A Review of Methods to Modify the PDMS Surface Wettability and Their Applications
Abstract
:1. Introduction
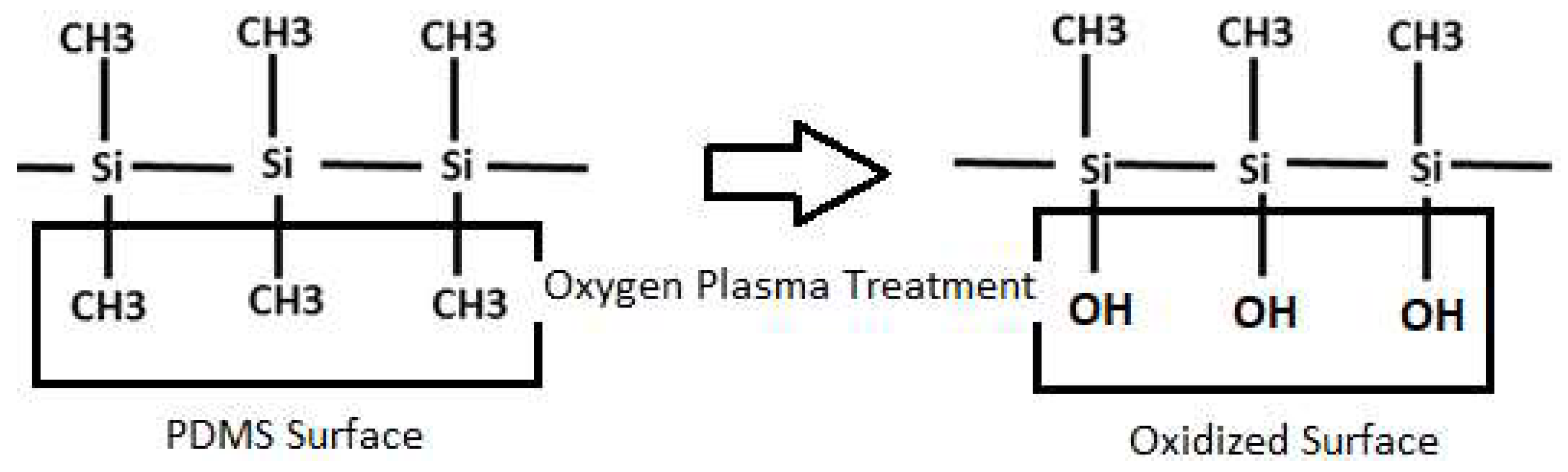
2. Oxygen Plasma Treatment
2.1. Principles of Oxygen Plasma Treatment and Applications of PDMS Treated with Oxygen Plasma
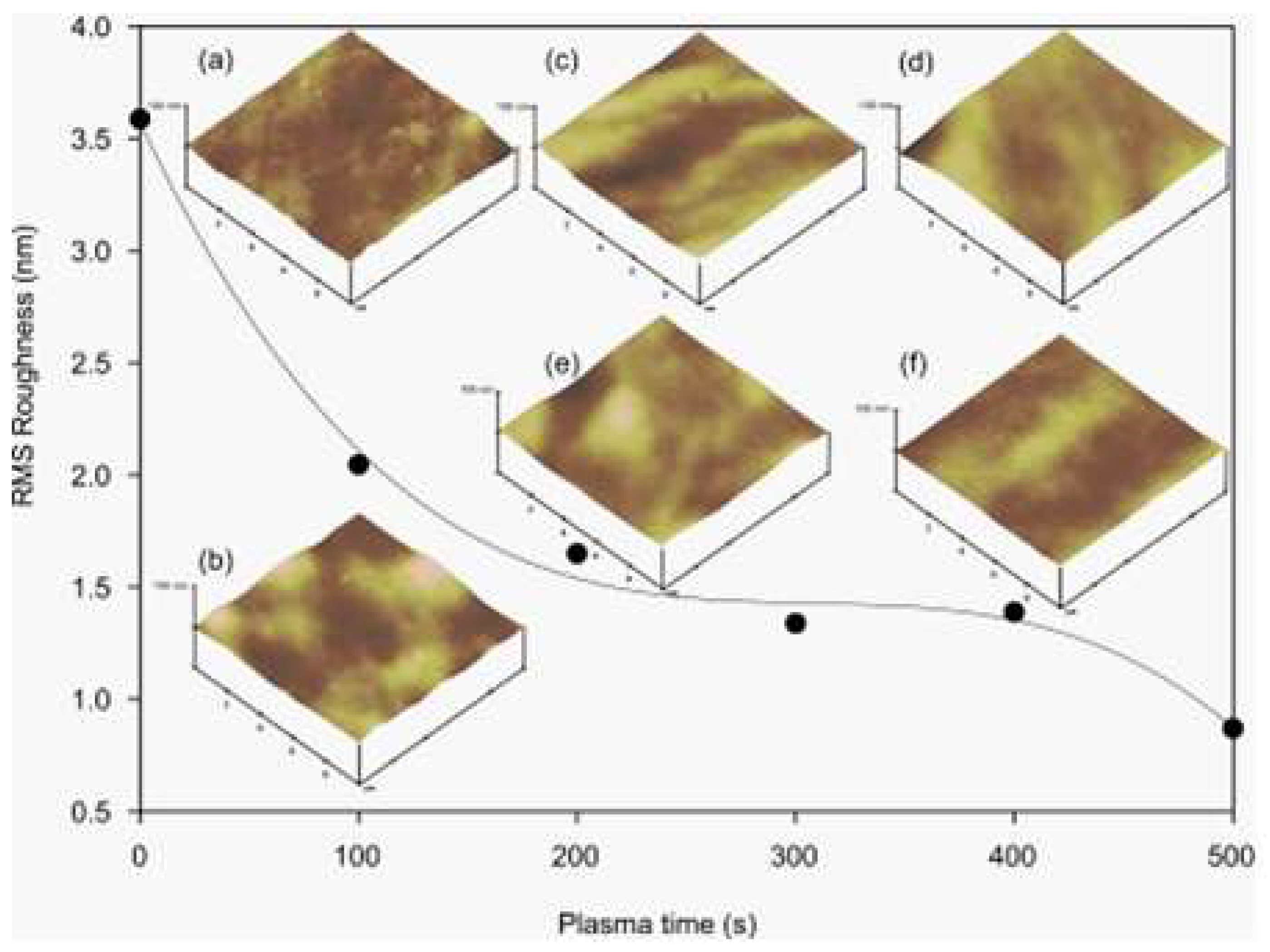
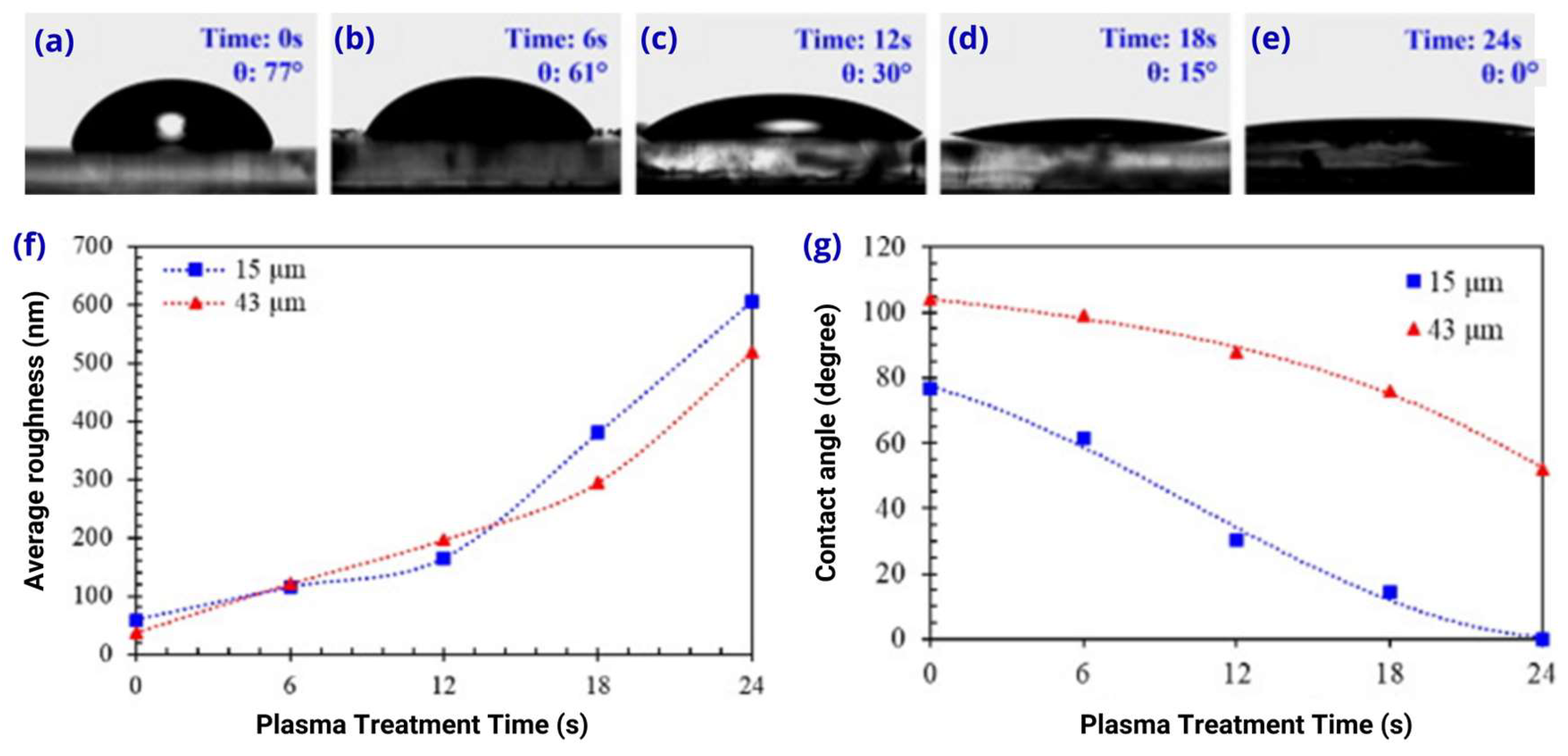
2.2. Effects of Oxygen Plasma Treatment on PDMS
3. UV-Ozone Treatment
4. Surfactant Addition
4.1. Effects of Surface Coating Treatment with Surfactants on PDMS
4.2. Applications of Surfactant-Treated PDMS
5. Incorporation of Nanomaterials
6. Modification of PDMS Surface Wettability for Microfluidic Applications
7. Promising Trends and Future Prospects
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nomenclature | Abbreviation | ||
| PDMS | Polydimethylsiloxane | θ | Contact angle |
| UV | Ultraviolet | ||
| L | Liquid | ||
| V | Steam | ||
| S | Solid | ||
| O2 | Oxygen | ||
| SiOH | Silanol group | ||
| SEM | Scanning electron microscopy | ||
| XPS–X-ray | Photoelectron spectroscopy | ||
| DC | Direct current | ||
| AFM | Atomic force microscopy | ||
| SRMJ | Scanning radical microjet | ||
| WCA | Water contact angle | ||
| CA | Contact angle | ||
| ccm | Cubic centimeters per minute | ||
| PEG | Polyethylene glycol | ||
| BSA | Bovine serum albumin | ||
| RF | Radio frequency | ||
| JKR | Johnson–Kendall–Roberts | ||
References
- Xia, Y.; Whitesides, G.M. Extending Microcontact Printing as a Microlithographic Technique. Langmuir 1997, 13, 2059–2067. [Google Scholar] [CrossRef]
- Ng, J.M.K.; Gitlin, I.; Stroock, A.D.; Whitesides, G.M. Components for Integrated Poly(Dimethylsiloxane) Microfluidic Systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar] [CrossRef] [PubMed]
- Friend, J.; Yeo, L. Fabrication of Microfluidic Devices Using Polydimethylsiloxane. Biomicrofluidics 2010, 4, 026502. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of Microfluidic Systems in Poly(Dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Dhall, A.; Masiello, T.; Gattu, S.; Strohmayer, M.; Butt, L.; Hemachandra, L.P.M.; Schujman, S.; Tokranova, N.; Khoury, J.; Papa Rao, S.; et al. Characterization and Neutral Atom Beam Surface Modification of a Clear Castable Polyurethane for Biomicrofluidic Applications. Surfaces 2019, 2, 100–116. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fujii, T.; Nojima, T. PDMS–Glass Hybrid Microreactor Array with Embedded Temperature Control Device. Application to Cell-Free Protein Synthesis. Lab Chip 2002, 2, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Bayraktaroglu, S.; Kizil, S.; Bulbul Sonmez, H. A Highly Reusable Polydimethylsiloxane Sorbents for Oil/Organic Solvent Clean-up from Water. J. Environ. Chem. Eng. 2021, 9, 106002. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kwon, T.-H.; Im, H.; Moon, D.-I.; Baek, D.J.; Seol, M.-L.; Duarte, J.P.; Choi, Y.-K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef] [PubMed]
- Turco, A.; Malitesta, C.; Barillaro, G.; Greco, A.; Maffezzoli, A.; Mazzotta, E. A Magnetic and Highly Reusable Macroporous Superhydrophobic/Superoleophilic PDMS/MWNT Nanocomposite for Oil Sorption from Water. J. Mater. Chem. A Mater. 2015, 3, 17685–17696. [Google Scholar] [CrossRef]
- Guo, H.; Yang, J.; Xu, T.; Zhao, W.; Zhang, J.; Zhu, Y.; Wen, C.; Li, Q.; Sui, X.; Zhang, L. A Robust Cotton Textile-Based Material for High-Flux Oil–Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 13704–13713. [Google Scholar] [CrossRef]
- Cao, C.; Ge, M.; Huang, J.; Li, S.; Deng, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. Robust Fluorine-Free Superhydrophobic PDMS–Ormosil@fabrics for Highly Effective Self-Cleaning and Efficient Oil–Water Separation. J. Mater. Chem. A Mater. 2016, 4, 12179–12187. [Google Scholar] [CrossRef]
- Pan, G.; Xiao, X.; Ye, Z. Fabrication of Stable Superhydrophobic Coating on Fabric with Mechanical Durability, UV Resistance and High Oil-Water Separation Efficiency. Surf. Coat. Technol. 2019, 360, 318–328. [Google Scholar] [CrossRef]
- Zhou, J.; Khodakov, D.A.; Ellis, A.V.; Voelcker, N.H. Surface Modification for PDMS-Based Microfluidic Devices. Electrophoresis 2012, 33, 89–104. [Google Scholar] [CrossRef] [PubMed]
- van Poll, M.L.; Zhou, F.; Ramstedt, M.; Hu, L.; Huck, W.T.S. A Self-Assembly Approach to Chemical Micropatterning of Poly(Dimethylsiloxane). Angew. Chem. 2007, 119, 6754–6757. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas Sorption, Diffusion, and Permeation in Poly(Dimethylsiloxane). J. Polym. Sci. B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(Dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Sulym, I.Y.; Borysenko, M.V.; Goncharuk, O.V.; Terpilowski, K.; Sternik, D.; Chibowski, E.; Gun’ko, V.M. Structural and Hydrophobic–Hydrophilic Properties of Nanosilica/Zirconia Alone and with Adsorbed PDMS. Appl. Surf. Sci. 2011, 258, 270–277. [Google Scholar] [CrossRef]
- Gezer, P.G.; Brodsky, S.; Hsiao, A.; Liu, G.L.; Kokini, J.L. Modification of the Hydrophilic/Hydrophobic Characteristic of Zein Film Surfaces by Contact with Oxygen Plasma Treated PDMS and Oleic Acid Content. Colloids Surf. B Biointerfaces 2015, 135, 433–440. [Google Scholar] [CrossRef]
- He, Z.; Wang, N.; Yang, X.; Mu, L.; Wang, Z.; Su, J.; Luo, M.; Li, J.; Deng, F.; Lan, X. Antifouling Induced by Surface Wettability of Poly(Dimethyl Siloxane) and Its Nanocomposites. Nanotechnol. Rev. 2023, 12. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, U.P.; Lee, S.-J.; Kim, C.-L.; Lee, J.-W. Implementation of Endurable Superhydrophobic Surfaces through Dilution Rate Control of the PDMS Coating on Micro-Nano Surface Structures. Polymer 2023, 275, 125929. [Google Scholar] [CrossRef]
