Fabricating Precise and Smooth Microgroove Structures on Zr-Based Metallic Glass Using Jet-ECM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electrochemical Measurement Setup
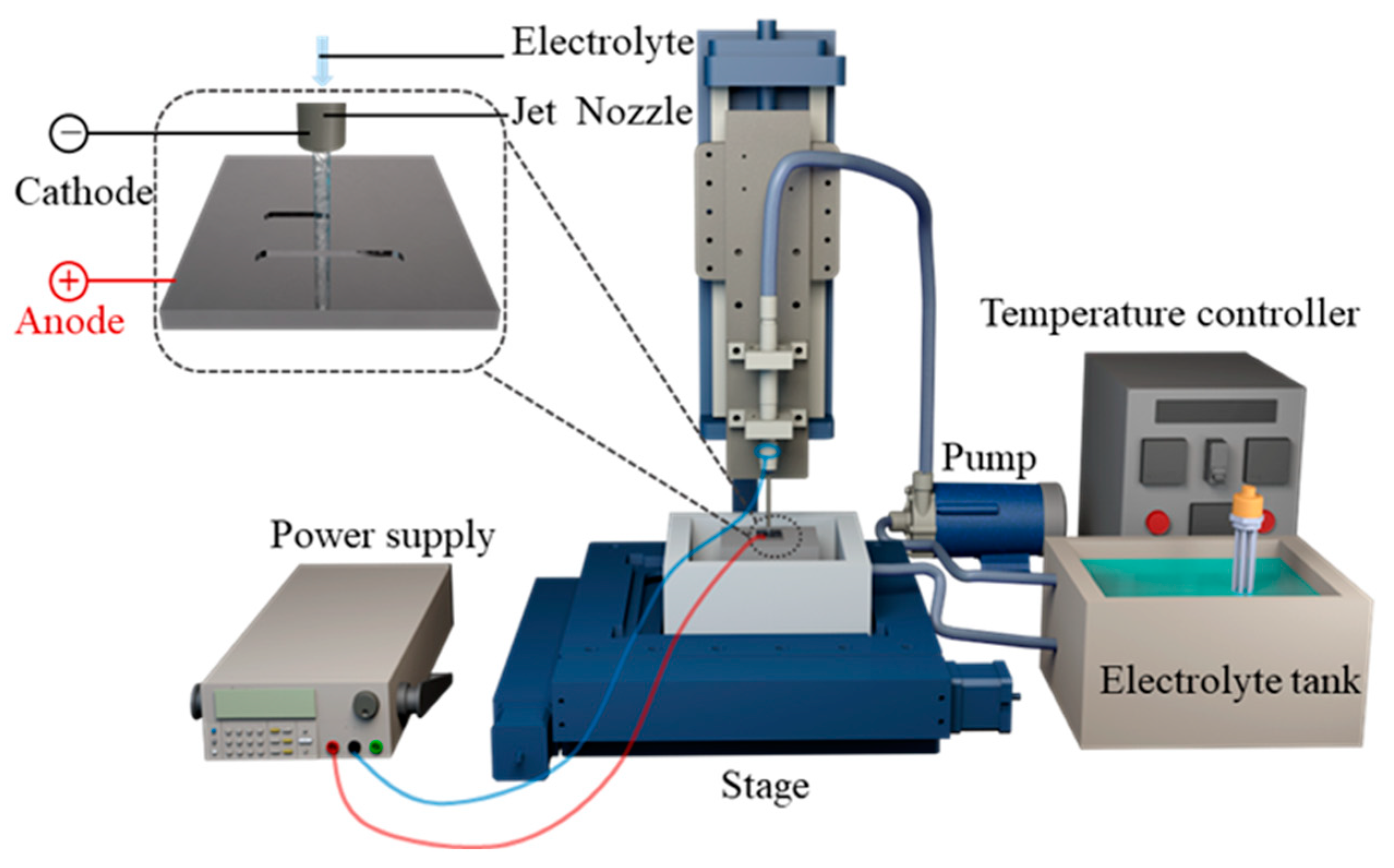
2.3. Experimental System for Jet-ECM
2.4. Test Equipment
3. Results and Discussion
3.1. Electrochemical Characteristic Analysis of the Vit1
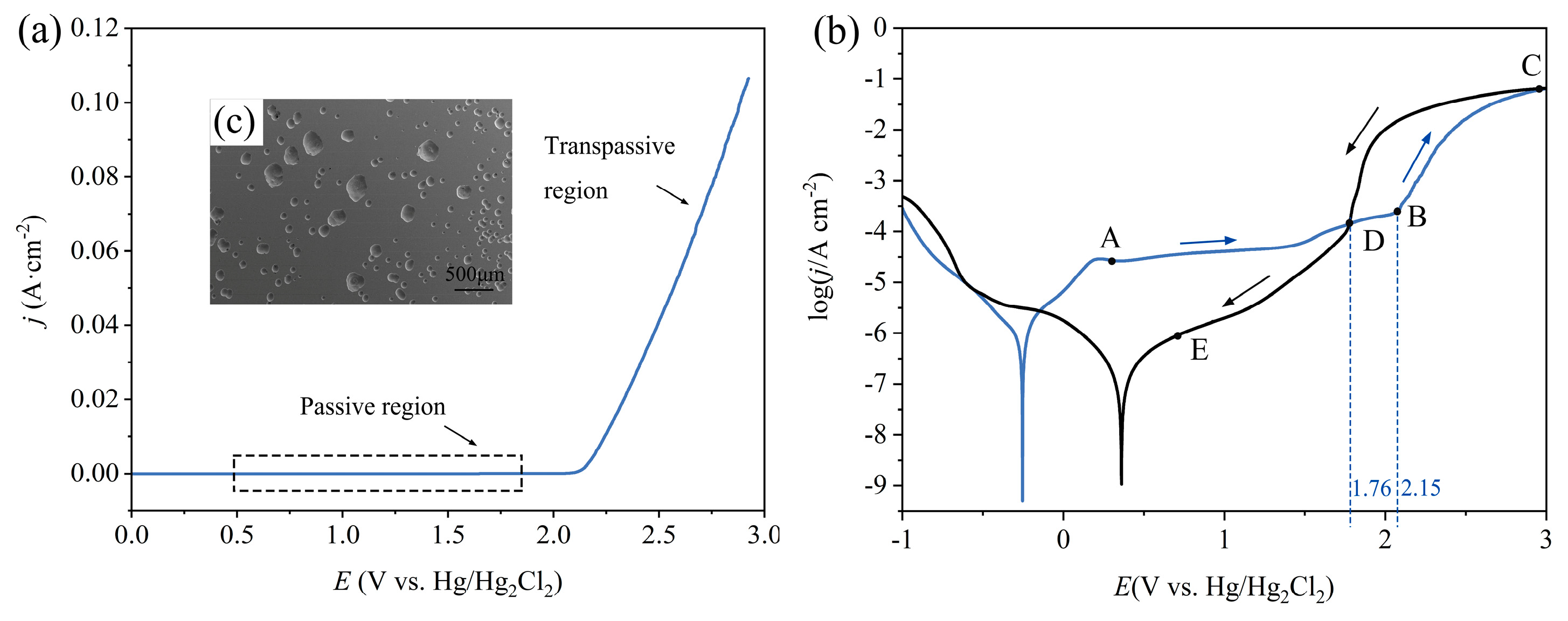
3.1.1. Anodic Polarization Curves
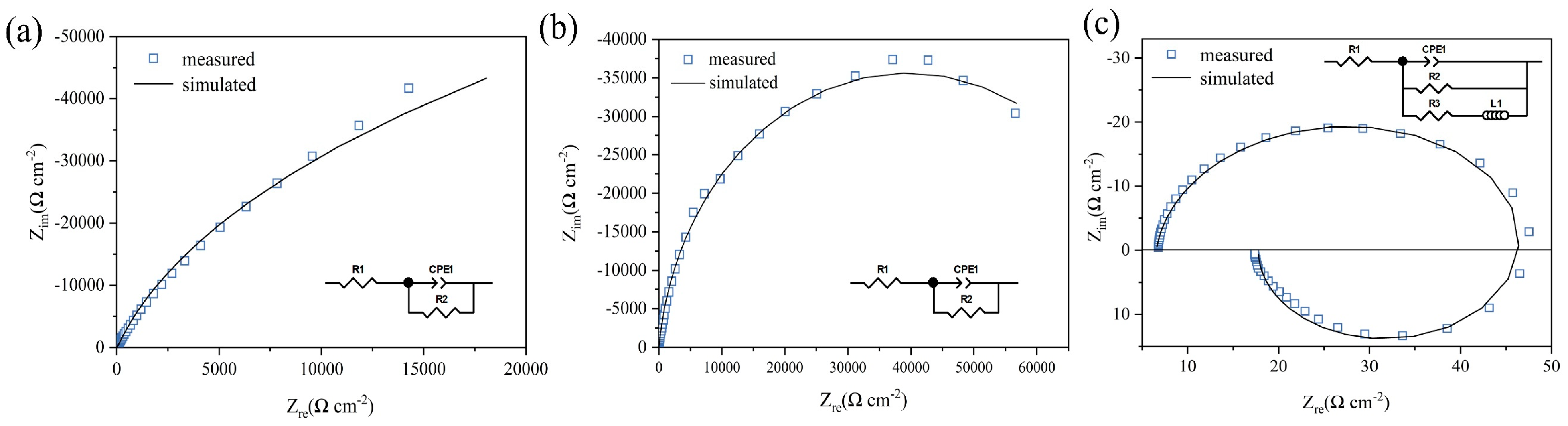
3.1.2. Electrochemical Impedance Spectroscopy (EIS)
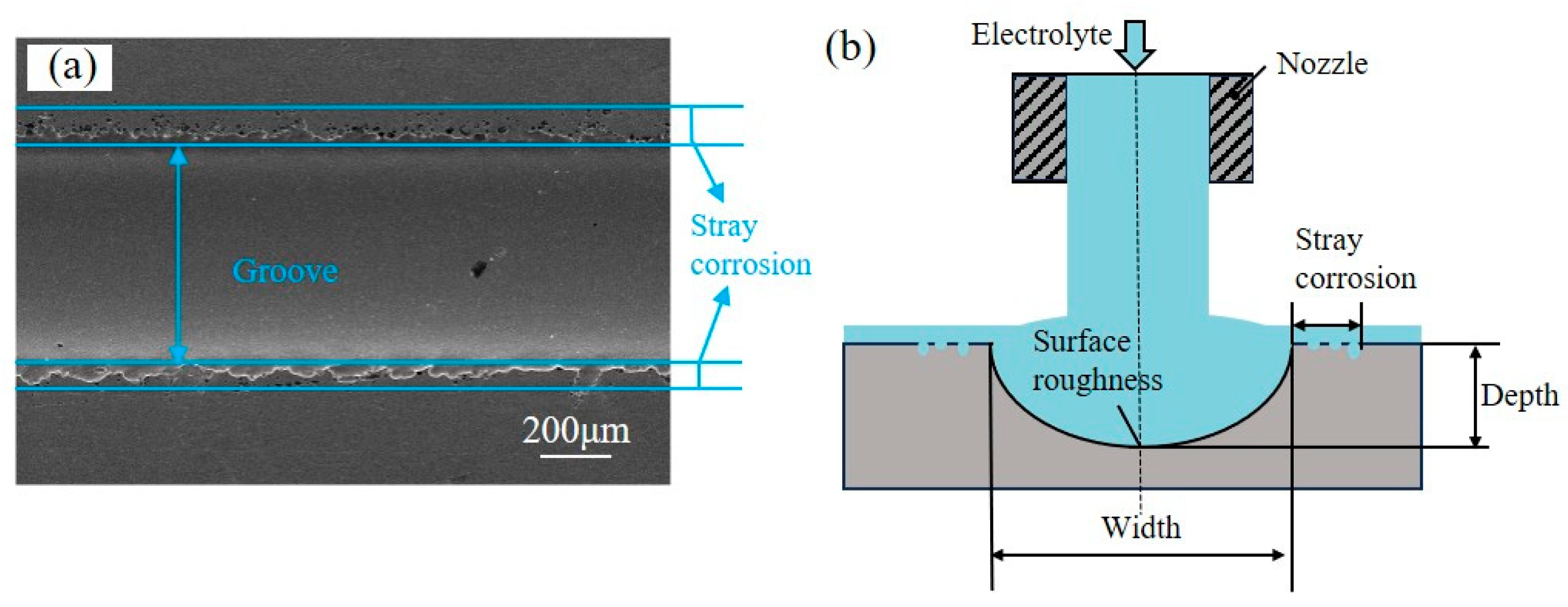
3.1.3. Discussion of the Material Removal Region in Jet-ECM
3.2. Parametric Effects of Microgroove Fabrication by Jet-ECM
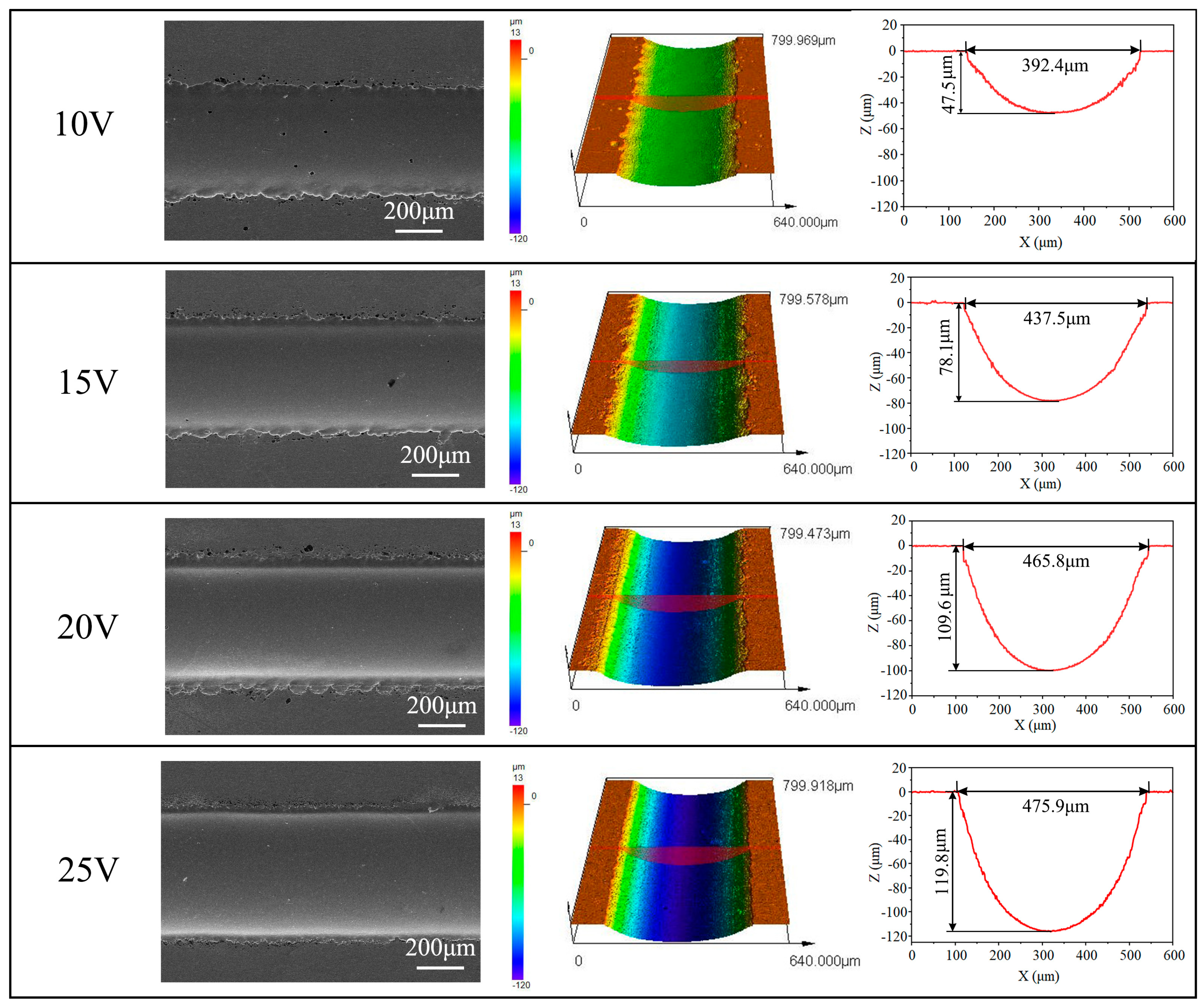
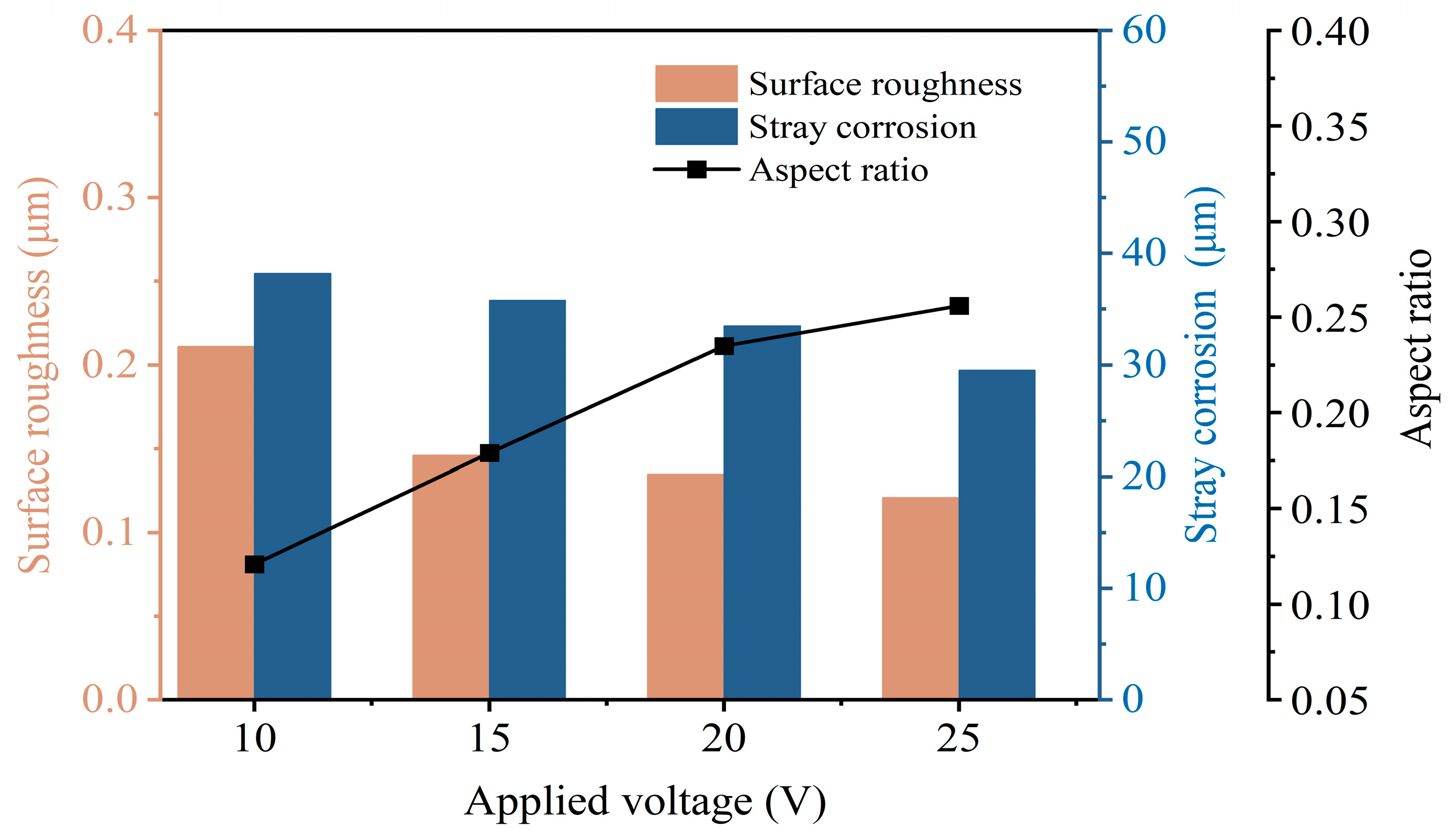
3.2.1. Effects of Applied Voltage
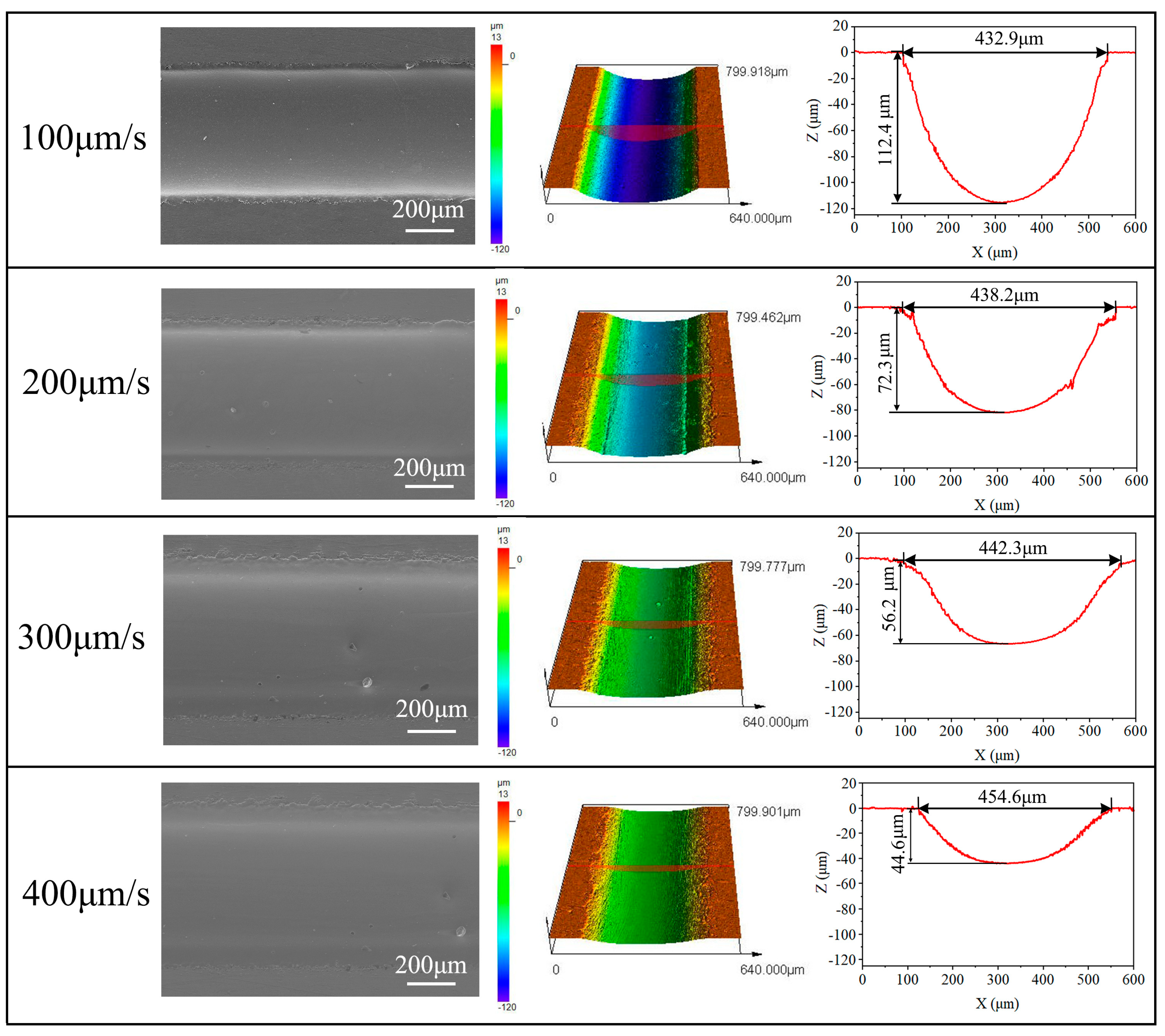
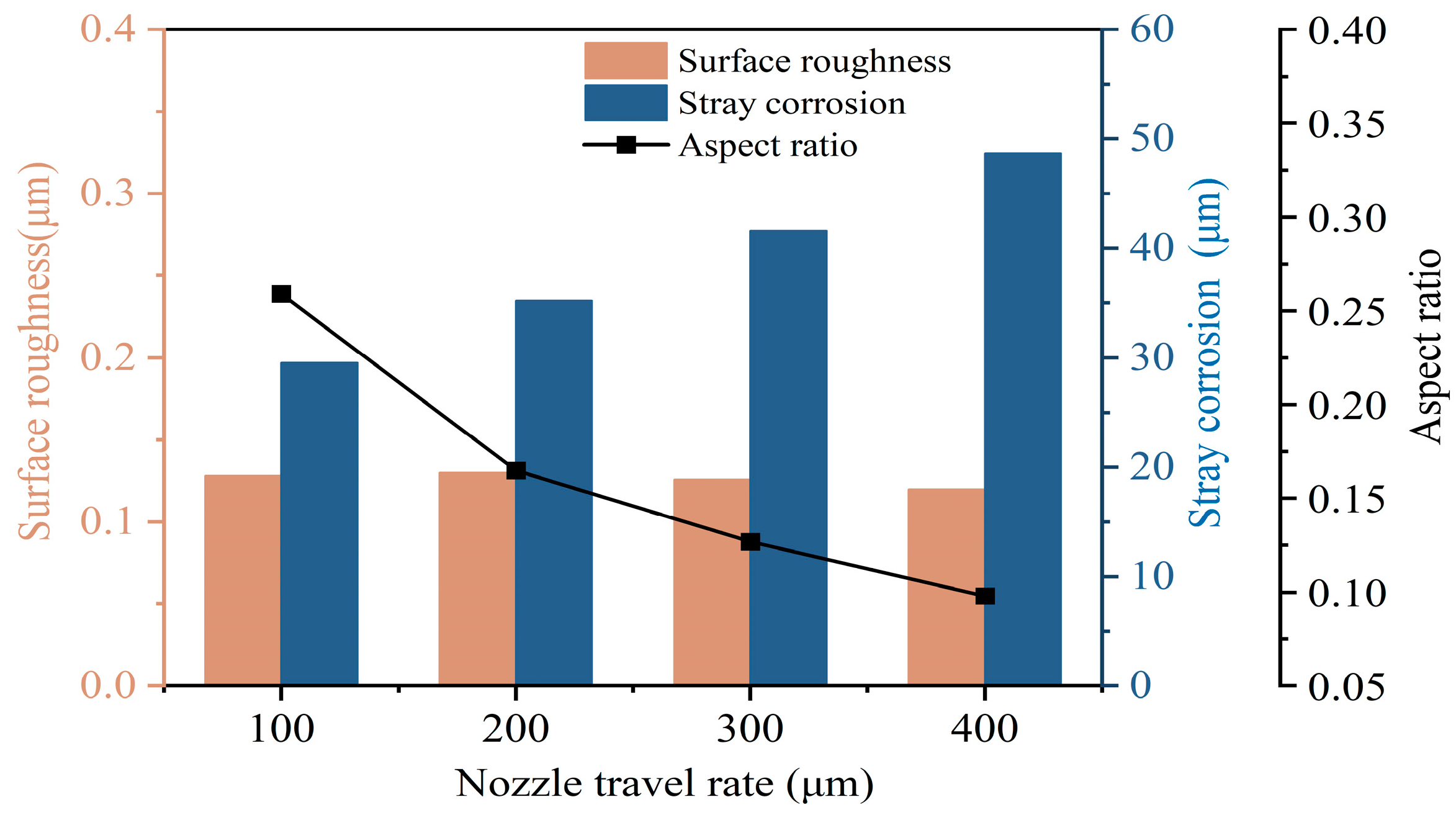
3.2.2. Effects of Nozzle Travel Rate
3.2.3. The Dissolution Mechanism of the Zr-based MG in NaNO3
3.3. Fabrication of Precise and Smooth Microgroove Structures
4. Discussion
- Electrochemical characteristics indicate that Zr-based MG exhibited passive, trans-passive, and re-passive performances. An applied voltage higher than the transpassivation potential is required for jet electrochemical machining of Zr-based MG.
- Jet-ECM can attain precise and smooth microgroove structures on the Zr-based metallic glass using a sodium nitrate electrolyte, with processing parameters including an applied voltage of 25 V, a nozzle travel rate of 100 μm/s, and a NaNO3 electrolyte concentration of 10 wt%.
