Viscosity Modeling for Blood and Blood Analog Fluids in Narrow Gap and High Reynolds Numbers Flows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Point and Model Development
- Starting Point
- Development of the Viscosity Model
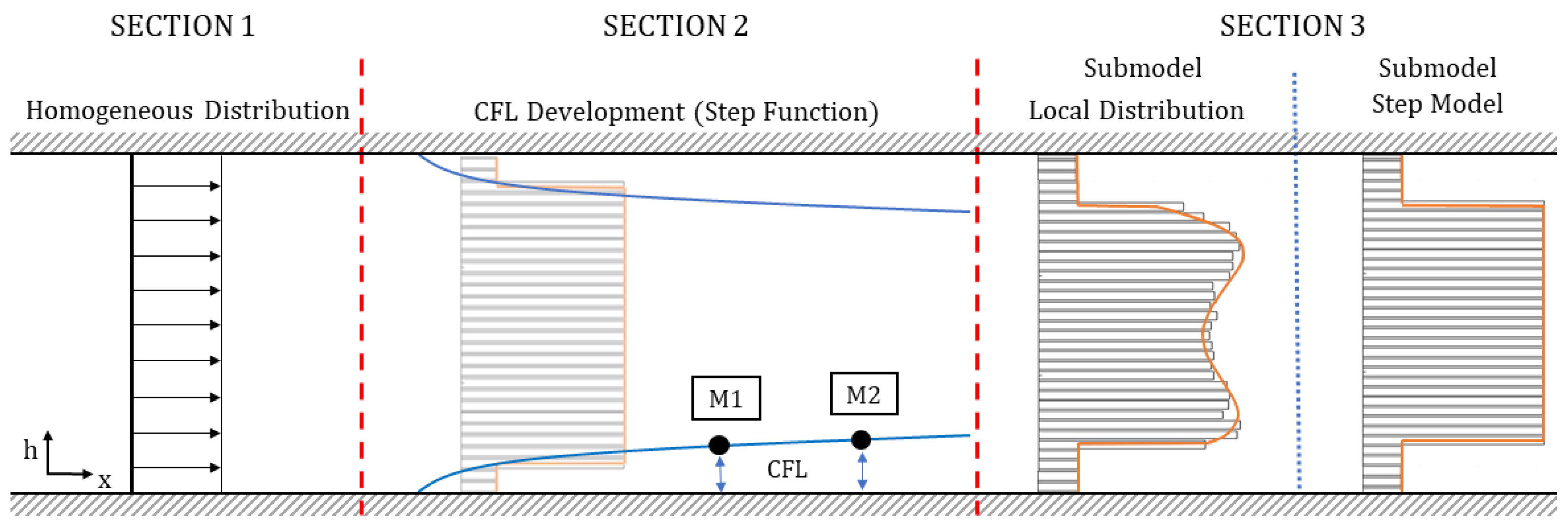
- Section 1—Homogeneous Distribution: The viscosity model is designed in such a way that the viscosity does not change only with channel height h, but also in the flow direction (x-axis), representing a particle-laden flow, which starts flowing from a homogeneous distribution. In Section 1, a homogeneous particle distribution is defined based on . It is assumed that this distribution will exist only over a short distance in flow direction, e.g., just for one micron. The definition