- Si, Y.; Dong, Z.; Jiang, L. Bioinspired Designs of Superhydrophobic and Superhydrophilic Materials. ACS Cent. Sci. 2018, 4, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yao, X.; Jiang, L. Recent Developments in Bio-Inspired Special Wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and Superhydrophilic Surfaces and Materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
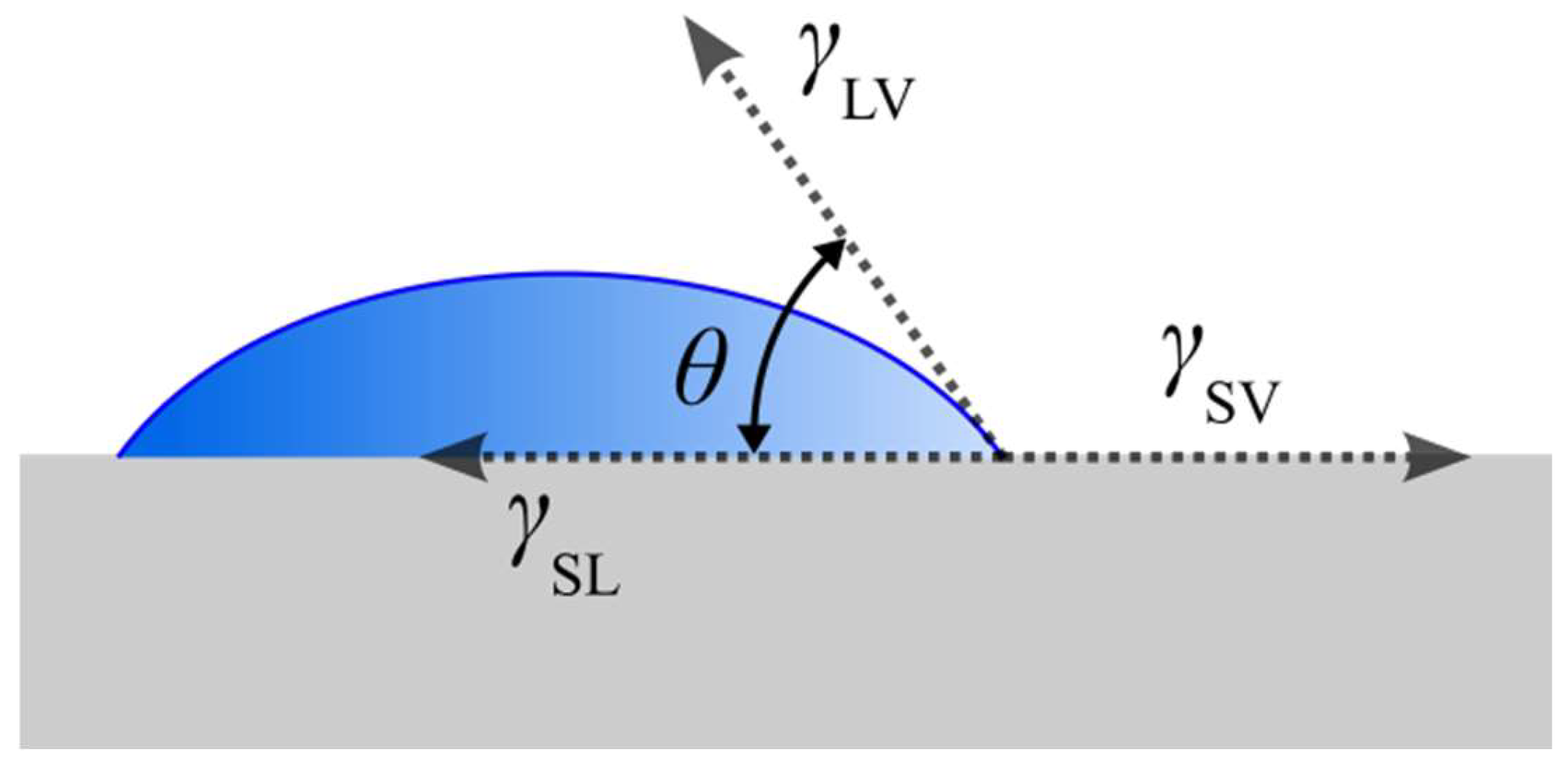
- Young, T. III. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1997, 95, 65–87. [Google Scholar] [CrossRef]
- Jothi Prakash, C.G.; Prasanth, R. Approaches to Design a Surface with Tunable Wettability: A Review on Surface Properties. J. Mater. Sci. 2020, 56, 108–135. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Britto Joseph, G.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic Surfaces: A Review on Fundamentals, Applications, and Challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Wettability of Conducting Polymers: From Superhydrophilicity to Superoleophobicity. Prog. Polym. Sci. 2014, 39, 656–682. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Zhao, Y.; Chen, C.-H. Planar Jumping-Drop Thermal Diodes. Appl. Phys. Lett. 2011, 99, 234105. [Google Scholar] [CrossRef]
- Jozanović, M.; Pukleš, I.; Sakač, N.; Carrilho, E.; Kilár, A.; Matasović, B.; Samardžić, M.; Budetić, M.; Kilár, F. Nanomaterials in Microchip Electrophoresis—A Review. TrAC Trends Anal. Chem. 2023, 165, 117111. [Google Scholar] [CrossRef]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent Developments in PDMS Surface Modification for Microfluidic Devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.S.; Kumar Sadasivuni, K.; Liu, S. Recent Advances in Durability of Superhydrophobic Self-Cleaning Technology: A Critical Review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Drelich, J.; Marmur, A. Physics and Applications of Superhydrophobic and Superhydrophilic Surfaces and Coatings. Surf. Innov. 2014, 2, 211–227. [Google Scholar] [CrossRef]
- Antonov, D.V.; Islamova, A.G.; Strizhak, P.A. Hydrophilic and Hydrophobic Surfaces: Features of Interaction with Liquid Drops. Materials 2023, 16, 5932. [Google Scholar] [CrossRef]
- Duangkanya, K.; Kopwitthaya, A.; Chanhorm, S.; Infahsaeng, Y. Oxygen Plasma Treatment Time Induced Hydrophilicity of Polydimethylsiloxane (PDMS) Thin Films for Liquid Lenses Application. Mater. Today Proc. 2022, 65, 2442–2445. [Google Scholar] [CrossRef]
- Shrirao, A.B.; Kung, F.H.; Yip, D.; Cho, C.H.; Townes-Anderson, E. Vacuum-Assisted Fluid Flow in Microchannels to Pattern Substrates and Cells. Biofabrication 2014, 6, 035016. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, M.; Bessoth, F.G.; Manz, A. Microfabricated Devices for Fluid Mixing and Their Application for Chemical Synthesis. Chem. Rec. 2001, 1, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Amerian, M.; Amerian, M.; Sameti, M.; Seyedjafari, E. Improvement of PDMS Surface Biocompatibility Is Limited by the Duration of Oxygen Plasma Treatment. J. Biomed. Mater. Res. A 2019, 107, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Eddington, D.T.; Puccinelli, J.P.; Beebe, D.J. Thermal Aging and Reduced Hydrophobic Recovery of Polydimethylsiloxane. Sens. Actuators B Chem. 2006, 114, 170–172. [Google Scholar] [CrossRef]
- Karthik, C.; Rajalakshmi, S.; Thomas, S.; Thomas, V. Intelligent Polymeric Biomaterials Surface Driven by Plasma Processing. Curr. Opin. Biomed. Eng. 2023, 26, 100440. [Google Scholar] [CrossRef]
- Vasilets, V.N.; Nakamura, K.; Uyama, Y.; Ogata, S.; Ikada, Y. Improvement of the Micro-Wear Resistance of Silicone by Vacuum Ultraviolet Irradiation. Polymer 1998, 39, 2875–2881. [Google Scholar] [CrossRef]
- Berdichevsky, Y.; Khandurina, J.; Guttman, A.; Lo, Y.-H. UV/Ozone Modification of Poly(Dimethylsiloxane) Microfluidic Channels. Sens. Actuators B Chem. 2004, 97, 402–408. [Google Scholar] [CrossRef]
- Pan, J.; Chiang, C.; Wang, X.; Bertani, P.; Ma, Y.; Cheng, J.; Talesara, V.; Lee, L.J.; Lu, W. Cell Membrane Damage and Cargo Delivery in Nano-Electroporation. Nanoscale 2023, 15, 4080–4089. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.-J.; Xu, J.-J.; Zhang, Q.; Chen, H.-Y. The Use of Poly(Dimethylsiloxane) Surface Modification with Gold Nanoparticles for the Microchip Electrophoresis. Talanta 2006, 69, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.-J.; Xu, J.-J.; Chen, H.-Y. Proteins Modification of Poly(Dimethylsiloxane) Microfluidic Channels for the Enhanced Microchip Electrophoresis. J. Chromatogr. A 2006, 1107, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, L.; Zhou, F.; Zhu, J.-J.; Zhang, J.-R. Electroosmotic Flow-Switchable Poly(Dimethylsiloxane) Microfluidic Channel Modified with Cysteine Based on Gold Nanoparticles. Talanta 2007, 73, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Bodas, D.; Khan-Malek, C. Hydrophilization and Hydrophobic Recovery of PDMS by Oxygen Plasma and Chemical Treatment—An SEM Investigation. Sens. Actuators B Chem. 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Hillborg, H.; Ankner, J.F.; Gedde, U.W.; Smith, G.D.; Yasuda, H.K.; Wikström, K. Crosslinked Polydimethylsiloxane Exposed to Oxygen Plasma Studied by Neutron Reflectometry and Other Surface Specific Techniques. Polymer 2000, 41, 6851–6863. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on Surface Wettability of Poly(Dimethyl) Siloxane (PDMS) and Glass under Oxygen-Plasma Treatment and Correlation with Bond Strength. J. Microelectromechanical Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Jiang, B.; White, A.; Ou, W.; Van Belleghem, S.; Stewart, S.; Shamul, J.G.; Rahaman, S.O.; Fisher, J.P.; He, X. Noncovalent Reversible Binding-Enabled Facile Fabrication of Leak-Free PDMS Microfluidic Devices without Plasma Treatment for Convenient Cell Loading and Retrieval. Bioact. Mater. 2022, 16, 346–358. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, H.; Chen, D.; Zhou, M.; Jiang, B.; Guo, H.; Chen, D.; Zhou, M. Microscale Investigation on the Wettability and Bonding Mechanism of Oxygen Plasma-Treated PDMS Microfluidic Chip. ApSS 2022, 574, 151704. [Google Scholar] [CrossRef]