- High geometric dimensional consistency and low surface roughness microhelical and micro-S structures can be fabricated, and their widths and depths are 433.7 ± 2.4 µm and 101.4 ± 1.6 µm, respectively. Their surface roughness is 0.118 ± 0.002 µm, which represents a significant improvement over the structures obtained by non-aqueous-based ECM processes reported earlier.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.; Chu, J.S.; Byrne, C.J.; Browne, D.J.; Gilchrist, M.D. Replication of Micro/Nano-Scale Features by Micro Injection Molding with a Bulk Metallic Glass Mold Insert. J. Micromech. Microeng. 2012, 22, 065019. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer Microfabrication Technologies for Microfluidic Systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Kukharenka, E.; Farooqui, M.M.; Grigore, L.; Kraft, M.; Hollinshead, N. Electroplating Moulds Using Dry Film Thick Negative Photoresist. J. Micromech. Microeng. 2003, 13, S67. [Google Scholar] [CrossRef]
- Bourne, G.R.; Bardt, J.; Sawyer, W.G.; Ziegert, J.; Zeenberg, D.; Schmitz, T. Closed Channel Fabrication Using Micromolding of Metallic Glass. J. Mater. Process. Technol. 2009, 209, 4765–4768. [Google Scholar] [CrossRef]
- Hupert, M.L.; Guy, W.J.; Llopis, S.D.; Shadpour, H.; Rani, S.; Nikitopoulos, D.E.; Soper, S.A. Evaluation of Micromilled Metal Mold Masters for the Replication of Microchip Electrophoresis Devices. Microfluid. Nanofluid 2006, 3, 1–11. [Google Scholar] [CrossRef]
