Surface Acoustic Wave Resonator Chip Setup for the Elimination of Interfering Conductivity Responses
Abstract
:1. Introduction
- No coating, i.e., no parylene C;
- Thin parylene C coating below the first Love mode resonance;
- Thick parylene C coating at the thickness of the first Love mode resonance;
- Thick parylene C coating at the thickness of the first Love mode and gold layer.
2. Materials and Methods
2.1. SAW Resonator Chip Setup and Coating
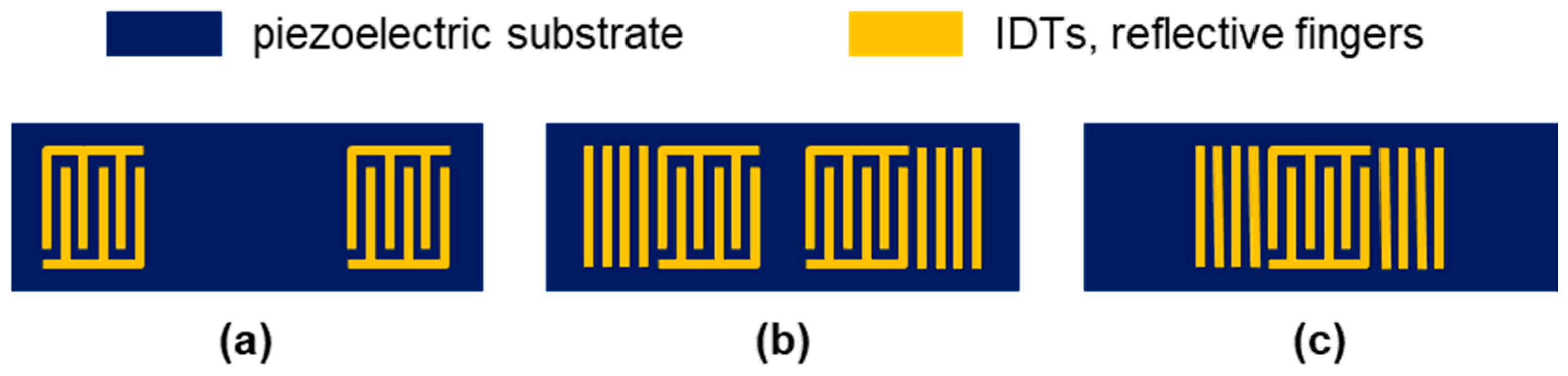
2.1.1. SAW Resonator Devices
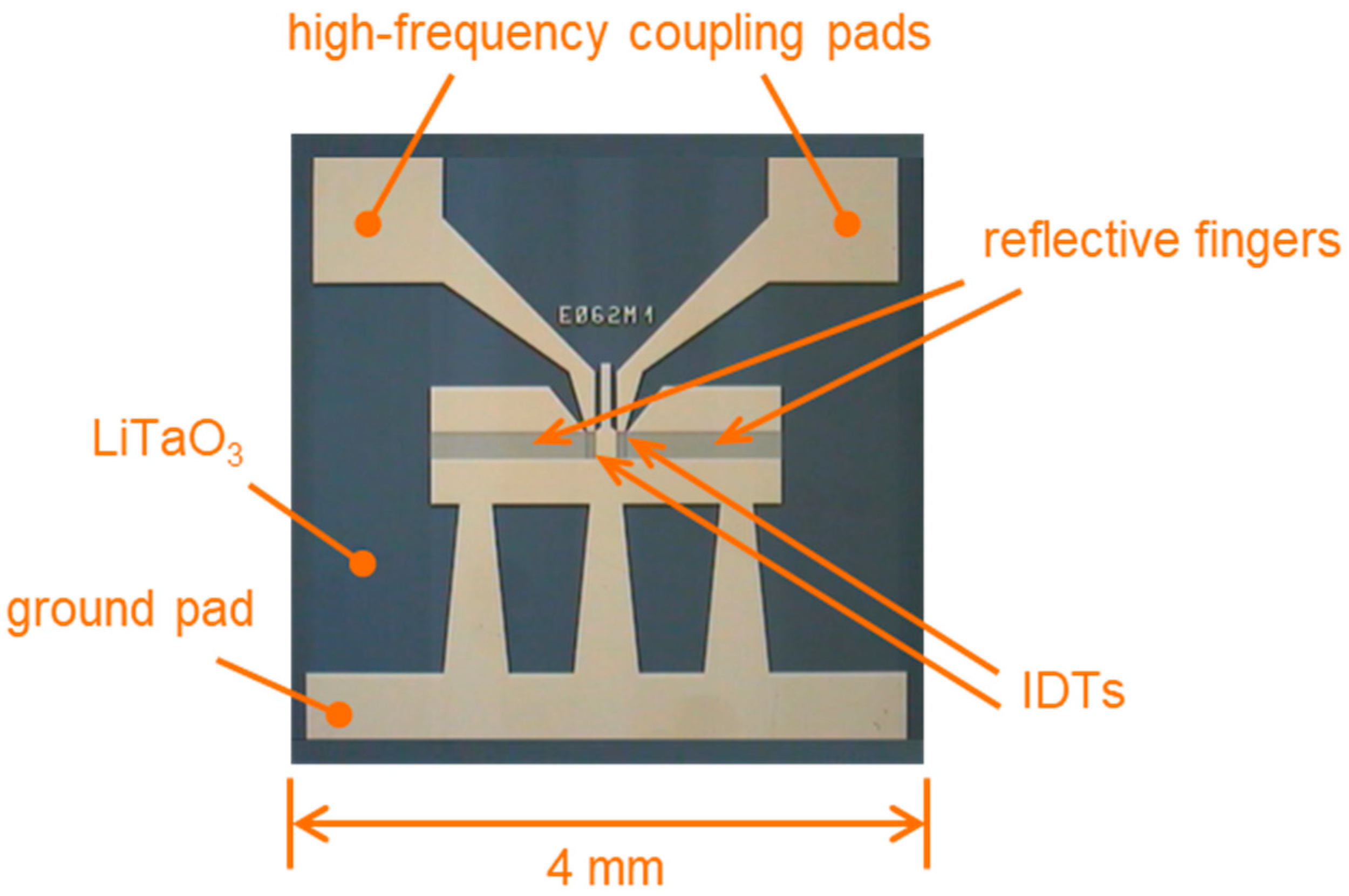
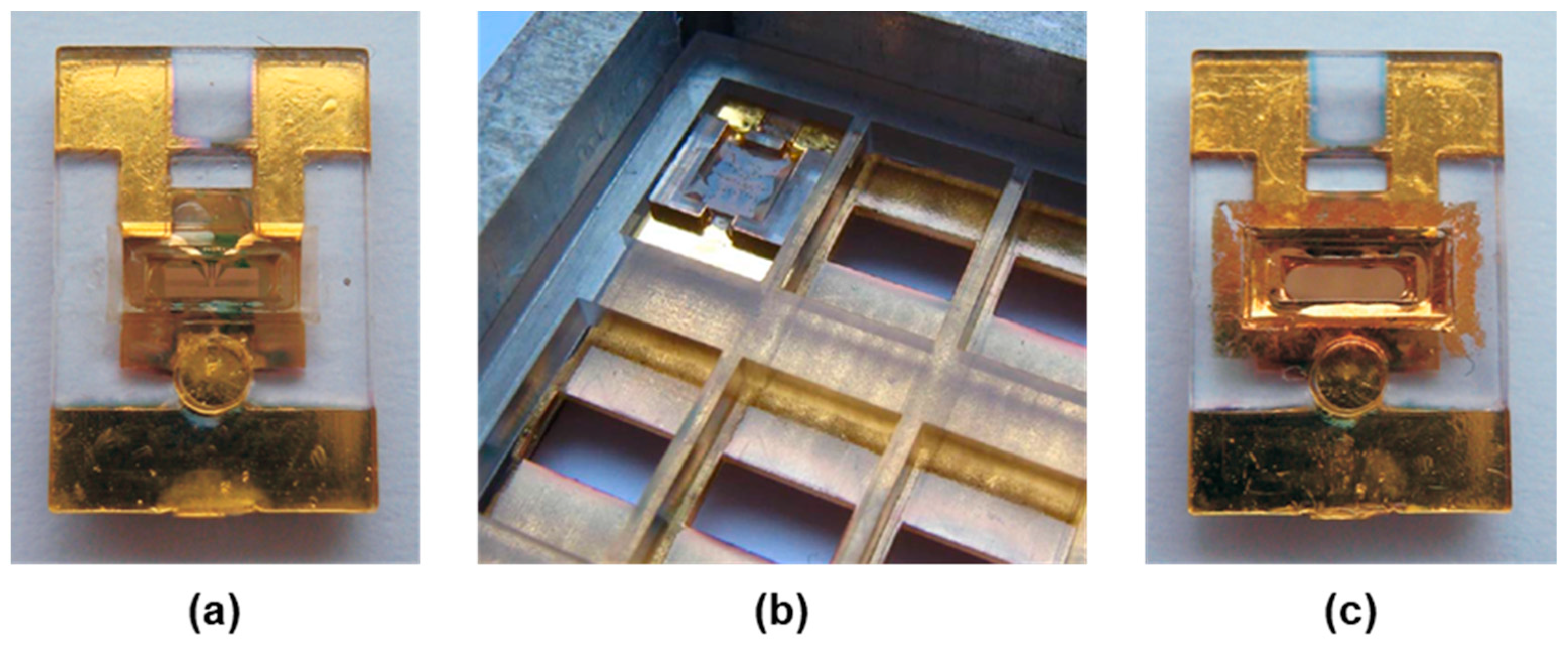
2.1.2. SAW Resonator Chips
2.1.3. Coating of the SAW Resonator Surfaces
2.2. SAW Resonator Chip Measurements
2.2.1. Operating Electronics
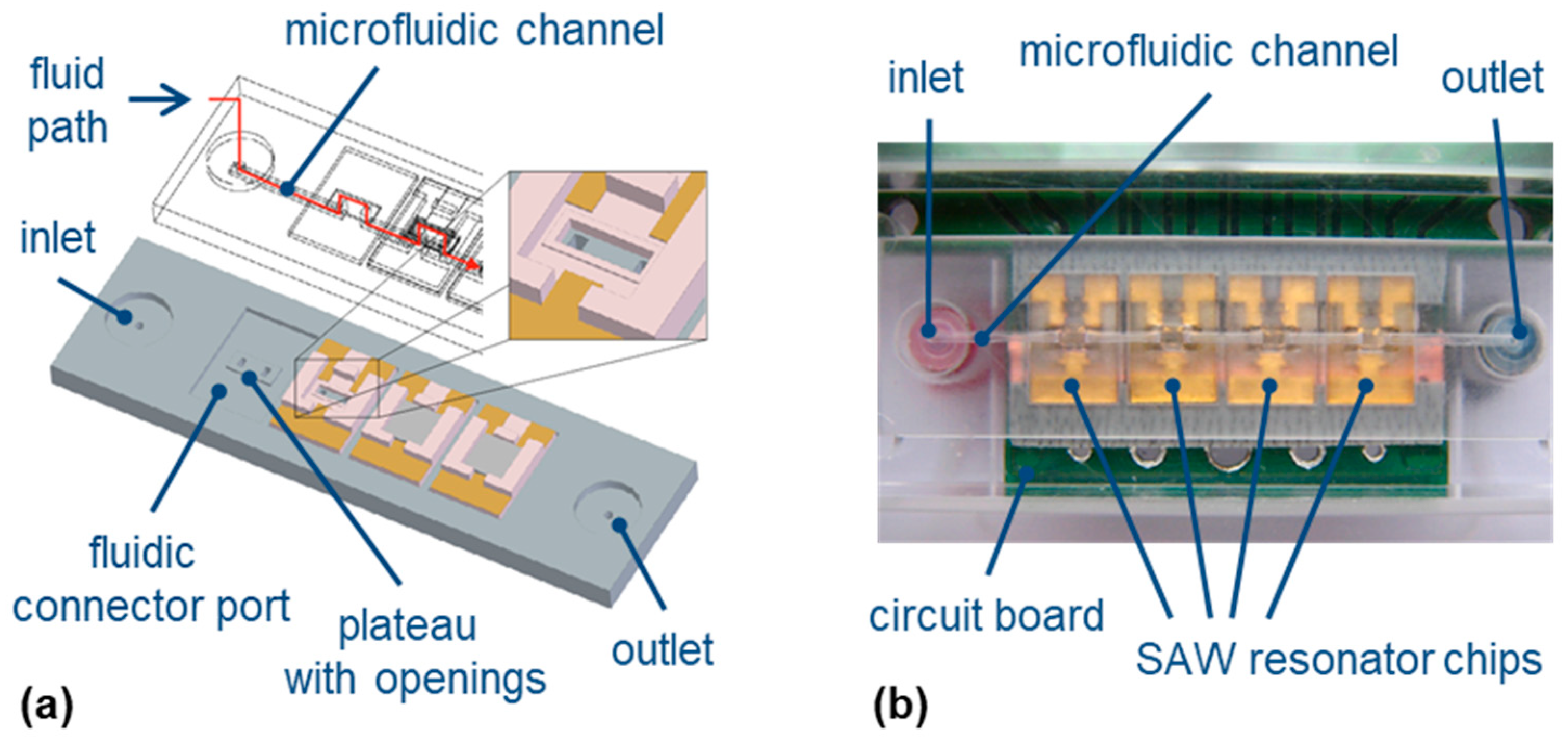
2.2.2. Microfluidic Array
2.2.3. Preparation of KCl Solutions and Conductivity Measurements
2.2.4. Measurement Procedures
- Thin polymer layer, i.e., 100 nm parylene C;
- Love wave guiding layer, i.e., 810 nm parylene C;
- Love wave guiding layer and gold film;
- No coating.
3. Results and Discussion
3.1. Estimated Electrical Impact of the KCl Solutions on the SAW Velocity
3.2. Impact of the KCl Solutions on the SAW Resonator Signal Response
3.2.1. SAW Resonator Measurement Signals
3.2.2. Impact of the SAW Resonator Coating on the Difference Frequency Shifts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Simulation of the Impact of Electrical Changes on the SAW Velocity
Appendix B. Correlation between SAW Velocity and SAW Frequency
Appendix C. Impact of Viscosity and Density on the SAW Velocity
References
- Mujahid, A.; Afzal, A.; Dickert, F.L. An overview of high frequency acoustic sensors-QCMs, SAWs and FBARs-chemical and biochemical applications. Sensors 2019, 19, 4395. [Google Scholar] [CrossRef] [PubMed]
