Novel Isolating Approaches to Circulating Tumor Cell Enrichment Based on Microfluidics: A Review
Abstract
:1. Introduction
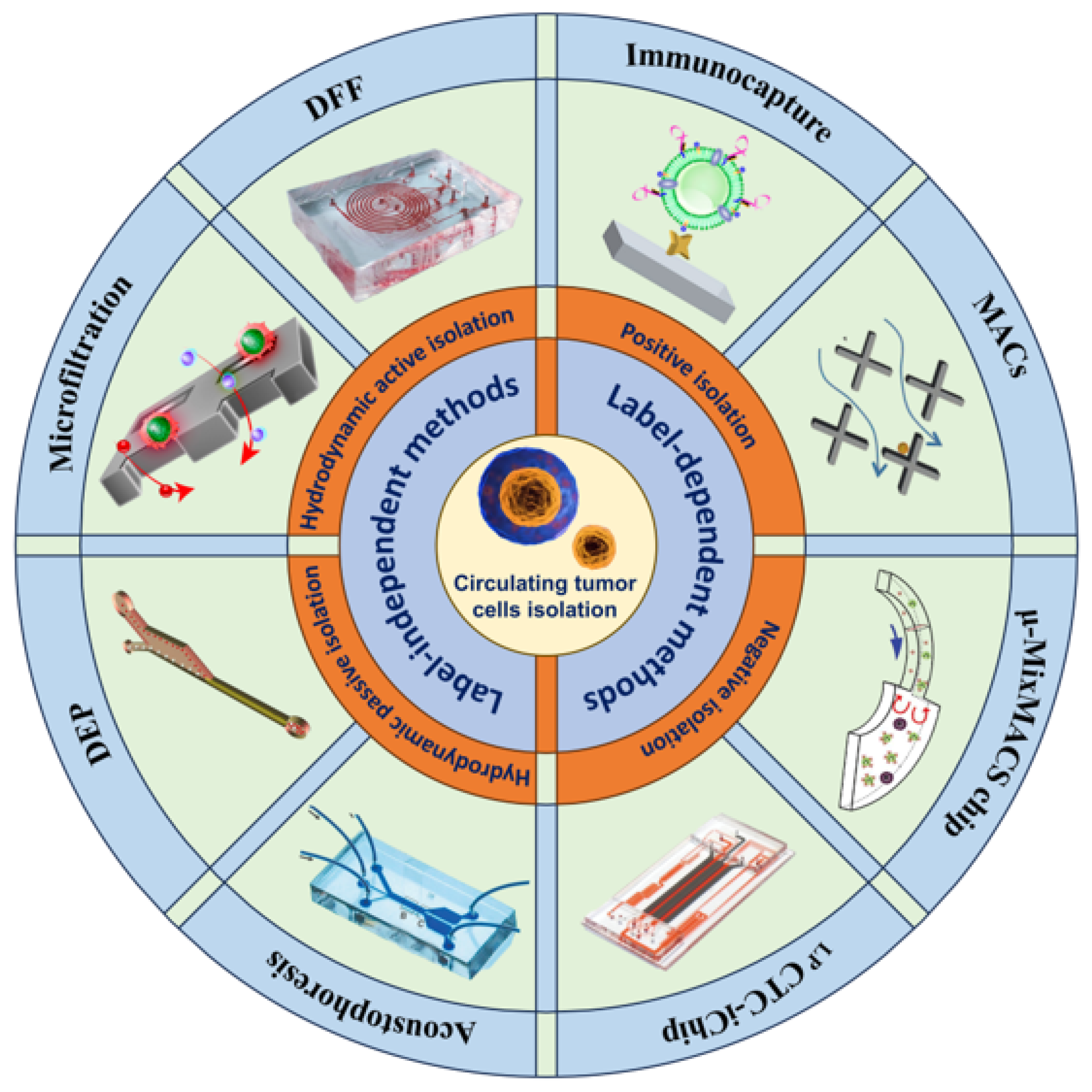
2. Label-Dependent Methods
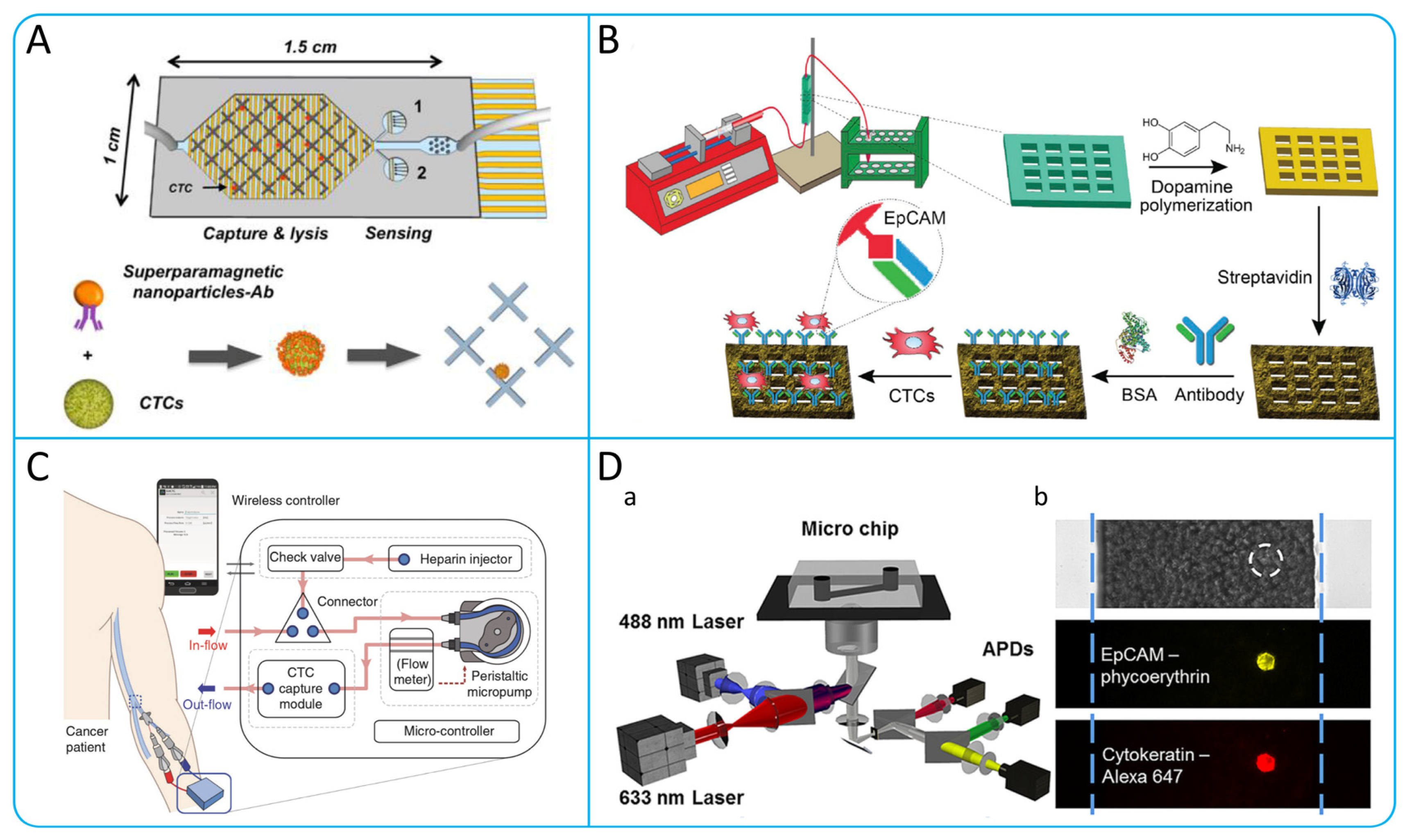
2.1. Immunocapture
2.1.1. Aptamer-Based CTCs Isolation
2.1.2. Antibody Functionalized Micropost Array
2.1.3. Chaotic Mixing Microchannel
2.1.4. Nanomaterials with High Surface Area Ratio

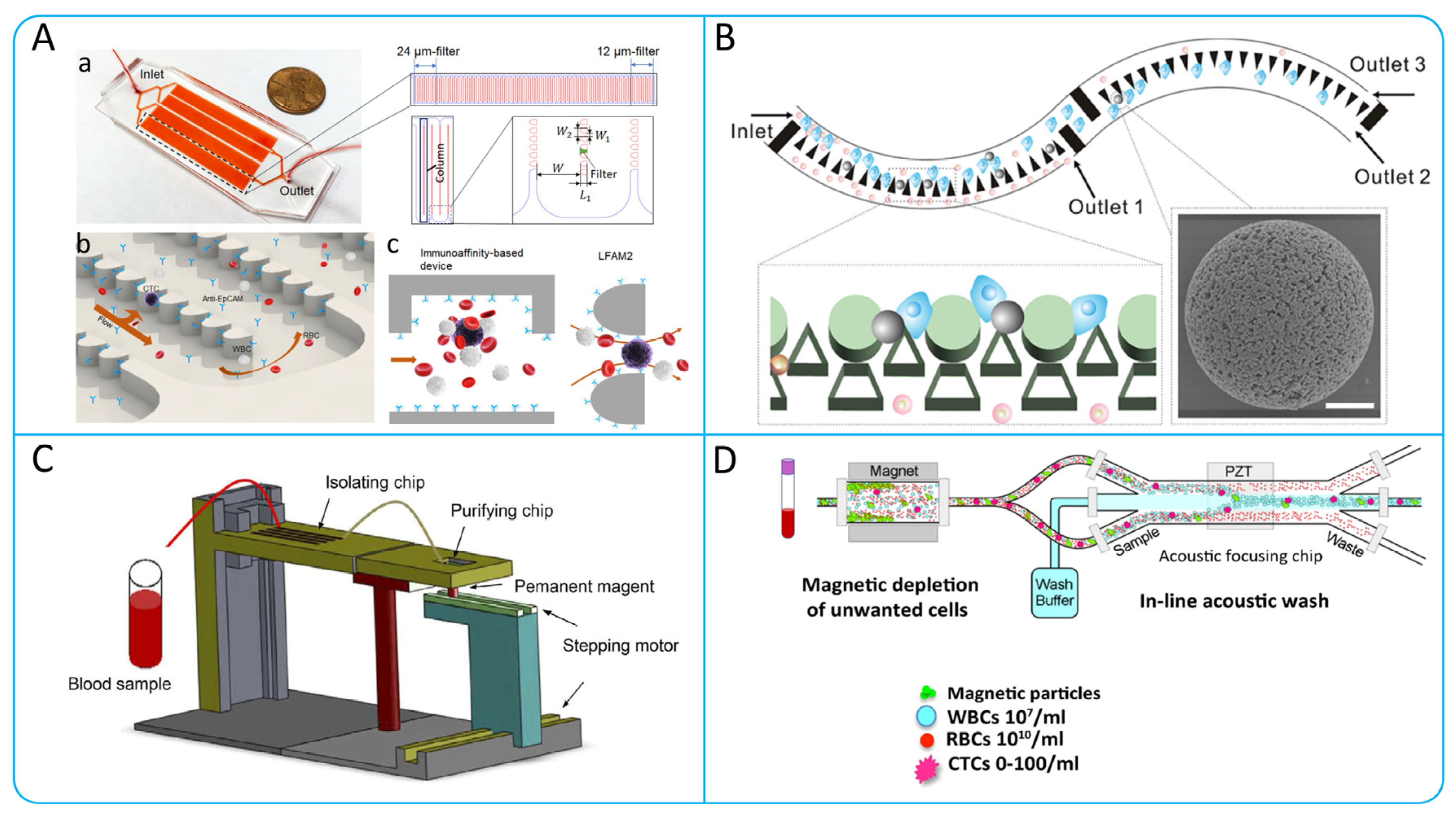
2.2. Immunomagnetic Capture
2.3. Immunofluorescence
| Isolation Technologies | Working Principle | Specific Methods | Advantages | Limitations | Cancer Cell Lines | Throughput (mL h−1) | Recovery | Reference |
|---|---|---|---|---|---|---|---|---|
| Immunocapture | Chaotic mixing | HB-Chip | Microvortex can be generated to enhance the interaction of CTCs with the antibody-coated chip surface. | CTCs are hard to release. | PC-3 | 1 | 91.8% 5.2% | [62] |
| Micropost array | OncoBean-Chip | Ultra-high-throughput processing of blood samples. | Relatively high production costs. | H1650, MCF-7 | 10 | >80% | [63] | |
| Nanomaterial | Nanovesicles | Has the advantage of a natural biomembrane. Minimizes blood cell adsorption. | Low throughput. | MCF-7, HCT116, LNCaP | 0.35 | >70% | [64] | |
| Aptamer | AP-Octopus-Chip | Multivalent aptamers improve CTCs capture efficiency. | Complicated to manufacture. Low throughput. | SW480, LNCap | 1 | 86.7%–89.4% | [48] | |
| Immunomagnetic capture | Velocity valley | VV-Chip | Flow rate of blood samples can be controlled. | CTCs are hard to release. | DU145 | 2 | 97% | [53] |
| Direct capture | High throughput. Convenient operation. | Collected CTCs have low activity. | COLO205 | 10 | 90% | [65] | ||
| Magnetic filtration | Ultra-high-throughput enrichment of CTCs. | Magnetic beads on CTCs are difficult to remove. | MCF-7 | 120 | 89% | [66] | ||
| Micromixer | μ-MixMACS | Increased WBCs depletion by generating micro-vortexes. | Lower purity of enriched CTCs. | MCF-7 | 18 | >80% | [67] | |
| Vivo enrichment | Magnetic wire | Enrichment of CTCs directly from human whole blood. | Somewhat invasive to the human body. | nH1650, NSCLC | 1200 | 49 ± 8% | [55] | |
| Immunofluorescence | Immunostaining | High sensitivity, high specificity, and visualization. | False-positive or false-negative results may occur. | MCF-7 | 2 | 94% | [61] |
3. Label-Independent Methods
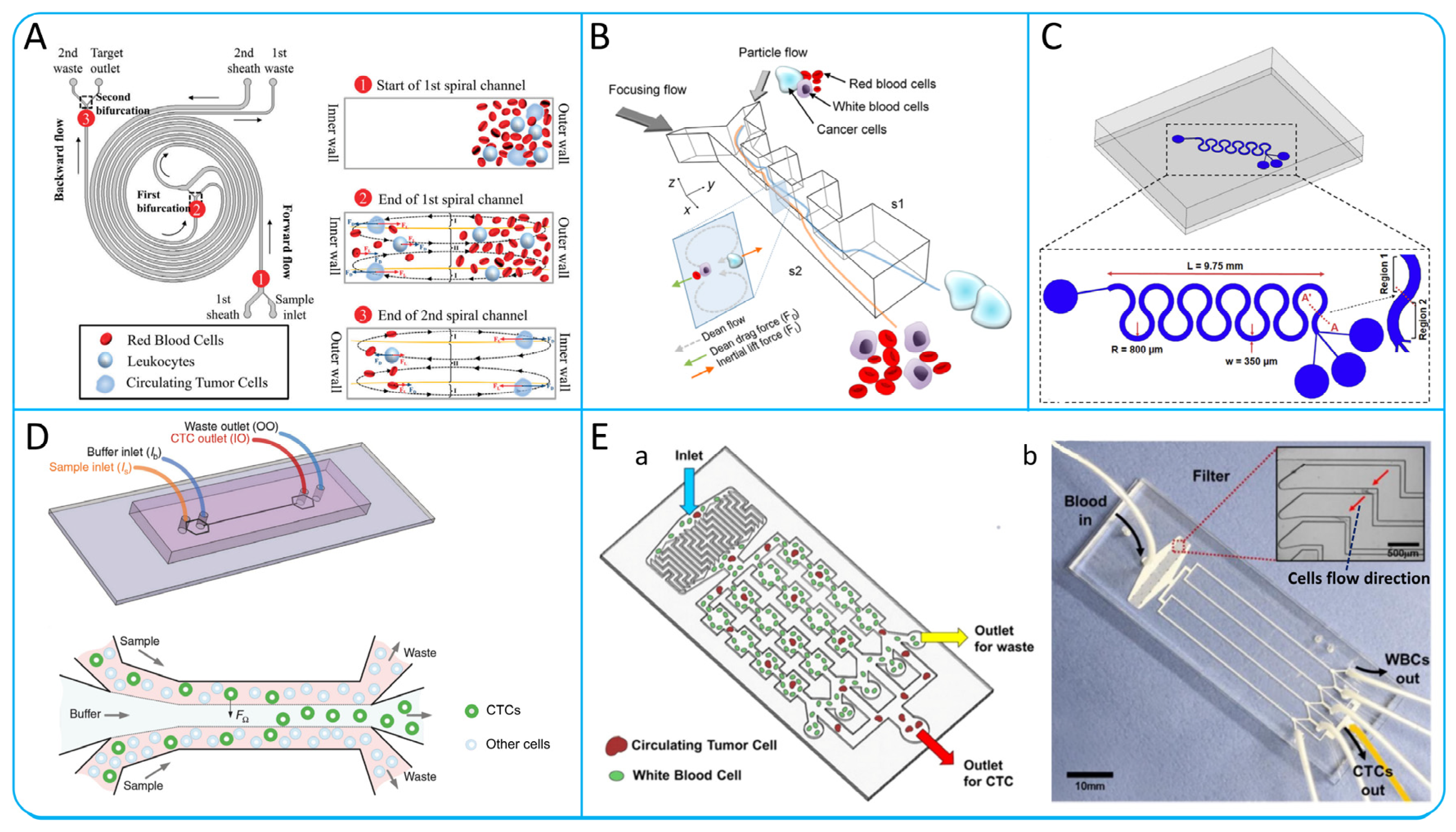
3.1. Hydrodynamic Passive Isolation
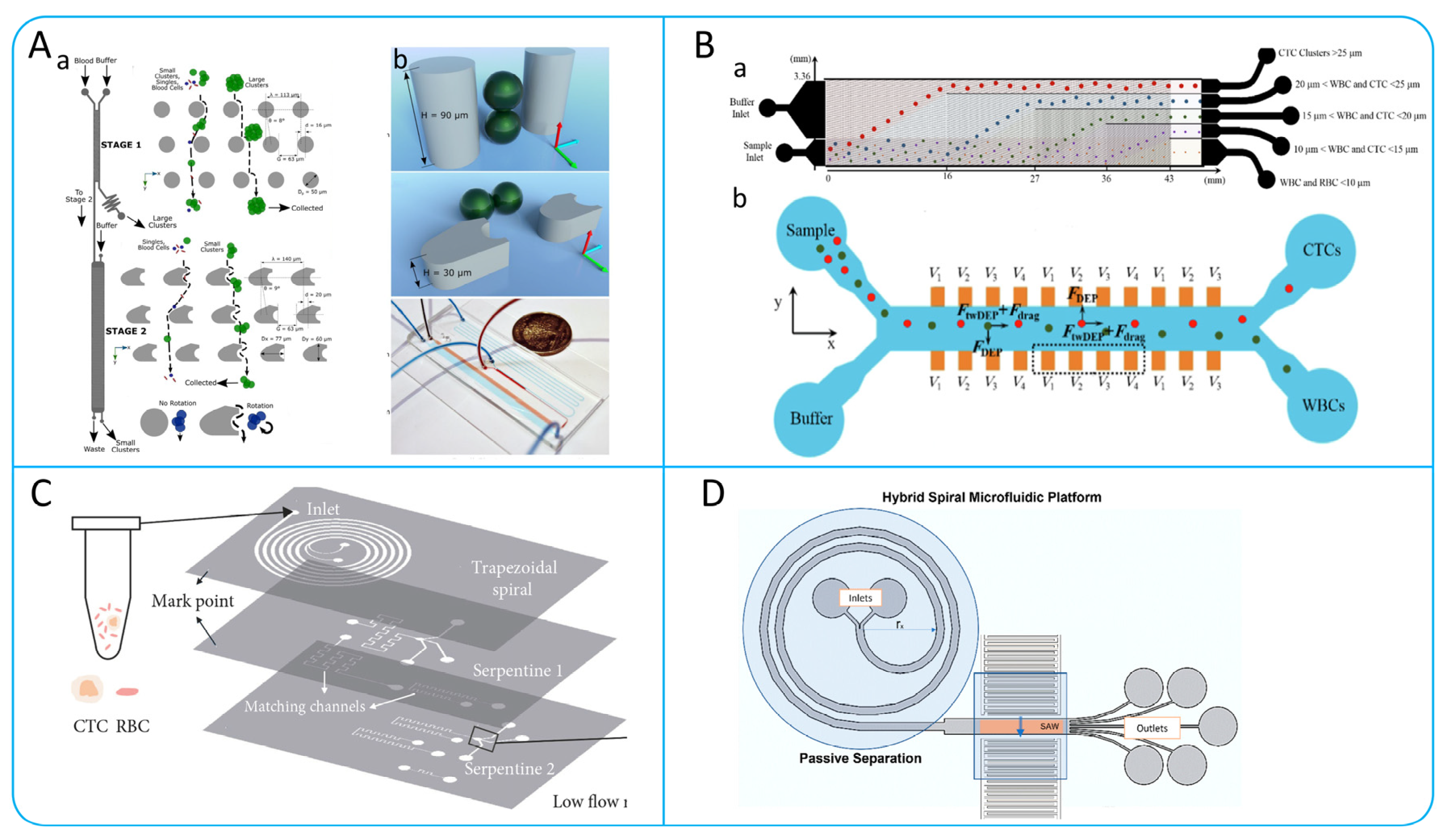
3.1.1. Dean Flow Fractionation (DFF) and Inertial Focusing
Spiral Microchannel
Contraction−Expansion Array (CEA) Microchannel
Serpentine Microchannel
Straight Microchannel
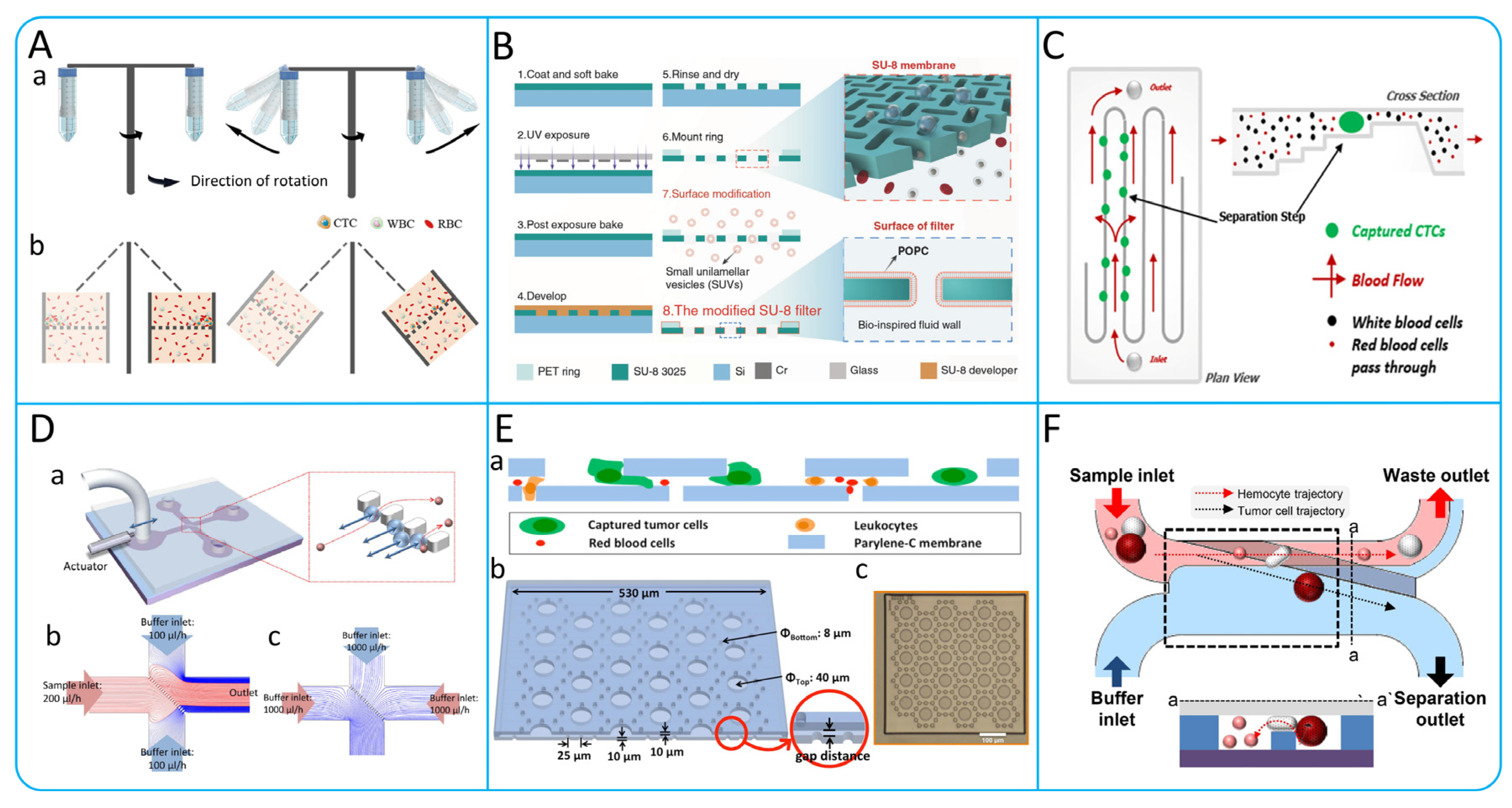
3.1.2. Microvortex
3.1.3. Deterministic Lateral Displacement

3.2. Microfiltration
3.3. Hydrodynamic Active Isolation
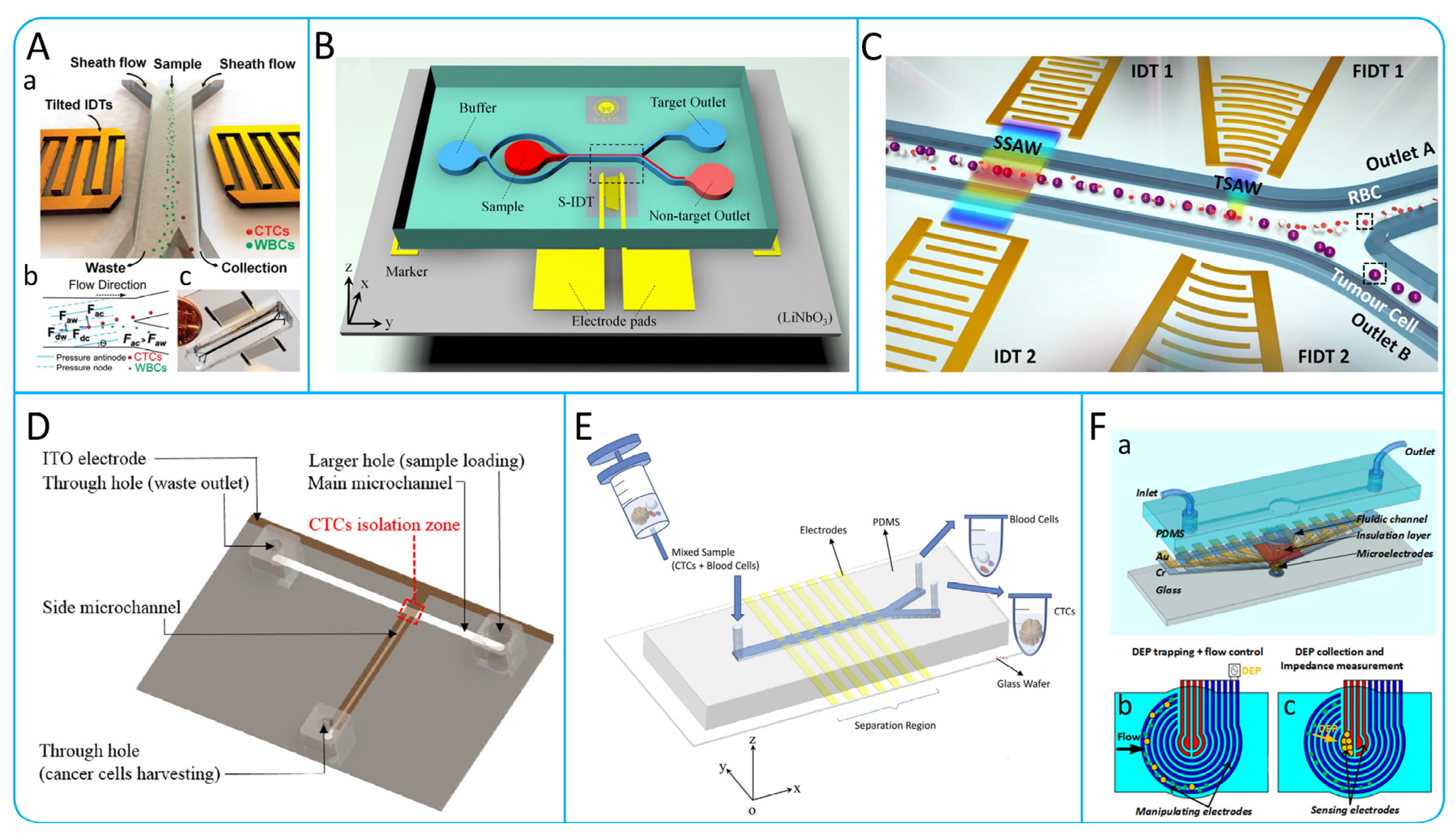
3.3.1. Acoustophoresis
3.3.2. Dielectrophoresis
| Isolation Technologies | Working Principle | Specific Methods | Advantages | Limitations | Cancer Cell Lines | Throughput (mL h−1) | Recovery | Reference |
|---|---|---|---|---|---|---|---|---|
| Hydrodynamics | Inertial focusing | DFF | Continuous collection, high throughput. | Need to dilute blood samples. | MCF-7 | 3 | 85% | [75] |
| CEA | Continuous high-throughput collection of CTCs under low shear stress. | Cell viability is greatly affected by flow velocity. Low purity. | MCF7, SK-BR-3 | 6 | 99.1% | [78] | ||
| P-MOFF | Excellent parallelizability, high throughput. | Need to dilute blood samples, which may cause loss of tumor cells. | MCF-7 | 36 | 91.60% | [79] | ||
| Symmetrical serpentine microchannel | Good parallelizability and portability. | Easy to lose smaller CTCs. | MEL | 36 | 94.90% | [82] | ||
| MFM | Without the need for complex sample preparation steps. | Higher shear stress. | NSCLC | 18 | >93% | [83] | ||
| Deterministic lateral displacement | Two-stage DLD strategy | Can be integrated online with other CTCS analysis or capture technologies. | This method has lower throughput and lower efficiency in processing samples. | MCF-7 | >0.25 | 99% | [118] | |
| Triangular Column Mirror Array | Ultra-high-throughput isolation of highly active CTCs. | CTCS clusters are hard to release. | MDA-MB-231, MCF10A | 600 | >85% | [91] | ||
| Microfluidic Vortex | Vortex Chip | Enrichment of active CTCs in a continuous, high-throughput manner. | Low isolation efficiency. | MCF-7, PC-3, A549 | 24 | 20.7% | [88] | |
| Vortex HT | Good parallelizability, high throughput. | Higher shear stress. | MCF-7 | 48 | >85% | [89] | ||
| Mechanical filtering | Size and deformation | CTCS cluster-chip | No pre-processing of blood samples required. | Lower sensitivity. | MCF-7 | 2.5 | >80% | [119] |
| Parsortix | Simple to operate with low cost | CTCs exhibit heterogeneity in size. | DU145 | 4 | 57.3% ± 8.3% | [104] | ||
| MCA | Simple microchannel structure and convenient operation. | It is inevitable to lose smaller tumor cells and may cause blockage. | NCI-H820, A549 | 2.5 | >70% | [70] | ||
| Slanted Weir | Continuous isolation of CTCs without blockage. | Accurate analysis of the inclination angle of the weir is required. | LM2, MDA-MB-231 | 2.5–3.8 | 97% | [108] | ||
| Microfluidic ratchet mechanism | CTCs captured with 25 times more than traditional Cell Search systems. | Relatively low throughput. | UM-UC-13 | 1 | >90% | [71] | ||
| 3D Palladium Filter | Can withstand high pressures. | Pores fusion and low-density distribution can reduce capture efficiency. | NCI N-87, COLM-5 | 120–150 | >85% | [100] | ||
| Dielectrophoresis | DEP | DEP-FFF | Continuous processing of whole blood cells with high sensitivity. | The dielectric properties may change due to treatment plan. | MDA-MB-231 | 1.2 | 70%–80% | [114] |
| ODEP | This system can achieve up to 100% purity of CTCs. | Low throughput and low recovery. | PC-3 | 0.15 | 41.5% | [115] | ||
| DEP-MOFF | Can continuously, quickly, and high-purity isolate CTCs. | During the isolation process, the system can cause cell loss. | MCF-7 | 7.5 | >75.18% | [120] | ||