of this length for the homogeneous distribution is empirical and is necessary for generating the CFL development discussed in Section 2.
- Section 2—CFL Development: The literature has shown that particle-laden flows exhibit a development length for particle migration to reach an equilibrium position and establish a steady-state CFL height [28].
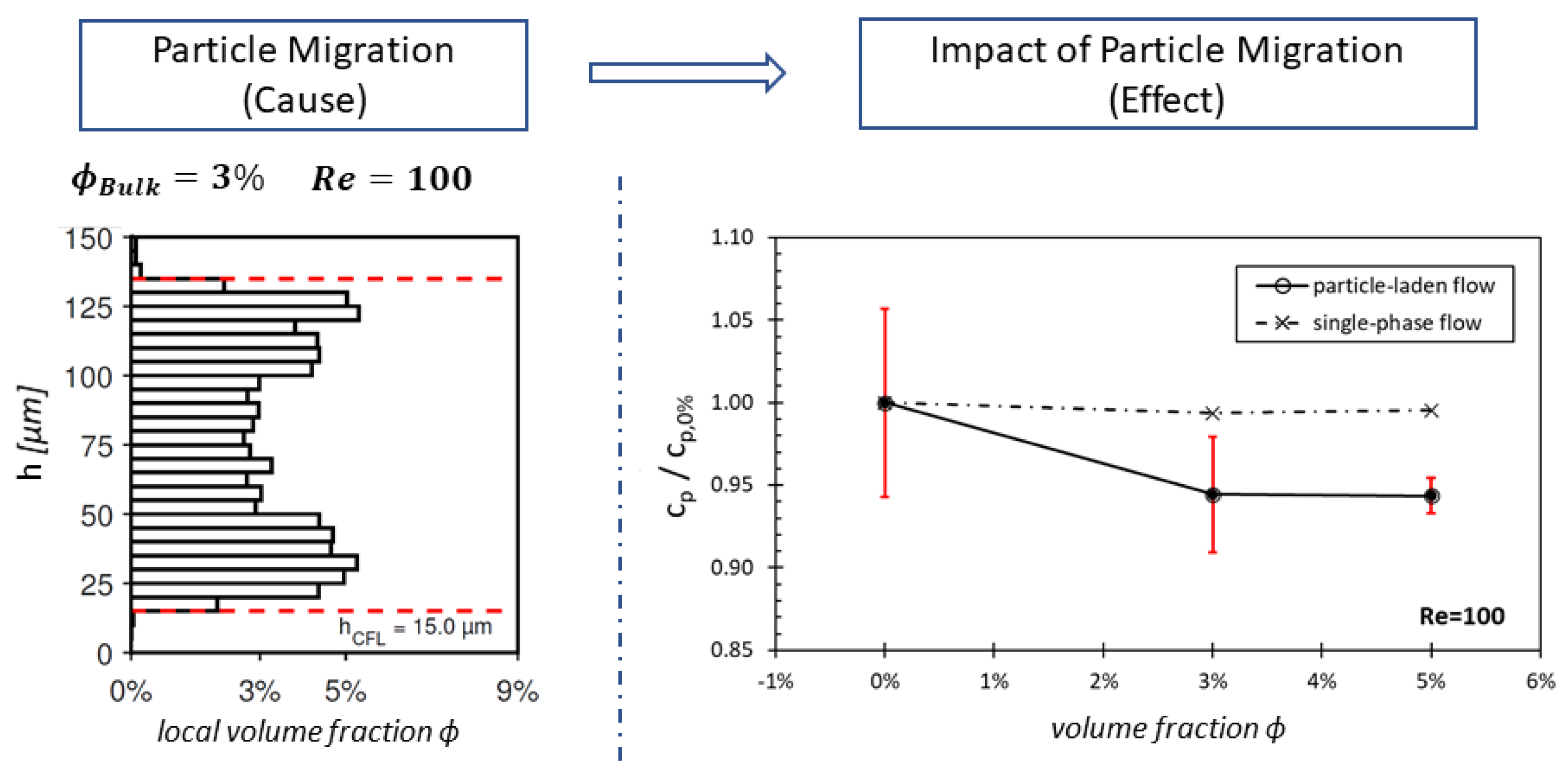
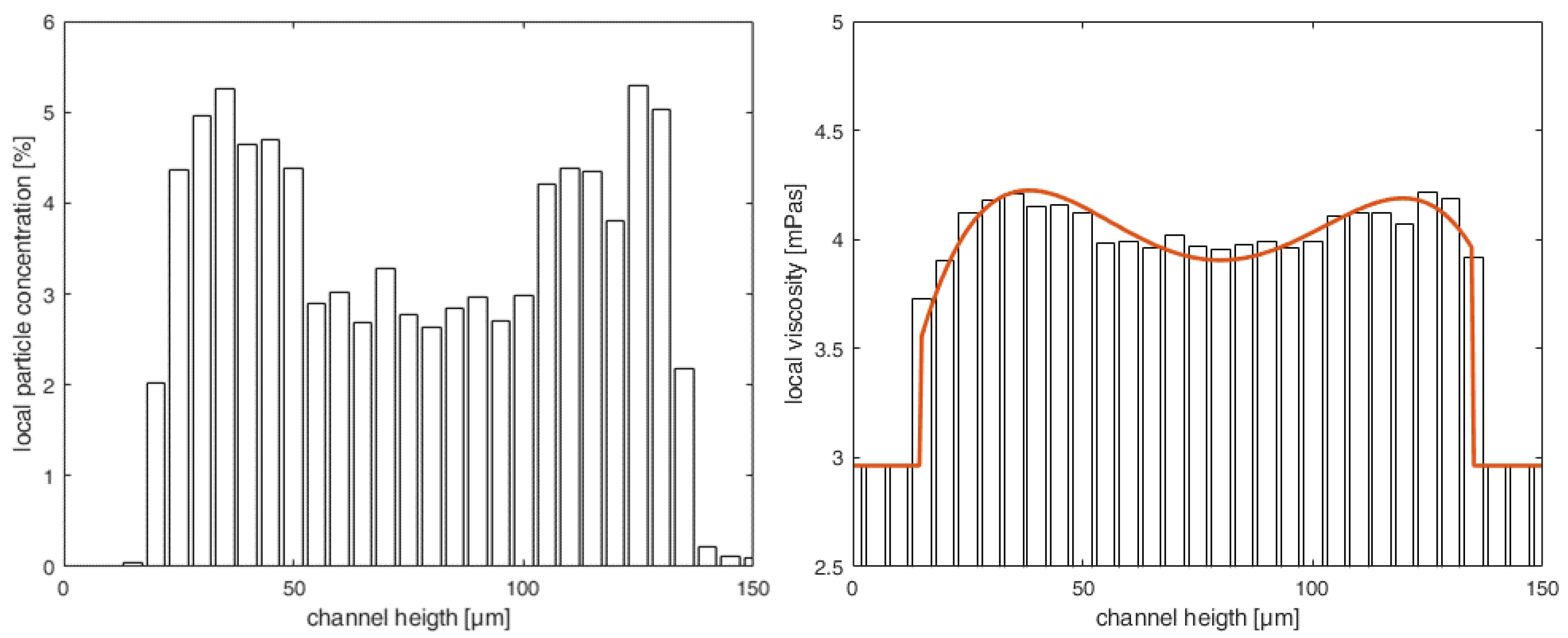
- Section 3—Heterogeneous distribution with steady-state CFL: Once the equilibrium position of the particles is attained and a steady-state CFL is established, the optically measured data from particle distributions (see Figure 3 and Figure 6) are utilized to define the heterogeneous viscosity distributions for the simulations.
