Alignment Control of Ferrite-Decorated Nanocarbon Material for 3D Printing
Abstract
1. Introduction
2. Experimental
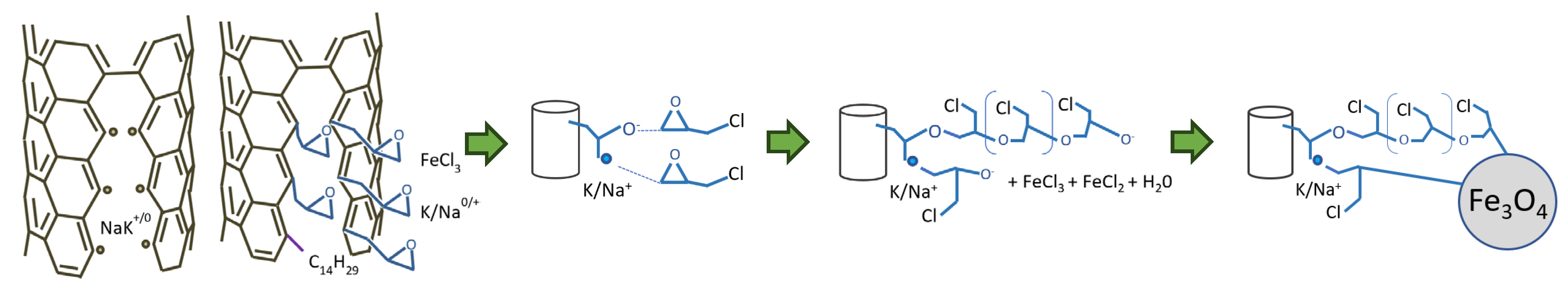
2.1. Synthesis of Decorated Carbon Materials
2.1.1. Materials
2.1.2. Unzipping Process
2.1.3. Attaching Epoxy Group to the Tubes
2.1.4. Attaching Magnetite Particles
2.1.5. Preparation for Analysis
2.1.6. Instruments
2.2. 3D Printing Photopolymer Formulation
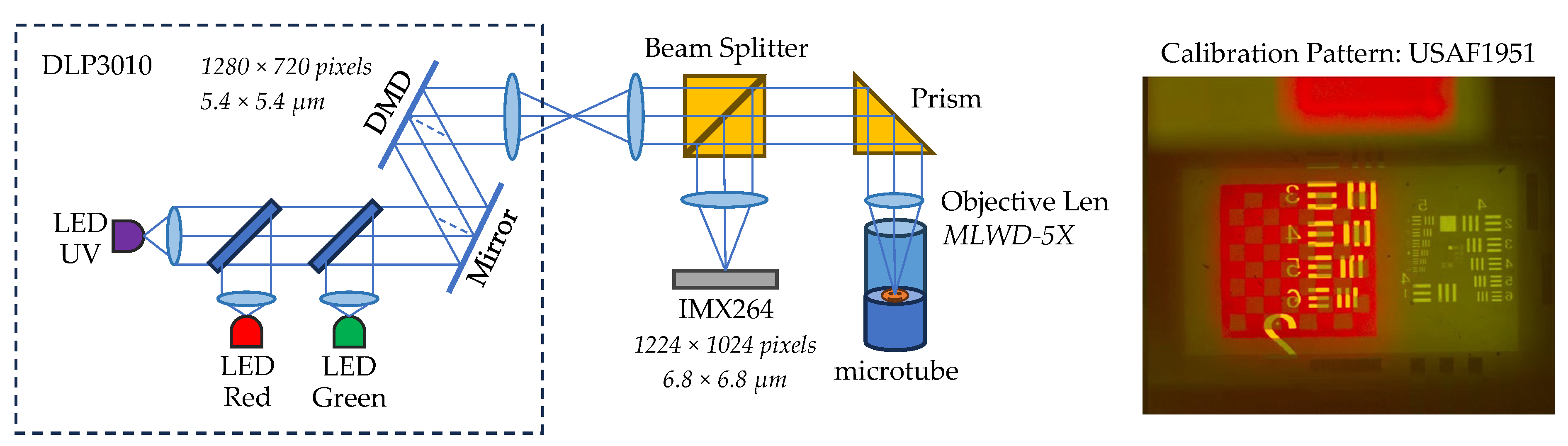
2.3. Microscopic 3D Printer Design
2.4. Optical System
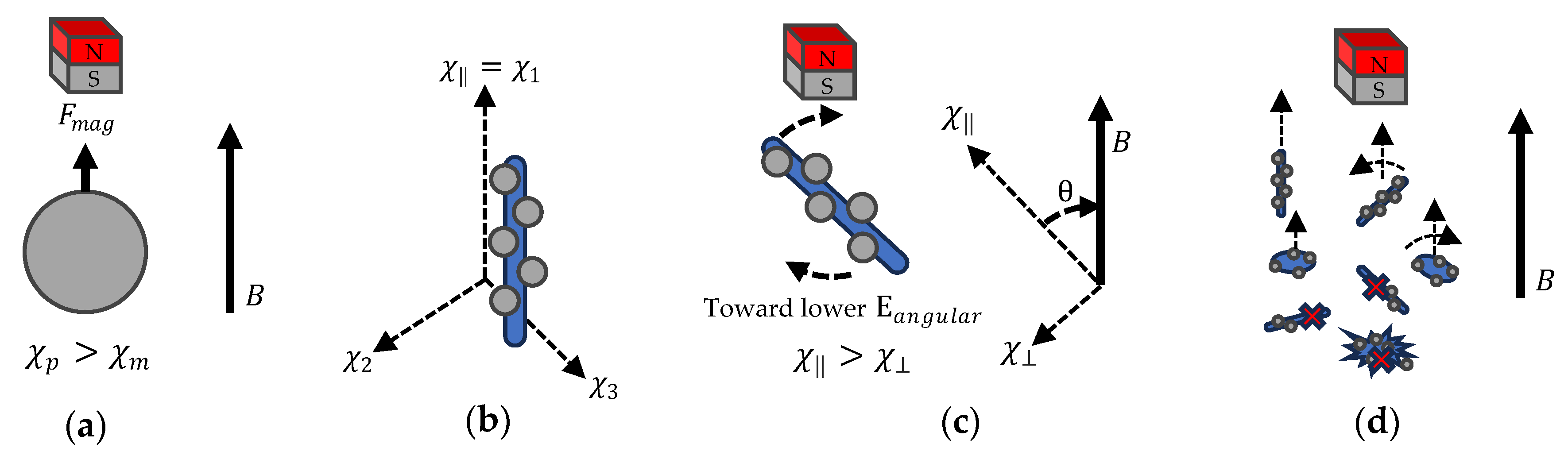
2.5. Magnetic Actuator
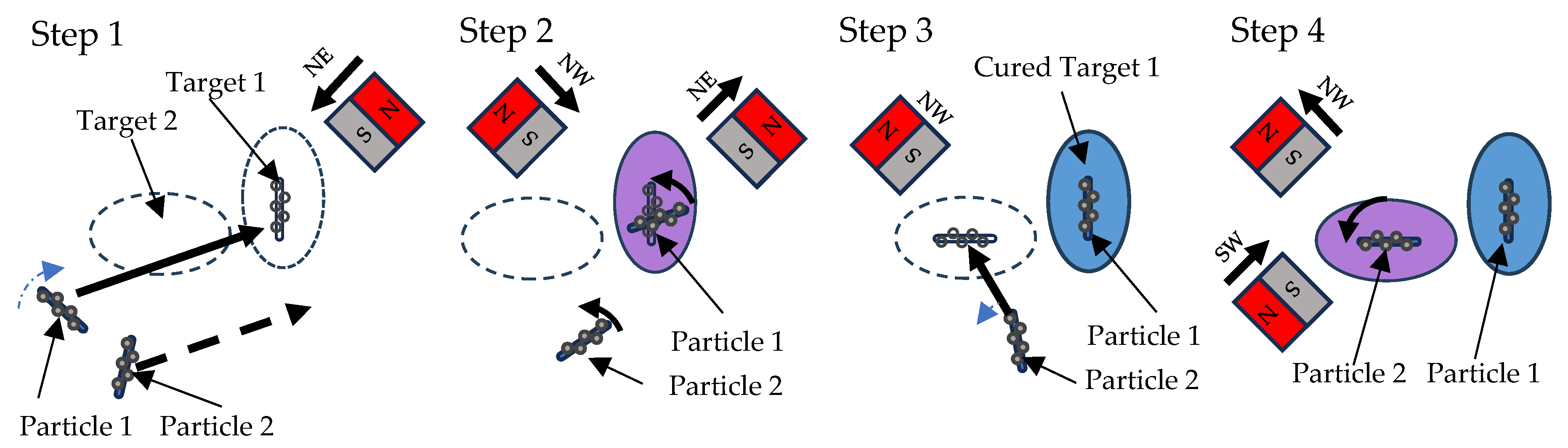
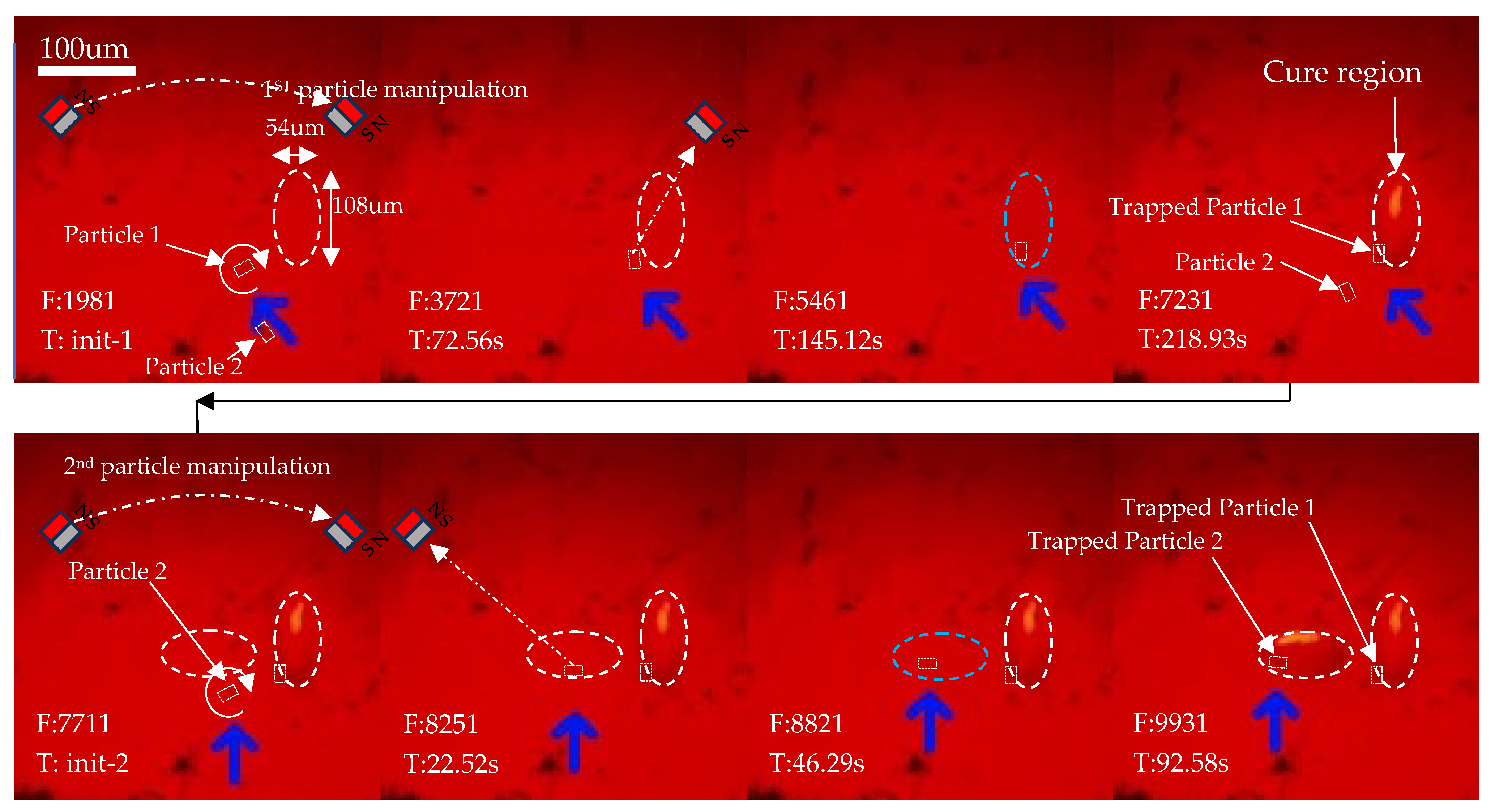
2.6. Particle Manipulation
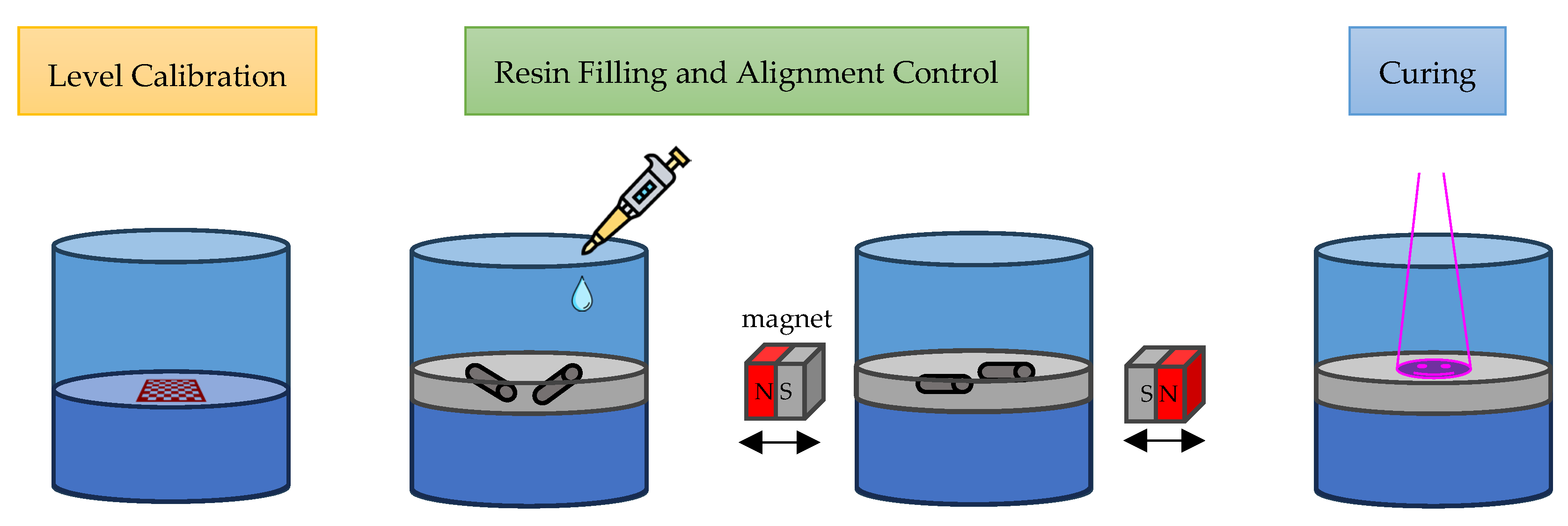
2.7. Printing Procedure
3. Results and Discussion
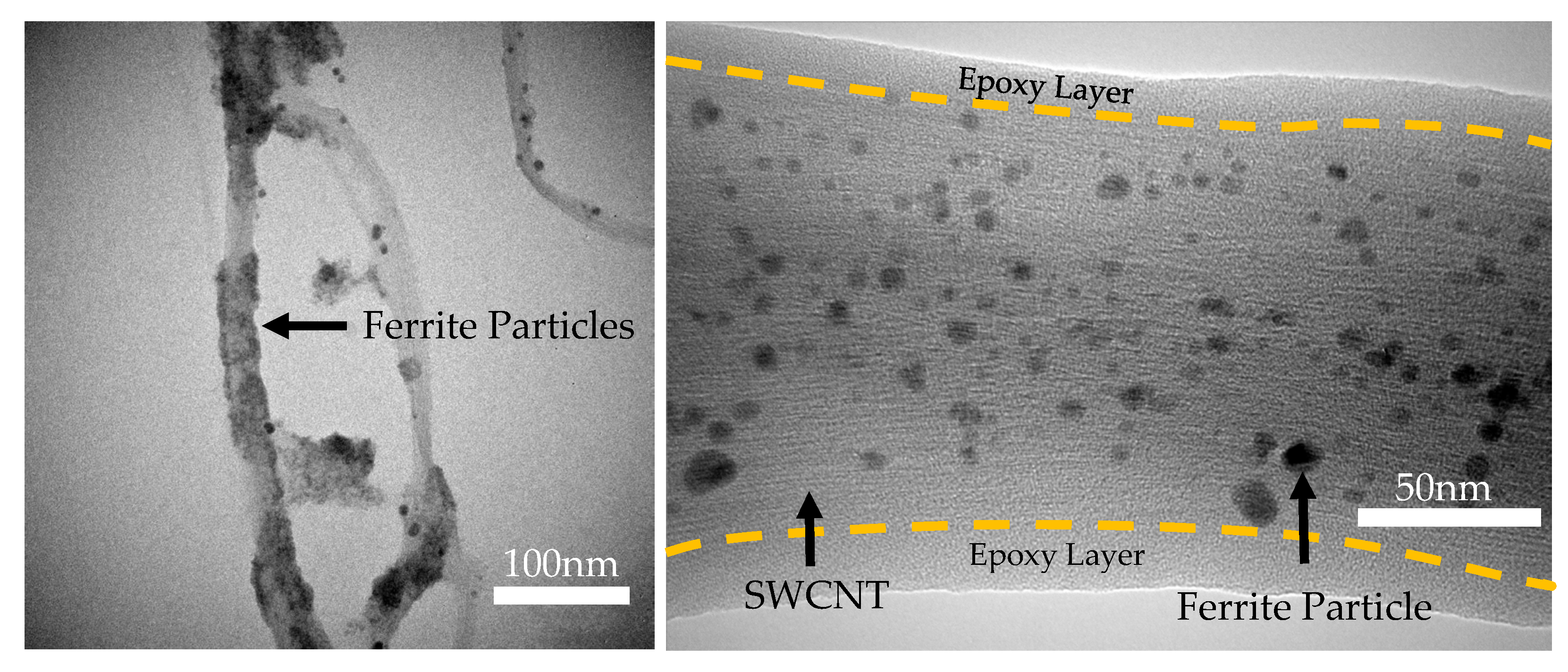
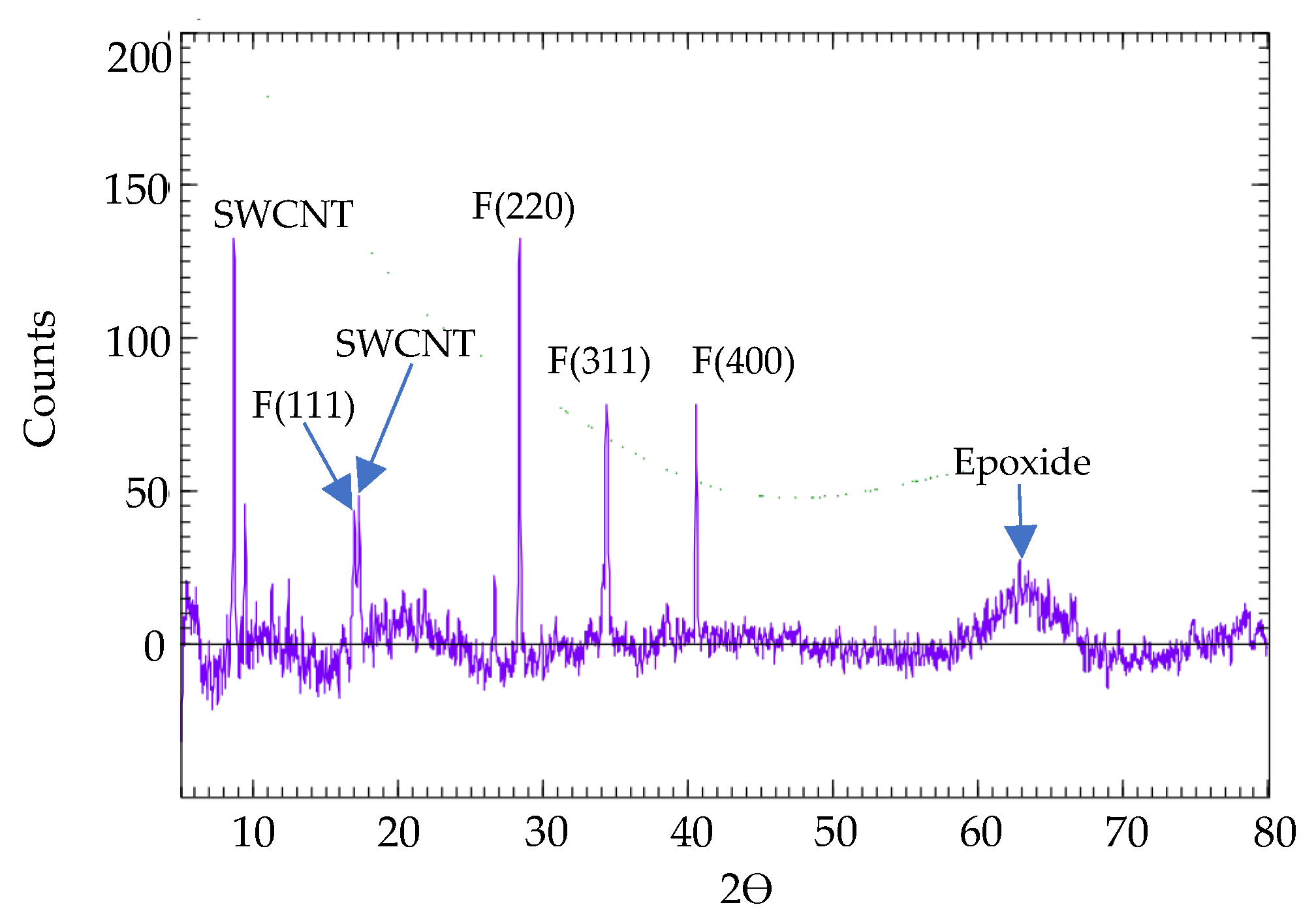
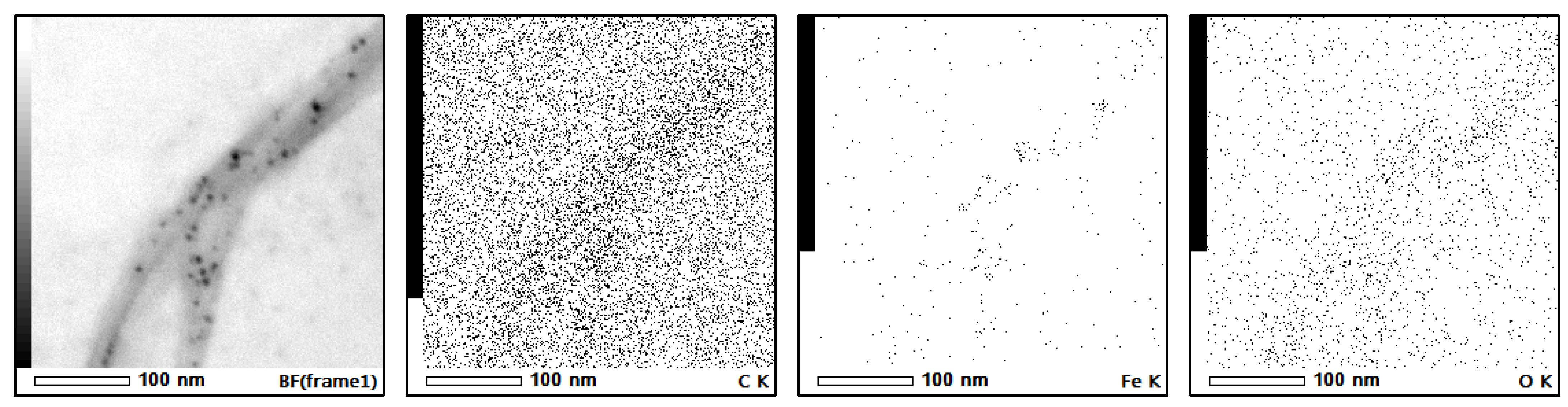
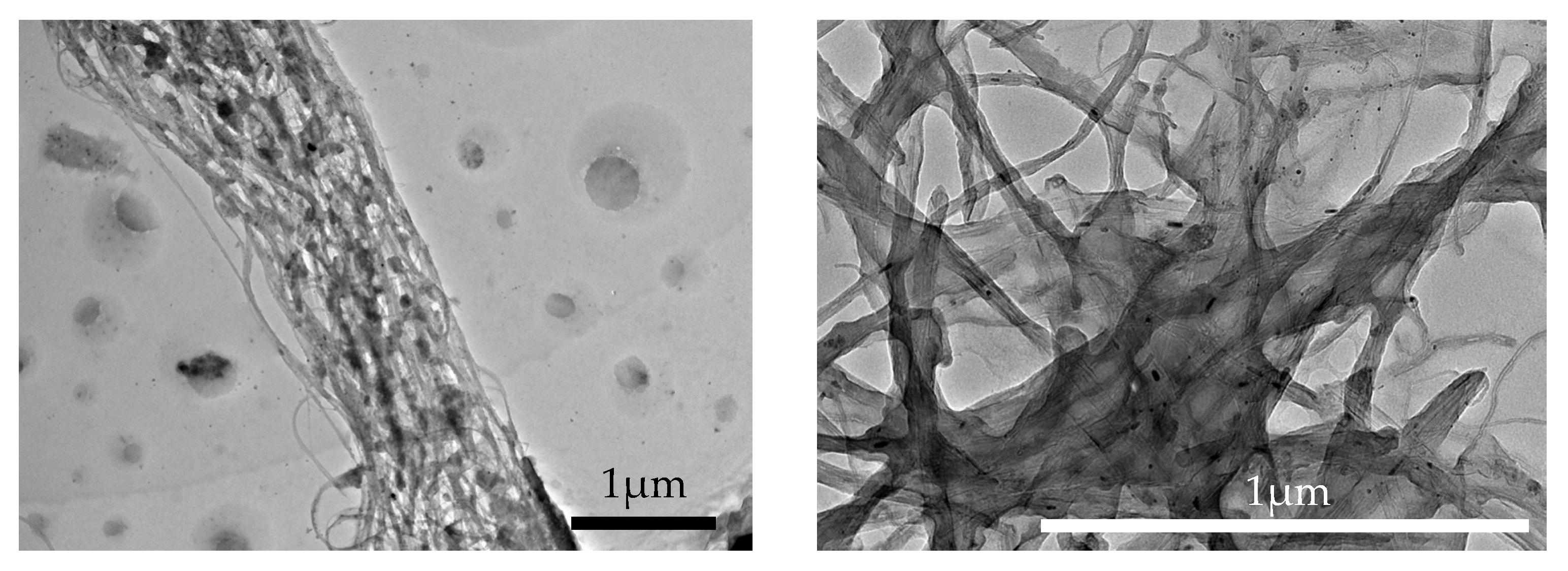
3.1. Attachment of Ferrite Nanoparticles
3.2. Formulating Photopolymer for 3D Printing
3.3. 3D Printing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Theoretical Calculation of the Enhancement in Anisotropic 3D Printing
References
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.L.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The Status, Challenges, and Future of Additive Manufacturing in Engineering. Comput.-Aided Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Residori, S.; Dul, S.; Pegoretti, A.; Fambri, L.; Pugno, N.M. Three Dimensional Printing of Multiscale Carbon Fiber-Reinforced Polymer Composites Containing Graphene or Carbon Nanotubes. Nanomaterials 2022, 12, 2064. [Google Scholar] [CrossRef]