| Acoustophoresis | Aoustic radiation force | Acoustophoresis Chip | Label-free isolation with high sensitivity. | Low throughput and lack of long-term stability of equipment. | DU145 | 4.2 | >83.7% | [109] |
| taSSAW | Excellent biocompatibility, simple design, and label free automated operation. | Any instability of the pressure source may lead to deviations. | MCF-7, UACC903 | 1.2 | >83% | [110] | ||
| Hybrid PDMS–glass microchannel | Maintaining the integrity, function and viability of CTCs. | The equipment process is complex, and the cost is high. | PC-3, LnCaP | 7.5 | >86% | [112] |
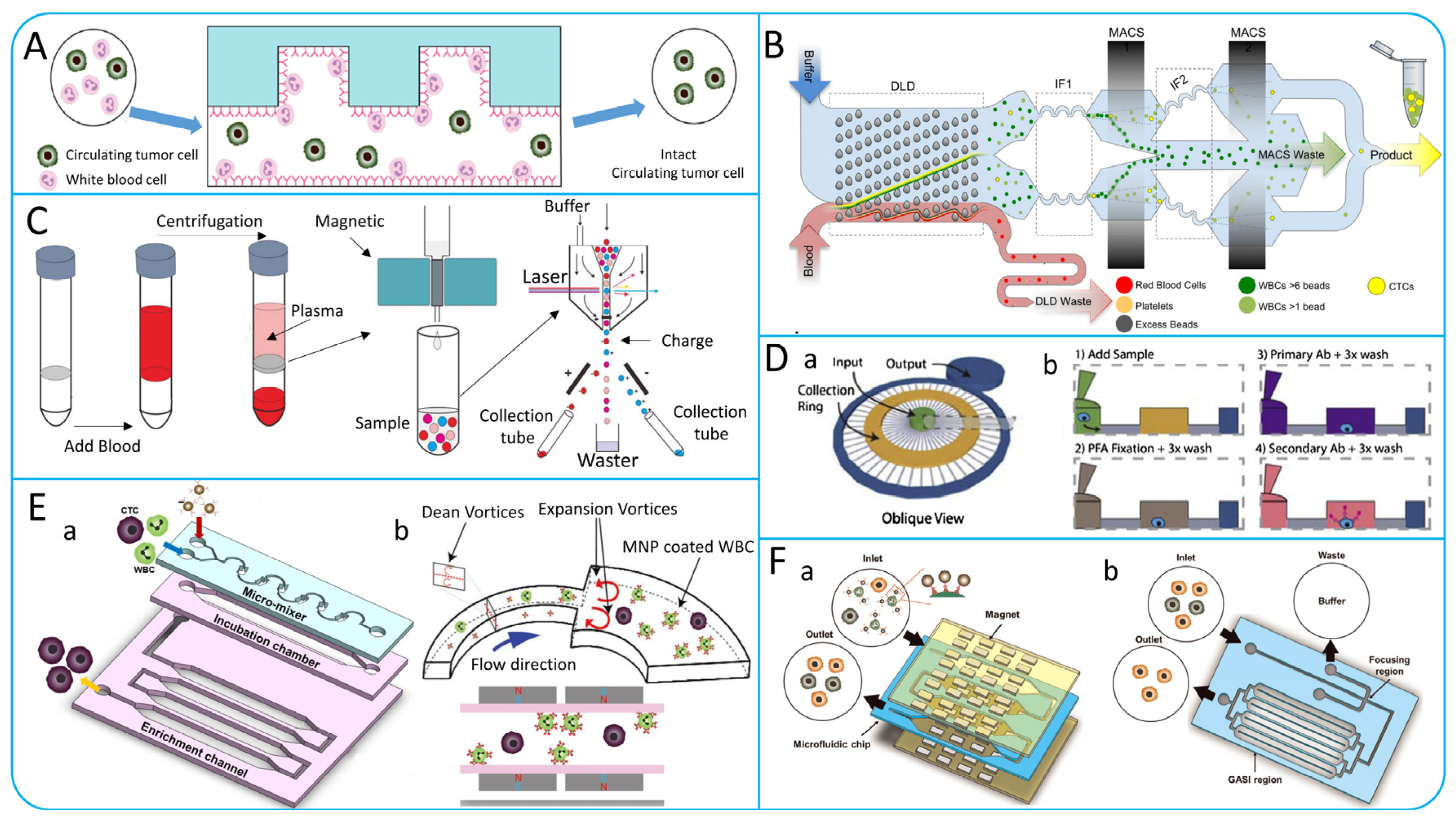
4. Multi-Step CTCs Isolation Methods
4.1. Multi-Step Isolation Based on Physical Principles
4.2. Multi-Step Isolation Based on Biological and Physical Principles
5. Negative Enrichment of CTCs
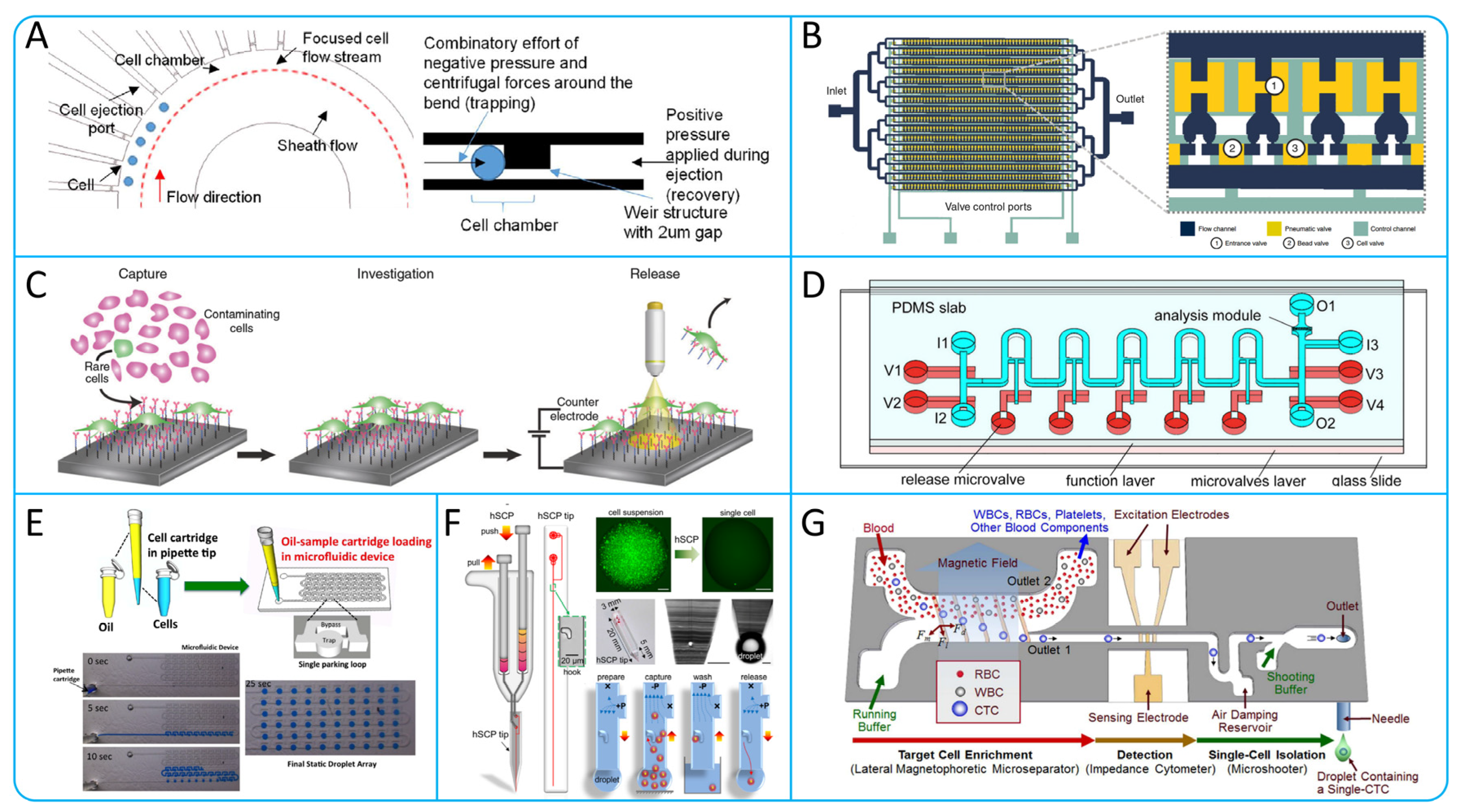
6. Microfluidic Technologies for Isolating Single CTCs
7. Specific Clinical Application of CTCs
8. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pantel, K.; Speicher, M.R. The biology of circulating tumor cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Asghar, W.; Wan, Y.; Ilyas, A.; Bachoo, R.; Kim, Y.-t.; Iqbal, S.M. Electrical fingerprinting, 3D profiling and detection of tumor cells with solid-state micropores. Lab A Chip 2012, 12, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Harouaka, R.A.; Zhou, M.-D.; Yeh, Y.-T.; Khan, W.J.; Das, A.; Liu, X.; Christ, C.C.; Dicker, D.T.; Baney, T.S.; Kaifi, J.T.; et al. Flexible Micro Spring Array Device for High-Throughput Enrichment of Viable Circulating Tumor Cells. Clin. Chem. 2014, 60, 323–333. [Google Scholar] [CrossRef]
- Strilic, B.; Offermanns, S. Intravascular survival and extravasation of tumor cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Oliver, T.G. Partners in Crime: Neutrophil&–CTC Collusion in Metastasis. Trends Immunol. 2019, 40, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Heeke, S.; Mograbi, B.; Alix-Panabières, C.; Hofman, P. Never travel alone: The crosstalk of circulating tumor cells and the blood microenvironment. Cells 2019, 8, 714. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Delaunay, M.; Lusque, A.; Boubekeur, N.; Rouquette, I.; Clermont, E.; Mourlanette, J.; Gouin, S.; Dormoy, I.; Favre, G.; et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018, 120, 108–112. [Google Scholar] [CrossRef]
- Ilié, M.; Szafer-Glusman, E.; Hofman, V.; Chamorey, E.; Lalvée, S.; Selva, E.; Leroy, S.; Marquette, C.H.; Kowanetz, M.; Hedge, P.; et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann. Oncol. 2018, 29, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Alanko, J.; Mai, A.; Jacquemet, G.; Schauer, K.; Kaukonen, R.; Saari, M.; Goud, B.; Ivaska, J. Integrin endosomal signalling suppresses anoikis. Nat. Cell Biol. 2015, 17, 1412–1421. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Yelle, N.; Venugopal, C.; Singh, S.K. EMT: Mechanisms and therapeutic implications. Pharmacol. Ther. 2018, 182, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P.G.; Moreno-Bueno, G.; Portillo, F.; Cano, A. EMT: Present and future in clinical oncology. Mol. Oncol. 2017, 11, 718–738. [Google Scholar] [CrossRef]
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.-Y.; Bidard, F.-C. Circulating tumor cells: Clinical validity and utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kim, C.-J.; Sunkara, V.; Kim, M.-H.; Cho, Y.-K. Liquid biopsy in lung cancer: Clinical applications of circulating biomarkers (CTCs and ctDNA). Micromachines 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Ozbey, A.; Karimzadehkhouei, M.; Kocaturk, N.M.; Bilir, S.E.; Kutlu, O.; Gozuacik, D.; Kosar, A. Inertial focusing of cancer cell lines in curvilinear microchannels. Micro Nano Eng. 2019, 2, 53–63. [Google Scholar] [CrossRef]
- Girotti, M.R.; Gremel, G.; Lee, R.; Galvani, E.; Rothwell, D.; Viros, A.; Mandal, A.K.; Lim, K.H.J.; Saturno, G.; Furney, S.J. Application of sequencing, liquid biopsies, and patient-derived xenografts for personalized medicine in melanoma. Cancer Discov. 2016, 6, 286–299. [Google Scholar] [CrossRef]
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular analysis of circulating tumour cells—Biology and biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144. [Google Scholar] [CrossRef]
- Mitra, A.; Mishra, L.; Li, S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015, 6, 10697. [Google Scholar] [CrossRef]
- Hajba, L.; Guttman, A. Circulating tumor-cell detection and capture using microfluidic devices. TrAC Trends Anal. Chem. 2014, 59, 9–16. [Google Scholar] [CrossRef]
- Lei, K.F. A Review on Microdevices for Isolating Circulating Tumor Cells. Micromachines 2020, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wu, M.; Jia, Y.; Mou, R.; Li, X. EpCAM as a novel biomarker for survivals in prostate cancer patients. Front. Cell Dev. Biol. 2022, 10, 843604. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.G.; Fernández-Baldo, M.A.; Serrano, M.J.; Messina, G.A.; Lorente, J.A.; Raba, J. Epithelial cancer biomarker EpCAM determination in peripheral blood samples using a microfluidic immunosensor based in silver nanoparticles as platform. Sens. Actuators B Chem. 2015, 221, 248–256. [Google Scholar] [CrossRef]
- Mishima, Y.; Matsusaka, S.; Chin, K.; Mikuniya, M.; Minowa, S.; Takayama, T.; Shibata, H.; Kuniyoshi, R.; Ogura, M.; Terui, Y. Detection of HER2 amplification in circulating tumor cells of HER2-negative gastric cancer patients. Target. Oncol. 2017, 12, 341–351. [Google Scholar] [CrossRef]
- Perrier, A.; Gligorov, J.; Lefèvre, G.; Boissan, M. The extracellular domain of Her2 in serum as a biomarker of breast cancer. Lab. Investig. 2018, 98, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Backes, F.J.; Walker, C.J.; Goodfellow, P.J.; Hade, E.M.; Agarwal, G.; Mutch, D.; Cohn, D.E.; Suarez, A.A. Estrogen receptor-alpha as a predictive biomarker in endometrioid endometrial cancer. Gynecol. Oncol. 2016, 141, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H. Estrogen receptors as the novel therapeutic biomarker in non-small cell lung cancer. World J. Clin. Oncol. 2014, 5, 1020. [Google Scholar] [CrossRef]
