Study on the Effect of Nanoporous Copper Particle Size on Copper-Based Azide
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Equipment
2.2. Precursor Synthesis
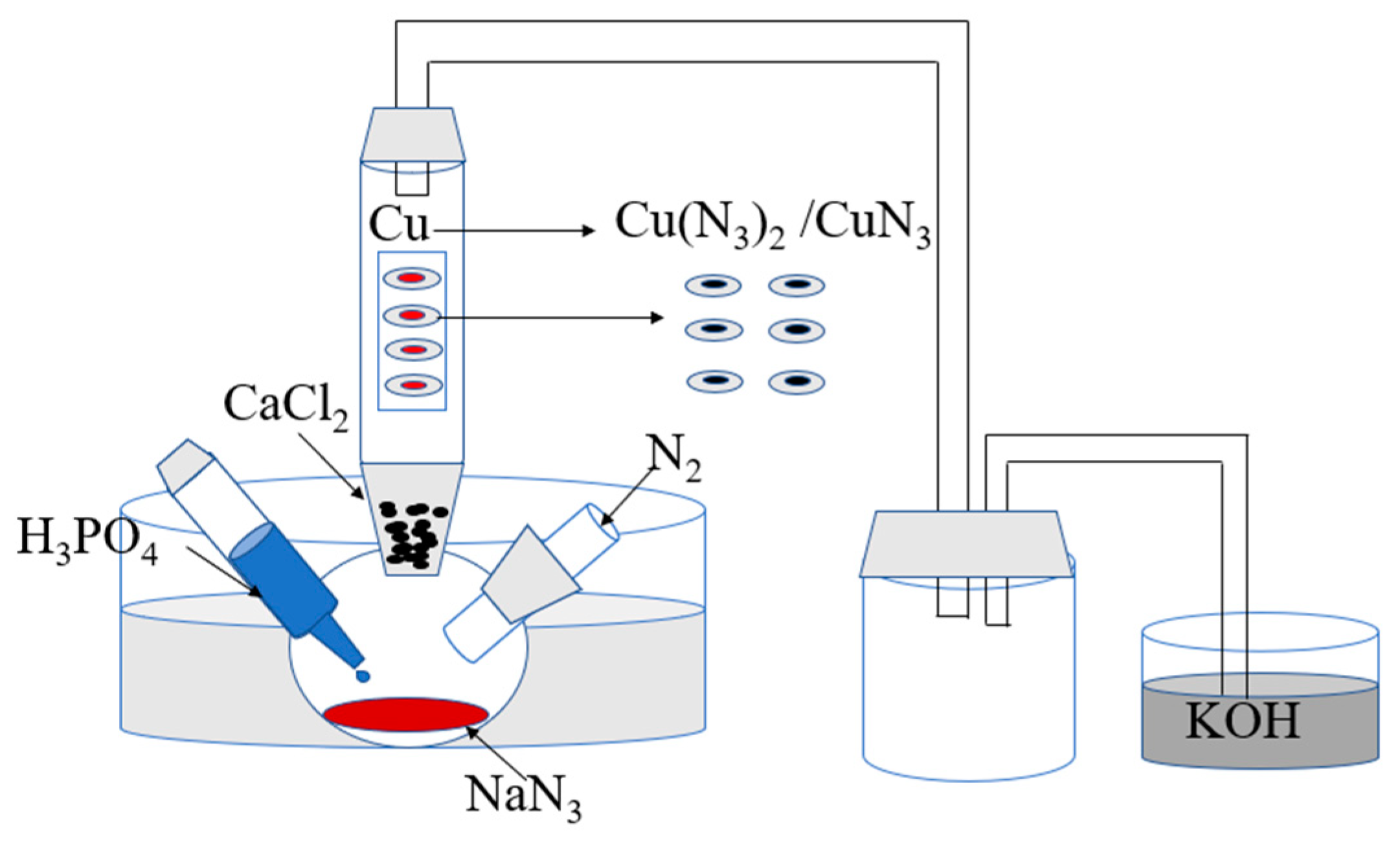
2.3. In Situ Reaction Process
2.4. Initiation Test of Copper-Based Azide Micro-Initiators
3. Results and Discussion