- Länge, K. Bulk and surface acoustic wave sensor arrays for multi-analyte detection: A review. Sensors 2019, 19, 5382. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Spulber, I.; Gardner, J.W. Surface acoustic wave electronic tongue for robust analysis of sensory components. Sens. Actuators B-Chem. 2015, 207, 1147–1153. [Google Scholar] [CrossRef]
- Yang, Y.; Dejous, C.; Hallil, H. Trends and applications of surface and bulk acoustic wave devices: A review. Micromachines 2023, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Banerjee, S. Surface acoustic wave (SAW) sensors: Physics, materials, and applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef] [PubMed]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Gaso, M.I.; March-Iborra, C.; Montoya-Baides, A.; Arnau-Vives, A. Surface generated acoustic wave biosensors for the detection of pathogens: A review. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef] [PubMed]
- Cheeke, J.D.N. Fundamentals and Applications of Ultrasonic Waves, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Wang, X.Y.; Du, L.L.; Cheng, L.N.; Zhai, S.P.; Zhang, C.; Wang, W.; Liang, Y.; Yang, D.C.; Chen, Q.; Lei, G. Pd/Ni nanowire film coated SAW hydrogen sensor with fast response. Sens. Actuators B-Chem. 2022, 351, 130952. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Kondoh, J.; Saito, K.; Shiokawa, S.; Suzuki, H. Simultaneous measurements of liquid properties using multichannel shear horizontal surface acoustic wave microsensor. Jpn. J. Appl. Phys. Part 1—Regul. Pap. Brief Commun. Rev. Pap. 1996, 35, 3093–3096. [Google Scholar] [CrossRef]
- Nomura, T.; Saitoh, A.; Horikoshi, Y. Measurement of acoustic properties of liquid using liquid flow SH-SAW sensor system. Sens. Actuators B-Chem. 2001, 76, 69–73. [Google Scholar] [CrossRef]