- Kramer, R.C.L.N.; Verlinden, E.J.; Angeloni, L.; van den Heuvel, A.; Fratila-Apachitei, L.E.; van der Maarel, S.M.; Ghatkesar, M.K. Multiscale 3D-Printing of Microfluidic AFM Cantilevers. Lab Chip 2020, 20, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tan, Y.H.; Ding, J.; Guan, C. 3D Printing of Next-Generation Electrochemical Energy Storage Devices: From Multiscale to Multimaterial. Energy Environ. Mater. 2022, 5, 427–438. [Google Scholar] [CrossRef]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Z.; Song, X.; Zhang, Z.; Zhang, J.; Shung, K.K.; Zhou, Q.; Chen, Y. Biomimetic Anisotropic Reinforcement Architectures by Electrically Assisted Nanocomposite 3D Printing. Adv. Mater. 2017, 29, 1605750. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, J.P.; Rodriguez, J.N.; Zhu, C.; Worsley, M.A.; Wu, A.S.; Kanarska, Y.; Horn, J.D.; Duoss, E.B.; Ortega, J.M.; Elmer, W.; et al. 3D-Printing of Meso-Structurally Ordered Carbon Fiber/Polymer Composites with Unprecedented Orthotropic Physical Properties. Sci. Rep. 2017, 7, 43401. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Cho, S.-Y.; Chong, S.; Koh, H.-J.; Kim, D.W.; Kim, J.; Jung, H.-T. Edge-Functionalized Graphene Nanoribbon Chemical Sensor: Comparison with Carbon Nanotube and Graphene. ACS Appl. Mater. Interfaces 2018, 10, 42905–42914. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Yang, Y.; Kim, G.; Gim, D.; Ha, M. Anisotropy in Magnetic Materials for Sensors and Actuators in Soft Robotic Systems. Nanoscale 2024, 16, 6778–6819. [Google Scholar] [CrossRef]