- Todenhoefer, T.; Hennenlotter, J.; Feyerabend, S.; Aufderklamm, S.; Mischinger, J.; Kühs, U.; Gerber, V.; Fetisch, J.; Schilling, D.; Hauch, S. Preliminary experience on the use of the Adnatest® system for detection of circulating tumor cells in prostate cancer patients. Anticancer Res. 2012, 32, 3507–3513. [Google Scholar]
- Wu, F.; Chen, X.; Wang, S.; Zhou, R.; Wang, C.; Yu, L.; Zheng, J.; Yang, C.; Hou, X. Green Synthesized Liquid-like Dynamic Polymer Chains with Decreased Nonspecific Adhesivity for High-Purity Capture of Circulating Tumor Cells. CCS Chem. 2023, 6, 507–517. [Google Scholar] [CrossRef]
- Khetani, S.; Mohammadi, M.; Nezhad, A.S. Filter-based isolation, enrichment, and characterization of circulating tumor cells. Biotechnol. Bioeng. 2018, 115, 2504–2529. [Google Scholar] [CrossRef]
- Wu, F.; Kong, X.; Liu, Y.; Wang, S.; Chen, Z.; Hou, X. Microfluidic-based isolation of circulating tumor cells with high-efficiency and high-purity. Chin. Chem. Lett. 2024, 35, 109754. [Google Scholar] [CrossRef]
- Dharmasiri, U.; Balamurugan, S.; Adams, A.A.; Okagbare, P.I.; Obubuafo, A.; Soper, S.A. Highly efficient capture and enumeration of low abundance prostate cancer cells using prostate-specific membrane antigen aptamers immobilized to a polymeric microfluidic device. Electrophoresis 2009, 30, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, S.; Sun, Y.; Tan, T.; Zheng, J.; Zhou, Y.; Yu, S.; Zhang, J.-R.; Zhu, J.-J. A Detachable Magnetic Nanodevice for the Efficient Capture and Subtype Identification of Circulating Tumor Cells. Adv. Funct. Mater. 2024, 2401333. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, C.; Xu, L.; Zhang, Z.; Li, X.; Hu, H.; Cheng, S.; Zhou, W.; Huang, M.; Fong, A.; et al. Enhanced and Differential Capture of Circulating Tumor Cells from Lung Cancer Patients by Microfluidic Assays Using Aptamer Cocktail. Small 2016, 12, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.G.; Abate, M.F.; Song, Y.; Zhu, Z.; Yan, F.; Xu, Y.; Wang, X.; Li, Q.; Yang, C. Isolation, Detection, and Antigen-Based Profiling of Circulating Tumor Cells Using a Size-Dictated Immunocapture Chip. Angew. Chem. Int. Ed. 2017, 56, 10681–10685. [Google Scholar] [CrossRef] [PubMed]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic Mixer for Microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Wang, S.; Thomas, A.; Lee, E.; Yang, S.; Cheng, X.; Liu, Y. Highly efficient and selective isolation of rare tumor cells using a microfluidic chip with wavy-herringbone micro-patterned surfaces. Analyst 2016, 141, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Glia, A.; Deliorman, M.; Sukumar, P.; Janahi, F.K.; Samara, B.; Brimmo, A.T.; Qasaimeh, M.A. Herringbone Microfluidic Probe for Multiplexed Affinity-Capture of Prostate Circulating Tumor Cells. Adv. Mater. Technol. 2021, 6, 2100053. [Google Scholar] [CrossRef]
- Chen, G.D.; Fachin, F.; Fernandez-Suarez, M.; Wardle, B.L.; Toner, M. Nanoporous elements in microfluidics for multiscale manipulation of bioparticles. Small 2011, 7, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, K.; Liu, J.; Yu, Z.T.F.; Xu, X.; Zhao, L.; Lee, T.; Lee, E.K.; Reiss, J.; Lee, Y.K. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew. Chem. 2011, 123, 3140–3144. [Google Scholar] [CrossRef]
- Chen, G.D.; Fachin, F.; Colombini, E.; Wardle, B.L.; Toner, M. Nanoporous micro-element arrays for particle interception in microfluidic cell separation. Lab A Chip 2012, 12, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Chen, J.-F.; Lu, Y.-T.; Zhang, Y.; Song, J.; Hou, S.; Ke, Z.; Tseng, H.-R. Nanostructure Embedded Microchips for Detection, Isolation, and Characterization of Circulating Tumor Cells. Acc. Chem. Res. 2014, 47, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Shanker, A.; Wang, Y.; Kozminsky, M.; Jin, Q.; Palanisamy, N.; Burness, M.L.; Azizi, E.; Simeone, D.M.; Wicha, M.S. Tunable thermal-sensitive polymer-graphene oxide composite for efficient capture and release of viable circulating tumor cells. Adv. Mater. 2016, 28, 4891. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhao, L.; Shen, Q.; Yu, J.; Ng, C.; Kong, X.; Wu, D.; Song, M.; Shi, X.; Xu, X. Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells. Angew. Chem. 2013, 125, 3463–3467. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, X.; Guo, C.; Ma, B.; Liu, Z.; Du, Y.; Wang, B.; Li, N.; Huang, X.; Ou, L. Genetically Engineered Cell Membrane-Coated Magnetic Nanoparticles for High-Performance Isolation of Circulating Tumor Cells. Adv. Funct. Mater. 2024, 34, 2304426. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Y.; Huang, M.; Wang, W.; Wang, Y.; Cheng, J.; Lei, Z.; Zhu, Z.; Yang, C. Bioinspired Engineering of a Multivalent Aptamer-Functionalized Nanointerface to Enhance the Capture and Release of Circulating Tumor Cells. Angew. Chem. Int. Ed. 2019, 58, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Chen, T.; Tan, W.; Fan, Z.H. Multivalent DNA Nanospheres for Enhanced Capture of Cancer Cells in Microfluidic Devices. ACS Nano 2013, 7, 7067–7076. [Google Scholar] [CrossRef]
- Pipatwatcharadate, C.; Iyer, P.R.; Pissuwan, D. Recent Update Roles of Magnetic Nanoparticles in Circulating Tumor Cell (CTC)/Non-CTC Separation. Pharmaceutics 2023, 15, 2482. [Google Scholar] [CrossRef]
- Riethdorf, S.; Fritsche, H.; Müller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Jänicke, F.; et al. Detection of Circulating Tumor Cells in Peripheral Blood of Patients with Metastatic Breast Cancer: A Validation Study of the CellSearch System. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Bai, M.; Yang, K.; Huo, Y.; Wang, Z.; Tian, X.; Cheng, K.; Zhang, F.; Yang, Y.; Dong, M.; et al. Size effect of dynamic bioactive magnetic particles on regulated isolation of tumor cells. Appl. Surf. Sci. 2024, 660, 159959. [Google Scholar] [CrossRef]