- Manouchehri, M. A Comprehensive Review on State-of-the-Art Antifouling Super(Wetting and Anti-Wetting) Membranes for Oily Wastewater Treatment. Adv. Colloid Interface Sci. 2024, 323, 103073. [Google Scholar] [CrossRef] [PubMed]
- Lawton, R.A.; Price, C.R.; Runge, A.F.; Doherty, W.J.; Saavedra, S.S. Air Plasma Treatment of Submicron Thick PDMS Polymer Films: Effect of Oxidation Time and Storage Conditions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 253, 213–215. [Google Scholar] [CrossRef]
- Morra, M.; Occhiello, E.; Marola, R.; Garbassi, F.; Humphrey, P.; Johnson, D. On the Aging of Oxygen Plasma-Treated Polydimethylsiloxane Surfaces. J. Colloid Interface Sci. 1990, 137, 11–24. [Google Scholar] [CrossRef]
- Tóth, A.; Bertóti, I.; Blazsó, M.; Bánhegyi, G.; Bognar, A.; Szaplonczay, P. Oxidative Damage and Recovery of Silicone Rubber Surfaces. I. X-Ray Photoelectron Spectroscopic Study. J. Appl. Polym. Sci. 1994, 52, 1293–1307. [Google Scholar] [CrossRef]
- Chen, I.-J.; Lindner, E. The Stability of Radio-Frequency Plasma-Treated Polydimethylsiloxane Surfaces. Langmuir 2007, 23, 3118–3122. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Shevkoplyas, S.S.; Zasońska, B.; Szymborski, T.; Garstecki, P.; Whitesides, G.M. Formation of Bubbles and Droplets in Parallel, Coupled Flow-Focusing Geometries. Small 2008, 4, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Nguyen, N.T.; Chua, Y.C.; Kang, T.G. Oxygen Plasma Treatment for Reducing Hydrophobicity of a Sealed Polydimethylsiloxane Microchannel. Biomicrofluidics 2010, 4, 032204. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Moreno, J.A.; Ávila-Ortega, A.; Oliva, A.I.; Avilés, F.; Cauich-Rodríguez, J. V Effect of Wettability and Surface Roughness on the Adhesion Properties of Collagen on PDMS Films Treated by Capacitively Coupled Oxygen Plasma. Appl. Surf. Sci. 2015, 349, 763–773. [Google Scholar] [CrossRef]
- Yang, S.Y.; Bai, B.C.; Kim, Y.R. Effective Surface Structure Changes and Characteristics of Activated Carbon with the Simple Introduction of Oxygen Functional Groups by Using Radiation Energy. Surfaces 2024, 7, 12–25. [Google Scholar] [CrossRef]
- Kim, J.; Chaudhury, M.K.; Owen, M.J.; Orbeck, T. The Mechanisms of Hydrophobic Recovery of Polydimethylsiloxane Elastomers Exposed to Partial Electrical Discharges. J. Colloid Interface Sci. 2001, 244, 200–207. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Formation of More Stable Hydrophilic Surfaces of PDMS by Plasma and Chemical Treatments. Microelectron. Eng. 2006, 83, 1277–1279. [Google Scholar] [CrossRef]
- Grace, J.M.; Gerenser, L.J. Plasma Treatment of Polymers. J. Dispers. Sci. Technol. 2003, 24, 305–341. [Google Scholar] [CrossRef]
- Hegemann, D.; Brunner, H.; Oehr, C. Plasma Treatment of Polymers for Surface and Adhesion Improvement. Nucl. Instrum. Methods Phys. Res. B 2003, 208, 281–286. [Google Scholar] [CrossRef]
- Owen, M.J.; Smith, P.J. Plasma Treatment of Polydimethylsiloxane. J. Adhes. Sci. Technol. 1994, 8, 1063–1075. [Google Scholar] [CrossRef]
- Vogler, E.A. Surface Modification for Biocompatibility. In Engineered Biomimicry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 189–220. ISBN 978-0-12-415995-2. [Google Scholar]
- Bagiatis, V.; Critchlow, G.W.; Price, D.; Wang, S. The Effect of Atmospheric Pressure Plasma Treatment (APPT) on the Adhesive Bonding of Poly(Methyl Methacrylate) (PMMA)-to-Glass Using a Polydimethylsiloxane (PDMS)-Based Adhesive. Int. J. Adhes. Adhes. 2019, 95, 102405. [Google Scholar] [CrossRef]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A Practical Guide to Rapid-Prototyping of PDMS-Based Microfluidic Devices: A Tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with Designer Functionalities—Properties, Modifications Strategies, and Applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Ruzi, M.; Celik, N.; Onses, M.S. Superhydrophobic Coatings for Food Packaging Applications: A Review. Food Packag. Shelf Life 2022, 32, 100823. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, Q.; Chen, G.; Fang, Y.; Kurilova, O.; Liu, Z.; Li, S.; Chen, J. Advances in Wearable Respiration Sensors. Mater. Today 2024, 72, 140–162. [Google Scholar] [CrossRef]
- de Camargo, J.S.G.; de Menezes, A.J.; da Cruz, N.C.; Rangel, E.C.; Delgado-Silva, A.D.O. Morphological and Chemical Effects of Plasma Treatment with Oxygen (O2) and Sulfur Hexafluoride (SF6) on Cellulose Surface. Mater. Res. 2018, 20, 842–850. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-Surface Modification of Biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Tan, H.M.L.; Fukuda, H.; Akagi, T.; Ichiki, T. Surface Modification of Poly(Dimethylsiloxane) for Controlling Biological Cells’ Adhesion Using a Scanning Radical Microjet. Thin Solid Films 2007, 515, 5172–5178. [Google Scholar] [CrossRef]
- Pal, C.A.; Angaru, G.K.R.; Lingamdinne, L.P.; Choi, Y.-L.; Momin, Z.H.; Kulkarni, R.; Koduru, J.R.; Chang, Y.-Y. Fabrication of Plasma-Treated Superhydrophobic Polydimethylsiloxane (PDMS)—Coatėd Melamine Sponge for Enhanced Adhesion Capability and Sustainable Oil/Water Separation. Sep. Purif. Technol. 2024, 330, 125483. [Google Scholar] [CrossRef]
- Almutairi, Z.; Ren, C.L.; Simon, L. Evaluation of Polydimethylsiloxane (PDMS) Surface Modification Approaches for Microfluidic Applications. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 406–412. [Google Scholar] [CrossRef]
- Zhu, X.; Mills, K.L.; Peters, P.R.; Bahng, J.H.; Liu, E.H.; Shim, J.; Naruse, K.; Csete, M.E.; Thouless, M.D.; Takayama, S. Fabrication of Reconfigurable Protein Matrices by Cracking. Nat. Mater. 2005, 4, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Akther, F.; Yakob, S.B.; Nguyen, N.-T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Yuan, C.; Muisener, R.J.; Boulares, A.; Koberstein, J.T. Conversion of Some Siloxane Polymers to Silicon Oxide by UV/Ozone Photochemical Processes. Chem. Mater. 2000, 12, 1591–1596. [Google Scholar] [CrossRef]