- Beiderbeck, D.; Deradjat, D.; Minshall, T. The Impact of Additive Manufacturing Technologies on Industrial Spare Parts Strategies. 2018. Available online: https://www.repository.cam.ac.uk/items/bb47e878-52d1-4e14-ac41-7925f73d3df5 (accessed on 12 May 2018).
- Gebisa, A.W.; Lemu, H.G. A Case Study on Topology Optimized Design for Additive Manufacturing. IOP Conf. Ser. Mater. Sci. Eng. 2017, 276, 012026. [Google Scholar] [CrossRef]
- Kazakis, G.; Kanellopoulos, I.; Sotiropoulos, S.; Lagaros, N.D. Topology Optimization Aided Structural Design: Interpretation, Computational Aspects and 3D Printing. Heliyon 2017, 3, e00431. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cao, R.; Wu, G.; Huang, W.; Chen, Z.; Yang, X.; Tu, Y. From Ultratough Artificial Nacre to Elastomer: Poly(n-Butyl Acrylate) Grafted Graphene Oxide Nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 88, 156–164. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, Q.; Duanmu, L.; Wu, S.; Liu, Q.; Li, B.; Zhou, X. Bioinspired Composites Reinforced with Ordered Steel Fibers Produced via a Magnetically Assisted 3D Printing Process. J. Mater. Sci. 2020, 55, 15510–15522. [Google Scholar] [CrossRef]
- Martin, J.; Fiore, B.; Erb, R. Designing Bioinspired Composite Reinforcement Architectures via 3D Magnetic Printing. Nat. Commun. 2015, 6, 8641. [Google Scholar] [CrossRef] [PubMed]
- Clay, G.; Song, H.; Nielsen, J.; Stasiak, J.; Khavari, M.; Jander, A.; Dhagat, P. 3D Printing Magnetic Material with Arbitrary Anisotropy. NIP Digit. Fabr. Conf. 2015, 31, art00068_1. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Chou, V.H.Y.; Behera, R.P.; Le Ferrand, H. Magnetically Assisted Drop-on-Demand 3D Printing of Microstructured Multimaterial Composites. Nat. Commun. 2022, 13, 5015. [Google Scholar] [CrossRef]
