The Effects of Nucleating Agents and Processing on the Crystallization and Mechanical Properties of Polylactic Acid: A Review
Abstract
1. Introduction
2. Overview of Polylactic Acid (PLA)
3. PLA Crystallization
- Heterogeneous nucleation, initiated by nucleating agents, is more commonly observed in PLA. Nucleating agents provide sites where PLA chains can organize and form crystalline nuclei. These agents can be inorganic materials, such as talc; organic compounds, such as sorbitol; or a specific PLLA-PDLA matrix known as stereocomplex PLA. Nucleating agents in the PLA polymer matrix serve as crystallization catalysts, reducing the energy barrier for nucleation [41,43]. Once nucleation sites are formed during the nucleation step, crystalline domains grow in the polymer matrix. PLA exhibits more than one form or phase of crystalline structures. The most common structure is the α-form. These crystalline domains consist of regularly arranged polymer chains, which increase the PLA strength and stiffness compared to amorphous regions. Depending on the nucleating agent added, processing techniques, and crystallization conditions, PLA can present four distinct crystalline forms, as summarized in Table 1.
| Crystalline Form | Crystal System | Cell Parameters | |||||
|---|---|---|---|---|---|---|---|
| a (nm) | b (nm) | c (nm) | α (°) | β (°) | γ (°) | ||
| α [43] | Orthorhombic | 10.05 | 0.61 | 2.88 | 90 | 90 | 90 |
| α and α′ [44] | Pseudo-orthorhombic | 1.07 | 0.645 | 2.78 | 90 | 90 | 90 |
| β [45] | Orthorhombic | 1.031 | 1.821 | 0.9 | 90 | 90 | 90 |
| β [46] | Trigonal | 1.052 | 1.052 | 0.88 | 90 | 90 | 90 |
| γ [47] | Orthorhombic | 0.995 | 0.625 | 0.88 | 90 | 90 | 90 |
| SC [48] | Triclinic | 0.916 | 0.916 | 0.87 | 109 | 109 | 110 |
| SC [49] | Triclinic | 1.498 | 1.498 | 0.87 | 90 | 90 | 120 |
4. Material Innovations in PLA
4.1. Inorganic Additives
4.1.1. Mineral Materials
4.1.2. Nanoparticles
4.1.3. Zeolites
4.2. Organic Nucleating Agents
4.2.1. Micromolecular Nucleating Agents
Amide Compounds
4.2.2. Organic Salt
4.2.3. Other Macromolecular Materials
5. Processing Optimization Techniques
5.1. Annealing
| PLA Blend | Annealing Technique | Crystallinity (%) | Mechanical Properties | Reference | ||
|---|---|---|---|---|---|---|
| Impact Strength (J/m2) | Yield Stress (MPa) | Young’s Modulus (GPa) | ||||
| PLA–PCL | 25 °C | 19.4 | 5.9 | - | - | [144] |
| 110 °C | 42.5 | 26.8 | - | - | ||
| PLA–TPPE | 25 °C | 3.8 | 20.3 | - | - | [145] |
| 110 °C | 31 | 53.5 | - | - | ||
| PLA | 70 °C | 0 | 28 | - | - | [146] |
| 80 °C | 47.1 | 54 | - | - | ||
| 90 °C | 61.5 | 63 | - | - | ||
| 100 °C | 39.2 | 31 | - | - | ||
| PLLA | 25 °C | 2.5 | - | 47 | - | [147] |
| 90 °C | 40 | - | 68 | - | ||
| 120 °C | 53.9 | - | 59 | - | ||
| 140 °C | 60.9 | - | 54 | - | ||
| PDLA | 25 °C | 2 | - | 46 | - | |
| 90 °C | 31 | - | 59 | - | ||
| 120 °C | 35 | - | 59 | - | ||
| 135 °C | 37 | - | 52 | - | ||
| PLLA | water | 5.3 | - | 64.7 | 3.4 | [148] |
| air | 6.9 | - | 70.6 | 3.4 | ||
| annealed | 51.2 | - | 67.3 | 3.5 | ||
| PDLA | water | 11.2 | - | 64.2 | 3.4 | |
| air | 34 | - | 69.3 | 3.4 | ||
| annealed | 39.6 | - | 70 | 3.8 | ||
5.2. Shear-Induced Processing Techniques
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rydh, C.J.; Sun, M. Life cycle inventory data for materials grouped according to environmental and material properties. J. Clean. Prod. 2005, 13, 1258–1268. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Zhang, N. A Review of Microinjection Moulding of Polymeric Micro Devices. Micromachines 2022, 13, 1530. [Google Scholar] [CrossRef]
- Helanto, K.; Matikainen, L.; Talja, R.; Rojas, O.J. Bio-based polymers for sustainable packaging and biobarriers: A critical review. BioResources 2019, 14, 4902–4951. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.-X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2021, 7, 83–103. [Google Scholar] [CrossRef]
- Hosseinnezhad, R.; Vozniak, I.; Morawiec, J.; Galeski, A.; Dutkiewicz, S. In situ generation of sustainable PLA-based nanocomposites by shear induced crystallization of nanofibrillar inclusions. RSC Adv. 2019, 9, 30370–30380. [Google Scholar] [CrossRef]
- Kuang, T.; Esmaeili, A.; Ehsani, M. Eco-friendly biodegradable polymers: Sustainable future. Polym. Renew. Resour. 2022, 13, 71–79. [Google Scholar] [CrossRef]
- Mtibe, A.; Motloung, M.P.; Bandyopadhyay, J.; Ray, S.S. Synthetic Biopolymers and Their Composites: Advantages and Limitations—An Overview. Macromol. Rapid Commun. 2021, 42, 2100130. [Google Scholar] [CrossRef]
- Morini, A.A.; Ribeiro, M.J.; Hotza, D. Early-stage materials selection based on embodied energy and carbon footprint. Mater. Des. 2019, 178, 107861. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Abdy, C.; Zhang, Y.; Wang, J.; Yang, Y.; Artamendi, I.; Allen, B. Pyrolysis of polyolefin plastic waste and potential applications in asphalt road construction: A technical review. Resour. Conserv. Recycl. 2022, 180, 106213. [Google Scholar] [CrossRef]
- Gao, P.; Masato, D.; Kundu, A.; Coulter, J.P. An Investigation on the Efficacy of Orotic Acid as a Bio-Nucleating Agent for Poly-Lactic Acid under Quiescent Condition and Injection Molding. Micromachines 2022, 13, 2186. [Google Scholar] [CrossRef]
- Gao, P.; Kundu, A.; Coulter, J. Vibration-assisted injection molding: An efficient process for enhanced crystallinity development and mechanical characteristics for poly lactic acid. Int. J. Adv. Manuf. Technol. 2022, 121, 3111–3124. [Google Scholar] [CrossRef]
- Ghosh, S.; Viana, J.; Reis, R.; Mano, J. Effect of processing conditions on morphology and mechanical properties of injection-molded poly(L-lactic acid). Polym. Eng. Sci. 2007, 47, 1141–1147. [Google Scholar] [CrossRef]
- Tábi, T.; Sajó, I.E.; Szabó, F.; Luyt, A.S.; Kovacs, J.G. Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Joseph, B.; James, J.; Kalarikkal, N.; Thomas, S. Recycling of medical plastics. Adv. Ind. Eng. Polym. Res. 2021, 4, 199–208. [Google Scholar] [CrossRef]
- Auras, R. Poly(Lactic Acid); Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Lactic Acid. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lactic-Acid (accessed on 10 April 2024).