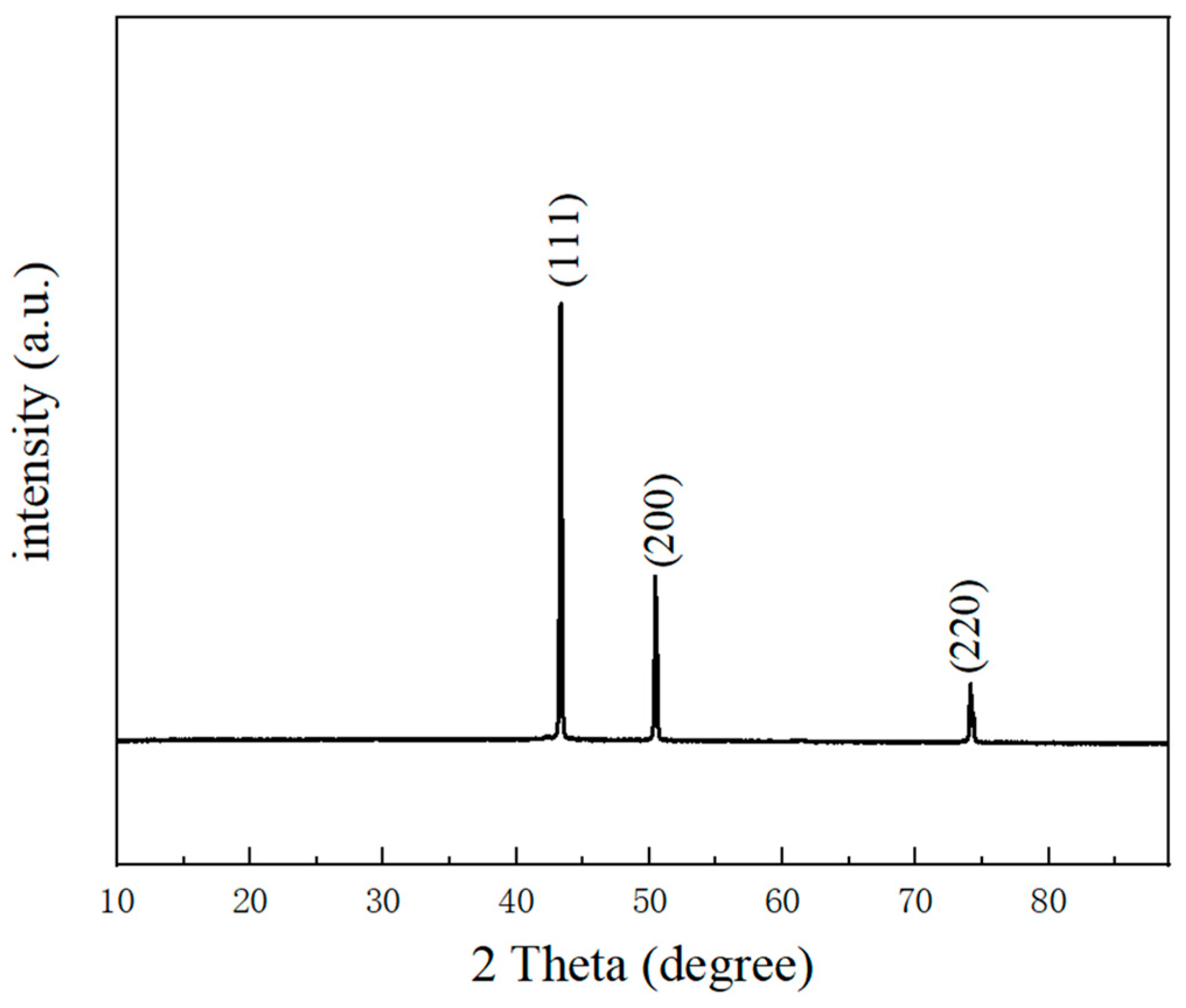
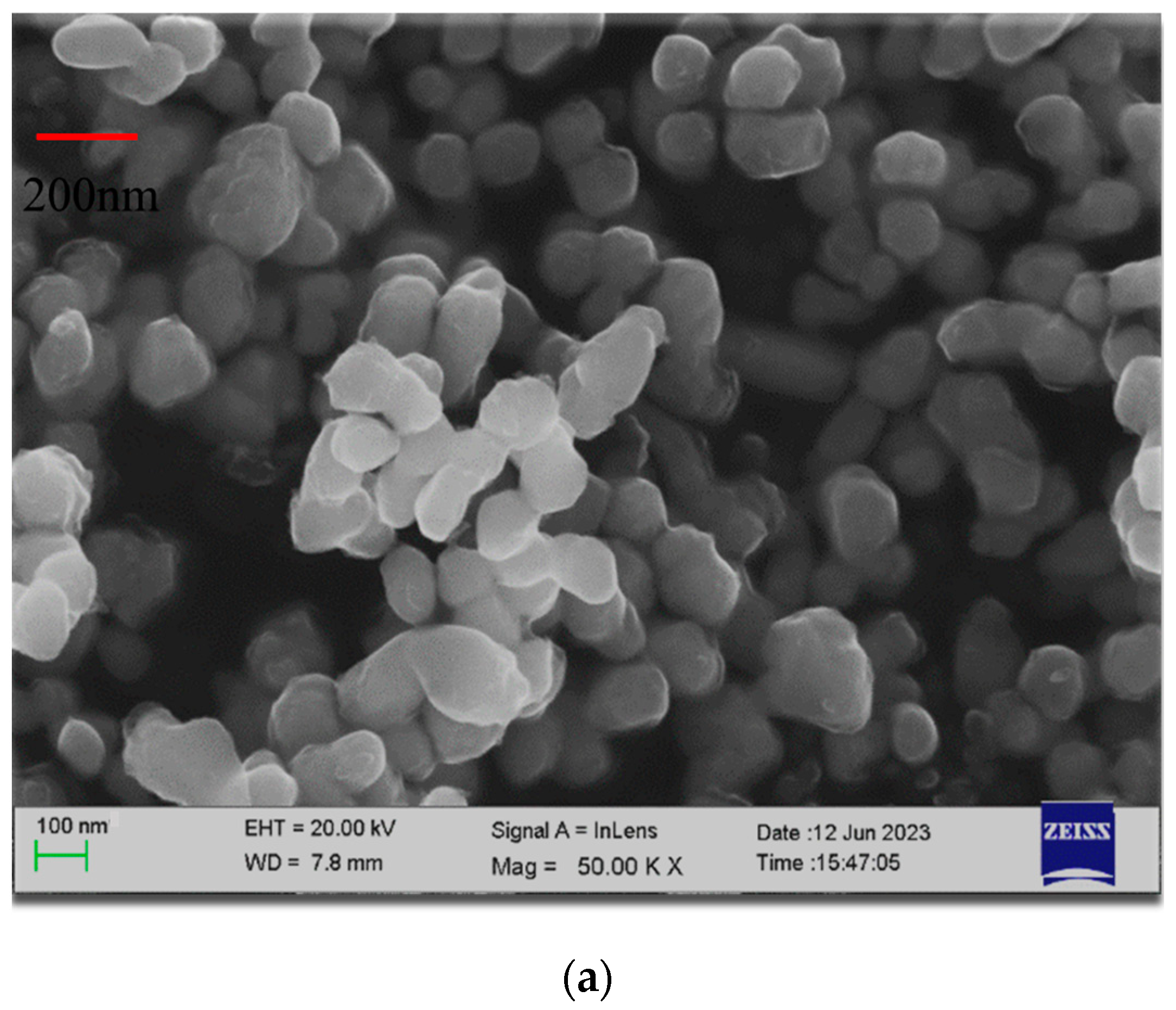
3.1. Nanoporous Copper
3.2. Copper-Based Azide
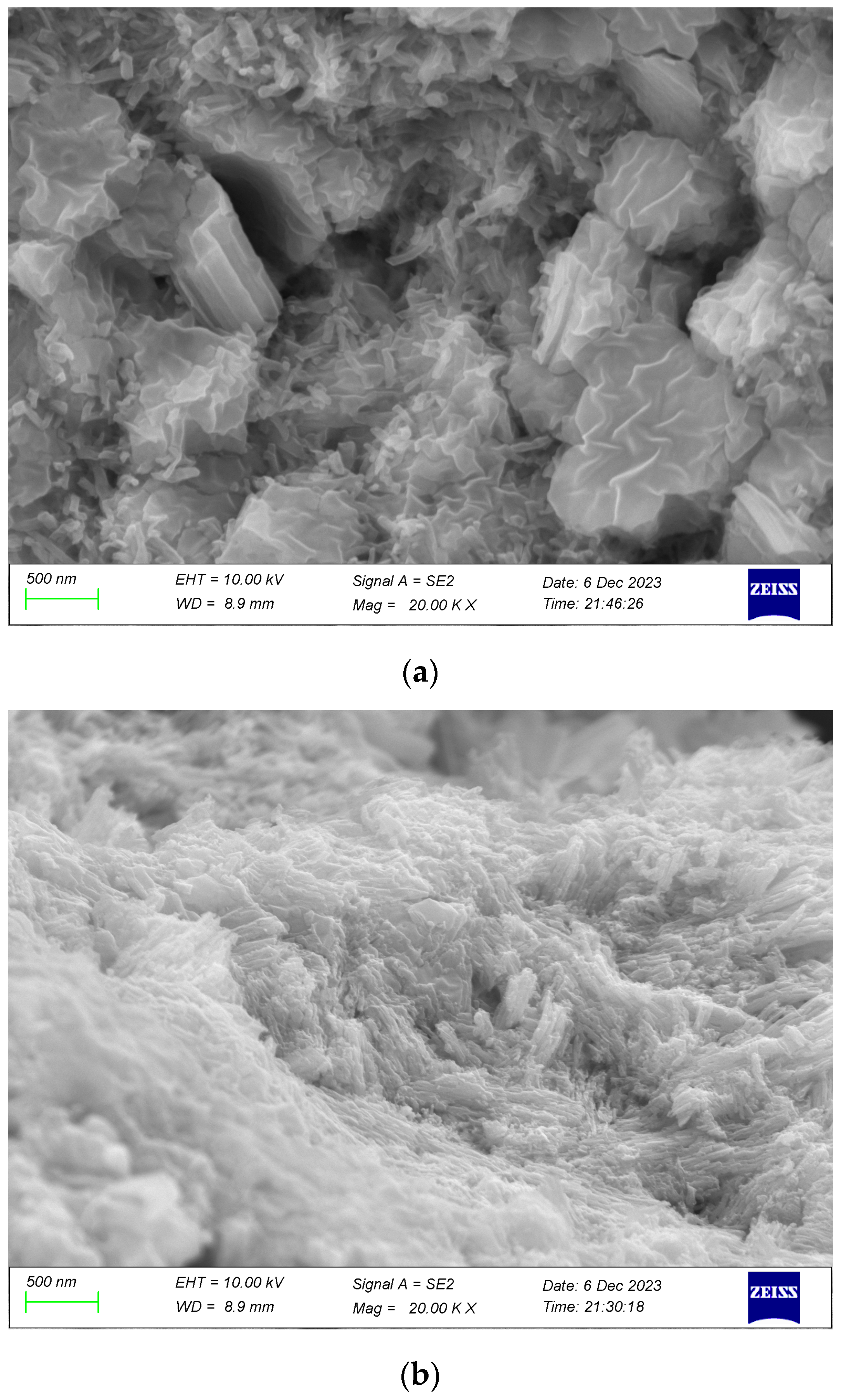
3.2.1. Morphological Analysis of Copper-Based Azide
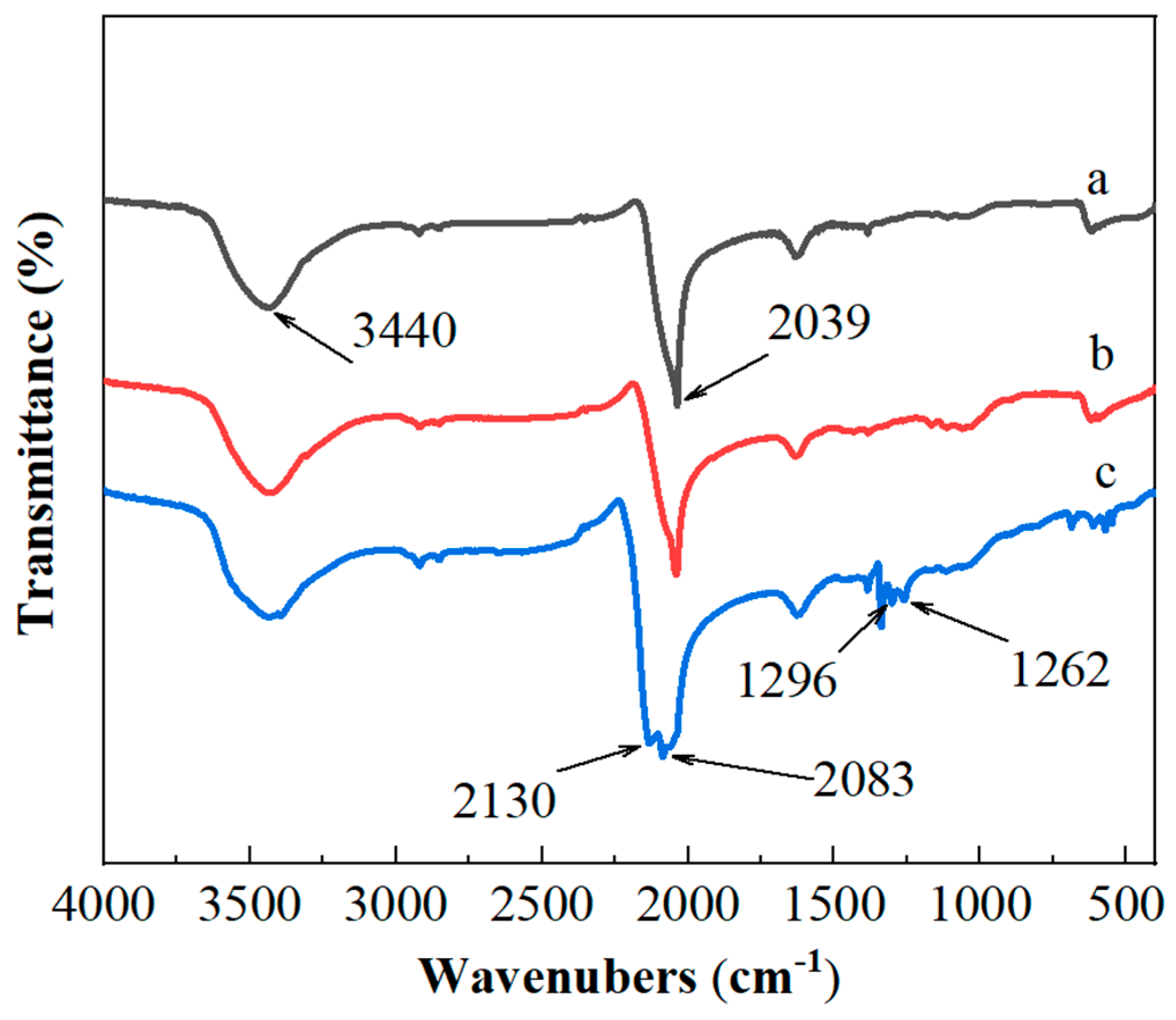
3.2.2. Composition Analysis of Copper-Based Azide
3.2.3. Compositional Analysis and Bulk Energy Density of Copper-Based Azide
3.3. Initiation Test of Copper-Based Azide Micro-Initiators
3.4. Discussion
4. Conclusions
- (1)
- Doping copper acetate during the preparation of nanoporous copper via the sintered copper oxalate method was found to reduce the particle size of the nanoporous copper. By carefully controlling the addition of copper acetate, nanoporous copper particles with sizes of around 30 nm and 60 nm were successfully obtained.
- (2)
- The gas–solid reaction principle was employed to elucidate the diverse morphologies of the reaction products derived from precursors with different particle sizes. The composition of the copper-based azide was analyzed, and the bulk energy density was calculated. A balance was achieved between precursor size, loading density and reaction depth, with the optimal particle size for the nanoporous copper precursor deemed to be 60 nm.