- Cole, M.; Sehra, G.; Gardner, J.W.; Varadan, V.K. Development of smart tongue devices for measurement of liquid properties. IEEE Sens. J. 2004, 4, 543–550. [Google Scholar] [CrossRef]
- Kano, K.; Kogai, T.; Yoshimura, N.; Yatsuda, H.; Kondoh, J.; Shiokawa, S. Measurement characteristics of flow-type shear-horizontal surface acoustic wave sensor for direct methanol fuel cells. Jpn. J. Appl. Phys. 2012, 51, 07GC20. [Google Scholar] [CrossRef]
- Kondoh, J.; Nakayama, K.; Kuznetsova, I. Study of frequency dependence of shear horizontal surface acoustic wave sensor for engine oil measurements. Sens. Actuators A-Phys. 2021, 325, 112503. [Google Scholar] [CrossRef]
- Nomura, T.; Yasuda, T.; Furukawa, S. Liquid sensor using two-port surface acoustic wave resonator. Jpn. J. Appl. Phys. Part 1—Regul. Pap. Short Notes Rev. Pap. 1992, 31, 78–81. [Google Scholar] [CrossRef]
- Powell, D.A.; Kalantar-Zadeh, K.; Wlodarski, W.; Ippolito, S.J. Layered surface acoustic wave chemical and bio-sensors. In Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pishko, M.V., Eds.; American Scientific Publishers: Valencia, CA, USA, 2006; Volume 5, pp. 245–262. [Google Scholar]
- Nomura, T.; Yasuda, T. Surface acoustic wave liquid sensors based on one-port resonator. Jpn. J. Appl. Phys. Part 1—Regul. Pap. Short Notes Rev. Pap. 1993, 32, 2372–2375. [Google Scholar] [CrossRef]
- Rapp, B.E.; Schickling, B.; Prokop, J.; Piotter, V.; Rapp, M.; Länge, K. Design and integration of a generic disposable array-compatible sensor housing into an integrated disposable indirect microfluidic flow injection analysis system. Biomed. Microdevices 2011, 13, 909–922. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Micromachining of parylene C for bioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Bender, F.; Länge, K.; Barié, N.; Kondoh, J.; Rapp, M. On-line monitoring of polymer deposition for tailoring the waveguide characteristics of Love-wave biosensors. Langmuir 2004, 20, 2315–2319. [Google Scholar] [CrossRef]