- Mohamadi, R.M.; Ivanov, I.; Stojcic, J.; Nam, R.K.; Sargent, E.H.; Kelley, S.O. Sample-to-Answer Isolation and mRNA Profiling of Circulating Tumor Cells. Anal. Chem. 2015, 87, 6258–6264. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.-Y.; Wang, X.; Sweet, E.; Liu, N.; Gong, X.; Lin, L. 3D printed microfluidic devices for circulating tumor cells (CTCs) isolation. Biosens. Bioelectron. 2020, 150, 111900. [Google Scholar] [CrossRef] [PubMed]
- Vermesh, O.; Aalipour, A.; Ge, T.J.; Saenz, Y.; Guo, Y.; Alam, I.S.; Park, S.-m.; Adelson, C.N.; Mitsutake, Y.; Vilches-Moure, J.; et al. An intravascular magnetic wire for the high-throughput retrieval of circulating tumour cells in vivo. Nat. Biomed. Eng. 2018, 2, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Kefayat, A.; Sartipzadeh, O.; Molaabasi, F.; Amiri, M.; Gholami, R.; Mirzadeh, M.; Shokati, F.; Khandaei, M.; Ghahremani, F.; Poursamar, S.A.; et al. Microfluidic System Consisting of a Magnetic 3D-Printed Microchannel Filter for Isolation and Enrichment of Circulating Tumor Cells Targeted by Anti-HER2/MOF@Ferrite Core–Shell Nanostructures: A Theranostic CTC Dialysis System. Anal. Chem. 2024, 96, 4377–4384. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Wang, Y.; Oliver, C.R.; Thamm, D.H.; Cooling, L.; Paoletti, C.; Smith, K.J.; Nagrath, S.; Hayes, D.F. A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nat. Commun. 2019, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Sato, K. Microdevice in Cellular Pathology: Microfluidic Platforms for Fluorescence in situ Hybridization and Analysis of Circulating Tumor Cells. Anal. Sci. 2015, 31, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.Y.; Spence, C.; Scherer, A.; Arnold, F.H.; Quake, S.R. A microfabricated fluorescence-activated cell sorter. Nat. Biotechnol. 1999, 17, 1109–1111. [Google Scholar] [CrossRef]
- Navin, N.; Kendall, J.; Troge, J.; Andrews, P.; Rodgers, L.; McIndoo, J.; Cook, K.; Stepansky, A.; Levy, D.; Esposito, D.; et al. Tumour evolution inferred by single-cell sequencing. Nature 2011, 472, 90–94. [Google Scholar] [CrossRef]
- Zhao, M.; Schiro, P.G.; Kuo, J.S.; Koehler, K.M.; Sabath, D.E.; Popov, V.; Feng, Q.; Chiu, D.T. An Automated High-Throughput Counting Method for Screening Circulating Tumor Cells in Peripheral Blood. Anal. Chem. 2013, 85, 2465–2471. [Google Scholar] [CrossRef] [PubMed]
- Stott, S.L.; Hsu, C.-H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef] [PubMed]
- Murlidhar, V.; Zeinali, M.; Grabauskiene, S.; Ghannad-Rezaie, M.; Wicha, M.S.; Simeone, D.M.; Ramnath, N.; Reddy, R.M.; Nagrath, S. A Radial Flow Microfluidic Device for Ultra-High-Throughput Affinity-Based Isolation of Circulating Tumor Cells. Small 2014, 10, 4895–4904. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, H.; Qu, X.; Shi, X.; Yang, J.; Huang, M.; Zhang, J.; Zhang, H.; Song, J.; Zhu, L.; et al. Fluidic Multivalent Membrane Nanointerface Enables Synergetic Enrichment of Circulating Tumor Cells with High Efficiency and Viability. J. Am. Chem. Soc. 2020, 142, 4800–4806. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Huang, Y.-Y.; Lane, N.; Huebschman, M.; Uhr, J.W.; Frenkel, E.P.; Zhang, X. Microchip-based immunomagnetic detection of circulating tumor cells. Lab A Chip 2011, 11, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-L.; Huang, W.; Jalal, S.I.; Chan, B.-D.; Mahmood, A.; Shahda, S.; O’Neil, B.H.; Matei, D.E.; Savran, C.A. Circulating tumor cell detection using a parallel flow micro-aperture chip system. Lab A Chip 2015, 15, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Hyun, K.-A.; Kim, S.-I.; Jung, H.-I. An integrated microfluidic chip for one-step isolation of circulating tumor cells. Sens. Actuators B Chem. 2017, 238, 1144–1150. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, T.; Iinuma, H.; Shimamura, T.; Imoto, S.; Sugimachi, K.; Ishii, H.; Iwatsuki, M.; Ota, D.; Ohkuma, M.; Iwaya, T. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial–mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 2013, 73, 2059–2069. [Google Scholar] [CrossRef]
- Yagi, S.; Koh, Y.; Akamatsu, H.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; Endo, K.; Nakamura, S.; Higuchi, M.; et al. Development of an automated size-based filtration system for isolation of circulating tumor cells in lung cancer patients. PLoS ONE 2017, 12, e0179744. [Google Scholar] [CrossRef]
- Park, E.S.; Jin, C.; Guo, Q.; Ang, R.R.; Duffy, S.P.; Matthews, K.; Azad, A.; Abdi, H.; Todenhöfer, T.; Bazov, J.; et al. Continuous Flow Deformability-Based Separation of Circulating Tumor Cells Using Microfluidic Ratchets. Small 2016, 12, 1909–1919. [Google Scholar] [CrossRef]
- Gascoyne, P.R.C.; Shim, S. Isolation of Circulating Tumor Cells by Dielectrophoresis. Cancers 2014, 6, 545–579. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating tumor cell technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Luo, G.; Du, W.; Kong, T.; Liu, C.; Liu, Z. Recent Advances in Microfluidic Platforms Applied in Cancer Metastasis: Circulating Tumor Cells’ (CTCs) Isolation and Tumor-On-A-Chip. Small 2020, 16, 1903899. [Google Scholar] [CrossRef]
- Hou, H.W.; Warkiani, M.E.; Khoo, B.L.; Li, Z.R.; Soo, R.A.; Tan, D.S.-W.; Lim, W.-T.; Han, J.; Bhagat, A.A.S.; Lim, C.T. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 2013, 3, 1259. [Google Scholar] [CrossRef]
- Li, B.-W.; Wei, K.; Liu, Q.-Q.; Sun, X.-G.; Su, N.; Li, W.-M.; Shang, M.-Y.; Li, J.-M.; Liao, D.; Li, J. Enhanced separation efficiency and purity of circulating tumor cells based on the combined effects of double sheath fluids and inertial focusing. Front. Bioeng. Biotechnol. 2021, 9, 750444. [Google Scholar] [CrossRef]
- Abdulla, A.; Liu, W.; Gholamipour-Shirazi, A.; Sun, J.; Ding, X. High-Throughput Isolation of Circulating Tumor Cells Using Cascaded Inertial Focusing Microfluidic Channel. Anal. Chem. 2018, 90, 4397–4405. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Shin, J.H.; Bae, C.Y.; Choi, S.; Park, J.-K. Label-Free Cancer Cell Separation from Human Whole Blood Using Inertial Microfluidics at Low Shear Stress. Anal. Chem. 2013, 85, 6213–6218. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.-A.; Kwon, K.; Han, H.; Kim, S.-I.; Jung, H.-I. Microfluidic flow fractionation device for label-free isolation of circulating tumor cells (CTCs) from breast cancer patients. Biosens. Bioelectron. 2013, 40, 206–212. [Google Scholar] [CrossRef]