- Saotome, Y.; Imai, K.; Shioda, S.; Shimizu, S.; Zhang, T.; Inoue, A. The Micro-Nanoformability of Pt-Based Metallic Glass and the Nanoforming of Three-Dimensional Structures. Intermetallics 2002, 10, 1241–1247. [Google Scholar] [CrossRef]
- Gong, Y.D.; Liu, Y.; Sun, Y.; Wen, X.L.; Li, Q.; Qu, S.S.; Cai, M. Experimental and Emulational Investigations into Grinding Characteristics of Zr-Based Bulk Metallic Glass (BMG) Using Microgrinding. Int. J. Adv. Manuf. Technol. 2018, 97, 3431–3451. [Google Scholar]
- Inoue, A. Stabilization of Metallic Supercooled Liquid and Bulk Amorphous Alloys. Acta Materialia 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Wei, Z.; Miao, H.T.; Li, Y.H.; Chang, C.T.; Xie, G.Q.; Jia, X.J. Glass-Forming Ability and Thermoplastic Formability of Ferromagnetic (Fe, Co, Ni)75P10C10B5 Metallic Glasses. J. Alloys Compd. 2017, 707, 57–62. [Google Scholar]
- Bakkal, M.; Shih, A.J.; McSpadden, S.B.; Scattergood, R.O. Thrust Force, Torque, and Tool Wear in Drilling the Bulk Metallic Glass. Int. J. Mach. Tools Manuf. 2005, 45, 863–872. [Google Scholar] [CrossRef]
- Bakkal, M.; Shih, A.J.; McSpadden, S.B.; Liu, C.T.; Scattergood, R.O. Light Emission, Chip Morphology, and Burr Formation in Drilling the Bulk Metallic Glass. Int. J. Mach. Tools Manuf. 2005, 45, 741–752. [Google Scholar] [CrossRef]
- Williams, E.; Lavery, N. Laser Processing of Bulk Metallic Glass: A Review. J. Mater. Process. Technol. 2017, 247, 73–91. [Google Scholar] [CrossRef]