- Wu, H.Y.; Xiong, X.M.; Zu, H.F.; Wang, J.H.C.; Wang, Q.M. Theoretical analysis of a Love wave biosensor in liquid with a viscoelastic wave guiding layer. J. Appl. Phys. 2017, 121, 054501. [Google Scholar] [CrossRef]
- Newton, M.I.; McHale, G.; Martin, F. Experimental study of Love wave devices with thick guiding layers. Sens. Actuators A-Phys. 2004, 109, 180–185. [Google Scholar] [CrossRef]
- Rapp, B.E.; Voigt, A.; Dirschka, M.; Länge, K. Deposition of ultrathin parylene C films in the range of 18 nm to 142 nm: Controlling the layer thickness and assessing the closeness of the deposited films. Thin Solid Film. 2012, 520, 4884–4888. [Google Scholar] [CrossRef]
- Länge, K.; Blaess, G.; Voigt, A.; Götzen, R.; Rapp, M. Integration of a surface acoustic wave biosensor in a microfluidic polymer chip. Biosens. Bioelectron. 2006, 22, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, S.; Kögel, S.; Brunner, Y.; Schmieg, B.; Ewald, C.; Kirschhöfer, F.; Brenner-Weiss, G.; Länge, K. Surface acoustic wave (SAW) resonators for monitoring conditioning film formation. Sensors 2015, 15, 11873–11888. [Google Scholar] [CrossRef] [PubMed]
- Länge, K.; Gruhl, F.J.; Rapp, M. Surface acoustic wave (SAW) biosensors: Coupling of sensing layers and measurement. Methods Mol. Biol. 2013, 949, 491–505. [Google Scholar] [PubMed]
- Shcherbakov, V.V. Regularity of conductivity of concentrated aqueous solutions of strong electrolytes. Russ. J. Electrochem. 2009, 45, 1292–1295. [Google Scholar] [CrossRef]
- Marcus, Y. Evaluation of the static permittivity of aqueous electrolytes. J. Solut. Chem. 2013, 42, 2354–2363. [Google Scholar] [CrossRef]
