, 383, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, D.; Liu, G.; Mwizerwa, J.P.; Zhang, Q.; Zhao, Y.; Xu, X.; Yao, X. Sulfide solid electrolytes for all-solid-state lithium batteries: Structure, conductivity, stability and application. Energy Storage Mater. 2018, 14, 58–74. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. First principles study on electrochemical and chemical stability of solid electrolyte–electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A 2016, 4, 3253–3266. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, Y.J. Li3PO4 Coated Li[Ni0.75Co0.1Mn0.15]O2 Cathode for All-Solid-State Batteries Based on Sulfide Electrolyte. J. Electrochem. Sci. Technol. 2022, 13, 407–415. [Google Scholar] [CrossRef]
- Nagao, M.; Hayashi, A.; Tatsumisago, M.; Kanetsuku, T.; Tsuda, T.; Kuwabata, S. In situ SEM study of a lithium deposition and dissolution mechanism in a bulk-type solid-state cell with a Li2S–P2S5 solid electrolyte. Phys. Chem. Chem. Phys. 2013, 15, 18600–18606. [Google Scholar] [CrossRef] [PubMed]
- Porz, L.; Swamy, T.; Sheldon, B.W.; Rettenwander, D.; Frömling, T.; Thaman, H.L.; Berendts, S.; Uecker, R.; Carter, W.C.; Chiang, Y.M. Mechanism of Lithium Metal Penetration through Inorganic Solid Electrolytes. Adv. Energy Mater. 2017, 7, 1701003. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Z.; Li, X.; Liu, X.; Wang, H.; Ma, W.; Zhang, L.; Zhu, L.; Zhang, X. Growth of lithium-indium dendrites in all-solid-state lithium-based batteries with sulfide electrolytes. Nat. Commun. 2021, 12, 6968. [Google Scholar] [CrossRef]
- Camacho-Forero, L.E.; Balbuena, P.B. Exploring interfacial stability of solid-state electrolytes at the lithium-metal anode surface. J. Power Sources 2018, 396, 782–790. [Google Scholar] [CrossRef]
- Wenzel, S.; Weber, D.A.; Leichtweiss, T.; Busche, M.R.; Sann, J.; Janek, J. Interphase formation and degradation of charge transfer kinetics between a lithium metal anode and highly crystalline Li7P3S11 solid electrolyte. Solid State Ion. 2016, 286, 24–33. [Google Scholar] [CrossRef]
- Wenzel, S.; Sedlmaier, S.J.; Dietrich, C.; Zeier, W.G.; Janek, J. Interfacial reactivity and interphase growth of argyrodite solid electrolytes at lithium metal electrodes. Solid State Ion. 2018, 318, 102–112. [Google Scholar] [CrossRef]
- Hood, Z.D.; Mane, A.U.; Sundar, A.; Tepavcevic, S.; Zapol, P.; Eze, U.D.; Adhikari, S.P.; Lee, E.J.; Sterbinsky, G.E.; Elam, J.W.; et al. Multifunctional Coatings on Sulfide-Based Solid Electrolyte Powders with Enhanced Processability, Stability, and Performance for Solid-State Batteries. Adv. Mater. 2023, 35, 2300673. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.P.; Mo, Y.F.; Richards, W.D.; Miara, L.; Lee, H.S.; Ceder, G. Phase stability, electrochemical stability and ionic conductivity of the Li10±1MP2X12 (M = Ge, Si, Sn, Al or P, and X = O, S or Se) family of superionic conductors. Energy Environ. Sci. 2013, 6, 148–156. [Google Scholar] [CrossRef]