- Mirley, C.L.; Koberstein, J.T. A Room Temperature Method for the Preparation of Ultrathin SiOx Films from Langmuir-Blodgett Layers. Langmuir 1995, 11, 1049–1052. [Google Scholar] [CrossRef]
- Li, H.; Chiang, C.-L.; Kwak, K.J.; Wang, X.; Doddi, S.; Ramanathan, L.V.; Cho, S.M.; Hou, Y.-C.; Cheng, T.-S.; Mo, X.; et al. Extracellular Vesicular Analysis of Glypican 1 MRNA and Protein for Pancreatic Cancer Diagnosis and Prognosis. Adv. Sci. 2024, 11, 2306373. [Google Scholar] [CrossRef]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface Modification of Sylgard-184 Poly(Dimethyl Siloxane) Networks by Ultraviolet and Ultraviolet/Ozone Treatment. J. Colloid Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Tsougeni, K.; Tserepi, A.; Boulousis, G.; Constantoudis, V.; Gogolides, E. Tunable Poly(Dimethylsiloxane) Topography in O2 or Ar Plasmas for Controlling Surface Wetting Properties and Their Ageing. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2007, 46, 744–750. [Google Scholar] [CrossRef]
- Fu, Y.J.; Qui, H.Z.; Liao, K.S.; Lue, S.J.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Effect of UV-Ozone Treatment on Poly(Dimethylsiloxane) Membranes: Surface Characterization and Gas Separation Performance. Langmuir 2010, 26, 4392–4399. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Rivera, J.; Hirasaki, G.J.; Biswal, S.L. Wettability Control and Patterning of PDMS Using UV–Ozone and Water Immersion. J. Colloid Interface Sci. 2011, 363, 371–378. [Google Scholar] [CrossRef]
- Song, J.; Tranchida, D.; Vancso, G.J. Contact Mechanics of UV/Ozone-Treated PDMS by AFM and JKR Testing: Mechanical Performance from Nano- to Micrometer Length Scales. Macromolecules 2008, 41, 6757–6762. [Google Scholar] [CrossRef]
- Liu, C.; Xu, H.; Huo, B.; Wang, J.; Wang, Z.; Chen, X.; Meng, F.; Sun, C.; Wang, Y. Research Progress of PVDF Based Piezoelectric Polymer Composites in Water Pollution Remediation. J. Water Process Eng. 2023, 55, 104181. [Google Scholar] [CrossRef]
- Hashimoto, Y. Surface Modification of Polymers by Vacuum Ultraviolet Illumination Containing Low Wavelength below 160 Nm and Microfluidic Applications of Irradiated Polycarbonate. Polymer 2023, 287, 126439. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W. Effective Strategies to Enhance Ultraviolet Barrier Ability in Biodegradable Polymer-Based Films/Coatings for Fruit and Vegetable Packaging. Trends Food Sci. Technol. 2023, 139, 104139. [Google Scholar] [CrossRef]
- Oláh, A.; Hillborg, H.; Vancso, G.J. Hydrophobic Recovery of UV/Ozone Treated Poly(Dimethylsiloxane): Adhesion Studies by Contact Mechanics and Mechanism of Surface Modification. Appl. Surf. Sci. 2005, 239, 410–423. [Google Scholar] [CrossRef]
- Fatona, A.; Chen, Y.; Reid, M.; Brook, M.A.; Moran-Mirabal, J.M. One-Step in-Mould Modification of PDMS Surfaces and Its Application in the Fabrication of Self-Driven Microfluidic Channels. Lab Chip 2015, 15, 4322–4330. [Google Scholar] [CrossRef]
- Hu, S.; Ren, X.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N. Cross-Linked Coatings for Electrophoretic Separations in Poly(Dimethylsiloxane) Microchannels. Electrophoresis 2003, 24, 3679–3688. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Brook, M.A. Facile Functionalization of PDMS Elastomer Surfaces Using Thiol–Ene Click Chemistry. Langmuir 2013, 29, 12432–12442. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Chen, C. PDMS-ZnO Nano-Composite Enhanced the Hydrophobicn, Self-Cleaning, and Mechanical Property of Packaging Corrugated Paper. Inorg. Chem. Commun. 2023, 158, 111508. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces; Interscience Publishers: New York, NY, USA, 1967; Volume 150, ISBN 0306090120. [Google Scholar]
- Seo, J.; Lee, L.P. Effects on Wettability by Surfactant Accumulation/Depletion in Bulk Polydimethylsiloxane (PDMS). Sens. Actuators B Chem. 2006, 119, 192–198. [Google Scholar] [CrossRef]
- Nam, G.; Yoon, S.H. Predicting the Temporal Wetting of Porous, Surfactant-Added Polydimethylsiloxane (PDMS). J. Colloid Interface Sci. 2019, 556, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, L.; Bonakdar, S.; Taghipour, M.; Renaud, P.; Baharvand, H. Modification of PDMS to Fabricate PLGA Microparticles by a Double Emulsion Method in a Single Microfluidic Device. Lab Chip 2016, 16, 2596–2600. [Google Scholar] [CrossRef]
- Soriano-Jerez, Y.; Gourlaouen, E.; Zeriouh, O.; Cerón-García, M.D.C.; Arrabal-Campos, F.M.; Ruiz-Martínez, C.; Fernández, I.; Gallardo-Rodríguez, J.J.; García-Camacho, F.; Molina-Grima, E.; et al. Role of Dynamic Surface Tension of Silicone Polyether Surfactant-Based Silicone Coatings on Protein Adsorption: An Insight on the ‘Ambiguous’ Interfacial Properties of Fouling Release Coatings. Prog. Org. Coat. 2024, 186, 108079. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; Wiley: Hoboken, NJ, USA, 2012; Available online: https://www.wiley.com/en-us/Surfactants+and+Interfacial+Phenomena%2C+4th+Edition-p-9780470541944 (accessed on 16 May 2024).
- Zdziennicka, A.; Jańczuk, B. Adsorption of Cetyltrimethylammonium Bromide and Propanol Mixtures with Regard to Wettability of Polytetrafluoroethylene. I. Adsorption at Aqueous Solution–Air Interface. J. Colloid Interface Sci. 2008, 317, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Boxshall, K.; Wu, M.-H.; Cui, Z.; Cui, Z.; Watts, J.F.; Baker, M.A. Simple Surface Treatments to Modify Protein Adsorption and Cell Attachment Properties within a Poly(Dimethylsiloxane) Micro-Bioreactor. Surf. Interface Anal. 2006, 38, 198–201. [Google Scholar] [CrossRef]
- Entering a New Era of Surfactants—Evonik Industries. Available online: https://household-care.evonik.com/en/products/biosurfactants (accessed on 16 May 2024).