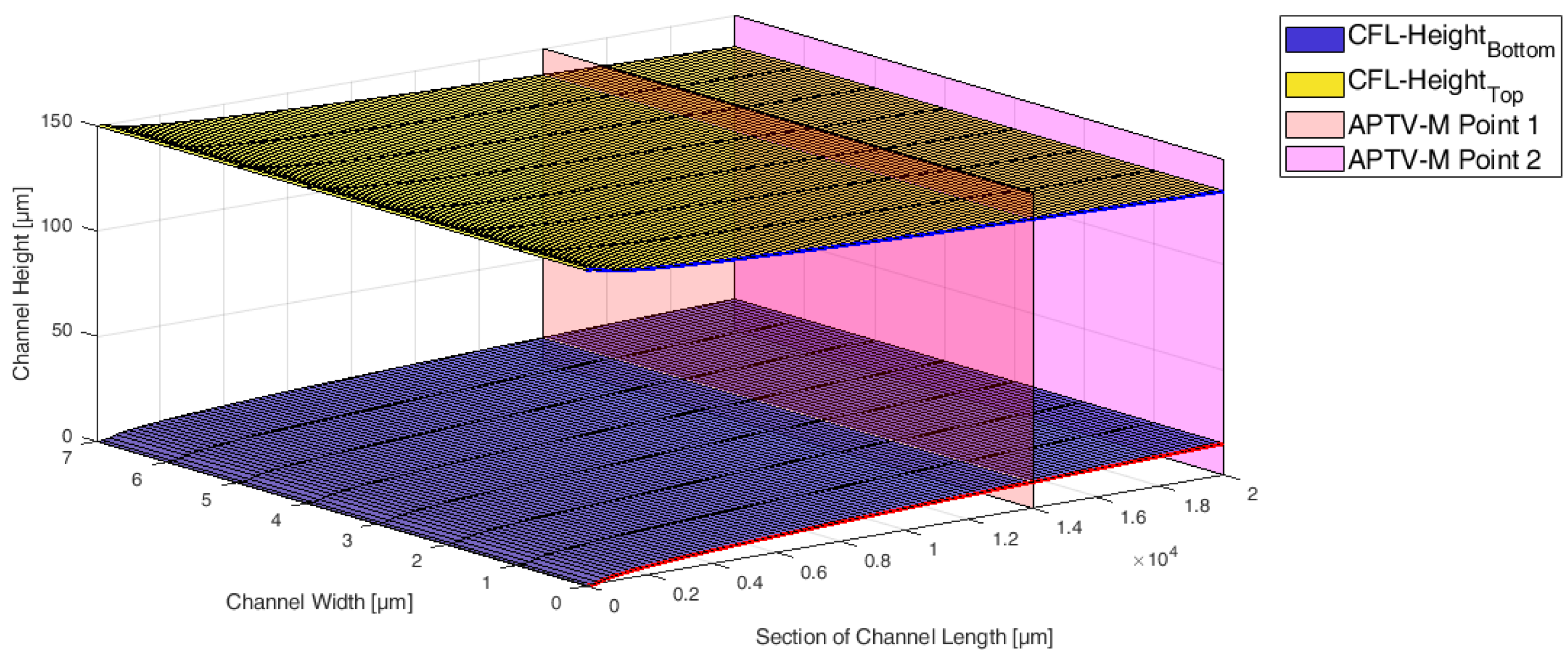
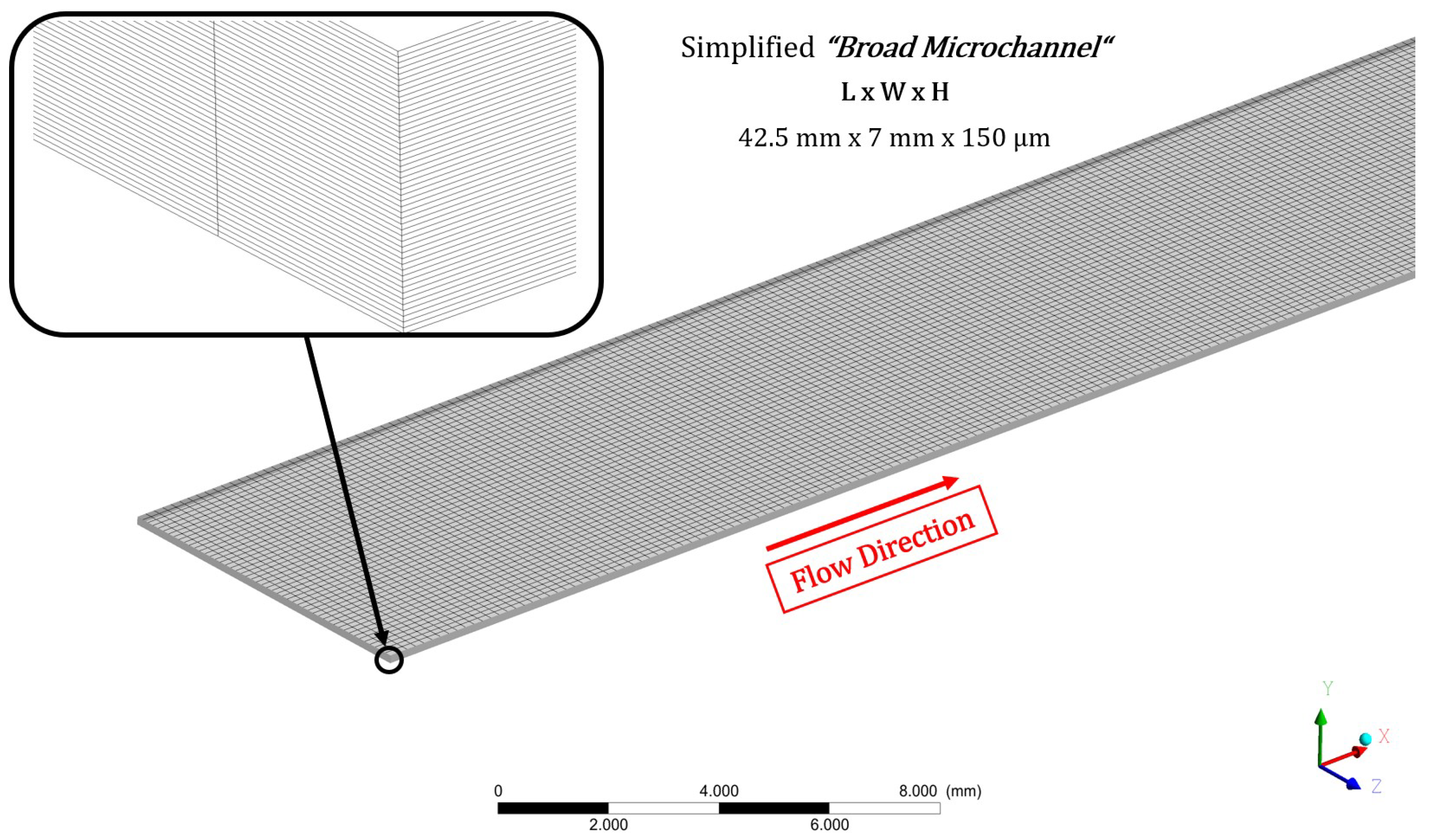
2.2. Numerical Setup for the Simulations with the Novel Viscosity Model
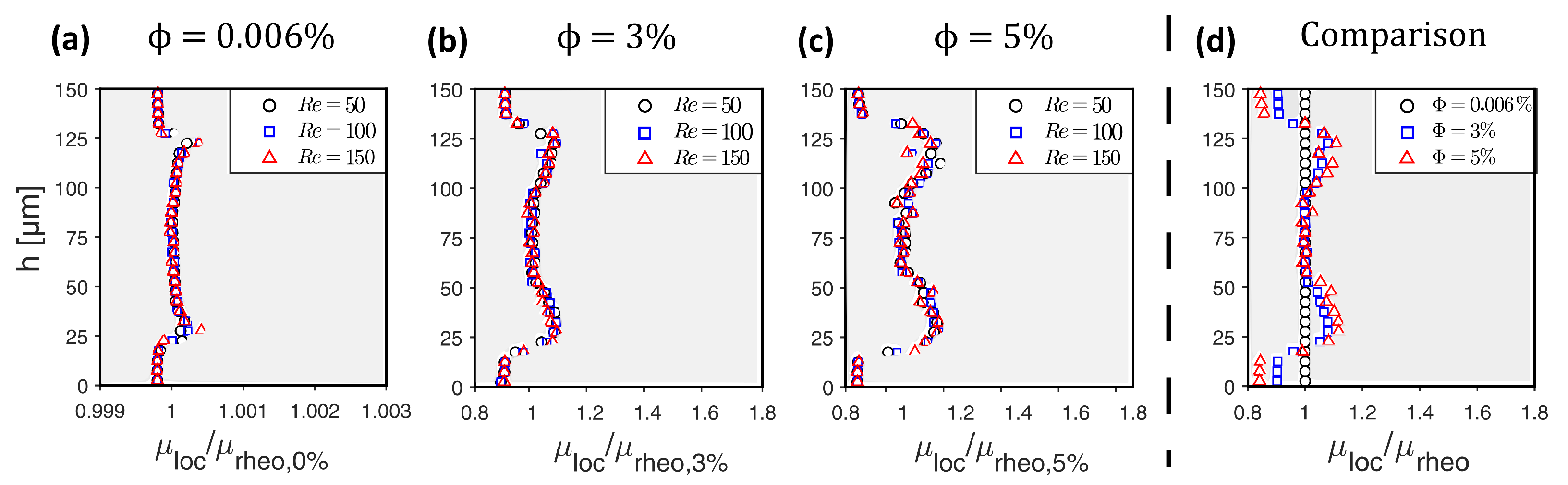
2.3. Testing and Validation of the Viscosity Model
3. Results and Discussion
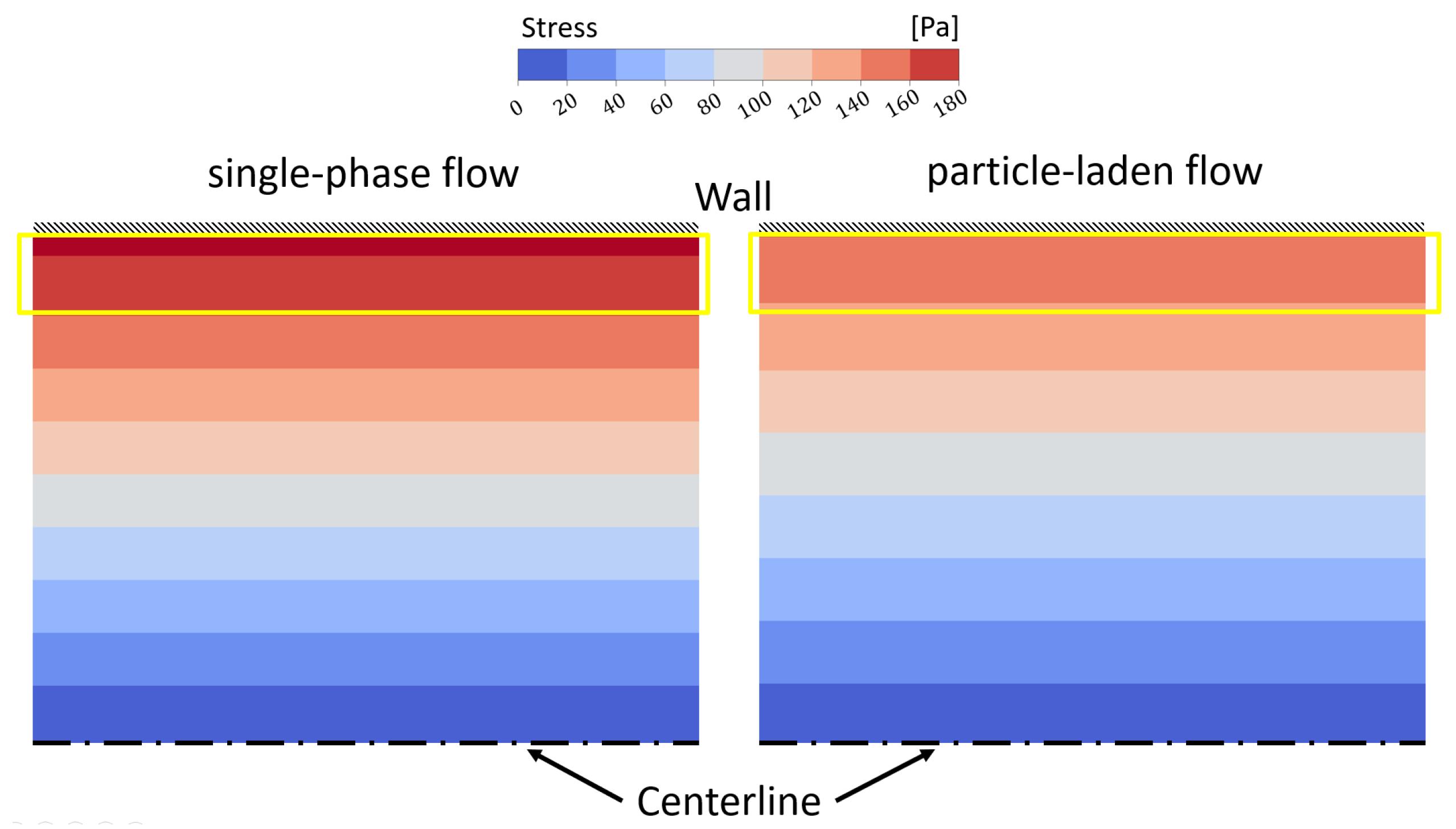
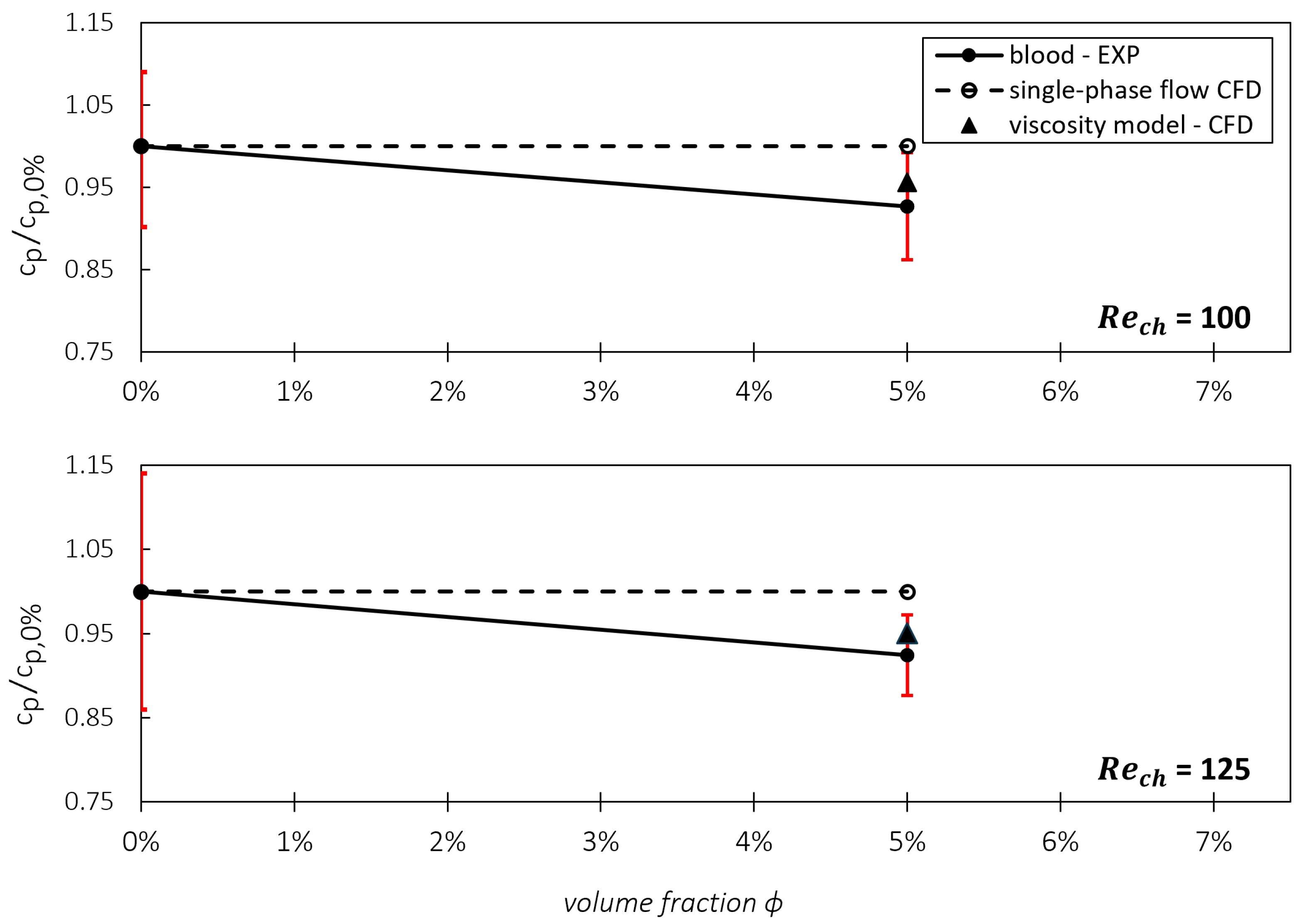
Testing and Validating the Viscosity Model against Experimental Data