- Sun, Y.; Suzuki, K.; Hara, K.; Hori, S.; Yano, T.-A.; Hara, M.; Hirayama, M.; Kanno, R. Oxygen substitution effects in Li10GeP2S12 solid electrolyte. J. Power Sources 2016, 324, 798–803. [Google Scholar] [CrossRef]
- Suzuki, K.; Sakuma, M.; Hori, S.; Nakazawa, T.; Nagao, M.; Yonemura, M.; Hirayama, M.; Kanno, R. Synthesis, structure, and electrochemical properties of crystalline Li–P–S–O solid electrolytes: Novel lithium-conducting oxysulfides of Li10GeP2S12 family. Solid State Ion. 2016, 288, 229–234. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714. [Google Scholar] [CrossRef]
- Choi, H.M.; Jun, S.J.; Lee, J.; Ryu, M.-H.; Shin, H.; Jung, K.-N. UV-cured Polymer Solid Electrolyte Reinforced using a Ceramic-Polymer Composite Layer for Stable Solid-State Li Metal Batteries. J. Electrochem. Sci. Technol. 2023, 14, 85–95. [Google Scholar] [CrossRef]
- Xia, W.H.; Xu, B.Y.; Duan, H.A.; Tang, X.Y.; Guo, Y.P.; Kang, H.M.; Li, H.; Liu, H.Z. Reaction mechanisms of lithium garnet pellets in ambient air: The effect of humidity and CO2. J. Am. Chem. Soc. 2017, 100, 2832–2839. [Google Scholar] [CrossRef]
- Sharafi, A.; Yu, S.H.; Naguib, M.; Lee, M.; Ma, C.; Meyer, H.M.; Nanda, J.; Chi, M.F.; Siegel, D.J.; Sakamoto, J. Impact of air exposure and surface chemistry on Li-Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 2017, 5, 13475–13487. [Google Scholar] [CrossRef]
- Cheng, L.; Crumlin, E.J.; Chen, W.; Qiao, R.M.; Hou, H.M.; Lux, S.F.; Zorba, V.; Russo, R.; Kostecki, R.; Liu, Z.; et al. The origin of high electrolyte-electrode interfacial resistances in lithium cells containing garnet type solid electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 18294–18300. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide Solid Electrolyte with Favorable Mechanical Property for All-Solid-State Lithium Battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; He, W.J.; Ding, L.X.; Wang, S.Q.; Wang, H.H. Enhancing interfacial contact in all solid state batteries with a cathode-supported solid electrolyte membrane framework. Energy Environ. Sci. 2019, 12, 938–944. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Q.; Ning, T.; Liu, T.; Luo, Y.; He, X.; Luo, Z.; Lu, A. Critical challenges and progress of solid garnet electrolytes for all-solid-state batteries. Mater. Today. Chem. 2020, 18, 100368. [Google Scholar] [CrossRef]
- Li, Y.T.; Chen, X.; Dolocan, A.; Cui, Z.M.; Xin, S.; Xue, L.G.; Xu, H.H.; Park, K.; Goodenough, J.B. Garnet Electrolyte with an Ultralow Interfacial Resistance for Li-Metal Batteries. J. Am. Chem. Soc. 2018, 140, 6448–6455. [Google Scholar] [CrossRef]