- Stålgren, J.J.R. Adsorption of Surfactants at the Solid-Liquid Interface: A Quartz Crystal Microbalance Study. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2002. [Google Scholar]
- Lee, S.; Iten, R.; Müller, M.; Spencer, N.D. Influence of Molecular Architecture on the Adsorption of Poly(Ethylene Oxide)−Poly(Propylene Oxide)−Poly(Ethylene Oxide) on PDMS Surfaces and Implications for Aqueous Lubrication. Macromolecules 2004, 37, 8349–8356. [Google Scholar] [CrossRef]
- Gökaltun, A.; Kang, Y.B.; Yarmush, M.L.; Usta, O.B.; Asatekin, A. Simple Surface Modification of Poly(Dimethylsiloxane) via Surface Segregating Smart Polymers for Biomicrofluidics. Sci. Rep. 2019, 9, 7377. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Singh, A.K. Advancement in Utilization of Bio-Based Materials Including Cellulose, Lignin, Chitosan for Bio-Inspired Surface Coatings with Special Wetting Behavior: A Review on Fabrication and Applications. Int. J. Biol. Macromol. 2023, 246, 125709. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Man, J.; Qiu, Y.; Wang, J.; Liu, J.; Li, R.; Zhang, Y.; Li, J.; Li, J.; Chen, Y. Design, Preparation, and Characterization of Lubricating Polymer Brushes for Biomedical Applications. Acta Biomater. 2023. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.-H.; Bao, N.; Xu, J.-J.; Chen, H.-Y. A Dynamically Modified Microfluidic Poly(Dimethylsiloxane) Chip with Electrochemical Detection for Biological Analysis. Electrophoresis 2002, 23, 3558–3566. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, G.; Munroe, M.; Tang, T.; Oleschuk, R.; Westra, K.; Harrison, D.J. Electrokinetic Control of Fluid Flow in Native Poly(Dimethylsiloxane) Capillary Electrophoresis Devices. Electrophoresis 2000, 21, 107–115. [Google Scholar] [CrossRef]
- Lim, C.S.; Von Lau, E.; Kee, K.E.; Hung, Y.M. A Comparative Study of Superhydrophobicity of 0D/1D/2D Thermally Functionalized Carbon Nanomaterials. Ceram. Int. 2021, 47, 30331–30342. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I. Modification of the Wettability of Polymer Surfaces Using Nanoparticles. Prog. Org. Coat. 2014, 77, 331–338. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Shen, Y. Chemical Modification on Top of Nanotopography to Enhance Surface Properties of PDMS. Surf. Coat. Technol. 2012, 206, 2161–2167. [Google Scholar] [CrossRef]
- Alameda, M.T.; Osorio, M.R.; Hernández, J.J.; Rodríguez, I. Multilevel Hierarchical Topographies by Combined Photolithography and Nanoimprinting Processes to Create Surfaces with Controlled Wetting. ACS Appl. Nano Mater. 2019, 2, 4727–4733. [Google Scholar] [CrossRef]
- Wen, H.; Raza, S.; Wang, P.; Zhu, Z.; Zhang, J.; Huang, W.; Liang, L.; Hu, H.; Deng, L.; Liu, C. Robust Super Hydrophobic Cotton Fabrics Functionalized with Ag and PDMS for Effective Antibacterial Activity and Efficient Oil–Water Separation. J. Environ. Chem. Eng. 2021, 9, 106083. [Google Scholar] [CrossRef]
- Barthwal, S.; Barthwal, S.; Singh, B.; Bahadur Singh, N. Multifunctional and Fluorine-Free Superhydrophobic Composite Coating Based on PDMS Modified MWCNTs/ZnO with Self-Cleaning, Oil-Water Separation, and Flame Retardant Properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124776. [Google Scholar] [CrossRef]
- Sadler, E.; Crick, C.R. Suction or Gravity-Fed Oil-Water Separation Using PDMS-Coated Glass Filters. Sustain. Mater. Technol. 2021, 29, e00321. [Google Scholar] [CrossRef]
