Fabrication of Cost-Effective Microchip-Based Device Using Sandblasting Technique for Real-Time Multiplex PCR Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Portable Microdevice
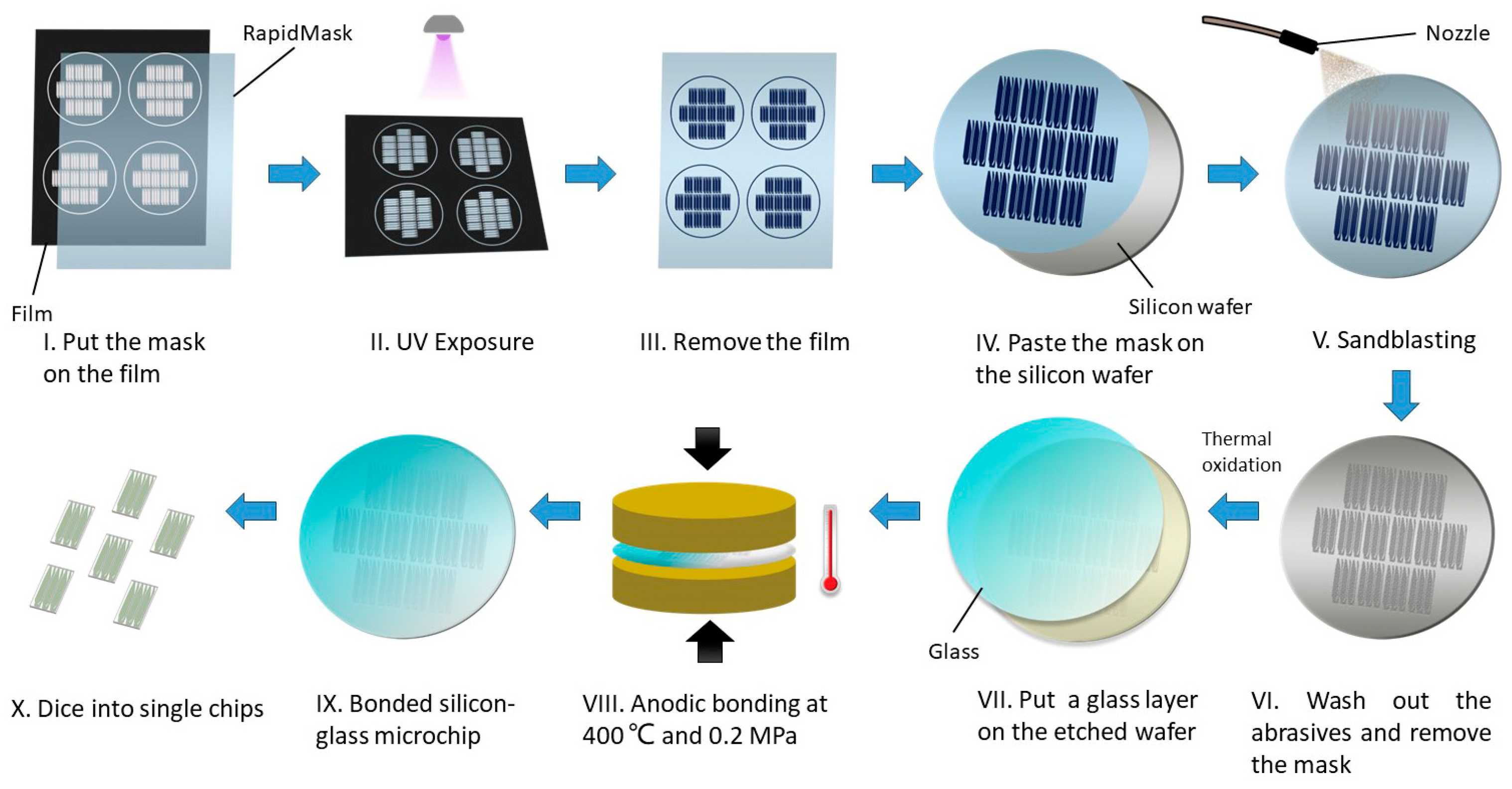
2.2. Fabrication of the Glass-Silicon Microfluidic Chip