- Wu, J.F.; Pu, B.W.; Wang, D.; Shi, S.Q.; Zhao, N.; Guo, X.X.; Guo, X. In Situ Formed Shields Enabling Li2CO3-Free Solid Electrolytes: A New Route to Uncover the Intrinsic Lithiophilicity of Garnet Electrolytes for Dendrite-Free Li-Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Lu, Y.; Huang, X.; Su, J.; Sun, C.; Jin, J.; Wen, Z. Acid induced conversion towards a robust and lithiophilic interface for Li–Li7La3Zr2O12 solid-state batteries. J. Mater. Chem. A 2019, 7, 14565–14574. [Google Scholar] [CrossRef]
- Sharafi, A.; Kazyak, E.; Davis, A.L.; Yu, S.; Thompson, T.; Siegel, D.J.; Dasgupta, N.P.; Sakamoto, J. Surface Chemistry Mechanism of Ultra-Low Interfacial Resistance in the Solid-State Electrolyte Li7La3Zr2O12. Chem. Mater. 2017, 29, 7961–7968. [Google Scholar] [CrossRef]
- Ma, X.; Xu, Y.; Zhang, B.; Xue, X.; Wang, C.; He, S.; Lin, J.; Yang, L. Garnet Si–Li7La3Zr2O12 electrolyte with a durable, low resistance interface layer for all-solid-state lithium metal batteries. J. Power Sources 2020, 453, 227881. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, Y.; Grundish, N.; Xin, S.; Wang, S.; You, Y.; Wu, N.; Gao, J.; Cui, Z.; Li, Y. Polymer lithium-garnet interphase for an all-solid-state rechargeable battery. Nano Energy 2018, 53, 926–931. [Google Scholar] [CrossRef]
- Zhou, W.D.; Wang, S.F.; Li, Y.T.; Xin, S.; Manthiram, A.; Goodenough, J.B. Plating a Dendrite-Free Lithium Anode with a Polymer/Ceramic/Polymer Sandwich Electrolyte. J. Am. Chem. Soc. 2016, 138, 9385–9388. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gong, Y.; Fu, K.; He, X.; Hitz, G.T.; Dai, J.; Pearse, A.; Liu, B.; Wang, H.; Rubloff, G.; et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 2017, 16, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, Y.; Liu, B.; Fu, K.; Yao, Y.; Hitz, E.; Li, Y.; Dai, J.; Xu, S.; Luo, W.; et al. Conformal, Nanoscale ZnO Surface Modification of Garnet-Based Solid-State Electrolyte for Lithium Metal Anodes. Nano Lett. 2017, 17, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Gong, Y.; Zhu, Y.; Fu, K.K.; Dai, J.; Lacey, S.D.; Wang, C.; Liu, B.; Han, X.; Mo, Y.; et al. Transition from Superlithiophobicity to Superlithiophilicity of Garnet Solid-State Electrolyte. J. Am. Chem. Soc. 2016, 138, 12258–12262. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, J.; Munakata, H.; Kanamura, K. Effect of Gold Layer on Interface Resistance between Lithium Metal Anode and Li6.25Al0.25La3Zr2O12 Solid Electrolyte. J. Electrochem. Soc. 2017, 164, A1022–A1025. [Google Scholar] [CrossRef]
- Bocharova, V.; Sokolov, A.P. Perspectives for polymer electrolytes: A view from fundamentals of ionic conductivity. Macromolecules 2020, 53, 4141–4157. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef]