- Vickers, J.A.; Caulum, M.M.; Henry, C.S. Generation of Hydrophilic Poly(Dimethylsiloxane) for High-Performance Microchip Electrophoresis. Anal. Chem. 2006, 78, 7446–7452. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.L.; McDonald, A.; Gourley, P.L.; Sasaki, D.Y. Poly(Dimethylsiloxane) Thin Films as Biocompatible Coatings for Microfluidic Devices: Cell Culture and Flow Studies with Glial Cells. J. Biomed. Mater. Res. A 2005, 72A, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Long, H.P.; Lai, C.C.; Chung, C.K. Polyethylene Glycol Coating for Hydrophilicity Enhancement of Polydimethylsiloxane Self-Driven Microfluidic Chip. Surf. Coat. Technol. 2017, 320, 315–319. [Google Scholar] [CrossRef]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic Surface Modification of PDMS for Droplet Microfluidics Using a Simple, Quick, and Robust Method via PVA Deposition. Microsyst. Nanoeng. 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chung, C.K. PDMS Microfabrication and Design for Microfluidics and Sustainable Energy Application: Review. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef] [PubMed]
- Holczer, E.; Fürjes, P. Effects of Embedded Surfactants on the Surface Properties of PDMS; Applicability for Autonomous Microfluidic Systems. Microfluid Nanofluidics 2017, 21, 81. [Google Scholar] [CrossRef]
- Vilčáková, J.; Moučka, R.; Svoboda, P.; Ilčíková, M.; Kazantseva, N.; Hřibová, M.; Mičušík, M.; Omastová, M. Effect of Surfactants and Manufacturing Methods on the Electrical and Thermal Conductivity of Carbon Nanotube/Silicone Composites. Molecules 2012, 17, 13157–13174. [Google Scholar] [CrossRef]
- Wu, Z.; Hjort, K. Surface Modification of PDMS by Gradient-Induced Migration of Embedded Pluronic. Lab Chip 2009, 9, 1500–1503. [Google Scholar] [CrossRef]
- Gonçalves, M.; Gonçalves, I.M.; Borges, J.; Faustino, V.; Soares, D.; Vaz, F.; Minas, G.; Lima, R.; Pinho, D. Polydimethylsiloxane Surface Modification of Microfluidic Devices for Blood Plasma Separation. Polymers 2024, 16, 1416. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Lee, S.H.; Jun, B.H.; Lee, S.H. Lithography Technology for Micro- and Nanofabrication. Adv. Exp. Med. Biol. 2021, 1309, 217–233. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.J.; Sedlack, A.J.H.; Bhatia, P.; Jensen, C.J.; Quintana-Puebla, A.; Paranjape, M. Systematic Characterization of Hydrophilized Polydimethylsiloxane. J. Microelectromechanical Syst. 2020, 29, 1216–1224. [Google Scholar] [CrossRef]
- Krishna, M.G.; Vinjanampati, M.; Purkayastha, D.D. Metal Oxide Thin Films and Nanostructures for Self-Cleaning Applications: Current Status and Future Prospects. Eur. Phys. J. Appl. Phys. 2013, 62, 30001. [Google Scholar] [CrossRef]
- Wang, M.; Kovacik, P.; Gleason, K.K. Chemical Vapor Deposition of Thin, Conductive, and Fouling-Resistant Polymeric Films. Langmuir 2017, 33, 10623–10631. [Google Scholar] [CrossRef]
- Maturos, T.; Wisitsora-at, A.; Lomas, T.; Sappat, A.; Tuantranont, A. Oxygen Plasma Treatment of Sputtered TiO2 Thin Film for Surface Modification of PDMS. In Proceedings of the 2007 7th IEEE Conference on Nanotechnology (IEEE NANO), Hong Kong, China, 2–5 August 2007; pp. 911–913. [Google Scholar]
- Maheshwari, N.; Kottantharayil, A.; Kumar, M.; Mukherji, S. Long Term Hydrophilic Coating on Poly(Dimethylsiloxane) Substrates for Microfluidic Applications. Appl. Surf. Sci. 2010, 257, 451–457. [Google Scholar] [CrossRef]