- (3)
- A copper azide micro-detonator was designed to propel the flying fragments of CL-20 explosives and HNS-IV explosives for detonation testing. The experimental outcomes demonstrated that reducing the particle size of the nanoporous copper significantly enhanced the output performance. A charge thickness of 0.5 mm and a charge mass of 0.8 mg ensured stable and reliable detonation of both the CL-20 explosives and HNS-IV explosives.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ejeian, F.; Azadi, S.; Razmjou, A.; Orooji, Y.; Kottapalli, A.; Warkiani, M.E.; Asadnia, M. Design and applications of MEMS flow sensors: A review. Sens. Actuators A 2019, 295, 483–502. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, L.; Han, J.M.; Li, H.; Huo, J. Fabrication of a nanoscale homogeneous lead azide@carbon fiber film with low electrostatic sensitivity by in situ synthesis. New J. Chem. 2021, 45, 11780. [Google Scholar] [CrossRef]
- Li, L.; Yan, Z.; Tong, W.; Hu, C.; Wang, S.; Li, H.; Yang, L.; Han, J.-M. Construction of high energy nanoscale lead azide composite with improved flame sensibility from intercalated hydroxide. Inorg. Chem. 2023, 63, 474–484. [Google Scholar] [CrossRef]
- Hawkes, A.S.; Winkler, C.A. The thermal explosion of lead azide. Can. J. Res. 2011, 25, 548–565. [Google Scholar] [CrossRef]
- Ma, J.; Tang, J.; Yang, H.; Yi, Z.; Wu, G.; Zhu, S.; Zhang, W.; Li, Y.; Cheng, G. Polynitro-functionalized triazolylfurazanate triaminoguanidine: Novel green primary explosive with insensitive nature. ACS Appl. Mater. Interfaces 2019, 11, 26053–26059. [Google Scholar] [CrossRef]
- Szimhardt, N.; Wurzenberger, M.H.; Beringer, A.; Daumann, L.J.; Stierstorfer, J. Coordination chemistry with 1-methyl-5H-tetrazole: Cocrystallization, laser-ignition, lead-free primary explosives—One ligand, three goals. J. Mater. Chem. A 2017, 5, 23753. [Google Scholar] [CrossRef]
- He, C.; Shreeve JN, M. Potassium 4, 5-Bis(dinitromethyl) furoxanate: A green primary explosive with a positive oxygen balance. Angew. Chem. 2016, 128, 782–785. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, B.; Li, M.; Wu, X. A miniature device for shock initiation of hexanitrostilbene by high-speed flyer. Propellants Explos. Pyrotech. 2016, 41, 864–869. [Google Scholar] [CrossRef]
- Wu, X.; Li, M.; Zeng, Q.; Hao, Y.; Ren, J. In-situ synthesis of copper azide chips and investigation of their initiation ability. Chem. Eng. J. 2022, 427, 131952. [Google Scholar] [CrossRef]
- Matyas, R.; Selesovsky, J.; Musil, T. Sensitivity to friction for primary explosives. J. Hazard. Mater. 2012, 213–214, 236–241. [Google Scholar] [CrossRef]
- Pelletier, V.; Bhattacharyya, S.; Knoke, I.; Forohar, F.; Bichay, M.; Gogotsi, Y. Copper azide confined inside templated carbon nanotubes. Adv. Funct. Mater. 2010, 20, 3168–3174. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Wang, S.; Song, N.; Chen, Y.; Tong, W.; Han, Y.; Yang, L.; Wang, B. Metal-organic framework templated synthesis of copper azide as the primary explosive with low electrostatic sensitivity and excellent initiation ability. Adv. Mater. 2016, 28, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, J.; Zhang, Y.; Yan, Z.; Velasco, E.; Yang, L.; Wang, B.; Zang, S.-Q. Fabrication of copper azide film through metal-organic framework for micro initiator applications. ACS Appl. Mater. Interfaces 2019, 27, 8081–8088. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, M.; Zeng, Q.; Wu, X. Copper azide fabricated by NPC precursor with proper density. Appl. Surf. Sci. 2018, 442, 38–44. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Bai, Y.; Zhang, R. Preparation and characterization of copper azide nanowire array. Mater. Lett. 2012, 89, 176–179. [Google Scholar] [CrossRef]