2.3. Temperature Features of the Microheater-Based Reaction Module
2.4. Fluorescence Reliability of the Optical Module
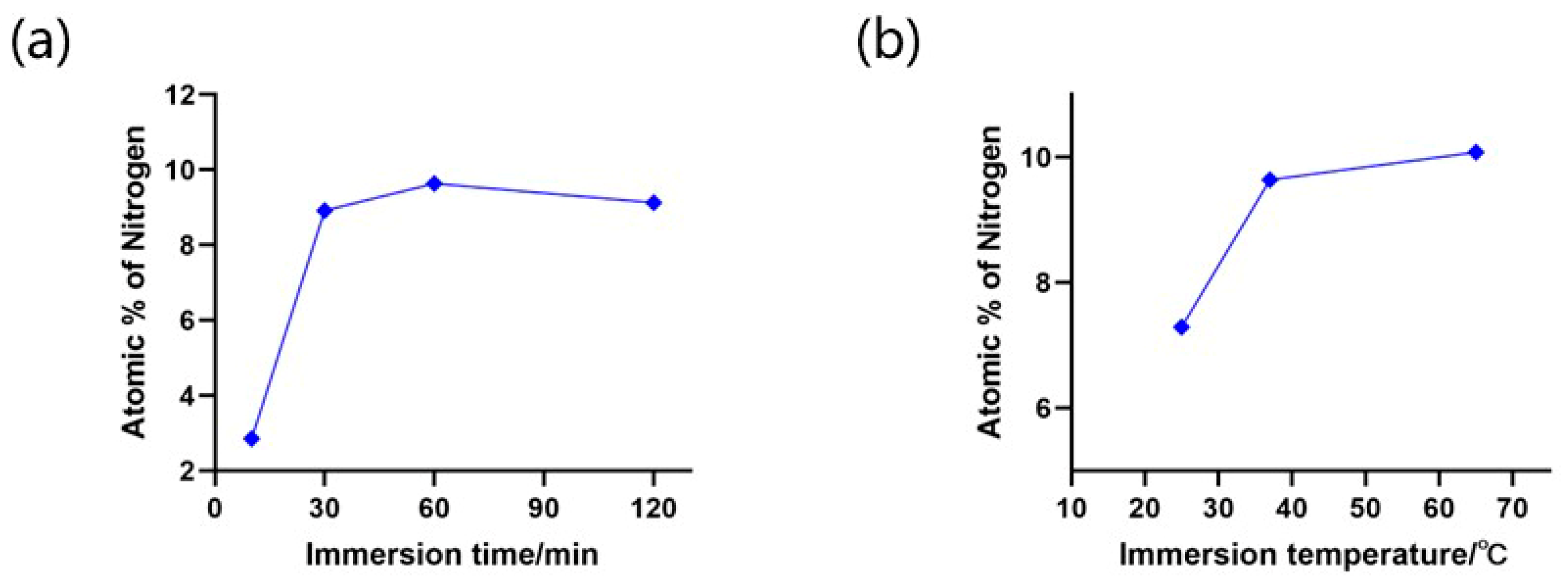
2.5. Process Optimization of the BSA Dip-Coating and Surface Characterization of the Microchips
2.6. COVID-19 PCR Detection Performed on the DRIE and Sandblasted Microchips