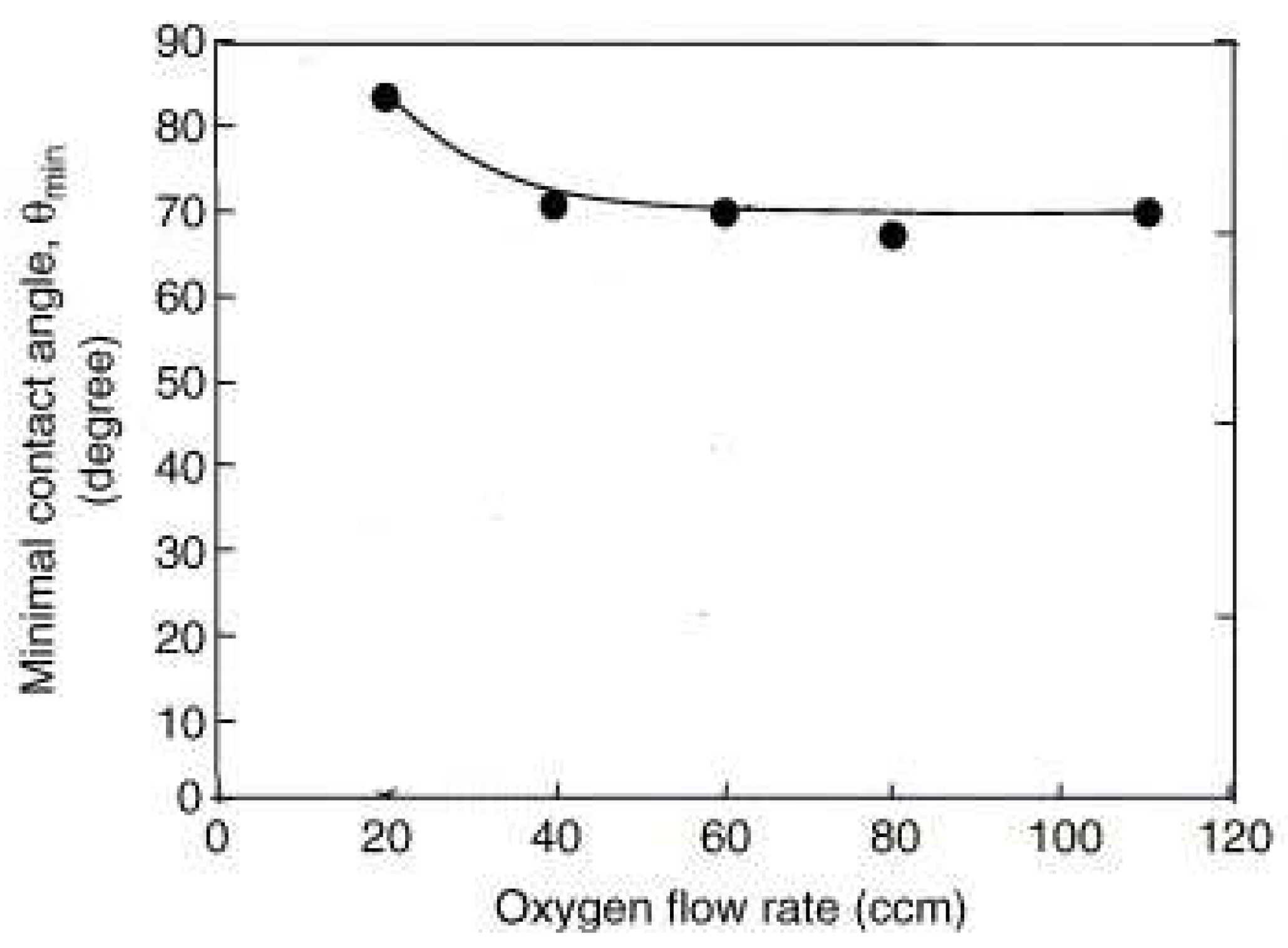

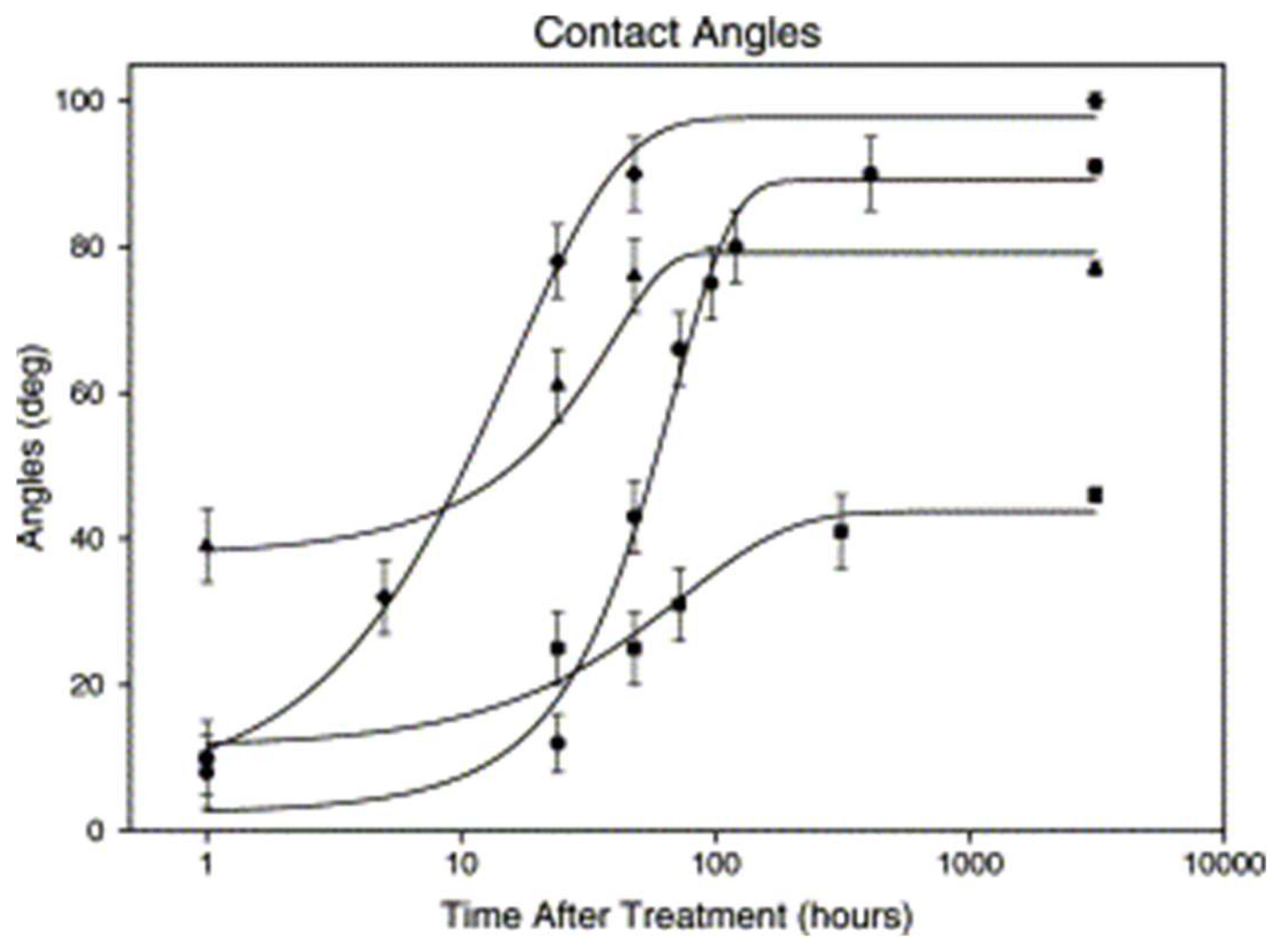

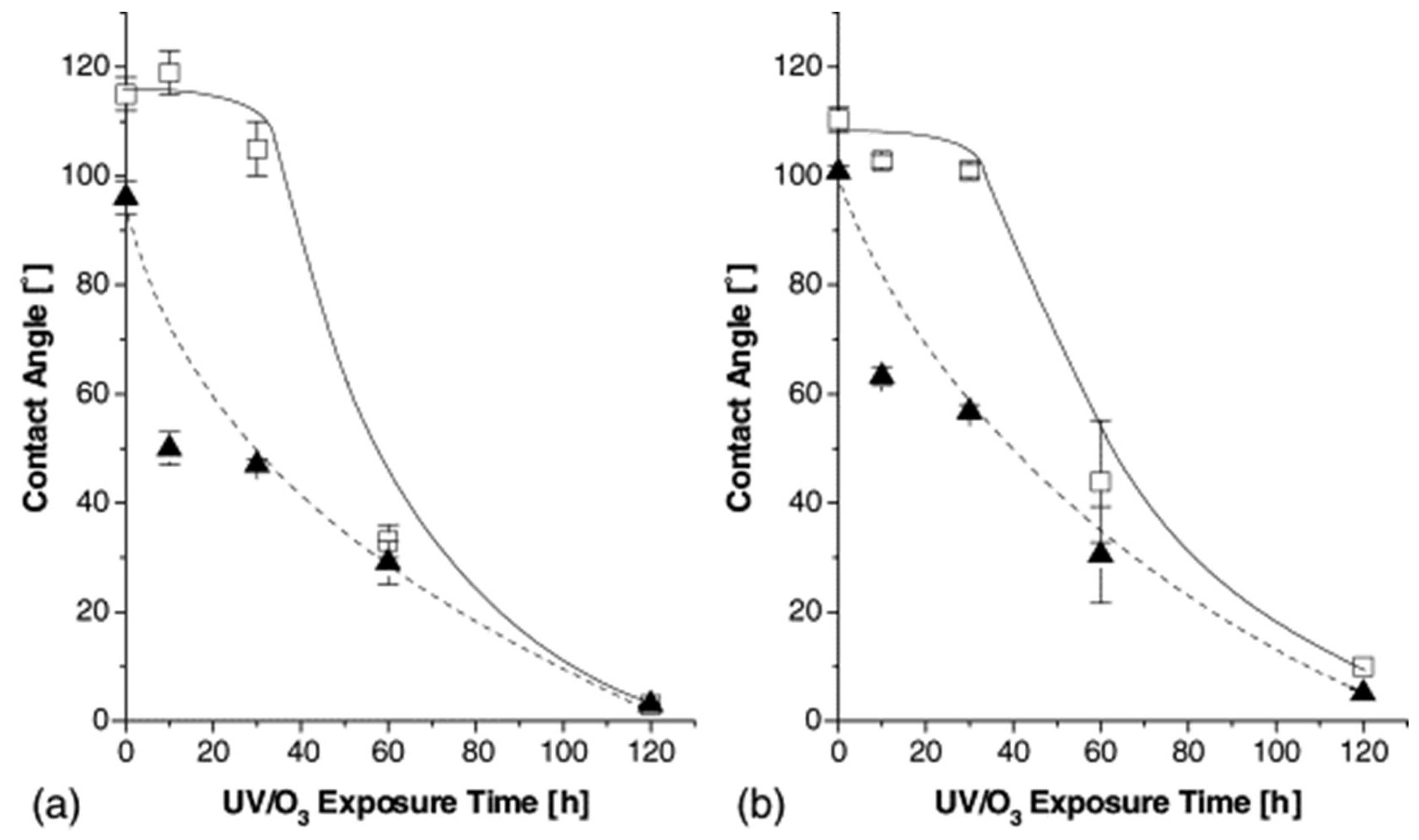
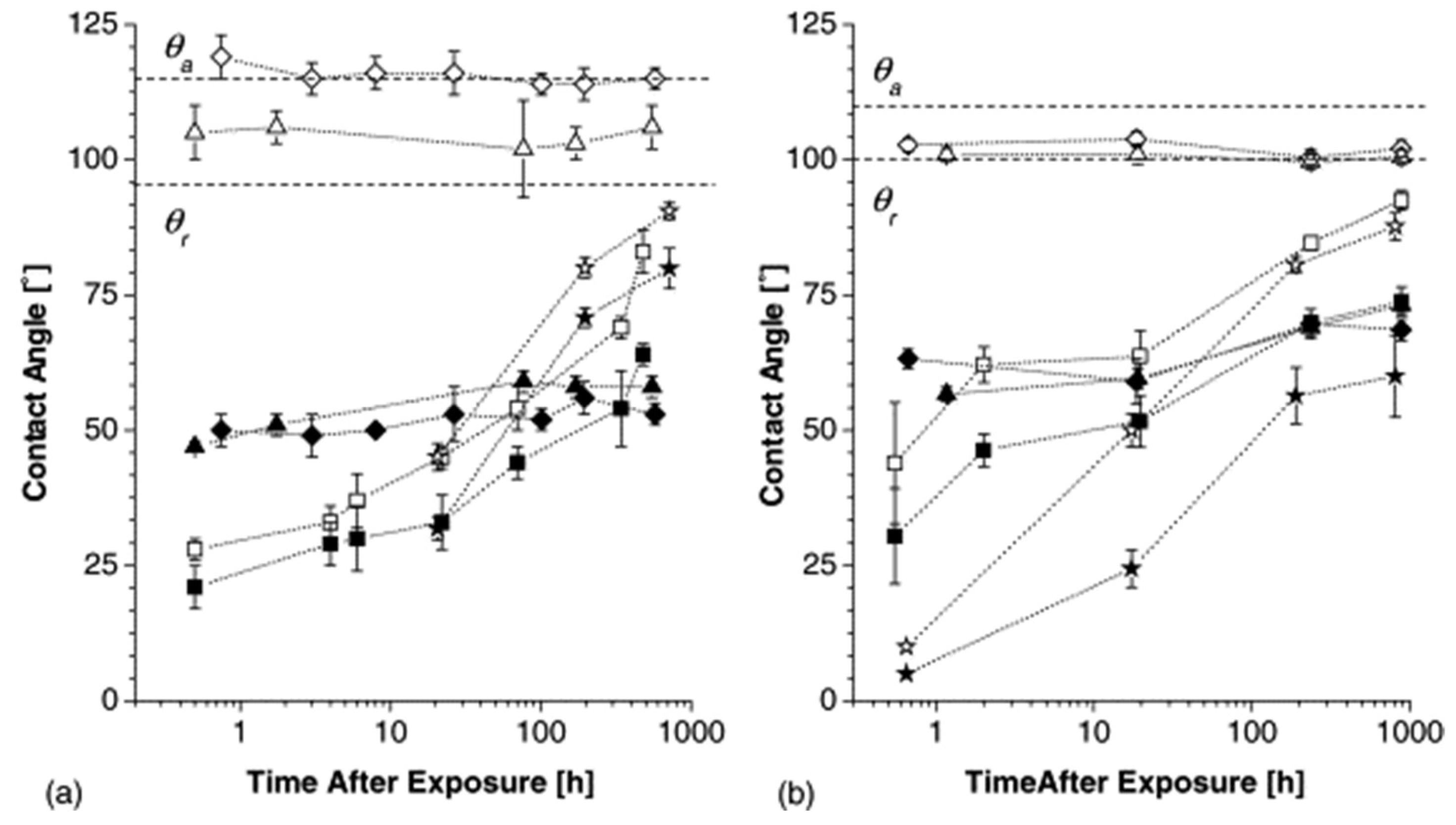

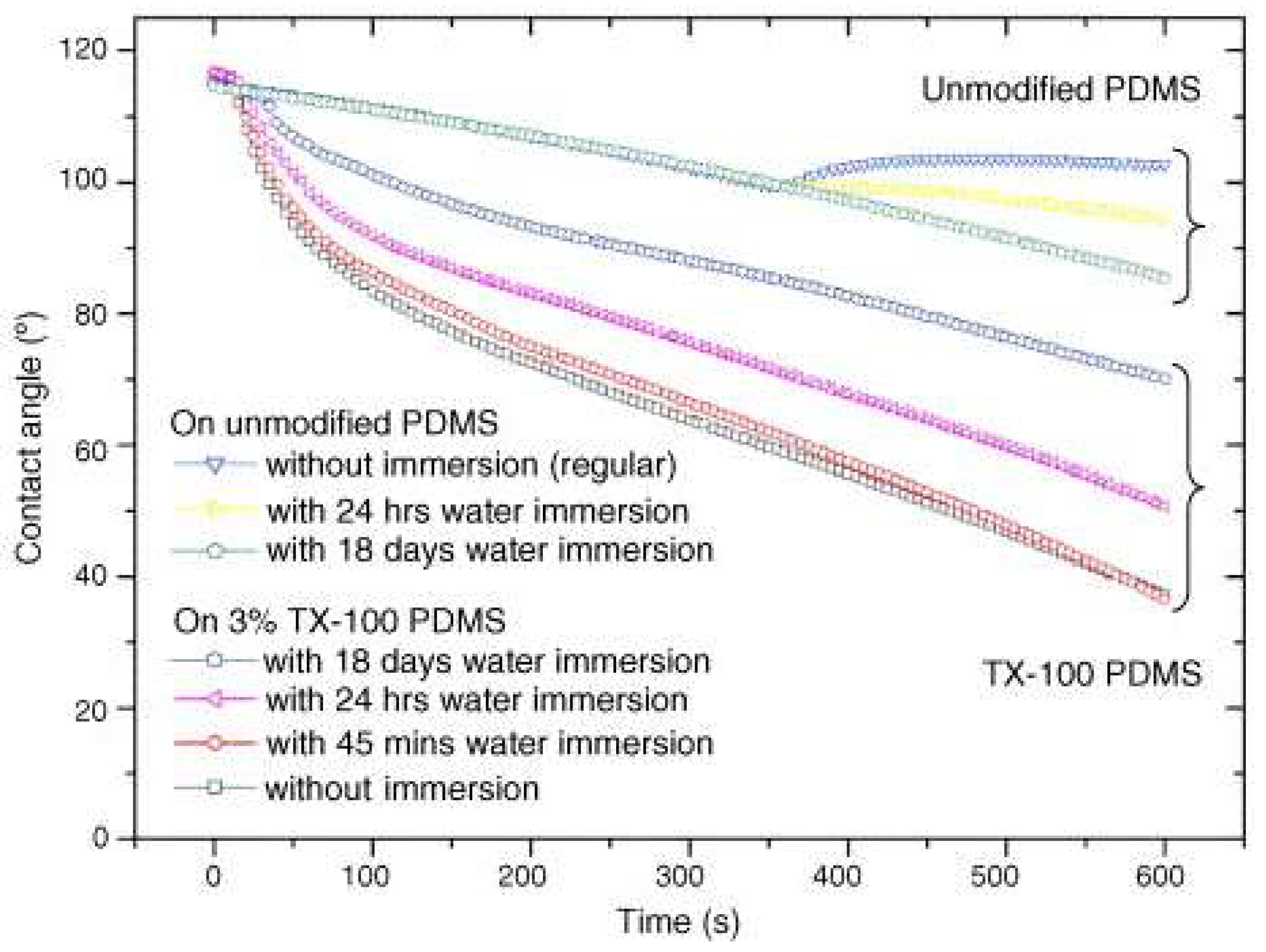
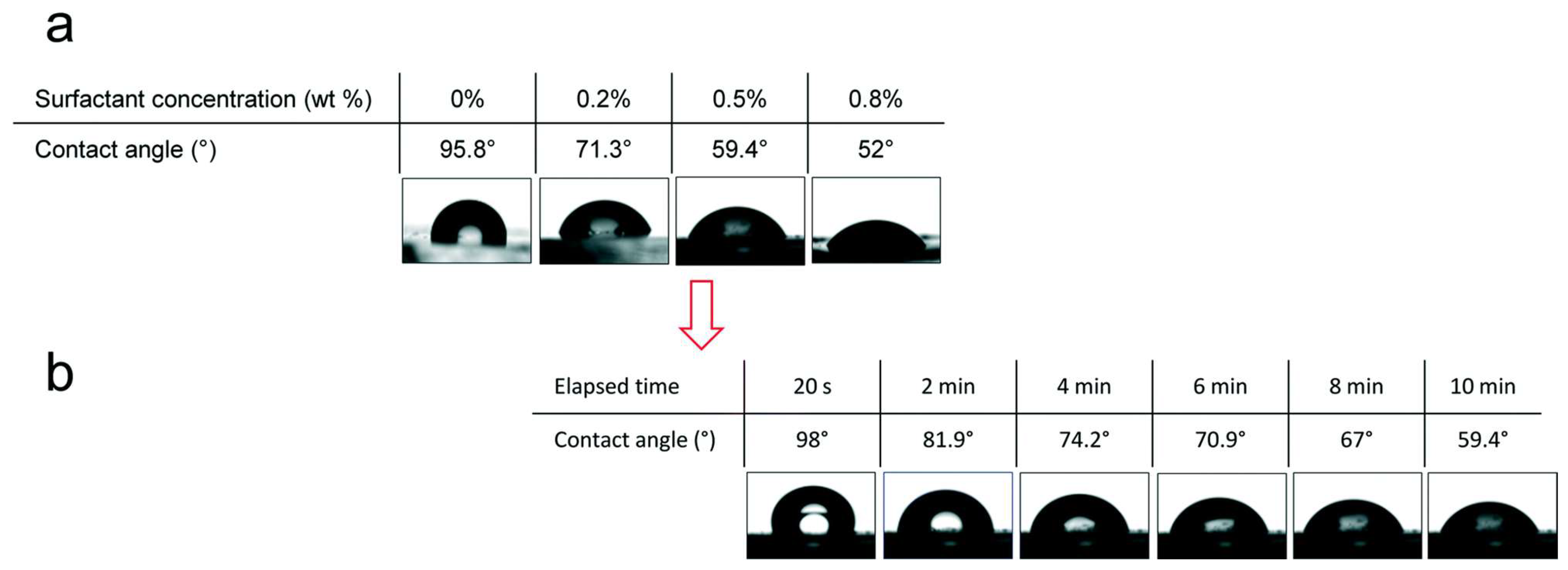

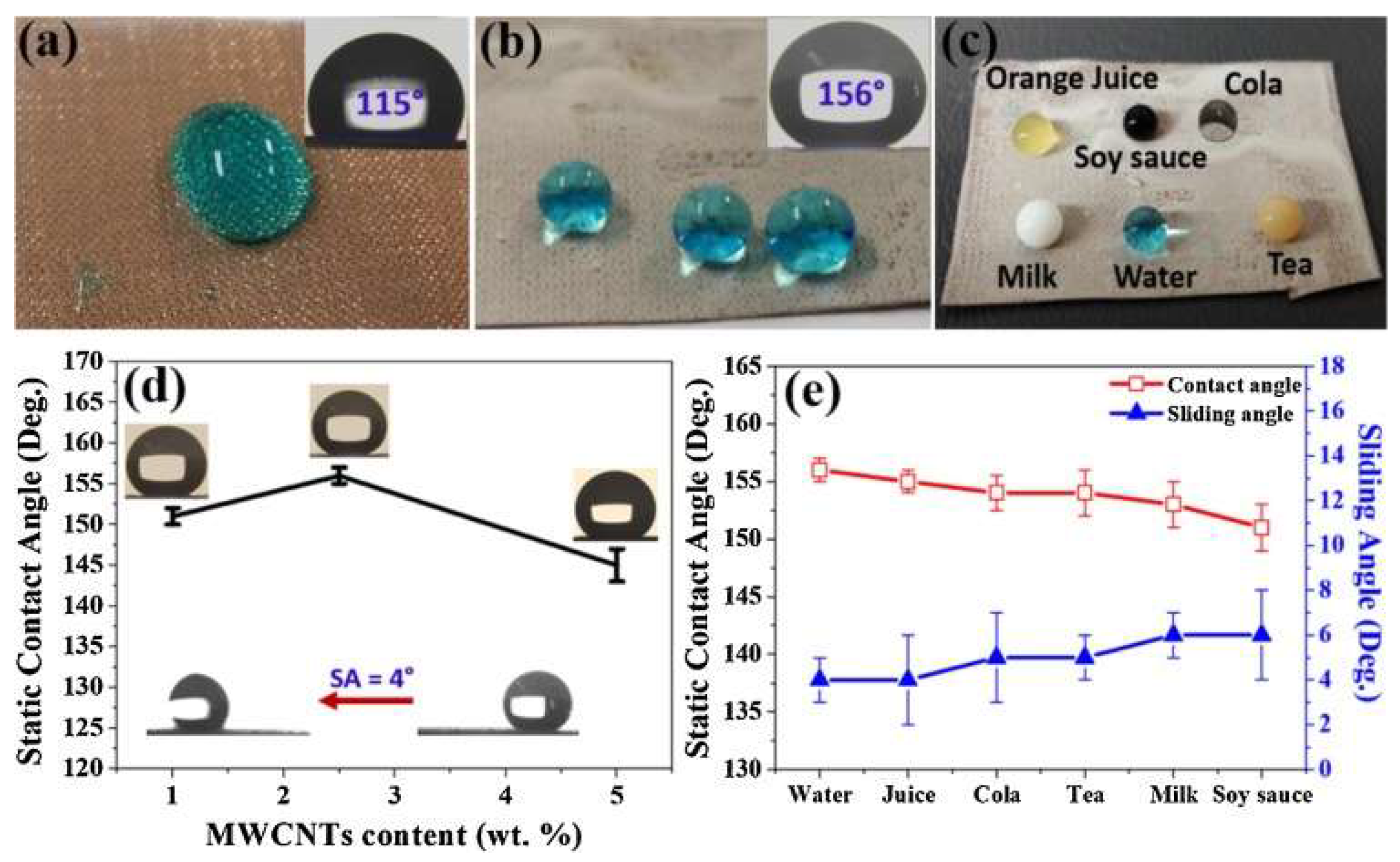
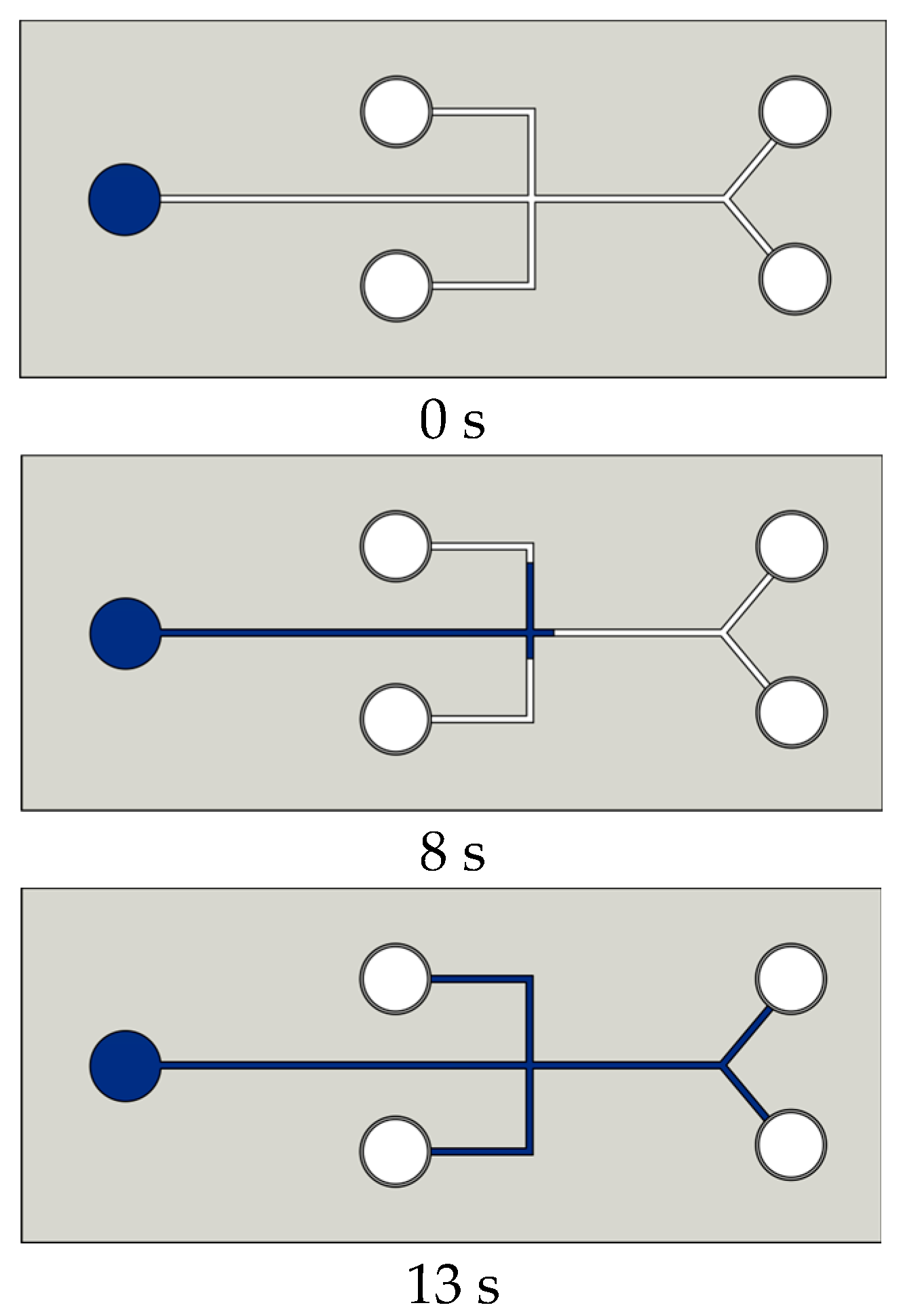
| Methods | WCA (and Over Time) ≈ | Advantages | Disadvantages | Applications | Ref. |
|---|---|---|---|---|---|
| Oxygen plasma treatment | 17°–46° (50°–115°, after 6 h) * | Low cost, ease of implementation, and compatibility with sensitive materials | Fast hydrophobic recovery and limitations on penetration depth | Microfluidic devices | [39] |
| Oxygen plasma treatment | 40°–101, 17° * | Improvement of polydimethylsiloxane (PDMS) surface biocompatibility as the most used biomaterial in maxillofacial prostheses for intraoral defects | [33] | ||
| Oxygen plasma treatment (SRMJ) | 70° | Biological cells adhered more easily to surfaces | Controlling biological cells’ attachment on biocompatible polymer material | [40] | |
| UV-ozone treatment | 10°–40° (40°–95°, after 30 days) * | Quick process and room temperature operation | Temporary surface modification and potential for degradation | Microfluidic | [41] |
| UV-ozone treatment | 7°, after 240 min UV/O treatment | [42] | |||
| Surfactant addition | 18°–68° (Table 2) | Ease of application; immediate effect and versatility | Uniformity challenges and potential leaching | Microfluidic, microfluidic biomedical, and non-toxic antibiofouling coatings | [43] |
| Incorporation of nanomaterials | <150° | Long-lasting modification and improvement of mechanical properties | Dispersion challenges, ecological problems, and high costs | Antibacterial activity and oil–water separation | [44] |
| Incorporation of nanomaterials | <150° | Self-cleaning, oil–water separation, and flame-retardant properties | [45] | ||
| Incorporation of nanomaterials | 158° | Oil–water separation | [46] |
| Surfactant | WCA (Before Soaking) | WCA (After Soaking) | WCA (After 11 Days or More) | WCA (21 °C) | WCA (60 °C) | WCA (80 °C) | WCA (100 °C) | Chemical Structure | Morphology before Soaking |
|---|---|---|---|---|---|---|---|---|---|
| Unmodified PDMS | 109° | - | - | - | - | - | - | (-Si(CH3)2-O-)n | - |
| Sodium dodecyl sulphate (SDS) | 57° | 114° | - | - | - | - | - | CH3(CH2)11OSO3Na | Opaque and roughened surfaces |
| Cetyl trimethylammonium bromide (CTAB) | 38° | 106° | - | - | - | - | - | C16H33N(CH3)3Br | Opaque and roughened surfaces |
| Tween 20 | 39° | 91° | Complete reversal | - | - | - | - | CH3(CH2)18(OCH2CH2)nOH | Optically clear and lowest surface roughness |
| Silsurf A008-UP | 20° | 63° | Complete reversal | ≈40° | ≈49° | ≈67° | ≈68° | - | Optically clear and surfaces presenting small dimples |
| * Alkyl (o-Wet) | 43° | 36° | - | ≈40° | ≈48° | ≈53° | ≈68° | (PEG) − (PDMS) − (PEG) − (Alkyl) | Smooth surfaces with depressions (microns wide) |
| * Siloxane (n-Wet) | 47° | 27° | - | ≈38° | ≈36° | ≈38° | ≈40° | (PEG) − (PDMS) − (PEG) − (Si-O) | Smooth surfaces with depressions (sub-micron wide) |
| * Siloxane (a-Wet) {has a more highly branched siloxane} | 22° | 25° | 40° | ≈21° | ≈18° | ≈19° | ≈19° | (PEG)−(PDMS)− (PEG)−(Si-O) branched | - |