- Pradana, Y.; Ferara, A.; Aminnudin, A.; Wahono, W.; Jang, S.C. The Effect of Discharge Current and Pulse-On Time on Biocompatible Zr-Based BMG Sinking-EDM. Open Engineering 2020, 10, 401–407. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.Y.; Zhang, T.; Zheng, L.J.; Zhu, X.G. High Performance Cutting of Zr-Based Bulk Metallic Glass: A Review of Chip Formation. Procedia CIRP 2018, 77, 421–424. [Google Scholar] [CrossRef]
- Schuster, R.; Kirchner, V.; Allongue, P.; Ertl, G. Electrochemical Micromachining. Science 2000, 289, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.F.; Chen, S.L.; Lin, M.H.; Ou, S.F.; Lin, W.T.; Huang, M.-S. Crystallization and Carbonization of an Electrical Discharge Machined Zr-Based Bulk Metallic Glass Alloy. J. Mater. Res. 2013, 28, 3177–3184. [Google Scholar] [CrossRef]
- Huang, H.; Yan, J.W. Microstructural Changes of Zr-Based Metallic Glass during Micro-Electrical Discharge Machining and Grinding by a Sintered Diamond Tool. J. Alloys Compd. 2016, 688, 14–21. [Google Scholar] [CrossRef]
- Huang, H.; Yan, J.W. On the Surface Characteristics of a Zr-Based Bulk Metallic Glass Processed by Microelectrical Discharge Machining. Appl. Surf. Sci. 2015, 355, 1306–1315. [Google Scholar] [CrossRef]
- Koza, J.A.; Sueptitz, R.; Uhlemann, M.; Schultz, L.; Gebert, A. Electrochemical Micromachining of a Zr-Based Bulk Metallic Glass Using a Micro-Tool Electrode Technique. Intermetallics 2011, 19, 437–444. [Google Scholar] [CrossRef]