- Chen, R.; Qu, W.; Guo, X.; Li, L.; Wu, F. The pursuit of solid-state electrolytes for lithium batteries: From comprehensive insight to emerging horizons. Mat. Horiz. 2016, 3, 487–516. [Google Scholar] [CrossRef]
- Sun, X.-G.; Kerr, J.B. Synthesis and characterization of network single ion conductors based on comb-branched polyepoxide ethers and lithium bis (allylmalonato) borate. Macromolecules 2006, 39, 362–372. [Google Scholar] [CrossRef]
- Irfan, M.; Atif, M.; Yang, Z.; Zhang, W. Recent advances in high performance conducting solid polymer electrolytes for lithium-ion batteries. J. Power Sources 2021, 486, 229378. [Google Scholar] [CrossRef]
- Ye, L.; Feng, Z. Polymer electrolytes as solid solvents and their applications. In Polymer electrolytes; Elsevier: Amsterdam, The Netherlands, 2010; pp. 550–582. [Google Scholar]
- Ramesh, S.; Liew, C.-W.; Morris, E.; Durairaj, R. Effect of PVC on ionic conductivity, crystallographic structural, morphological and thermal characterizations in PMMA–PVC blend-based polymer electrolytes. Thermochim. Acta 2010, 511, 140–146. [Google Scholar] [CrossRef]
- Nicotera, I.; Coppola, L.; Oliviero, C.; Castriota, M.; Cazzanelli, E. Investigation of ionic conduction and mechanical properties of PMMA–PVdF blend-based polymer electrolytes. Solid State Ion. 2006, 177, 581–588. [Google Scholar] [CrossRef]
- Wheatle, B.K.; Fuentes, E.F.; Lynd, N.A.; Ganesan, V. Design of polymer blend electrolytes through a machine learning approach. Macromolecules 2020, 53, 9449–9459. [Google Scholar] [CrossRef]
- Przyłuski, J.; Wieczorek, W. Copolymer electrolytes. Solid State Ion. 1992, 53, 1071–1076. [Google Scholar] [CrossRef]
- Liu, F.; Li, T.; Yang, Y.; Yan, J.; Li, N.; Xue, J.; Huo, H.; Zhou, J.; Li, L. Investigation on the Copolymer Electrolyte of Poly (1, 3-dioxolane-co-formaldehyde). Macromol. Rapid Commun. 2020, 41, 2000047. [Google Scholar] [CrossRef]
- Chen, Z.; Steinle, D.; Nguyen, H.-D.; Kim, J.-K.; Mayer, A.; Shi, J.; Paillard, E.; Iojoiu, C.; Passerini, S.; Bresser, D. High-energy lithium batteries based on single-ion conducting polymer electrolytes and Li[Ni0.8Co0.1Mn0.1]O2 cathodes. Nano Energy 2020, 77, 105129. [Google Scholar] [CrossRef]
- Gao, J.; Wang, C.; Han, D.-W.; Shin, D.-M. Single-ion conducting polymer electrolytes as a key jigsaw piece for next-generation battery applications. Chem. Sci. 2021, 12, 13248–13272. [Google Scholar] [CrossRef] [PubMed]
- Porcarelli, L.; Shaplov, A.S.; Bella, F.; Nair, J.R.; Mecerreyes, D.; Gerbaldi, C. Single-ion conducting polymer electrolytes for lithium metal polymer batteries that operate at ambient temperature. ACS Energy Lett. 2016, 1, 678–682. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single lithium-ion conducting solid polymer electrolytes: Advances and perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Rolland, J.; Poggi, E.; Vlad, A.; Gohy, J.-F. Single-ion diblock copolymers for solid-state polymer electrolytes. Polymer 2015, 68, 344–352. [Google Scholar] [CrossRef]