| Surfactant | Binding Type | Bond Strength | Bond Stability | Notes | References |
|---|---|---|---|---|---|
| Sodium dodecyl sulphate (SDS) | Ionic | Strong | Long-term | Bond strength and stability may vary depending on concentration and chain length. | [100] |
| Cetyl trimethylammonium bromide (CTAB) | Ionic (similar to SDS) | Strong | Long-term | Specific information on binding type, strength, and stability may require further research or data from the supplier. | [101] |
| Tween 20 | Non-ionic (hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may vary depending on the specific alkyl surfactant. | [102] |
| Silsurf A008-UP | Non-ionic (likely hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may vary depending on the specific siloxane surfactant. | [103] |
| * Alkyl (o-Wet) | Non-ionic (hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may vary depending on the specific siloxane surfactant. | [104] |
| * Siloxane (n-Wet) | Non-ionic (hydrophobic interactions, Van der Waals forces) | Moderate | Medium-term | Bond strength and stability may vary depending on concentration and temperature. | [105] |
| Siloxane (a-Wet) | Non-ionic (hydrophobic interactions, Van der Waals forces) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research. | [106] |
| Triton X-100 | Non-ionic (hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research. | [106] |
| Silwet L-77 | Non-ionic (hydrophobic interactions, Van der Waals forces) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research. | [98] |
| CMS-626 | Non-ionic (likely hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research. | [99] |
| DBE-224 | Non-ionic (likely hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research. | [99] |
| DBE-311 | Non-ionic (likely hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research. | [99] |
| DBE-814 | Non-ionic (likely hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research. | [99] |
| DBE-821 | Non-ionic (likely hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research or data from the supplier. | [99] |
| DBE-C25 | Non-ionic (likely hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research. | [99] |
| DOWSIL™ OFX-0400 | Non-ionic (likely hydrogen bonding, hydrophobic interactions) | Moderate | Medium-term | Specific information on binding type, strength, and stability may require further research or data from the supplier. | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, L.B.; Afonso, I.S.; Nobrega, G.; Barbosa, L.G.; Lima, R.A.; Ribeiro, J.E. A Review of Methods to Modify the PDMS Surface Wettability and Their Applications. Micromachines 2024, 15, 670. https://doi.org/10.3390/mi15060670
Neves LB, Afonso IS, Nobrega G, Barbosa LG, Lima RA, Ribeiro JE. A Review of Methods to Modify the PDMS Surface Wettability and Their Applications. Micromachines. 2024; 15(6):670. https://doi.org/10.3390/mi15060670
Chicago/Turabian StyleNeves, Lucas B., Inês S. Afonso, Glauco Nobrega, Luiz G. Barbosa, Rui A. Lima, and João E. Ribeiro. 2024. "A Review of Methods to Modify the PDMS Surface Wettability and Their Applications" Micromachines 15, no. 6: 670. https://doi.org/10.3390/mi15060670
APA StyleNeves, L. B., Afonso, I. S., Nobrega, G., Barbosa, L. G., Lima, R. A., & Ribeiro, J. E. (2024). A Review of Methods to Modify the PDMS Surface Wettability and Their Applications. Micromachines, 15(6), 670. https://doi.org/10.3390/mi15060670