- U.S. Food and Drug Administration. Inventory of Effective Food Contact Substance (FCS) Notifications. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=FCN (accessed on 14 March 2024).
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.-C.; Södergård, A. From lactic acid to poly(lactic acid) (PLA): Characterization and analysis of PLA and its precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Lei, Z. Synthesis of high molecular weight polylactic acid from aqueous lactic acid co-catalyzed by tin(II)chloride dihydrate and succinic anhydride. Chin. Sci. Bull. 2005, 50, 2390. [Google Scholar] [CrossRef]
- Woo, S.I.; Kim, B.O.; Jun, H.S.; Chang, H.N. Polymerization of aqueous lactic acid to prepare high molecular weight poly(lactic acid) by chain-extending with hexamethylene diisocyanate. Polym. Bull. 1995, 35, 415–421. [Google Scholar] [CrossRef]
- Proikakis, C.S.; Tarantili, P.A.; Andreopoulos, A.G. Synthesis and Characterization of Low Molecular Weight Polylactic Acid. J. Elastomers Plast. 2002, 34, 49–63. [Google Scholar] [CrossRef]
- Robert, J.L.; Aubrecht, K.B. Ring-Opening Polymerization of Lactide To Form a Biodegradable Polymer. J. Chem. Educ. 2008, 85, 258. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K. Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). Materials 2016, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Sawyer, D.J. Bioprocessing–no longer a field of dreams. Macromol. Symp. 2003, 201, 271–282. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Williams, J.S.; Lewis, D.N. Melt rheology of poly(lactic acid): Entanglement and chain architecture effects. J. Rheol. 1999, 43, 1141–1155. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Modification of Poly(lactic acid) Films: Enhanced Wettability from Surface-Confined Photografting and Increased Degradation Rate Due to an Artifact of the Photografting Process. Macromolecules 2004, 37, 9151–9159. [Google Scholar] [CrossRef]
- Tsuji, H. Autocatalytic hydrolysis of amorphous-made polylactides: Effects of l-lactide content, tacticity, and enantiomeric polymer blending. Polymer 2002, 43, 1789–1796. [Google Scholar] [CrossRef]
- Ghosh, S.; Viana, J.C.; Reis, R.L.; Mano, J.F. Oriented morphology and enhanced mechanical properties of poly(l-lactic acid) from shear controlled orientation in injection molding. Mater. Sci. Eng. A 2008, 490, 81–89. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Foglia, F.; De Meo, A.; Iozzino, V.; Volpe, V.; Pantani, R. Isothermal crystallization of PLA: Nucleation density and growth rates of α and α′ phases. Can. J. Chem. Eng. 2020, 98, 1998–2007. [Google Scholar] [CrossRef]
- Boruvka, M.; Behalek, L.; Lenfeld, P.; Brdlik, P.; Habr, J.; Wongmanee, S.; Bobek, J.; Pechociakova, M. Solid and microcellular polylactide nucleated with PLA stereocomplex and cellulose nanocrystals. J. Therm. Anal. Calorim. 2020, 142, 695–713. [Google Scholar] [CrossRef]
- Longo, A.; Di Maio, E.; Di Lorenzo, M.L. Heterogeneous Bubble Nucleation by Homogeneous Crystal Nuclei in Poly(l-Lactic Acid) Foaming. Macromol. Chem. Phys. 2022, 223, 2100428. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, B.; Fan, B.; Sun, H.; Zhang, H. Enhanced Nonisothermal Crystallization and Heat Resistance of Poly(l-lactic acid) by d-Sorbitol as a Homogeneous Nucleating Agent. ACS Macro Lett. 2021, 10, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhou, Z.; Yu, S.; Chen, Z.; Xiang, H.; Zhu, M. The synergistic effect of heterogeneous nucleation and stress-induced crystallization on supramolecular structure and performances of poly(lactic acid) melt-spun fibers. Int. J. Biol. Macromol. 2023, 226, 1579–1587. [Google Scholar] [CrossRef]
- Kobayashi, J.; Asahi, T.; Ichiki, M.; Oikawa, A.; Suzuki, H.; Watanabe, T.; Fukada, E.; Shikinami, Y. Structural and optical properties of poly lactic acids. J. Appl. Phys. 1995, 77, 2957–2973. [Google Scholar] [CrossRef]
- De Santis, P.; Kovacs, A.J. Molecular conformation of poly(s-lactic acid). Biopolymers 1968, 6, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Brinke, G.T.; Zugenmaier, P. Crystal Structure, Conformation And Morphology Of Solution-Spun Poly(l-Lactide) Fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- Puiggali, J.; Ikada, Y.; Tsuji, H.; Cartier, L.; Okihara, T.; Lotz, B. The frustrated structure of poly(l-lactide). Polymer 2000, 41, 8921–8930. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Ikada, Y.; Tsuji, H.; Puiggali, J.; Lotz, B. Epitaxial crystallization and crystalline polymorphism of polylactides. Polymer 2000, 41, 8909–8919. [Google Scholar] [CrossRef]