- Gebert, A.; Gostin, P.F.; Sueptitz, R.; Oswald, S.; Abdi, S.; Uhlemann, M.; Eckert, J. Polarization Studies of Zr-Based Bulk Metallic Glasses for Electrochemical Machining. J. Electrochem. Soc. 2014, 161, E66. [Google Scholar] [CrossRef]
- Cole, K.M.; Kirk, D.W.; Singh, C.V.; Thorpe, S.J. Optimizing Electrochemical Micromachining Parameters for Zr-Based Bulk Metallic Glass. J. Manuf. Process. 2017, 25, 227–234. [Google Scholar] [CrossRef]
- Guo, C.; Wu, B.; Xu, B.; Wu, S.; Shen, J.; Wu, X. Investigation of Pulse Electrochemical Machining of Zr-Based Bulk Metallic Glasses in NaNO3 -Ethylene Glycol Electrolyte. J. Electrochem. Soc. 2021, 168, 071502. [Google Scholar] [CrossRef]
- Guo, C.; He, J.; Zhuang, W.; Li, K.; Li, D. Fabrication of Dimples by Jet-ECM of Zr-Based Bulk Metallic Glasses with NaCl-Ethylene Glycol Electrolyte. Micromachines 2023, 14, 2196. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhou, A.; He, J.; Xiao, H.; Li, D. An Investigation in Sub-Millimeter Channel Fabrication by the Non-Aqueous Electrolyte Jet Machining of Zr-Based Bulk Metallic Glasses. Micromachines 2023, 14, 2232. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.S.; Zeng, Y.B.; Yang, T.; Meng, L.C. The Dissolution Characteristics and Wire Electrochemical Micromachining of Metallic Glass Ni82Cr7Si5Fe3B3. J. Manuf. Process. 2020, 58, 884–893. [Google Scholar] [CrossRef]
- Zhu, D.; Zeng, Y.B.; Xu, Z.Y.; Zhang, X.Y. Precision Machining of Small Holes by the Hybrid Process of Electrochemical Removal and Grinding. CIRP Annals 2011, 60, 247–250. [Google Scholar] [CrossRef]