- Xie, R.Z.; Ren, X.M.; Liu, L.; Xue, Y.; Fu, D.X.; Zhang, R. Research on design and firing performance of Si-based detonator. Def. Technol. 2014, 10, 34–39. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Zeng, Q.; Wu, X. Monolithic nanoporous copper fabricated through decomposition and sintering of oxalate. Micro Nano Lett. 2016, 11, 378–381. [Google Scholar] [CrossRef]
- Ye, B.Y.; Song, C.K.; Huang, H.; Li, Q.B.; An, C.W.; Wang, J.Y. Direct ink writing of 3D-Honeycombed CL-20 structures with low critical size. Def. Technol. 2020, 16, 588e95. [Google Scholar] [CrossRef]
- Ren, J.; Wang, J.; Zhang, W.; Wu, X.; Li, S.; Li, M.; Zeng, Q. Morphological evolution mechanism of copper-based azide during gas-solid azidation process: Experiment and thermodynamic analysis. Vacuum 2023, 217, 112564. [Google Scholar] [CrossRef]
- Matyâa, R.; Pachmâ, H.J. Primary Explosives; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ren, J.; Li, Y.; Li, M.; Wu, X.; Wang, J.; Zeng, Q. Simplified quantitative analysis method and its application in the in-situ synthesized copper-based azide chips. Def. Technol. 2023, 32, 309–316. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Wang, Y.; Han, R.; Chen, J.; Zhang, R.; Chu, E. In-situ preparation of copper azide by direct ink writing. Mater. Lett. 2019, 238, 130–133. [Google Scholar] [CrossRef]
- Xie, R.; Chu, E.; Dai, X.; Su, Q.; Xue, Y.; Ren, X.; Liu, L.; Liu, W. Design and Detonation Transfer/explosion Interruption Performance of Micro Initiation Train. Acta Armamentarii. 2021, 42, 1178–1184. [Google Scholar]
- He, X.; Yang, L.; Dong, H.; Lv, Z.; Yan, N. Design of a Microflyer Driven by a Microsized Charge Combined with an Initiation Criterion. Micromachines 2023, 14, 312. [Google Scholar] [CrossRef] [PubMed]




| Sample | α Cu(N3)2/% | α CuN3/% | α/% | Ev/J·mm−3 |
|---|---|---|---|---|
| a | 11.32 | 78.04 | 90.36 | 6.52 |
| b | 34.31 | 60.25 | 94.56 | 8.46 |
| c | 36.56 | 56.26 | 92.82 | 8.50 |
| Sample | Explosive | Thickness of CA (mm) | Mass of CA (mg) | Detonation or Not |
|---|---|---|---|---|
| a | CL-20 | 0.5 | 0.8 | no |
| CL-20 | 1.0 | 1.6 | yes | |
| HNS-IV | 0.5 | 0.8 | no | |
| HNS-IV | 1.0 | 1.6 | yes | |
| b | CL-20 | 0.5 | 0.8 | yes |
| HNS-IV | 0.5 | 0.8 | yes | |
| c | CL-20 | 0.5 | 0.8 | yes |
| HNS-IV | 0.5 | 0.8 | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ren, J.; Li, S.; Li, M.; Zeng, Q. Study on the Effect of Nanoporous Copper Particle Size on Copper-Based Azide. Micromachines 2024, 15, 462. https://doi.org/10.3390/mi15040462
Wang J, Ren J, Li S, Li M, Zeng Q. Study on the Effect of Nanoporous Copper Particle Size on Copper-Based Azide. Micromachines. 2024; 15(4):462. https://doi.org/10.3390/mi15040462
Chicago/Turabian StyleWang, Jiabao, Jie Ren, Shuang Li, Mingyu Li, and Qingxuan Zeng. 2024. "Study on the Effect of Nanoporous Copper Particle Size on Copper-Based Azide" Micromachines 15, no. 4: 462. https://doi.org/10.3390/mi15040462