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.-I.; Tsuji, H.; Hyon, S.-H.; Ikada, Y. Crystal structure of stereocomplex of poly(l-lactide) and poly(d-lactide). J. Macromol. Sci. Part B 1991, 30, 119–140. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(l-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Investigation of phase transitional behavior of poly(L-lactide)/poly(D-lactide) blend used to prepare the highly-oriented stereocomplex. Macromolecules 2007, 40, 1049–1054. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous crystallization and multiple melting behavior of poly(l-lactide): Molecular weight dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Shao, J.; Xiang, S.; Bian, X.; Sun, J.; Li, G.; Chen, X. Remarkable melting behavior of PLA stereocomplex in linear PLLA/PDLA blends. Ind. Eng. Chem. Res. 2015, 54, 2246–2253. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Lotz, B. Triangular Polymer Single Crystals: Stereocomplexes, Twins, and Frustrated Structures. Macromolecules 1997, 30, 6313–6322. [Google Scholar] [CrossRef]
- Han, L.; Pan, P.; Shan, G.; Bao, Y. Stereocomplex crystallization of high-molecular-weight poly(l-lactic acid)/poly(d-lactic acid) racemic blends promoted by a selective nucleator. Polymer 2015, 63, 144–153. [Google Scholar] [CrossRef]
- Ma, P.; Shen, T.; Xu, P.; Dong, W.; Lemstra, P.J.; Chen, M. Superior Performance of Fully Biobased Poly(lactide) via Stereocomplexation-Induced Phase Separation: Structure versus Property. ACS Sustain. Chem. Eng. 2015, 3, 1470–1478. [Google Scholar] [CrossRef]
- Muthukumar, M. Modeling Polymer Crystallization BT—Interphases and Mesophases in Polymer Crystallization III; Allegra, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 241–274. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Müller, A.J. Estimation of the nucleation and crystal growth contributions to the overall crystallization energy barrier. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1478–1487. [Google Scholar] [CrossRef]
- Welch, P.; Muthukumar, M. Molecular Mechanisms of Polymer Crystallization from Solution. Phys. Rev. Lett. 2001, 87, 218302. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S. Activation Energies and Temperature Dependencies of the Rates of Crystallization and Melting of Polymers. Polymers 2020, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the crystallinity and mechanical properties of PLA by nucleation and annealing. Polym. Degrad. Stab. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Youbing, L.; Xueqin, G.; Shaoyan, Y.; Yi, Y.; Jie, Z.; Wenti, K.; Kaizhi, S. The relationship between mechanical properties and morphology of vibration-injection-molded polyethylene. Polym. Int. 2005, 54, 240–245. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2007, 107, 2246–2255. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Following the morphological and thermal properties of PLA/PEO blends containing carbon nanotubes (CNTs) during hydrolytic degradation. Compos. Part B Eng. 2019, 175, 107132. [Google Scholar] [CrossRef]
- Arrieta, M.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J. Multifunctional PLA–PHB/cellulose nanocrystal films: Processing, structural and thermal properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Mazuki, N.; Nagao, Y.; Kufian, M.; Samsudin, A. The influences of PLA into PMMA on crystallinity and thermal properties enhancement-based hybrid polymer in gel properties. Mater. Today Proc. 2020, 49, 3105–3111. [Google Scholar] [CrossRef]
- Vidović, E.; Faraguna, F.; Jukić, A. Influence of inorganic fillers on PLA crystallinity and thermal properties. J. Therm. Anal. Calorim. 2017, 127, 371–380. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Sammut, P.; Park, C.B. Evidence of a dual network/spherulitic crystalline morphology in PLA stereocomplexes. Polymer 2012, 53, 5816–5824. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Righetti, M.C.; Gazzano, M.; Lazzeri, A. Effect of nucleating agents on crystallinity and properties of poly (lactic acid) (PLA). Eur. Polym. J. 2017, 93, 822–832. [Google Scholar] [CrossRef]
- Castillo, R.; Müller, A. Crystallization and morphology of biodegradable or biostable single and double crystalline block copolymers. Prog. Polym. Sci. 2009, 34, 516–560. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Chakoli, A.N.; Sui, J.; Amirian, M.; Cai, W. Crystallinity of biodegradable polymers reinforced with functionalized carbon nanotubes. J. Polym. Res. 2011, 18, 1249–1259. [Google Scholar] [CrossRef]