- Kokkinis, D.; Schaffner, M.; Studart, A.R. Multimaterial Magnetically Assisted 3D Printing of Composite Materials. Nat. Commun. 2015, 6, 8643. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhou, X.; Guo, R.; Yuan, X.; Chen, H.; Abrahams, I.; Zhang, D. 3D Printing of Anisotropic Polymer Nanocomposites with Aligned BaTiO3 Nanowires for Enhanced Energy Density. Mater. Adv. 2020, 1, 14–19. [Google Scholar] [CrossRef]
- Herren, B.; Gu, T.; Tang, Q.; Saha, M.; Liu, Y. 3D Printing and Stretching Effects on Alignment Microstructure in PDMS/CNT Nanocomposites; American Society of Mechanical Engineers Digital Collection: Nee York, NY, USA, 2020. [Google Scholar]
- Goh, G.L.; Agarwala, S.; Yeong, W.Y. Directed and On-Demand Alignment of Carbon Nanotube: A Review toward 3D Printing of Electronics. Adv. Mater. Interfaces 2019, 6, 1801318. [Google Scholar] [CrossRef]
- Acquah, S.F.A.; Leonhardt, B.E.; S. Nowotarski, M.; Magi, J.M.; Chambliss, K.A.; S.Venzel, T.E.; Delekar, S.D.; Al-Hariri, L.A. Carbon Nanotubes and Graphene as Additives in 3D Printing. In Carbon Nanotubes-Current Progress of Their Polymer Composites; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Shekhirev, M.; Sinitskii, A. Solution Synthesis of Atomically Precise Graphene Nanoribbons. Phys. Sci. Rev. 2017, 2, 20160108. [Google Scholar] [CrossRef]