- Park, J.-S.; Jung, H.-I. Multiorifice Flow Fractionation: Continuous Size-Based Separation of Microspheres Using a Series of Contraction/Expansion Microchannels. Anal. Chem. 2009, 81, 8280–8288. [Google Scholar] [CrossRef]
- Sim, T.S.; Kwon, K.; Park, J.C.; Lee, J.-G.; Jung, H.-I. Multistage-multiorifice flow fractionation (MS-MOFF): Continuous size-based separation of microspheres using multiple series of contraction/expansion microchannels. Lab A Chip 2011, 11, 93–99. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Sluyter, R.; Li, W.; Alici, G.; Nguyen, N.-T. Inertial particle separation by differential equilibrium positions in a symmetrical serpentine micro-channel. Sci. Rep. 2014, 4, 4527. [Google Scholar] [CrossRef]
- Zhou, J.; Kulasinghe, A.; Bogseth, A.; O’Byrne, K.; Punyadeera, C.; Papautsky, I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019, 5, 8. [Google Scholar] [CrossRef]
- Dhar, M.; Wong, J.; Karimi, A.; Che, J.; Renier, C.; Matsumoto, M.; Triboulet, M.; Garon, E.B.; Goldman, J.W.; Rettig, M.B.; et al. High efficiency vortex trapping of circulating tumor cells. Biomicrofluidics 2015, 9, 064116. [Google Scholar] [CrossRef] [PubMed]
- Renier, C.; Pao, E.; Che, J.; Liu, H.E.; Lemaire, C.A.; Matsumoto, M.; Triboulet, M.; Srivinas, S.; Jeffrey, S.S.; Rettig, M.; et al. Label-free isolation of prostate circulating tumor cells using Vortex microfluidic technology. NPJ Precis. Oncol. 2017, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.; Seth, P.; Bhat, R.; Sen, P. Vortex chip incorporating an orthogonal turn for size-based isolation of circulating cells. Anal. Chim. Acta 2021, 1159, 338423. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Papautsky, I. Vortex-aided inertial microfluidic device for continuous particle separation with high size-selectivity, efficiency, and purity. Biomicrofluidics 2013, 7, 044119. [Google Scholar] [CrossRef] [PubMed]
- Sollier, E.; Go, D.E.; Che, J.; Gossett, D.R.; O’Byrne, S.; Weaver, W.M.; Kummer, N.; Rettig, M.; Goldman, J.; Nickols, N.; et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab A Chip 2014, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yu, V.; Dhar, M.; Renier, C.; Matsumoto, M.; Heirich, K.; Garon, E.B.; Goldman, J.; Rao, J.; Sledge, G.W. Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology. Oncotarget 2016, 7, 12748. [Google Scholar] [CrossRef]
- Raillon, C.; Che, J.; Thill, S.; Duchamp, M.; Desbiolles, B.X.E.; Millet, A.; Sollier, E.; Renaud, P. Toward Microfluidic Label-Free Isolation and Enumeration of Circulating Tumor Cells from Blood Samples. Cytom. Part A 2019, 95, 1085–1095. [Google Scholar] [CrossRef]
- Loutherback, K.; D’Silva, J.; Liu, L.; Wu, A.; Austin, R.H.; Sturm, J.C. Deterministic separation of cancer cells from blood at 10 mL/min. AIP Adv. 2012, 2, 042107. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Kumar, R.; Panwala, F.C.; Shakeel, P.M. Design and analysis of an optimized microfluidic channel for isolation of circulating tumor cells using deterministic lateral displacement technique. Complex Intell. Syst. 2020, 6, 711–720. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kumar, R.; Al-Turjman, F. A Novel Approach for Tuning of Fluidic Resistance in Deterministic Lateral Displacement Array for Enhanced Separation of Circulating Tumor Cells. Cogn. Comput. 2022, 14, 1660–1676. [Google Scholar] [CrossRef]
- Tang, H.; Niu, J.; Pan, X.; Jin, H.; Lin, S.; Cui, D. Topology optimization based deterministic lateral displacement array design for cell separation. J. Chromatogr. A 2022, 1679, 463384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, R.; Li, Y.; Liu, J.; Wang, P.; Xia, X.; Qin, L. Integrated Microfluidic Chip for Efficient Isolation and Deformability Analysis of Circulating Tumor Cells. Adv. Biosyst. 2018, 2, 1800200. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, F.; Du, J.; Shu, W.; Feng, H.; Xu, X.; Chen, Y. Rapid isolation of cancer cells using microfluidic deterministic lateral displacement structure. Biomicrofluidics 2013, 7, 011801. [Google Scholar] [CrossRef]
- Wei, J.; Song, H.; Shen, Z.; He, Y.; Xu, X.; Zhang, Y.; Li, B.N. Numerical Study of Pillar Shapes in Deterministic Lateral Displacement Microfluidic Arrays for Spherical Particle Separation. IEEE Trans. NanoBioscience 2015, 14, 660–667. [Google Scholar] [CrossRef]
- Yamada, M.; Seko, W.; Yanai, T.; Ninomiya, K.; Seki, M. Slanted, asymmetric microfluidic lattices as size-selective sieves for continuous particle/cell sorting. Lab A Chip 2017, 17, 304–314. [Google Scholar] [CrossRef]
- Ahasan, K.; Landry, C.M.; Chen, X.; Kim, J.-H. Effect of angle-of-attacks on deterministic lateral displacement (DLD) with symmetric airfoil pillars. Biomed. Microdevices 2020, 22, 42. [Google Scholar] [CrossRef]
- Yusa, A.; Toneri, M.; Masuda, T.; Ito, S.; Yamamoto, S.; Okochi, M.; Kondo, N.; Iwata, H.; Yatabe, Y.; Ichinosawa, Y.; et al. Development of a New Rapid Isolation Device for Circulating Tumor Cells (CTCs) Using 3D Palladium Filter and Its Application for Genetic Analysis. PLoS ONE 2014, 9, e88821. [Google Scholar] [CrossRef]
- Han, J.; Lu, C.; Shen, M.; Sun, X.; Mo, X.; Yang, G. Fast, Reusable, Cell Uniformly Distributed Membrane Filtration Device for Separation of Circulating Tumor Cells. ACS Omega 2022, 7, 20761–20767. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Zhang, Z.; Guo, Z.; Zhang, C.; Wang, Z.; Guo, Q.; Li, C.; Li, C.; Yao, J.; et al. Integrated microdevice with a windmill-like hole array for the clog-free, efficient, and self-mixing enrichment of circulating tumor cells. Microsyst. Nanoeng. 2022, 8, 23. [Google Scholar] [CrossRef]