- Wang, J.; Ayari, M.A.; Khandakar, A.; Chowdhury, M.E.H.; Zaman, S.A.U.; Rahman, T.; Vaferi, B. Estimating the Relative Crystallinity of Biodegradable Polylactic Acid and Polyglycolide Polymer Composites by Machine Learning Methodologies. Polymers 2022, 14, 527. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, X.; Song, Y.; de Vos, S.; Wang, R.; Joziasse, C.A.; Liu, G.; Wang, D. Effect of nucleating agents on the strain-induced crystallization of poly(l-lactide). Polymer 2015, 65, 223–232. [Google Scholar] [CrossRef]
- Kolstad, J.J. Crystallization kinetics of poly(l-lactide-co-meso-lactide). J. Appl. Polym. Sci. 1996, 62, 1079–1091. [Google Scholar] [CrossRef]
- Refaa, Z.; Boutaous, M.; Xin, S.; Fulchiron, R. Synergistic effects of shear flow and nucleating agents on the crystallization mechanisms of Poly (Lactic Acid). J. Polym. Res. 2017, 24, 18. [Google Scholar] [CrossRef]
- Tsuji, H.; Takai, H.; Fukuda, N.; Takikawa, H. Non-isothermal crystallization behavior of poly(l-lactic acid) in the presence of various additives. Macromol. Mater. Eng. 2006, 291, 325–335. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, P.; Xu, P.; Wang, R.; Dong, W.; Chen, M.; Joziasse, C. The crystallization behavior of poly(lactic acid) with different types of nucleating agents. Int. J. Biol. Macromol. 2018, 106, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Battegazzore, D.; Bocchini, S.; Frache, A. Crystallization kinetics of poly(lactic acid)-talc composites. Express Polym. Lett. 2011, 5, 849–858. [Google Scholar] [CrossRef]
- Fowlks, A.C.; Narayan, R. The effect of maleated polylactic acid (PLA) as an interfacial modifier in PLA-talc composites. J. Appl. Polym. Sci. 2010, 118, 2810–2820. [Google Scholar] [CrossRef]
- Jain, S.; Misra, M.; Mohanty, A.K.; Ghosh, A.K. Thermal, mechanical and rheological behavior of poly (lactic acid)/talc composites. J. Polym. Environ. 2012, 20, 1027–1037. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pérez-Camargo, R.A.; Fürst, C.; Kucharczyk, P.; Müller, A.J. Nucleating efficiency and thermal stability of industrial non-purified lignins and ultrafine talc in poly(lactic acid) (PLA). Polym. Degrad. Stab. 2017, 142, 244–254. [Google Scholar] [CrossRef]
- Yu, F.; Liu, T.; Zhao, X.; Yu, X.; Lu, A.; Wang, J. Effects of talc on the mechanical and thermal properties of polylactide. J. Appl. Polym. Sci. 2012, 125, E99–E109. [Google Scholar] [CrossRef]
- Kim, M.W.; Song, Y.S.; Youn, J.R. Effects of interfacial adhesion and crystallization on the thermoresistance of poly (lactic acid)/mica composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1817–1822. [Google Scholar] [CrossRef]
- Fukushima, K.; Giménez, E.; Cabedo, L.; Lagarón, J.; Feijoo, J. Biotic degradation of poly(dl-lactide) based nanocomposites. Polym. Degrad. Stab. 2012, 97, 1278–1284. [Google Scholar] [CrossRef]
- Girdthep, S.; Limwanich, W.; Punyodom, W. Non-isothermal cold crystallization, melting, and moisture barrier properties of silver-loaded kaolinite filled poly (lactic acid) films. Mater. Chem. Phys. 2022, 276, 125227. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, W.; Zhang, C.; Chen, X.; Duan, S.; Fu, H. Synthesis and electrospinning of multiscale-ordered PLA/LDH@ AgGB composite nanofibrous membrane for antibacterial and oil–water separation. J. Appl. Polym. Sci. 2022, 139, e52621. [Google Scholar] [CrossRef]
- Delpouve, N.; Saiter-Fourcin, A.; Coiai, S.; Cicogna, F.; Spiniello, R.; Oberhauser, W.; Passaglia, E. Effects of organo-LDH dispersion on thermal stability, crystallinity and mechanical features of PLA. Polymer 2020, 208, 122952. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Liu, S.; Cheng, X.; Zhang, C. CNT@LDH functionalized poly(lactic acid) membranes with super oil–water separation and real-time press sensing properties. Polym. Compos. 2022, 43, 6548–6559. [Google Scholar] [CrossRef]
- Li, K.; Bian, S.; Zhen, W.; Li, H.; Zhao, L. Performance, crystallization and rheological behavior of poly (lactic acid)/N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride intercalated vermiculite grafted poly (acrylamide) nanocomposites. React. Funct. Polym. 2021, 158, 104791. [Google Scholar] [CrossRef]
- Khammassi, S.; Tarfaoui, M.; Škrlová, K.; Měřínská, D.; Plachá, D.; Erchiqui, F. Poly (Lactic acid)(PLA)-Based nanocomposites: Impact of vermiculite, silver, and graphene oxide on thermal stability, isothermal crystallization, and local mechanical behavior. J. Compos. Sci. 2022, 6, 112. [Google Scholar] [CrossRef]