- Vo, T.H.; Shekhirev, M.; Kunkel, D.A.; Morton, M.D.; Berglund, E.; Kong, L.; Wilson, P.M.; Dowben, P.A.; Enders, A.; Sinitskii, A. Large-Scale Solution Synthesis of Narrow Graphene Nanoribbons. Nat. Commun. 2014, 5, 3189. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Hayashi, H.; Jippo, H.; Shiotari, A.; Ohtomo, M.; Sakakura, M.; Hieda, N.; Aratani, N.; Ohfuchi, M.; Sugimoto, Y.; et al. Small Bandgap in Atomically Precise 17-Atom-Wide Armchair-Edged Graphene Nanoribbons. Commun. Mater. 2020, 1, 36. [Google Scholar] [CrossRef]
- Genorio, B.; Lu, W.; Dimiev, A.M.; Zhu, Y.; Raji, A.-R.O.; Novosel, B.; Alemany, L.B.; Tour, J.M. In Situ Intercalation Replacement and Selective Functionalization of Graphene Nanoribbon Stacks. ACS Nano 2012, 6, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Genorio, B.; Peng, Z.; Lu, W.; Price Hoelscher, B.K.; Novosel, B.; Tour, J.M. Synthesis of Dispersible Ferromagnetic Graphene Nanoribbon Stacks with Enhanced Electrical Percolation Properties in a Magnetic Field. ACS Nano 2012, 6, 10396–10404. [Google Scholar] [CrossRef] [PubMed]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal Unzipping of Carbon Nanotubes to Form Graphene Nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Kosynkin, D.V.; Lu, W.; Sinitskii, A.; Pera, G.; Sun, Z.; Tour, J.M. Highly Conductive Graphene Nanoribbons by Longitudinal Splitting of Carbon Nanotubes Using Potassium Vapor. ACS Nano 2011, 5, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Sussman, A.; Zettl, A. Graphene Nanoribbons Obtained by Electrically Unwrapping Carbon Nanotubes. ACS Nano 2010, 4, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.M.; Khannanov, A.; Vakhitov, I.; Kiiamov, A.; Shukhina, K.; Tour, J.M. Revisiting the Mechanism of Oxidative Unzipping of Multiwall Carbon Nanotubes to Graphene Nanoribbons. ACS Nano 2018, 12, 3985–3993. [Google Scholar] [CrossRef]