- Porcarelli, L.; Manojkumar, K.; Sardon, H.; Llorente, O.; Shaplov, A.S.; Vijayakrishna, K.; Gerbaldi, C.; Mecerreyes, D. Single ion conducting polymer electrolytes based on versatile polyurethanes. Electrochim. Acta 2017, 241, 526–534. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Zhao, S.; Westover, A.S.; Belharouak, I.; Cao, P.F. Single-ion conducting polymer electrolytes for solid-state lithium–metal batteries: Design, performance, and challenges. Adv. Energy Mater. 2021, 11, 2003836. [Google Scholar] [CrossRef]





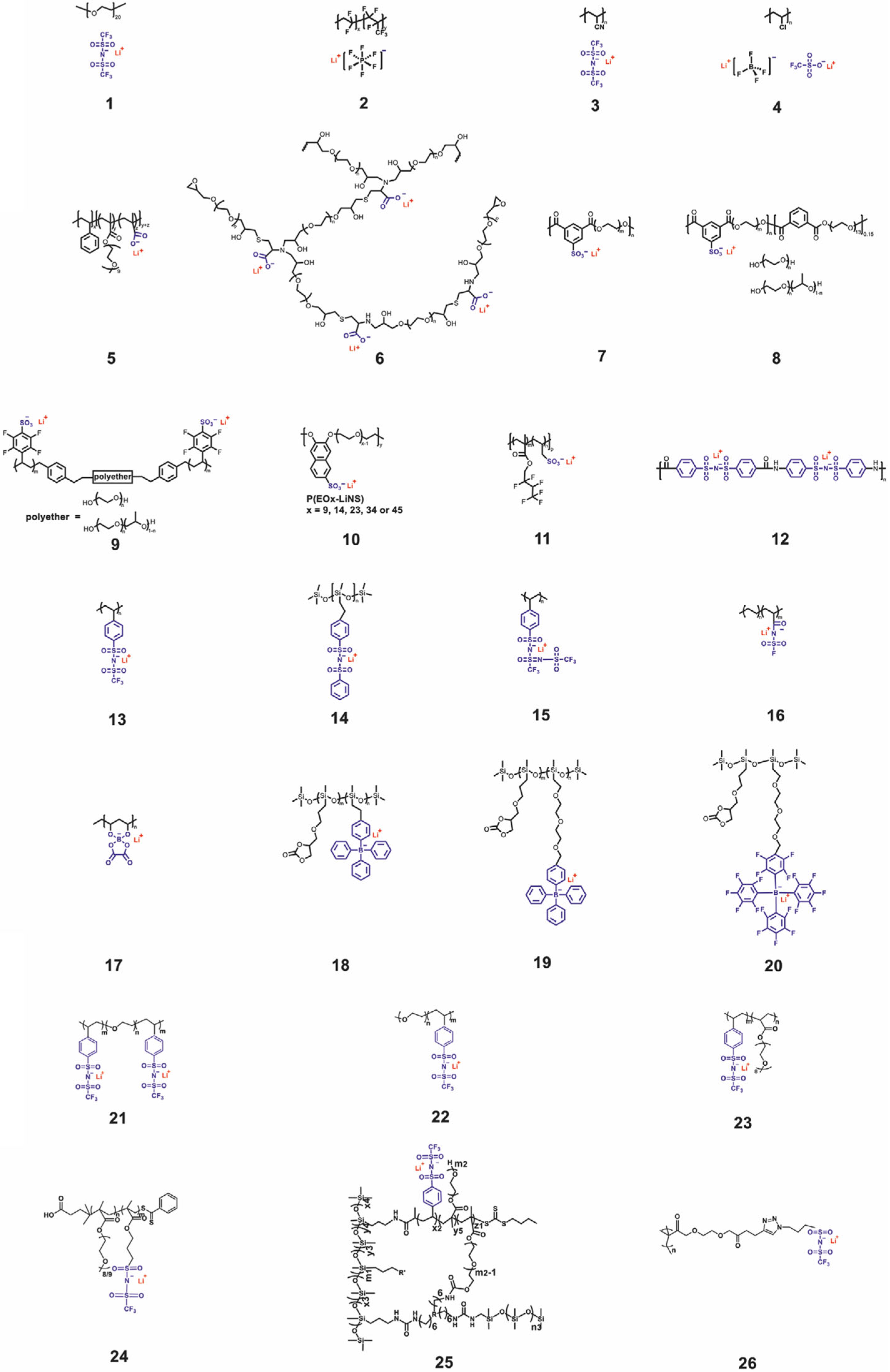
| Materials | Tg [°C] | tLi+ | Ionic Conductivity [S cm−1] | Testing Temperature [°C] | Mechanical Strength [MPa] |
|---|---|---|---|---|---|
| 1 | −57 | 0.29 | N/A | N/A | N/A |
| 2 | N/A | 0.46 | 8.9 × 10−4 | 25 | N/A |
| 3 | −105.2 | N/A | 2.5 × 10−3 | 23 | N/A |
| 4 | N/A | N/A | 2.6 × 10−3 | 25 | N/A |
| 5 | N/A | Close to unity | ≈10−8 | 20 | N/A |
| 6 | <0 | 0.86 | 1.2 × 10−4 | 85 | N/A |
| 7 | −37 | N/A | 10−6 | 25 | N/A |
| 8 | −20 | N/A | ≈10−5 | 25 | N/A |
| 9 | <0 | N/A | 1.4 × 10−5 | 60 | N/A |
| 10 | −38 | N/A | 5.5 × 10−6 | 120 | N/A |
| 11 | 17 | 0.92 | 10−4 | 80 | 7.1 |
| 12 | N/A | 0.92 | 1.4 × 10−4 | 25 | N/A |
| 13 | 152 | N/A | ≈10−5 | 41 | N/A |
| 14 | −110 | 0.89 | 7.2 × 10−4 | 25 | 5.8 |
| 15 | −47 | >0.9 | 7.6 × 10−6 | 25 | N/A |
| 16 | −30 | 0.91 | 5.84 × 10−4 | 25 | N/A |
| 17 | N/A | N/A | 6.11 × 10−6 | 25 | N/A |
| 18 | 10 | N/A | 10−8.2 | 25 | N/A |
| 19 | −17 | N/A | 10−7.0 | 25 | N/A |
| 20 | −16 | N/A | 10−6.9 | 25 | N/A |
| 21 | N/A | 0.85 | 1.35 × 10−5 | 60 | 10 |
| 22 | N/A | 0.95 | 3.8 × 10−4 | 90 | N/A |
| 23 | <0 | >0.9 | 10−4 | 60 | N/A |
| 24 | −61 | 0.83 | 2.3 × 10−6 | 25 | N/A |
| 25 | −17.5 | 0.79 | 4.5 × 10−7 | 30 | 0.37 |
| 26 | −27 to −14 | 0.79–0.99 | 10−5–10−4 | 90 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Yoon, T.; Chae, O.B. Strategies for Enhancing the Stability of Lithium Metal Anodes in Solid-State Electrolytes. Micromachines 2024, 15, 453. https://doi.org/10.3390/mi15040453
Lee H, Yoon T, Chae OB. Strategies for Enhancing the Stability of Lithium Metal Anodes in Solid-State Electrolytes. Micromachines. 2024; 15(4):453. https://doi.org/10.3390/mi15040453
Chicago/Turabian StyleLee, Hanbyeol, Taeho Yoon, and Oh B. Chae. 2024. "Strategies for Enhancing the Stability of Lithium Metal Anodes in Solid-State Electrolytes" Micromachines 15, no. 4: 453. https://doi.org/10.3390/mi15040453
APA StyleLee, H., Yoon, T., & Chae, O. B. (2024). Strategies for Enhancing the Stability of Lithium Metal Anodes in Solid-State Electrolytes. Micromachines, 15(4), 453. https://doi.org/10.3390/mi15040453