- Li, H.; Jilili, Y.; Zhen, W. Poly (lactic acid)/vermiculite-g-polyisoprene nanocomposites based on thiol-ene click chemistry: Performance, shear crystallization and Rheonaut technology analysis. Polym. Int. 2021, 70, 1570–1581. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- As’habi, L.; Jafari, S.H.; Khonakdar, H.A.; Häussler, L.; Wagenknecht, U.; Heinrich, G. Non-isothermal crystallization behavior of PLA/LLDPE/nanoclay hybrid: Synergistic role of LLDPE and clay. Thermochim. Acta 2013, 565, 102–113. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Wu, L.; Xu, B.; Zhang, Y.; Zhang, M. Nonisothermal cold crystallization behavior and kinetics of polylactide/clay nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1100–1113. [Google Scholar] [CrossRef]
- Pluta, M. Morphology and properties of polylactide modified by thermal treatment, filling with layered silicates and plasticization. Polymer 2004, 45, 8239–8251. [Google Scholar] [CrossRef]
- Nam, J.Y.; Sinha Ray, S.; Okamoto, M. Crystallization Behavior and Morphology of Biodegradable Polylactide/Layered Silicate Nanocomposite. Macromolecules 2003, 36, 7126–7131. [Google Scholar] [CrossRef]
- Day, M.; Nawaby, A.V.; Liao, X. A DSC study of the crystallization behaviour of polylactic acid and its nanocomposites. J. Therm. Anal. Calorim. 2006, 86, 623–629. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Qin, Y.; Guo, H. Effects of Processing Conditions and Plasticizing-Reinforcing Modification on the Crystallization and Physical Properties of PLA Films. Membranes 2021, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Soriano, E.; Hernández-Muñoz, P.; Gavara, R. Migration of Antimicrobial Silver from Composites of Polylactide with Silver Zeolites. J. Food Sci. 2010, 75, E186–E193. [Google Scholar] [CrossRef] [PubMed]
- Yuzay, I.E.; Auras, R.; Soto-Valdez, H.; Selke, S. Effects of synthetic and natural zeolites on morphology and thermal degradation of poly(lactic acid) composites. Polym. Degrad. Stab. 2010, 95, 1769–1777. [Google Scholar] [CrossRef]
- Yuzay, I.E.; Auras, R.; Selke, S. Poly(lactic acid) and zeolite composites prepared by melt processing: Morphological and physical–mechanical properties. J. Appl. Polym. Sci. 2010, 115, 2262–2270. [Google Scholar] [CrossRef]
- Cai, Y.-H. Influence of Ethylene bis-Stearamide on Crystallization Behaviour of Poly(l-lactide). Asian J. Chem. 2013, 25, 6219–6221. [Google Scholar] [CrossRef]
- Nam, J.Y.; Okamoto, M.; Okamoto, H.; Nakano, M.; Usuki, A.; Matsuda, M. Morphology and crystallization kinetics in a mixture of low-molecular weight aliphatic amide and polylactide. Polymer 2006, 47, 1340–1347. [Google Scholar] [CrossRef]
- Khwanpipat, T.; Seadan, M.; Suttiruengwong, S. Effect of PDLA and Amide Compounds as Mixed Nucleating Agents on Crystallization Behaviors of Poly (l-lactic Acid). Materials 2018, 11, 1139. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, C.; Liu, X.; Zhu, J. The crystallization behavior and mechanical properties of polylactic acid in the presence of a crystal nucleating agent. J. Appl. Polym. Sci. 2012, 125, 1108–1115. [Google Scholar] [CrossRef]
- Xing, Q.; Zhang, X.; Dong, X.; Liu, G.; Wang, D. Low-molecular weight aliphatic amides as nucleating agents for poly (l-lactic acid): Conformation variation induced crystallization enhancement. Polymer 2012, 53, 2306–2314. [Google Scholar] [CrossRef]
- Xing, Q.; Li, R.; Zhang, X.; Dong, X.; Wang, D.; Zhang, L. Tailoring crystallization behavior of poly (l-lactide) with a low molecular weight aliphatic amide. Colloid Polym. Sci. 2015, 293, 3573–3583. [Google Scholar] [CrossRef]
- Leoné, N.; Roy, M.; Saidi, S.; de Kort, G.; Hermida-Merino, D.; Wilsens, C.H.R.M. Improving processing, crystallization, and performance of poly-l-lactide with an amide-based organic compound as both plasticizer and nucleating agent. ACS Omega 2019, 4, 10376–10387. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Gui, B.; Gu, M.; Ren, J. Crystallization morphology and crystallization kinetics of poly(lactic acid): Effect of N-Aminophthalimide as nucleating agent. Polym. Bull. 2011, 67, 775–791. [Google Scholar] [CrossRef]
- He, D.; Wang, Y.; Shao, C.; Zheng, G.; Li, Q.; Shen, C. Effect of phthalimide as an efficient nucleating agent on the crystallization kinetics of poly(lactic acid). Polym. Test. 2013, 32, 1088–1093. [Google Scholar] [CrossRef]
- Su, L.; Zou, J.; Dong, S.; Hao, N.; Xu, H. Influence of different β-nucleation agents on poly (l-lactic acid): Structure, morphology, and dynamic mechanical behavior. RSC Adv. 2017, 7, 55364–55370. [Google Scholar] [CrossRef]