- Temnuch, N.; Suwattanamala, A.; Inpaeng, S.; Tedsree, K. Magnetite Nanoparticles Decorated on Multi-Walled Carbon Nanotubes for Removal of Cu2+ from Aqueous Solution. Environ. Technol. 2021, 42, 3572–3580. [Google Scholar] [CrossRef] [PubMed]
- Dehghani-Dashtabi, M.; Hekmatara, H.; Seyed-Yazdi, J. Highly Magnetic Nanocomposites Consist of Magnetite Nanoparticles, Graphene Oxide and Hyper-Branched Poly Citric Acid. Mater. Chem. Phys. 2019, 224, 271–278. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Zhou, J.; Cai, W.; Shen, B.; Jiang, Z. Magnetite Nanocrystals on Multiwalled Carbon Nanotubes as a Synergistic Microwave Absorber. J. Phys. Chem. C 2013, 117, 5446–5452. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Grzelczak, M.; Salgueiriño-Maceira, V.; Giersig, M.; Liz-Marzán, L.M.; Farle, M.; Sierazdki, K.; Diaz, R. Alignment of Carbon Nanotubes under Low Magnetic Fields through Attachment of Magnetic Nanoparticles. J. Phys. Chem. B 2005, 109, 19060–19063. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Thiel, W.; Hirsch, A. Reactivity of the Convex and Concave Surfaces of Single-Walled Carbon Nanotubes (SWCNTs) towards Addition Reactions: Dependence on the Carbon-Atom Pyramidalization. ChemPhysChem 2003, 4, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Bitaraf, M.; Amoozadeh, A. aUse of Epichlorohydrin as a New, Inexpensive and Highly Efficient Linker for Magnetization of Nano-metal Oxides: The Extension of Using a Covalent Linker as a Third Methodology. ChemistrySelect 2019, 4, 5954–5960. [Google Scholar] [CrossRef]