4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature and Abbreviations
| c | Bulk Velocity | [m/s] |
| Pressure Coefficient | [-] | |
| Normalized Pressure Coefficient | [-] | |
| Hydraulic Diameter | [m] | |
| Channel Height | [m] | |
| Height Cell-Free Layer | [m] | |
| L | Characteristic Length | [m] |
| Channel Reynolds Number | [-] | |
| Velocities | [m/s] | |
| w | Channel Width | [m] |
| Spatial Directions | [m] | |
| Apparent Viscosity | [mPas] | |
| Carrier Viscosity | [mPas] | |
| Viscosity of the Region with Particles | [mPas] | |
| Local Viscosity | [mPas] | |
| Bulk Viscosity (from the Rheometer) | [mPas] | |
| Density | [kg/m3] | |
| Shear Stress | [Pa] | |
| Wall Shear Stress | [Pa] | |
| Particle Volume Fraction | [%] | |
| Local Particle Distribution | [%] | |
| APTV | Astigmatism Particle Tracking Velocimetry | |
| BAF | Blood Analog Fluid | |
| CFD | Computational Fluid Dynamics | |
| CFL | Cell-Free Layer | |
| DNS | Direct Numerical Simulation | |
| EXP | Experiment | |
| PEG-water | Polyethylene Glycol–Water Mixture | |
| RBC | Red Blood Cell | |
| VAD | Ventricular Assist Device | |
| WSS | Wall Shear Stress |
References
- Shaping Europe’s Digital Future. Improved Treatment for Patients with Advanced Heart Failure. Available online: https://digital-strategy.ec.europa.eu/en/news/improved-treatment-patients-advanced-heart-failure (accessed on 24 October 2023).