- Xu, X.; Zhen, W.; Bian, S. Structure, performance and crystallization behavior of poly (lactic acid)/humic acid amide composites. Polym.-Plast. Technol. Eng. 2018, 57, 1858–1872. [Google Scholar] [CrossRef]
- Liu, P.; Zhen, W. Structure-property relationship, rheological behavior, and thermal degradability of poly (lactic acid)/fulvic acid amide composites. Polym. Adv. Technol. 2018, 29, 2192–2203. [Google Scholar] [CrossRef]
- Gui, Z.; Lu, C.; Cheng, S. Comparison of the effects of commercial nucleation agents on the crystallization and melting behaviour of polylactide. Polym. Test. 2013, 32, 15–21. [Google Scholar] [CrossRef]
- Bai, H.; Huang, C.; Xiu, H.; Zhang, Q.; Fu, Q. Enhancing mechanical performance of polylactide by tailoring crystal morphology and lamellae orientation with the aid of nucleating agent. Polymer 2014, 55, 6924–6934. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, W.; Deng, H.; Zhang, Q.; Fu, Q. Control of crystal morphology in poly(l-lactide) by adding nucleating agent. Macromolecules 2011, 44, 1233–1237. [Google Scholar] [CrossRef]
- Li, C.; Luo, S.; Wang, J.; Wu, H.; Guo, S.; Zhang, X. Conformational regulation and crystalline manipulation of PLLA through a self-assembly nucleator. Biomacromolecules 2017, 18, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, Y.; Qu, J.-P. Manufacturing high-performance polylactide by constructing 3D network crystalline structure with adding self-assembly nucleator. Ind. Eng. Chem. Res. 2022, 61, 4567–4578. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, H.; Liu, W.; Zhang, M.; Du, Z.; Wang, X. The synergistic effect of zinc oxide and phenylphosphonic acid zinc salt on the crystallization behavior of poly (lactic acid). Polym. Degrad. Stab. 2015, 122, 25–35. [Google Scholar] [CrossRef]
- Wittmann, J.; Lotz, B. Epitaxial crystallization of polymers on organic and polymeric substrates. Prog. Polym. Sci. 1990, 15, 909–948. [Google Scholar] [CrossRef]
- Yang, T.-C.; Hung, K.-C.; Wu, T.-L.; Wu, T.-M.; Wu, J.-H. A comparison of annealing process and nucleating agent (zinc phenylphosphonate) on the crystallization, viscoelasticity, and creep behavior of compression-molded poly (lactic acid) blends. Polym. Degrad. Stab. 2015, 121, 230–237. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Matykiewicz, D.; Skórczewska, K.; Lewandowski, K.; Andrzejewski, J.; Piasecki, A. Development of polylactide composites with improved thermomechanical properties by simultaneous use of basalt powder and a nucleating agent. Polym. Compos. 2020, 41, 2947–2957. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Crystallization behavior and morphology of polylactic acid (PLA) with aromatic sulfonate derivative. J. Appl. Polym. Sci. 2016, 133, 43673. [Google Scholar] [CrossRef]
- Jongpanya-Ngam, P.; Khankrua, R.; Seadan, M.; Suttiruengwong, S. Effect of synthesized sulfonate derivatives as nucleating agents on crystallization behavior of poly(lactic acid). Des. Monomers Polym. 2022, 25, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, P.; Najafi, N.; Kontopoulou, M. Advances in peroxide-initiated graft modification of thermoplastic biopolyesters by reactive extrusion. Can. J. Chem. Eng. 2021, 99, 1870–1884. [Google Scholar] [CrossRef]
- Yokohara, T.; Yamaguchi, M. Structure and properties for biomass-based polyester blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarría, G.; Eguiazábal, J.I. Melt processed PLA/PCL blends: Effect of processing method on phase structure, morphology, and mechanical properties. J. Appl. Polym. Sci. 2015, 132, 42641. [Google Scholar] [CrossRef]
- Liu, W.; Chen, P.; Wang, X.; Wang, F.; Wu, Y. Effects of Poly(butyleneadipate-co-terephthalate) as a Macromolecular Nucleating Agent on the Crystallization and Foaming Behavior of Biodegradable Poly(lactic acid). Cell. Polym. 2017, 36, 75–96. [Google Scholar] [CrossRef]
- Gao, P.; Alanazi, S.; Masato, D. Crystallization of Polylactic Acid with Organic Nucleating Agents under Quiescent Conditions. Polymers 2024, 16, 320. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, A.; Thomas, N. Talc as a nucleating agent and reinforcing filler in poly(lactic acid) composites. Polym. Eng. Sci. 2014, 54, 64–70. [Google Scholar] [CrossRef]