- Zhou, M.-D.; Hao, S.; Williams, A.J.; Harouaka, R.A.; Schrand, B.; Rawal, S.; Ao, Z.; Brenneman, R.; Gilboa, E.; Lu, B.; et al. Separable Bilayer Microfiltration Device for Viable Label-free Enrichment of Circulating Tumour Cells. Sci. Rep. 2014, 4, 7392. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mao, X.; Imrali, A.; Syed, F.; Mutsvangwa, K.; Berney, D.; Cathcart, P.; Hines, J.; Shamash, J.; Lu, Y.-J. Optimization and Evaluation of a Novel Size Based Circulating Tumor Cell Isolation System. PLoS ONE 2015, 10, e0138032. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, S.; Lee, J.; Choi, J.; Kim, R.-K.; Lee, S.-J.; Sul, O.; Lee, S.-B. Clogging-free microfluidics for continuous size-based separation of microparticles. Sci. Rep. 2016, 6, 26531. [Google Scholar] [CrossRef] [PubMed]
- Beattie, W.; Qin, X.; Wang, L.; Ma, H. Clog-free cell filtration using resettable cell traps. Lab A Chip 2014, 14, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, L.; Wang, J.-c.; Tu, Q.; Ren, L.; Wang, Y.; Wang, J. Dynamic trapping and high-throughput patterning of cells using pneumatic microstructures in an integrated microfluidic device. Lab A Chip 2012, 12, 1702–1709. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, J.; Ra, M.; Gwon, H.; Lee, S.; Kim, M.Y.; Yoo, K.-C.; Sul, O.; Kim, C.G.; Kim, W.-Y.; et al. Continuous Separation of Circulating Tumor Cells from Whole Blood Using a Slanted Weir Microfluidic Device. Cancers 2019, 11, 200. [Google Scholar] [CrossRef]
- Augustsson, P.; Magnusson, C.; Nordin, M.; Lilja, H.; Laurell, T. Microfluidic, Label-Free Enrichment of Prostate Cancer Cells in Blood Based on Acoustophoresis. Anal. Chem. 2012, 84, 7954–7962. [Google Scholar] [CrossRef]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.-H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef]
- Geng, W.; Liu, Y.; Yu, N.; Qiao, X.; Ji, M.; Niu, Y.; Niu, L.; Fu, W.; Zhang, H.; Bi, K.; et al. An ultra-compact acoustofluidic device based on the narrow-path travelling surface acoustic wave (np-TSAW) for label-free isolation of living circulating tumor cells. Anal. Chim. Acta 2023, 1255, 341138. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, P.-H.; Zhang, R.; Mao, Z.; Chen, C.; Kemeny, G.; Li, P.; Lee, A.V.; Gyanchandani, R.; Armstrong, A.J.; et al. Circulating Tumor Cell Phenotyping via High-Throughput Acoustic Separation. Small 2018, 14, 1801131. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, W.; Lin, Z.; Cai, F.; Li, F.; Wu, J.; Meng, L.; Niu, L.; Zheng, H. Sorting of tumour cells in a microfluidic device by multi-stage surface acoustic waves. Sens. Actuators B Chem. 2018, 258, 1174–1183. [Google Scholar] [CrossRef]
- Shim, S.; Stemke-Hale, K.; Tsimberidou, A.M.; Noshari, J.; Anderson, T.E.; Gascoyne, P.R.C. Antibody-independent isolation of circulating tumor cells by continuous-flow dielectrophoresis. Biomicrofluidics 2013, 7, 011807. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.-K.; Chou, W.-P.; Huang, S.-B.; Wang, H.-M.; Lin, Y.-C.; Hsieh, C.-H.; Wu, M.-H. Application of optically-induced-dielectrophoresis in microfluidic system for purification of circulating tumour cells for gene expression analysis- Cancer cell line model. Sci. Rep. 2016, 6, 32851. [Google Scholar] [CrossRef] [PubMed]
- Waheed, W.; Alazzam, A.; Mathew, B.; Christoforou, N.; Abu-Nada, E. Lateral fluid flow fractionation using dielectrophoresis (LFFF-DEP) for size-independent, label-free isolation of circulating tumor cells. J. Chromatogr. B 2018, 1087–1088, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-V.; Jen, C.-P. Impedance detection integrated with dielectrophoresis enrichment platform for lung circulating tumor cells in a microfluidic channel. Biosens. Bioelectron. 2018, 121, 10–18. [Google Scholar] [CrossRef]
- Au, S.H.; Edd, J.; Stoddard, A.E.; Wong, K.H.K.; Fachin, F.; Maheswaran, S.; Haber, D.A.; Stott, S.L.; Kapur, R.; Toner, M. Microfluidic Isolation of Circulating Tumor Cell Clusters by Size and Asymmetry. Sci. Rep. 2017, 7, 2433. [Google Scholar] [CrossRef]
- Sarioglu, A.F.; Aceto, N.; Kojic, N.; Donaldson, M.C.; Zeinali, M.; Hamza, B.; Engstrom, A.; Zhu, H.; Sundaresan, T.K.; Miyamoto, D.T.; et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat. Methods 2015, 12, 685–691. [Google Scholar] [CrossRef]
- Moon, H.-S.; Kwon, K.; Kim, S.-I.; Han, H.; Sohn, J.; Lee, S.; Jung, H.-I. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab A Chip 2011, 11, 1118–1125. [Google Scholar] [CrossRef]
- Bakhshi, M.S.; Rizwan, M.; Khan, G.J.; Duan, H.; Zhai, K. Design of a novel integrated microfluidic chip for continuous separation of circulating tumor cells from peripheral blood cells. Sci. Rep. 2022, 12, 17016. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, R.; Shamloo, A.; Akbari, J.; Tebon, P.; Dokmeci, M.R.; Ahadian, S. Design and simulation of an integrated centrifugal microfluidic device for CTCs separation and cell lysis. Micromachines 2020, 11, 699. [Google Scholar] [CrossRef]
- Varmazyari, V.; Ghafoorifard, H.; Habibiyan, H.; Ebrahimi, M.; Ghafouri-Fard, S. A microfluidic device for label-free separation sensitivity enhancement of circulating tumor cells of various and similar size. J. Mol. Liq. 2022, 349, 118192. [Google Scholar] [CrossRef]
- Xu, X.; Huang, X.; Sun, J.; Chen, J.; Wu, G.; Yao, Y.; Zhou, N.; Wang, S.; Sun, L. 3D-stacked multistage inertial microfluidic chip for high-throughput enrichment of circulating tumor cells. Cyborg Bionic Syst. 2022, 2022, 9829287. [Google Scholar] [CrossRef] [PubMed]
- Altay, R.; Yapici, M.K.; Koşar, A. A hybrid spiral microfluidic platform coupled with surface acoustic waves for circulating tumor cell sorting and separation: A numerical study. Biosensors 2022, 12, 171. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Huang, F.; Feng, H.; Shu, W.; Xu, X.; Chen, Y. High throughput capture of circulating tumor cells using an integrated microfluidic system. Biosens. Bioelectron. 2013, 47, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Amontree, J.; Varillas, J.; Zhang, J.; George, T.J.; Fan, Z.H. Incorporation of lateral microfiltration with immunoaffinity for enhancing the capture efficiency of rare cells. Sci. Rep. 2020, 10, 14210. [Google Scholar] [CrossRef]