- Eurotransplant Yearly Statistics Overview 2022. Available online: https://statistics.eurotransplant.org/index.php?search_type=overview&search_text=9023 (accessed on 23 October 2023).
- Thunberg, C.A.; Gaitan, B.D.; Arabia, F.A.; Cole, D.J.; Grigore, A.M. Ventricular assist devices today and tomorrow. J. Cardiothorac. Vasc. Anesth. 2010, 24, 656–680. [Google Scholar] [CrossRef] [PubMed]
- Perschall, M. Numerische Untersuchung des Wellenpumpenkonzeptes und der Mechanischen Herzunterstützung. Ph.D. Thesis, Karlsruher Institut für Technologie Fakultät für Maschinenbau, Karlsruhe, Germany, 2010. [Google Scholar] [CrossRef]
- Thamsen, B.; Granneger, M.; Kretzscher, U. Blood damage in ventricular assist devices. Int. J. Artif. Organs 2016, 39, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Torner, B.; Konnigk, L.; Hallier, S.; Kumar, J.; Witte, M.; Wurm, F.H. Large eddy simulation in a rotary blood pump: Viscous shear stress computation and comparison with unsteady Reynolds-averaged Navier-Stokes simulation. Int. J. Artif. Organs 2018, 41, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Vidakovic, S.; Ayre, P.; Woodard, J.; Lingard, N.; Tansley, G.; Reizes, J. Paradoxical effects of viscosity on the VentrAssist rotary blood pump. Artif. Organs 2000, 24, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Griffith, B.P.; Wu, Z.J. Computational characterization of flow and blood damage potential of the new maglev CH-VAD pump versus the HVAD and HeartMate II pumps. Int. J. Artif. Organs 2020, 43, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Antaki, J.F.; Diao, C.G.; Shu, F.J.; Wu, J.C.; Zhao, R.; Kameneva, M.V. Microhaemodynamics within the blade tip clearance of a centrifugal turbodynamic blood pump. Proc. Inst. Mech. Engineers. Part H J. Eng. Med. 2008, 222, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, Y.G.; Keramydas, A.T.; Anastasiou, A.D.; Mouza, A.A.; Paras, S.V. Experimental and Numerical Study of Blood Flow in m-vessels: Influence of the Fahraeus–Lindqvist Effect. Fluids 2019, 4, 143. [Google Scholar] [CrossRef]
- Gracka, M.; Lima, R.; Miranda, J.M.; Student, S.; Melka, B.; Ostrowski, Z. Red blood cells tracking and cell-free layer formation in a microchannel with hyperbolic contraction: A CFD model validation. Comput. Methods Programs Biomed. 2022, 226, 107117. [Google Scholar] [CrossRef] [PubMed]
- Fink, K. Microfluidic Analysis of Vertebrate Red Blood Cell Characteristics. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2016. [Google Scholar]
- Roselli, R.J. Biotransport: Principles and Applications; SpringerLink Bücher, Springer Science+Business Media LLC: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Tabaeikazerooni, S.H. Laminar and Turbulent Particle Laden Flows: A Numerical and Experimental Study. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2019. [Google Scholar]
- Knüppel, F.; Sun, A.; Wurm, F.H.; Hussong, J.; Torner, B. Effect of Particle Migration on the Stress Field in Microfluidic Flows of Blood Analog Fluids at High Reynolds Numbers. Micromachines 2023, 14, 1494. [Google Scholar] [CrossRef] [PubMed]
- microParticles GmbH. Polymethylmethacrylatpartikel (PMMA-F) & Polystyrolpartikel (PS-F). Available online: https://www.microparticles-shop.de (accessed on 30 May 2023).