- Xu, P.; Tian, H.; Han, L.; Yang, H.; Bian, J.; Pan, H.; Zhang, H. Improved heat resistance in poly (lactic acid)/ethylene butyl methacrylate glycidyl methacrylate terpolymer blends by controlling highly filled talc particles. J. Therm. Anal. Calorim. 2022, 147, 5719–5732. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Hejna, A.; Biskup, R.; Szulc, J.; Michałowski, S.; Piasecki, A.; Kloziński, A. The Effect of Surface Treatment with Isocyanate and Aromatic Carbodiimide of Thermally Expanded Vermiculite Used as a Functional Filler for Polylactide-Based Composites. Polymers 2021, 13, 890. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, K.; Li, P.; He, X.; Li, W.; Wu, Y. Effect of nano-SiO2 on the compatibility interface and properties of polylactic acid-grafted-bamboo fiber/polylactic acid composite. Int. J. Biol. Macromol. 2020, 157, 177–186. [Google Scholar] [CrossRef]
- Kawamoto, N.; Sakai, A.; Horikoshi, T.; Urushihara, T.; Tobita, E. Physical and mechanical properties of poly(L-lactic acid) nucleated by dibenzoylhydrazide compound. J. Appl. Polym. Sci. 2007, 103, 244–250. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.-N.; Huang, Z.-G.; Weng, Y.-X. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater. Des. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Duan, K.; Zhen, W. The synthesis of poly (lactic acid)-fulvic acid graft polymer and its effect on the crystallization and performance of poly (lactic acid). Polym. Technol. Mater. 2019, 58, 1875–1888. [Google Scholar] [CrossRef]
- Refaa, Z.; Boutaous, M.; Siginer, D.A. PLA crystallization kinetics and morphology development. Int. Polym. Process. 2018, 33, 336–344. [Google Scholar] [CrossRef]
- Pastorek, M.; Kovalcik, A. Effects of thermal annealing as polymer processing step on poly(lactic acid). Mater. Manuf. Process. 2018, 33, 1674–1680. [Google Scholar] [CrossRef]
- Srithep, Y.; Nealey, P.; Turng, L. Effects of annealing time and temperature on the crystallinity and heat resistance behavior of injection-molded poly(lactic acid). Polym. Eng. Sci. 2013, 53, 580–588. [Google Scholar] [CrossRef]
- Zisopol, D.G.; Portoaca, A.I.; Nae, I.; Ramadan, I. A Comparative Analysis of the Mechanical Properties of Annealed PLA. Eng. Technol. Appl. Sci. Res. 2022, 12, 8978–8981. [Google Scholar] [CrossRef]
- Chen, J.; Deng, C.; Hong, R.; Fu, Q.; Zhang, J. Effect of thermal annealing on crystal structure and properties of PLLA/PCL blend. J. Polym. Res. 2020, 27, 221. [Google Scholar] [CrossRef]
- Wang, S.; Pang, S.; Pan, L.; Xu, N.; Li, T. Isothermal Cold Crystallization, Heat Resistance, and Tensile Performance of Polylactide/Thermoplastic Polyester Elastomer (PLA/TPEE) Blends: Effects of Annealing and Reactive Compatibilizer. Polymers 2016, 8, 417. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Siqueira, D.D.; Araújo, E.M.; Wellen, R.M.R. Annealing efficacy on PLA. Insights on mechanical, thermomechanical and crystallinity characters. Momento 2021, 1–17. [Google Scholar] [CrossRef]
- Guinault, A.; Sollogoub, C.; Domenek, S.; Grandmontagne, A.; Ducruet, V. Influence of crystallinity on gas barrier and mechanical properties of pla food packaging films. Int. J. Mater. Form. 2010, 3, 603–606. [Google Scholar] [CrossRef]
- Sarasua, J.; Balerdi, P.; Maiza, I.; Arraiza, A.L. Crystallinity and mechanical properties of optically pure polylactides and their blends. Polym. Eng. Sci. 2005, 45, 745–753. [Google Scholar] [CrossRef]
- Rhoades, A.; Pantani, R. Poly(Lactic Acid): Flow-Induced Crystallization. In Thermal Properties of Bio-Based Polymers; Di Lorenzo, M.L., Androsch, R., Eds.; Springer International Publishing: Cham, Switerland, 2019; pp. 87–117. [Google Scholar] [CrossRef]
- Piccolo, L.; Puleo, K.; Sorgato, M.; Lucchetta, G.; Masato, D. Modeling the replication of submicron-structured surfaces by micro injection molding. Mater. Des. 2020, 198, 109272. [Google Scholar] [CrossRef]
- Kazmer, D.O.; Masato, D.; Piccolo, L.; Puleo, K.; Krantz, J.; Venoor, V.; Colon, A.; Limkaichong, J.; Dewar, N.; Babin, D.; et al. Multivariate Modeling of Mechanical Properties for Hot Runner Molded Bioplastics and a Recycled Polypropylene Blend. Sustainability 2021, 13, 8102. [Google Scholar] [CrossRef]
- Jalali, A.; Shahbikian, S.; Huneault, M.A.; Elkoun, S. Effect of molecular weight on the shear-induced crystallization of poly(lactic acid). Polymer 2017, 112, 393–401. [Google Scholar] [CrossRef]
- Jalali, A.; Huneault, M.A.; Nofar, M.; Lee, P.C.; Park, C.B. Effect of branching on flow-induced crystallization of poly (lactic acid). Eur. Polym. J. 2019, 119, 410–420. [Google Scholar] [CrossRef]
- Bojda, J.; Piorkowska, E. Shear-induced nonisothermal crystallization of two grades of PLA. Polym. Test. 2016, 50, 172–181. [Google Scholar] [CrossRef]
- Altpeter, H.; Bevis, M.J.; Grijpma, D.W.; Feijen, J. Non-conventional injection molding of poly(lactide) and poly(ε-caprolactone) intended for orthopedic applications. J. Mater. Sci. Mater. Med. 2004, 15, 175–184. [Google Scholar] [CrossRef]