- Zaitsev, B.D.; Kuznetsova, I.E.; Joshi, S.G. Anomalous resistoacoustic effect in a piezoelectric-conducting liquid structure. Tech. Phys. 2001, 46, 767–769. [Google Scholar] [CrossRef]
- Zaitsev, B.D.; Kuznetsova, I.E.; Teplykh, A.A. Anomalous resisto-acoustic effect for leaky surface acoustic waves. J. Appl. Phys. 2005, 97, 046102. [Google Scholar] [CrossRef]
- Smirnov, A.; Anisimkin, V.; Voronova, N.; Shamsutdinova, E.; Li, P.; Ezzin, H.; Qian, Z.H.; Ma, T.F.; Kuznetsova, I. Multimode design and piezoelectric substrate anisotropy use to improve performance of acoustic liquid sensors. Sensors 2022, 22, 7231. [Google Scholar] [CrossRef]
- Walstra, P.; Geurts, T.J.; Noomen, A.; Jellema, A.; van Boekel, M.A.J.S. Dairy Technology: Principles of Milk. Properties and Processes, 1st ed.; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues. 3. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed]
- Visser, K.R. Electric conductivity of stationary and flowing human blood at low frequencies. Med. Biol. Eng. Comput. 1992, 30, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Biosolve. PBS Buffer 10X (Sterile) Molecular Biology. Product Specification. Available online: https://shop.biosolve-chemicals.eu/detail.php?id=2093 (accessed on 12 January 2024).
- Zheng, Z.Y.; Fu, Y.; Liu, K.Z.; Xiao, R.; Wang, X.H.; Shi, H.B. Three-stage vertical distribution of seawater conductivity. Sci. Rep. 2018, 8, 9916. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, J.; Shiokawa, S. Liquid-phase microsensor based on surface acoustic wave devices. Electron. Commun. Jpn. Part II-Electron. 1998, 81, 9–17. [Google Scholar] [CrossRef]
- Loginova, D.V.; Lileev, A.S.; Lyashchenko, A.K. Dielectric properties of aqueous potassium chloride solutions as a function of temperature. Russ. J. Inorg. Chem. 2002, 47, 1426–1433. [Google Scholar]