- Persons, E.L. Studies on red blood cell diameter: III. The relative diameter of immature (reticulocytes) and adult red blood cells in health and anemia, especially in pernicious anemia. J. Clin. Investig. 1929, 7, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Tanishita, K.; Yamamoto, K. (Eds.) Vascular Engineering: New Prospects of Vascular Medicine and Biology with a Multidiscipline Approach, 1st ed.; Springer: Tokyo, Japan, 2015. [Google Scholar] [CrossRef]
- Lima, R.; Ishikawa, T.; Imai, Y.; Yamaguchi, T. Blood flow behavior in microchannels: Past, current and future trends. In Single and Two-Phase Flows on Chemical and Biomedical Engineering; Dias, R., Martins, A.A., Lima, R., Mata, T.M., Eds.; Bentham Science: Sharjah, United Arab Emirates, 2012; pp. 513–547. [Google Scholar] [CrossRef]
- Schenkel, T.; Halliday, I. Continuum Scale Non Newtonian Particle Transport Model for Hæmorheology. Mathematics 2021, 9, 2100. [Google Scholar] [CrossRef]
- Karmakar, A.; Burgreen, G.W.; Rydquist, G.; Antaki, J.F. A homogenized two-phase computational framework for meso- and macroscale blood flow simulations. Comput. Methods Programs Biomed. 2024, 247, 108090. [Google Scholar] [CrossRef]
- Calejo, J.; Pinho, D.; Galindo-Rosales, F.J.; Lima, R.; Campo-Deaño, L. Particulate Blood Analogues Reproducing the Erythrocytes Cell-Free Layer in a Microfluidic Device Containing a Hyperbolic Contraction. Micromachines 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Pinho, D.; Campo-Deano, L.; Lima, R.; Pinho, F.T. In vitro particulate analogue fluids for experimental studies of rheological and hemorheological behavior of glucose-rich RBC suspensions. Biomicrofluidics 2017, 11, 054105. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, P.; Hussong, J. On the calibration of Astigmatism particle tracking velocimetry for suspensions of different volume fractions. Exp. Fluids 2021, 62, 23. [Google Scholar] [CrossRef]
- Brockmann, P.; Symanczyk, C.; Ennayar, H.; Hussong, J. Utilizing APTV to investigate the dynamics of polydisperse suspension flows beyond the dilute regime. Exp. Fluids 2022, 63, 129. [Google Scholar] [CrossRef]
- Haruki, S.; Sukenaga, S.; Saito, N.; Nakashima, K. Viscosity Estimation of Spherical Particles Dispersed Suspension. High Temp. Mater. Process. 2011, 30, 405–409. [Google Scholar] [CrossRef]
- Knüppel, F. Viscosity Model for Particle-Laden Fluids (e.g., Blood) Up to 5% Volume Fraction in Micro Geometry Flows. Available online: https://rosdok.uni-rostock.de/resolve/id/rosdok_document_0000025412 (accessed on 6 June 2024).
- Snook, B.; Butler, J.E.; Guazzelli, É. Dynamics of shear-induced migration of spherical particles in oscillatory pipe flow. J. Fluid Mech. 2016, 786, 128–153. [Google Scholar] [CrossRef]
- Coupier, G.; Kaoui, B.; Podgorski, T.; Misbah, C. Noninertial lateral migration of vesicles in bounded Poiseuille flow. Phys. Fluids 2008, 20, 111702. [Google Scholar] [CrossRef]
- Knüppel, F.; Thomas, I.; Wurm, F.H.; Torner, B. Suitability of Different Blood-Analogous Fluids in Determining the Pump Characteristics of a Ventricular Assist Device. Fluids 2023, 8, 151. [Google Scholar] [CrossRef]
- Walter, G.; Herms, G. Einführung in die Behandlung von Meßfehlern; Universität Rostock, Fachbereich Physik: Rostock, Germany, 1998. [Google Scholar]
- Grabe, M. Measurement Uncertainties in Science and Technology; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]


| Input Variables | Units |
|---|---|
| Channel height (H) | m |
| CFL height () | m |
| Viscosity of the particle-laden fluid () | mPas |
| Viscosity of the carrier fluid () | mPas |
| Local particle distribution () | % |
| Fluid | Re | Wall Shear Stresses (WSS) | ||||
|---|---|---|---|---|---|---|
| Ø EXP | CFD—Single | CFD with Viscosity Model + | ||||
| Phase | Local Distribution | Step Model | ||||
| Blood Analog | 100 | 167 Pa | 189 Pa () | 171 Pa () | 171 Pa () | |
| 150 | 257 Pa | 283 Pa () | 257 Pa () | 257 Pa () | ||
| Fluid (BAF) | 50 | 166 Pa | 225 Pa () | 169 Pa () | 169 Pa () | |
| Blood | 100 | 42 Pa | 45 Pa () | − | 41 Pa () | |
| 125 | 55 Pa | 56 Pa () | − | 52 Pa () | ||
| 150 | 63 Pa | 67 Pa () | − | 61 Pa () | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knüppel, F.; Malchow, S.; Sun, A.; Hussong, J.; Hartmann, A.; Wurm, F.-H.; Torner, B. Viscosity Modeling for Blood and Blood Analog Fluids in Narrow Gap and High Reynolds Numbers Flows. Micromachines 2024, 15, 793. https://doi.org/10.3390/mi15060793
Knüppel F, Malchow S, Sun A, Hussong J, Hartmann A, Wurm F-H, Torner B. Viscosity Modeling for Blood and Blood Analog Fluids in Narrow Gap and High Reynolds Numbers Flows. Micromachines. 2024; 15(6):793. https://doi.org/10.3390/mi15060793
Chicago/Turabian StyleKnüppel, Finn, Sasha Malchow, Ang Sun, Jeanette Hussong, Alexander Hartmann, Frank-Hendrik Wurm, and Benjamin Torner. 2024. "Viscosity Modeling for Blood and Blood Analog Fluids in Narrow Gap and High Reynolds Numbers Flows" Micromachines 15, no. 6: 793. https://doi.org/10.3390/mi15060793