- Eder, G.; Janeschitz-Kriegl, H. Theory of shear-induced crystallization of polymer melts. Colloid Polym. Sci. 1988, 266, 1087–1094. [Google Scholar] [CrossRef]
- Boutaous, M.; Bourgin, P.; Zinet, M. Thermally and flow induced crystallization of polymers at low shear rate. J. Non-Newton. Fluid Mech. 2010, 165, 227–237. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Shen, K.; Fu, Q.; Zhang, J. Insight into shear-induced modification for improving processability of polymers: Effect of shear rate on the evolution of entanglement state. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 598–606. [Google Scholar] [CrossRef]
- Jay, F.; Haudin, J.; Monasse, B. Shear-induced crystallization of polypropylenes: Effect of molecular weight. J. Mater. Sci. 1999, 34, 2089–2102. [Google Scholar] [CrossRef]
- Lagasse, R.R.; Maxwell, B. An experimental study of the kinetics of polymer crystallization during shear flow. Polym. Eng. Sci. 1976, 16, 189–199. [Google Scholar] [CrossRef]
- Mykhaylyk, O.O.; Chambon, P.; Impradice, C.; Fairclough, J.P.A.; Terrill, N.J.; Ryan, A.J. Control of Structural Morphology in Shear-Induced Crystallization of Polymers. Macromolecules 2010, 43, 2389–2405. [Google Scholar] [CrossRef]
- Krantz, J.; Nieduzak, Z.; Kazmer, E.; Licata, J.; Ferki, O.; Gao, P.; Sobkowicz, M.J.; Masato, D. Investigation of pressure-controlled injection molding on the mechanical properties and embodied energy of recycled high-density polyethylene. Sustain. Mater. Technol. 2023, 36, e00651. [Google Scholar] [CrossRef]
- Bowen, N.; Guyer, C.; Rippon, T.; Daly, M.; Gao, P.; Galati, V.; Lograsso, S.; Johnston, S.; Masato, D. Mechanical and crystallization properties of hot runner injection molded virgin and recycled polypropylene. Polym. Eng. Sci. 2024, 64, 2241–2255. [Google Scholar] [CrossRef]
- Lucchetta, G.; Masato, D.; Sorgato, M. Optimization of mold thermal control for minimum energy consumption in injection molding of polypropylene parts. J. Clean. Prod. 2018, 182, 217–226. [Google Scholar] [CrossRef]
- Gao, P.; Krantz, J.; Ferki, O.; Nieduzak, Z.; Perry, S.; Masato, D.; Sobkowicz, M.J. Ultrahigh-Speed Extrusion of Recycled Film-Grade LDPE and Injection Molding Characterization. In Technology Innovation for the Circular Economy: Recycling, Remanufacturing, Design, System Analysis and Logistics; Wiley: Hoboken, NJ, USA, 2024; pp. 321–332. [Google Scholar]


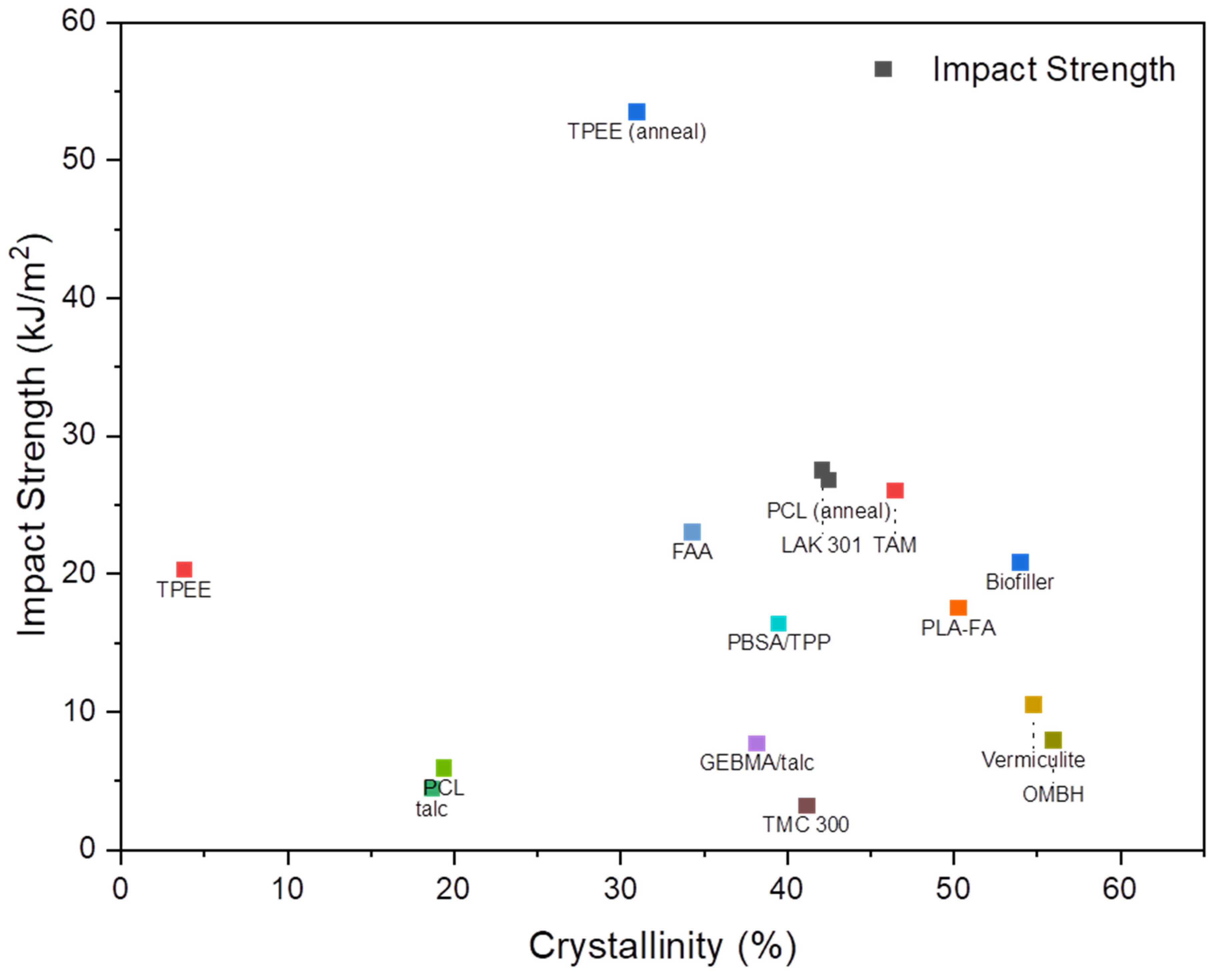
| PLA Blend | Crystallinity (%) | Processing, and Cooling Techniques * | Mechanical Properties | Reference | ||
|---|---|---|---|---|---|---|
| Impact Strength (kJ/m2) | Yield Stress (MPa) | Young’s Modulus (GPa) | ||||
| neat | 2 | CM, room temperature | 43.8 | 4.1 | [133] | |
| PLA–talc | 25 | 38 | 5.8 | |||
| neat | 3.1 | IM, 30 °C | 54.2 | 2.2 | [84] | |
| PLA–modified talc | 20.1 | 58.5 | 2 | |||
| neat | 18.7 | CM, 120 °C | 4.4 | 53 | [134] | |
| PLA–talc–GEBMA | 38.2 | 7.7 | 41.8 | |||
| neat | 49 | IM, 50 °C | 8 | [135] | ||
| Vermiculite | 54.8 | 10.5 | ||||
| neat | 25.3 | CM, room temperature | 14.5 | [136] | ||
| PLA–Biofiller–SiO2 | 28 | 20.1 | ||||
| neat | 26.1 | IM, 110 °C | 6.7 | [137] | ||
| PLA–TPP | 39.5 | 16.4 | ||||
| neat | 0.6 | CM, room temperature | 16.9 | 60.8 | [138] | |
| PLA–TMC328 | 20.0 | 24.2 | 58.6 | |||
| neat | 4.6 | IM, 75 °C | 9.5 | [139] | ||
| PLA–FA | 50.3 | 17.5 | ||||
| neat | 6.3 | IM, 65 °C | 10 | [116] | ||
| PLA–FAA | 34.3 | 23 | ||||
| neat | 5 | CM, 100 °C | 23 | [61] | ||
| PLA–LAK 301 | 42.1 | 27 | ||||
| PLA–TAM | 46.5 | 26 | ||||
| PLA–Biofiller | 54 | 16 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Masato, D. The Effects of Nucleating Agents and Processing on the Crystallization and Mechanical Properties of Polylactic Acid: A Review. Micromachines 2024, 15, 776. https://doi.org/10.3390/mi15060776
Gao P, Masato D. The Effects of Nucleating Agents and Processing on the Crystallization and Mechanical Properties of Polylactic Acid: A Review. Micromachines. 2024; 15(6):776. https://doi.org/10.3390/mi15060776
Chicago/Turabian StyleGao, Peng, and Davide Masato. 2024. "The Effects of Nucleating Agents and Processing on the Crystallization and Mechanical Properties of Polylactic Acid: A Review" Micromachines 15, no. 6: 776. https://doi.org/10.3390/mi15060776
APA StyleGao, P., & Masato, D. (2024). The Effects of Nucleating Agents and Processing on the Crystallization and Mechanical Properties of Polylactic Acid: A Review. Micromachines, 15(6), 776. https://doi.org/10.3390/mi15060776