- Kwart, H.; Goodman, A.L. The Acid-Catalyzed Ring Opening of Epichlorohydrin. J. Am. Chem. Soc. 1960, 82, 1947–1949. [Google Scholar] [CrossRef]
- Miguel-Sancho, N.; Bomatí-Miguel, O.; Colom, G.; Salvador, J.-P.; Marco, M.-P.; Santamaría, J. Development of Stable, Water-Dispersible, and Biofunctionalizable Superparamagnetic Iron Oxide Nanoparticles. Chem. Mater. 2011, 23, 2795–2802. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, Z.; Liu, C.; Liu, C.; Tan, T. Biodiesel Production Using Lipase Immobilized on Epoxychloropropane-Modified Fe3O4 Sub-Microspheres. Colloids Surf. B Biointerfaces 2016, 140, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Lari, G.M.; Pastore, G.; Mondelli, C.; Pérez-Ramírez, J. Towards Sustainable Manufacture of Epichlorohydrin from Glycerol Using Hydrotalcite-Derived Basic Oxides. Green Chem. 2018, 20, 148–159. [Google Scholar] [CrossRef]
- Kwon, J.-S.; Oh, J.H. Microfluidic Technology for Cell Manipulation. Appl. Sci. 2018, 8, 992. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Leow, C.H.; Toh, P.Y.; Low, S.C. Magnetophoresis of Iron Oxide Nanoparticles at Low Field Gradient: The Role of Shape Anisotropy. J. Colloid Interface Sci. 2014, 421, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Shen, F.; Hahn, Y.K.; Park, J.-H.; Park, J.-K. Magnetophoretic Sorting of Single Cell-Containing Microdroplets. Micromachines 2016, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.D.; Patrick, D.L. Organizing Carbon Nanotubes with Liquid Crystals. Available online: https://pubs.acs.org/doi/full/10.1021/nl025694j (accessed on 12 August 2018).