- Su, Y.; Tian, Q.; Pan, D.; Hui, L.; Chen, Y.; Zhang, Q.; Tian, W.; Yu, J.; Hu, S.; Gao, Y.; et al. Antibody-Functional Microsphere-Integrated Filter Chip with Inertial Microflow for Size–Immune-Capturing and Digital Detection of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2019, 11, 29569–29578. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, H.; Shu, W.; Tian, J.; Huang, Y.; Song, Y.; Wang, R.; Li, E.; Slamon, D.; Hou, D.; et al. An integrated microfluidic device for rapid and high-sensitivity analysis of circulating tumor cells. Sci. Rep. 2017, 7, 42612. [Google Scholar] [CrossRef]
- Bhagwat, N.; Dulmage, K.; Pletcher, C.H.; Wang, L.; DeMuth, W.; Sen, M.; Balli, D.; Yee, S.S.; Sa, S.; Tong, F.; et al. An integrated flow cytometry-based platform for isolation and molecular characterization of circulating tumor single cells and clusters. Sci. Rep. 2018, 8, 5035. [Google Scholar] [CrossRef]
- Topa, J.; Żaczek, A.J.; Markiewicz, A. Isolation of Viable Epithelial and Mesenchymal Circulating Tumor Cells from Breast Cancer Patients. In Single Cell Analysis: Methods and Protocols; Gužvić, M., Ed.; Springer: New York, NY, USA, 2024; pp. 43–52. [Google Scholar]
- Hyun, K.-A.; Lee, T.Y.; Jung, H.-I. Negative Enrichment of Circulating Tumor Cells Using a Geometrically Activated Surface Interaction Chip. Anal. Chem. 2013, 85, 4439–4445. [Google Scholar] [CrossRef]
- Hyun, K.-A.; Lee, T.Y.; Lee, S.H.; Jung, H.-I. Two-stage microfluidic chip for selective isolation of circulating tumor cells (CTCs). Biosens. Bioelectron. 2015, 67, 86–92. [Google Scholar] [CrossRef]
- Fachin, F.; Spuhler, P.; Martel-Foley, J.M.; Edd, J.F.; Barber, T.A.; Walsh, J.; Karabacak, M.; Pai, V.; Yu, M.; Smith, K.; et al. Monolithic Chip for High-throughput Blood Cell Depletion to Sort Rare Circulating Tumor Cells. Sci. Rep. 2017, 7, 10936. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, H.; Yu, T.; Xie, J.; Chen, S.; Dong, H.; Sinko, P.J.; Lian, S.; Xu, J.; Wang, J.; et al. Isolation and characterization of living circulating tumor cells in patients by immunomagnetic negative enrichment coupled with flow cytometry. Cancer 2015, 121, 3036–3045. [Google Scholar] [CrossRef]
- Casavant, B.P.; Mosher, R.; Warrick, J.W.; Maccoux, L.J.; Berry, S.M.F.; Becker, J.T.; Chen, V.; Lang, J.M.; McNeel, D.G.; Beebe, D.J. A negative selection methodology using a microfluidic platform for the isolation and enumeration of circulating tumor cells. Methods 2013, 64, 137–143. [Google Scholar] [CrossRef]
- Navin, N.E. Cancer genomics: One cell at a time. Genome Biol. 2014, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.; Biezuner, T.; Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 2013, 14, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Cahan, P.; Daley, G.Q. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat. Rev. Mol. Cell Biol. 2013, 14, 357–368. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Ma, W.F.; Hodonsky, C.J.; Turner, A.W.; Wong, D.; Song, Y.; Mosquera, J.V.; Ligay, A.V.; Slenders, L.; Gancayco, C.; Pan, H.; et al. Enhanced single-cell RNA-seq workflow reveals coronary artery disease cellular cross-talk and candidate drug targets. Atherosclerosis 2022, 340, 12–22. [Google Scholar] [CrossRef]
- Yang, X.; Kui, L.; Tang, M.; Li, D.; Wei, K.; Chen, W.; Miao, J.; Dong, Y. High-Throughput Transcriptome Profiling in Drug and Biomarker Discovery. Front. Genet. 2020, 11, 19. [Google Scholar] [CrossRef]
- Wimmers, F.; Donato, M.; Kuo, A.; Ashuach, T.; Gupta, S.; Li, C.; Dvorak, M.; Foecke, M.H.; Chang, S.E.; Hagan, T. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell 2021, 184, 3915–3935. [Google Scholar] [CrossRef]
- Citri, A.; Pang, Z.P.; Südhof, T.C.; Wernig, M.; Malenka, R.C. Comprehensive qPCR profiling of gene expression in single neuronal cells. Nat. Protoc. 2012, 7, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.; Tan, S.J.; Lim, C.L.; Lau, D.P.X.; Chua, Y.W.; Krisna, S.S.; Iyer, G.; Tan, G.S.; Lim, T.K.H.; Tan, D.S.W.; et al. Microfluidic enrichment for the single cell analysis of circulating tumor cells. Sci. Rep. 2016, 6, 22076. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Chen, Y.-C.; Lin, E.; Brien, R.; Jung, S.; Chen, Y.-T.; Lee, W.; Hao, Z.; Sahoo, S.; Min Kang, H.; et al. Hydro-Seq enables contamination-free high-throughput single-cell RNA-sequencing for circulating tumor cells. Nat. Commun. 2019, 10, 2163. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.G.; Yang, Y.; Ciampi, S.; Gupta, B.; Kimpton, K.; Mansfeld, F.M.; Kavallaris, M.; Gaus, K.; Gooding, J.J. A photoelectrochemical platform for the capture and release of rare single cells. Nat. Commun. 2018, 9, 2288. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, L.; Zhao, S.; Cheng, Z.; Qiu, S.; Lu, Y.; Wu, Z.; Abdel Wahab, A.H.A.; Mao, H.; Zhao, J. A microfluidic platform for high-purity separating circulating tumor cells at the single-cell level. Talanta 2019, 200, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Bithi, S.S.; Vanapalli, S.A. Microfluidic cell isolation technology for drug testing of single tumor cells and their clusters. Sci. Rep. 2017, 7, 41707. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.; Li, Y.; Li, S.Y.; Zu, Y.; Wang, Z.; Qin, L. Hand-Held and Integrated Single-Cell Pipettes. J. Am. Chem. Soc. 2014, 136, 10858–10861. [Google Scholar] [CrossRef]
- Kim, J.; Cho, H.; Han, S.-I.; Han, K.-H. Single-Cell Isolation of Circulating Tumor Cells from Whole Blood by Lateral Magnetophoretic Microseparation and Microfluidic Dispensing. Anal. Chem. 2016, 88, 4857–4863. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhao, W.; Zhang, J.; Chen, W.; Xie, T.; Wang, L.; Fan, W.; Xie, S.; Shen, J.; Zheng, H. Evaluation of the diagnostic value of circulating tumor cells with CytoSorter® CTC capture system in patients with breast cancer. Cancer Med. 2020, 9, 1638–1647. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Savenkov, O.; Asmus, E.J.; Ballman, K.V.; Scott, J.H.; Park, J.W.; Dickler, M.; Partridge, A.; Carey, L.A.; Winer, E.P.; et al. Clinical Significance of Circulating Tumor Cells in Hormone Receptor–positive Metastatic Breast Cancer Patients who Received Letrozole with or Without Bevacizumab. Clin. Cancer Res. 2020, 26, 4911–4920. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Liu, B.; Li, B.-Y.; Wang, T.; Chen, D.-Q. Effect of CTCs and INHBA level on the effect and prognosis of different treatment methods for patients with early breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12735–12740. [Google Scholar]