- Meng, L.C.; Zeng, Y.B.; Zhu, D. Investigation on Wire Electrochemical Micro Machining of Ni-Based Metallic Glass. Electrochim. Acta 2017, 233, 274–283. [Google Scholar] [CrossRef]
- Yang, T.; Li, Y.L.; Xu, Z.Y.; Zeng, Y.B. Electrochemical Cutting with Inner-Jet Electrolyte Flushing for Titanium Alloy (Ti-6Al-4V). Int. J. Adv. Manuf. Technol. 2021, 112, 2583–2592. [Google Scholar] [CrossRef]
- Hackert-Oschätzchen, M.; Meichsner, G.; Zinecker, M.; Martin, A.; Schubert, A. Micro Machining with Continuous Electrolytic Free Jet. Precis. Eng. 2012, 36, 612–619. [Google Scholar] [CrossRef]
- Liu, W.D.; Ao, S.S.; Li, Y.; Liu, Z.M.; Zhang, H.; Manladan, S.M.; Luo, Z.; Wang, Z.P. Effect of Anodic Behavior on Electrochemical Machining of TB6 Titanium Alloy. Electrochim. Acta 2017, 233, 190–200. [Google Scholar] [CrossRef]
- Wang, D.Y.; Zhu, Z.W.; Wang, N.F.; Zhu, D.; Wang, H.R. Investigation of the Electrochemical Dissolution Behavior of Inconel 718 and 304 Stainless Steel at Low Current Density in NaNO3 Solution. Electrochim. Acta 2015, 156, 301–307. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, N.S. Electrochemical Milling of TB6 Titanium Alloy in NaNO3 Solution. J. Electrochem. Soc. 2019, 166, E35–E49. [Google Scholar] [CrossRef]
- Bolzoni, F.M.; Diamanti, M.V.; Ormellese, M.; Pedeferri, M.P.; Pérez-Rosales, E. Characterisation of Titanium Oxide Films By Potentiodynamic Polarization And Electrochemical Impedance Spectroscopy. Corros. Eng. Sci. Technol. 2010, 45, 428–434. [Google Scholar]