- Kondoh, J.; Shiokawa, S. New application of shear horizontal surface acoustic wave sensors to identifying fruit juices. Jpn. J. Appl. Phys. Part 1—Regul. Pap. Short Notes Rev. Pap. 1994, 33, 3095–3099. [Google Scholar] [CrossRef]
- Reibel, J.; Stier, S.; Voigt, A.; Rapp, M. Influence of phase position on the performance of chemical sensors based on SAW device oscillators. Anal. Chem. 1998, 70, 5190–5197. [Google Scholar] [CrossRef]
- Ricco, A.J.; Martin, S.J. Thin metal film characterization and chemical sensors: Monitoring electronic conductivity, mass loading and mechanical properties with surface acoustic wave devices. Thin Solid Film. 1991, 206, 94–101. [Google Scholar] [CrossRef]






| Liquid | Potential Sensing Application | Conductivity [mS/cm] | Reference |
|---|---|---|---|
| Milk | Food sample | 4.0–5.5 1 | [34] |
| Blood | Clinical sample | ~7 2 | [35] |
| Blood plasma | Clinical sample | 15.7 2 | [36] |
| Phosphate-buffered saline (PBS) | Reference buffer | 10–20 1 | [37] |
| Sea water | Environmental sample | up to 60 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapp, B.E.; Voigt, A.; Dirschka, M.; Rapp, M.; Länge, K. Surface Acoustic Wave Resonator Chip Setup for the Elimination of Interfering Conductivity Responses. Micromachines 2024, 15, 501. https://doi.org/10.3390/mi15040501
Rapp BE, Voigt A, Dirschka M, Rapp M, Länge K. Surface Acoustic Wave Resonator Chip Setup for the Elimination of Interfering Conductivity Responses. Micromachines. 2024; 15(4):501. https://doi.org/10.3390/mi15040501
Chicago/Turabian StyleRapp, Bastian E., Achim Voigt, Marian Dirschka, Michael Rapp, and Kerstin Länge. 2024. "Surface Acoustic Wave Resonator Chip Setup for the Elimination of Interfering Conductivity Responses" Micromachines 15, no. 4: 501. https://doi.org/10.3390/mi15040501