- Basu, R. Effect of Carbon Nanotubes on the Field-Induced Nematic Switching. Appl. Phys. Lett. 2013, 103, 241906. [Google Scholar] [CrossRef]
- Lan, X.; Du, Y.; Liu, F.; Li, G. Development of Microrobot with Optical Magnetic Dual Control for Regulation of Gut Microbiota. Micromachines 2023, 14, 2252. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Yi, X.; Jin, S.; Chen, Y. Control of Self-Winding Microrobot Using an Electromagnetic Drive System: Integration of Movable Electromagnetic Coil and Permanent Magnet. Micromachines 2024, 15, 438. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xu, Q. A Survey of Recent Developments in Magnetic Microrobots for Micro-/Nano-Manipulation. Micromachines 2024, 15, 468. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cui, J.; Tian, K.; Han, Y. Three-Degrees-of-Freedom Orientation Manipulation of Small Untethered Robots with a Single Anisotropic Soft Magnet. Nat. Commun. 2023, 14, 7491. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Han, C.; Jeon, H. Submicrometer-Scale Pattern Generation via Maskless Digital Photolithography. Optica 2020, 7, 1788–1795. [Google Scholar] [CrossRef]
- Ivón Rodríguez-Villarreal, A.; Tarn, M.D.; Madden, L.A.; Lutz, J.B.; Greenman, J.; Samitier, J.; Pamme, N. Flow Focussing of Particles and Cells Based on Their Intrinsic Properties Using a Simple Diamagnetic Repulsion Setup. Lab Chip 2011, 11, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Watarai, H.; Namba, M. Magnetophoretic Behavior of Single Polystyrene Particles in Aqueous Manganese(II) Chloride. Anal. Sci. 2001, 17, 1233–1236. [Google Scholar] [CrossRef]
- Yamato, M.; Kimura, T. Magnetic Processing of Diamagnetic Materials. Polymers 2020, 12, 1491. [Google Scholar] [CrossRef] [PubMed]
- Masrour, R.; Hlil, E.K.; Hamedoun, M.; Benyoussef, A.; Mounkachi, O.; El Moussaoui, H. Electronic and Magnetic Structures of Fe3O4 Ferrimagnetic Investigated by First Principle, Mean Field and Series Expansions Calculations. J. Magn. Magn. Mater. 2015, 378, 37–40. [Google Scholar] [CrossRef]
- Reilly, C.A.; Swalen, J.D. Analysis of Complex NMR Spectra: Epichlorohydrin. J. Chem. Phys. 1961, 35, 1522–1523. [Google Scholar] [CrossRef]
- PubChem 1,2-Dimethoxyethane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8071 (accessed on 28 May 2024).
- Bertolucci, E.; Galletti, A.M.R.; Antonetti, C.; Marracci, M.; Tellini, B.; Piccinelli, F.; Visone, C. Chemical and Magnetic Properties Characterization of Magnetic Nanoparticles. In Proceedings of the 2015 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) Proceedings, Pisa, Italy, 11–14 May 2015; pp. 1492–1496. [Google Scholar]
- Zhuang, L.; Zhang, W.; Zhao, Y.; Shen, H.; Lin, H.; Liang, J. Preparation and Characterization of Fe3O4 Particles with Novel Nanosheets Morphology and Magnetochromatic Property by a Modified Solvothermal Method. Sci. Rep. 2015, 5, 9320. [Google Scholar] [CrossRef] [PubMed]
- Kovtyukhova, N.I.; Mallouk, T.E.; Pan, L.; Dickey, E.C. Individual Single-Walled Nanotubes and Hydrogels Made by Oxidative Exfoliation of Carbon Nanotube Ropes. J. Am. Chem. Soc. 2003, 125, 9761–9769. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonhaijaroen, N.; Sitthi-amorn, P.; Srituravanich, W.; Suanpong, K.; Ekgasit, S.; Pengprecha, S. Alignment Control of Ferrite-Decorated Nanocarbon Material for 3D Printing. Micromachines 2024, 15, 763. https://doi.org/10.3390/mi15060763
Boonhaijaroen N, Sitthi-amorn P, Srituravanich W, Suanpong K, Ekgasit S, Pengprecha S. Alignment Control of Ferrite-Decorated Nanocarbon Material for 3D Printing. Micromachines. 2024; 15(6):763. https://doi.org/10.3390/mi15060763
Chicago/Turabian StyleBoonhaijaroen, Narit, Pitchaya Sitthi-amorn, Werayut Srituravanich, Kwanrat Suanpong, Sanong Ekgasit, and Somchai Pengprecha. 2024. "Alignment Control of Ferrite-Decorated Nanocarbon Material for 3D Printing" Micromachines 15, no. 6: 763. https://doi.org/10.3390/mi15060763
APA StyleBoonhaijaroen, N., Sitthi-amorn, P., Srituravanich, W., Suanpong, K., Ekgasit, S., & Pengprecha, S. (2024). Alignment Control of Ferrite-Decorated Nanocarbon Material for 3D Printing. Micromachines, 15(6), 763. https://doi.org/10.3390/mi15060763