- Wang, J.T.; Xu, Z.Y.; Wang, J.; Zhu, D. Anodic Dissolution Characteristics of Inconel 718 in C6H5K3O7 and NaNO3 Solutions by Pulse Electrochemical Machining. Corros. Sci. 2021, 183, 109335. [Google Scholar] [CrossRef]
- Herraiz-Cardona, I.; Ortega, E.; Antón, J.G.; Pérez-Herranz, V. Assessment of the Roughness Factor Effect and the Intrinsic Catalytic Activity for Hydrogen Evolution Reaction on Ni-Based Electrodeposits. Int. J. Hydrogen Energy 2011, 36, 9428–9438. [Google Scholar] [CrossRef]
- Liu, G.D.; Tong, H.; Li, Y.; Tan, Q.F.; Zhu, Y.L. Passivation Behavior of S136H Steel in Neutral Electrolytes Composed of NaClO3 and NaNO3 and Its Influence on Micro Electrochemical Machining Performance. Mater. Today Commun. 2021, 29, 102762. [Google Scholar] [CrossRef]
- Schultze, J.W.; Lohrengel, M.M. Stability, Reactivity and Breakdown of Passive Films. Problems of Recent and Future Research. Electrochim. Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Rosenkranz, C.; Lohrengel, M.M.; Schultze, J.W. The Surface Structure during Pulsed ECM of Iron in NaNO3. Electrochim. Acta 2005, 50, 2009–2016. [Google Scholar] [CrossRef]
- Liu, W.D.; Luo, Z.; Li, Y.; Liu, Z.M.; Li, K.B.; Xu, J.X.; Ao, S.S. Investigation on Parametric Effects on Groove Profile Generated on Ti1023 Titanium Alloy by Jet Electrochemical Machining. Int. J. Adv. Manuf. Technol. 2019, 100, 2357–2370. [Google Scholar] [CrossRef]



| Parameters | Value |
|---|---|
| Specific conductance (Ms/m) | 0.52–0.53 |