- Deutsch, T.M.; Stefanovic, S.; Feisst, M.; Fischer, C.; Riedel, F.; Fremd, C.; Domschke, C.; Pantel, K.; Hartkopf, A.D.; Sutterlin, M. Cut-off analysis of CTC change under systemic therapy for defining early therapy response in metastatic breast cancer. Cancers 2020, 12, 1055. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ye, X.; Liu, Y.; Zhao, Y.; He, M.; Xiao, H. The combination of CTCs and CEA can help guide the management of patients with SPNs suspected of being lung cancer. BMC Cancer 2020, 20, 106. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lee, C.-L.; Wu, C.-F.; Fu, J.-Y.; Yang, C.-T.; Wen, C.-T.; Liu, Y.-H.; Liu, H.-P.; Hsieh, J.C.-H. Circulating tumor cells as a tool of minimal residual disease can predict lung cancer recurrence: A longitudinal, prospective trial. Diagnostics 2020, 10, 144. [Google Scholar] [CrossRef]
- Vasseur, A.; Kiavue, N.; Bidard, F.C.; Pierga, J.Y.; Cabel, L. Clinical utility of circulating tumor cells: An update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Hille, C.; Scher, H.I. Circulating Tumor Cells in Prostate Cancer: From Discovery to Clinical Utility. Clin. Chem. 2019, 65, 87–99. [Google Scholar] [CrossRef]
- Cani, A.K.; Hayes, D.F. Breast Cancer Circulating Tumor Cells: Current Clinical Applications and Future Prospects. Clin. Chem. 2024, 70, 68–80. [Google Scholar] [CrossRef]
- Abdalla, T.S.; Meiners, J.; Riethdorf, S.; König, A.; Melling, N.; Gorges, T.; Karstens, K.-F.; Izbicki, J.R.; Pantel, K.; Reeh, M. Prognostic value of preoperative circulating tumor cells counts in patients with UICC stage I-IV colorectal cancer. PLoS ONE 2021, 16, e0252897. [Google Scholar] [CrossRef] [PubMed]
- Garrel, R.; Mazel, M.; Perriard, F.; Vinches, M.; Cayrefourcq, L.; Guigay, J.; Digue, L.; Aubry, K.; Alfonsi, M.; Delord, J.-P.; et al. Circulating Tumor Cells as a Prognostic Factor in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: The CIRCUTEC Prospective Study. Clin. Chem. 2019, 65, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Zhou, L.; Liu, Z.; Tan, X. A combination of circulating tumor cells and CA199 improves the diagnosis of pancreatic cancer. J. Clin. Lab. Anal. 2022, 36, e24341. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, A.; Dall, K.; Brandt, B.; Geisen, R.; Röder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Longitudinal analysis of circulating tumor cells in colorectal cancer patients by a cytological and molecular approach: Feasibility and clinical application. Front. Oncol. 2021, 11, 646885. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-P.; Liu, S.-H.; Chen, C.-T.; Lv, L.; Li, D.; Liu, Q.-Y.; Liu, G.-L.; Wu, Y. Circulating tumor cells as a new predictive and prognostic factor in patients with small cell lung cancer. J. Cancer 2020, 11, 2113. [Google Scholar] [CrossRef]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.-i.; Morgan, B.; Trautwein, J.; et al. Inertial Focusing for Tumor Antigen–Dependent and –Independent Sorting of Rare Circulating Tumor Cells. Sci. Transl. Med. 2013, 5, 179ra147. [Google Scholar] [CrossRef]
| Clinical Applications | Methods | Cancer Type | Result Description | Reference |
|---|---|---|---|---|
| Early cancer detection | NE-imFISH and immunostaining | PDAC | When the cut-off value was 2 CTCs/3.2 mL, the ACU was 0.85, the specificity was 94%, and the sensitivity was 76%. | [164] |
| CytoSorter® microfluidic platform | BC | When the cut-off value was 2 CTCs/4 mL, the ACU was 0.86, the specificity was 95.4%, and the sensitivity was 76.56%. | [153] | |
| SE i-FISH | NSCLC | Sensitivity was 77.8 percent and specificity was 90 percent when CTCs (cut-off value 12 units) were combined with carcinoembryonic antigen (1.78 ng/mL) | [157] | |
| Therapeutic response monitoring | RT-qPCR and Immunofluorescence staining | CRC | Patients whose NYONE® test results were CTC-negative before surgery or whose CK20 mRNA relative expression was below the threshold after PCR analysis were tested for CTC immediately after surgery, and the results showed a significant increase. | [165] |
| Flow cytometry and EasySep | Early-staged lung cancer | When the cut-off value was greater than 3 CTCs/mL, the number of CTCs decreased after surgery. The increased number of CTCs was positively correlated with cancer recurrence | [158] | |
| Prognosis evaluation | Cell Search | MBC | progression-free survival: HR 1.79; overall survival: HR 2.72 | [154] |
| FISH and immunomagnetic enrichment | SCLC | The number of CTCs was correlated with lymph node metastasis (N), distant metastasis (M), TNM and NSE. High CTCS predicted poor prognosis, and the ROC curve AUC was always greater than 0.5. | [166] | |
| Cell Search | CRC | Multivariate analysis showed that only advanced age and preoperative CTCs detection were independent predictors of adverse OS. When the ratio is greater than 1 CTCs/7.5 mL, the overall survival and progression-free survival are shorter. | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Z.; Teng, X.; Liu, A.; Yang, W. Novel Isolating Approaches to Circulating Tumor Cell Enrichment Based on Microfluidics: A Review. Micromachines 2024, 15, 706. https://doi.org/10.3390/mi15060706
Qiao Z, Teng X, Liu A, Yang W. Novel Isolating Approaches to Circulating Tumor Cell Enrichment Based on Microfluidics: A Review. Micromachines. 2024; 15(6):706. https://doi.org/10.3390/mi15060706
Chicago/Turabian StyleQiao, Zezheng, Xiangyu Teng, Anqin Liu, and Wenguang Yang. 2024. "Novel Isolating Approaches to Circulating Tumor Cell Enrichment Based on Microfluidics: A Review" Micromachines 15, no. 6: 706. https://doi.org/10.3390/mi15060706
APA StyleQiao, Z., Teng, X., Liu, A., & Yang, W. (2024). Novel Isolating Approaches to Circulating Tumor Cell Enrichment Based on Microfluidics: A Review. Micromachines, 15(6), 706. https://doi.org/10.3390/mi15060706