| Young’s modulus (GPa) | 94.9 |
| Poisson ratio | 0.30 |
| Hardness (HV) | 568–619 |
| Parameters | Value |
|---|---|
| Material | Zr41.2Ti13.8Cu12.5Ni10.0Be22.5 |
| Tool electrode | SUS 304 nozzle |
| Inner diameter of nozzle | 220 ± 2 μm |
| Outer diameter of nozzle | 450 ± 3 μm |
| Electrolyte composition | 10 wt% NaNO3 |
| Machining gap (μm) | 200 |
| Electrolyte pressure (MPa) | 1 |
| Machining voltage (V) | 10, 15, 20, 25, |
| Nozzle travel rate (μm/s) | 100, 200, 300, 400 |
| Temperature of electrolyte (°C) | 25 ± 5 |
| Potential (V) | R1 (Ω cm−2) | Q1 × 10−6 (snΩ−1 cm−2) | n1 | R2 (Ω cm−2) | R3 (Ω cm−2) | L1 (H cm−2) |
|---|---|---|---|---|---|---|
| 0.1 | 16.36 | 17.96 | 0.91 | 182,770 | - | - |
| 1 | 8.49 | 8.13 | 0.93 | 78,989 | - | - |
| 2.1 | 6.55 | 10.21 | 0.93 | 44.18 | 15.06 | 0.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Ming, P.; Niu, S.; Yang, G.; Cheng, K. Fabricating Precise and Smooth Microgroove Structures on Zr-Based Metallic Glass Using Jet-ECM. Micromachines 2024, 15, 497. https://doi.org/10.3390/mi15040497
Li D, Ming P, Niu S, Yang G, Cheng K. Fabricating Precise and Smooth Microgroove Structures on Zr-Based Metallic Glass Using Jet-ECM. Micromachines. 2024; 15(4):497. https://doi.org/10.3390/mi15040497
Chicago/Turabian StyleLi, Dongdong, Pingmei Ming, Shen Niu, Guangbin Yang, and Kuaile Cheng. 2024. "Fabricating Precise and Smooth Microgroove Structures on Zr-Based Metallic Glass Using Jet-ECM" Micromachines 15, no. 4: 497. https://doi.org/10.3390/mi15040497