2.7. Multiplex PCR Assay Protocol
3. Results and Discussion
3.1. Thermal Cycle Performance
3.2. Fluorescence Collection Performance
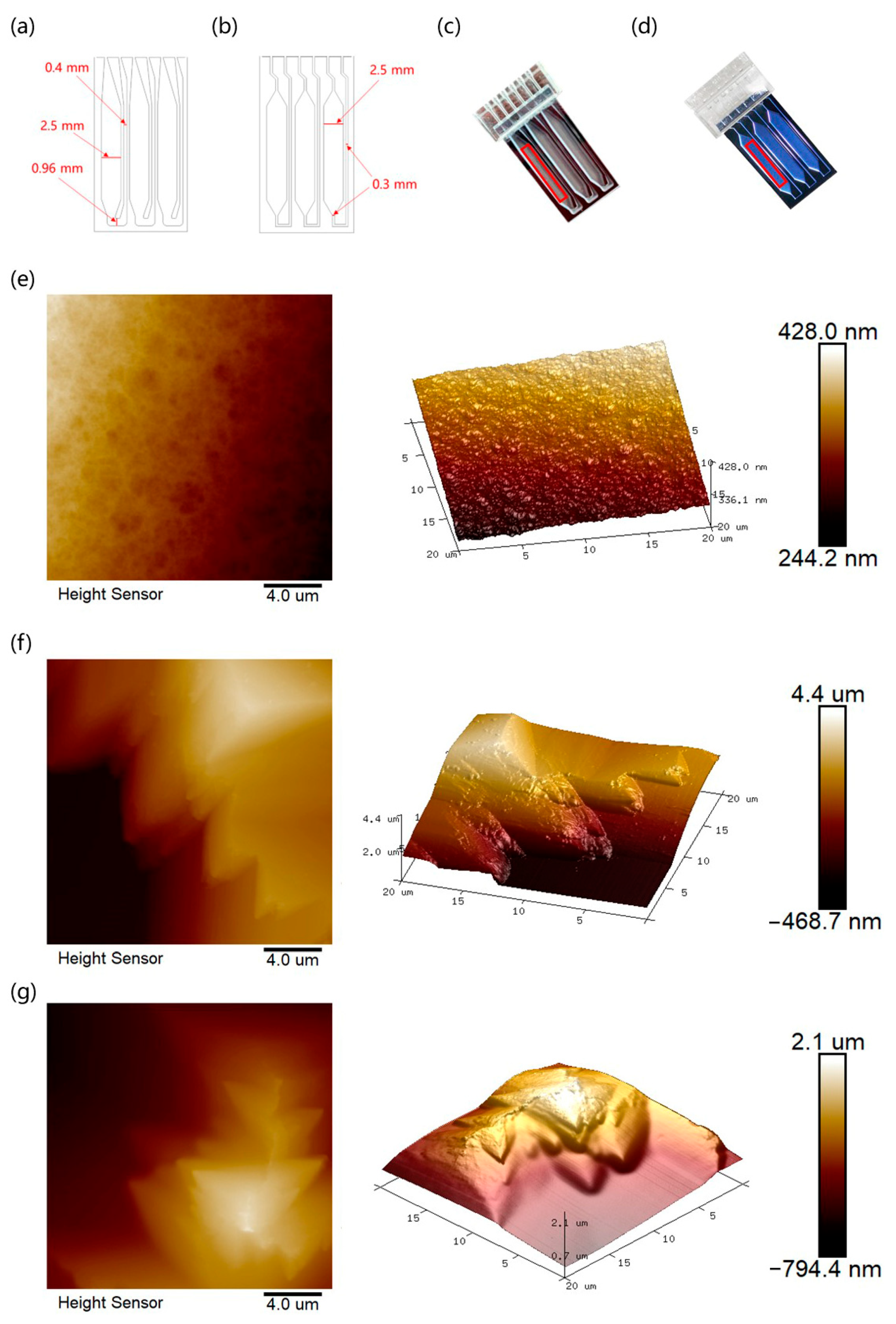
3.3. AFM Scanning and Roughness Analysis of the Microchips
3.4. BSA Coating Optimization and SEM Analysis
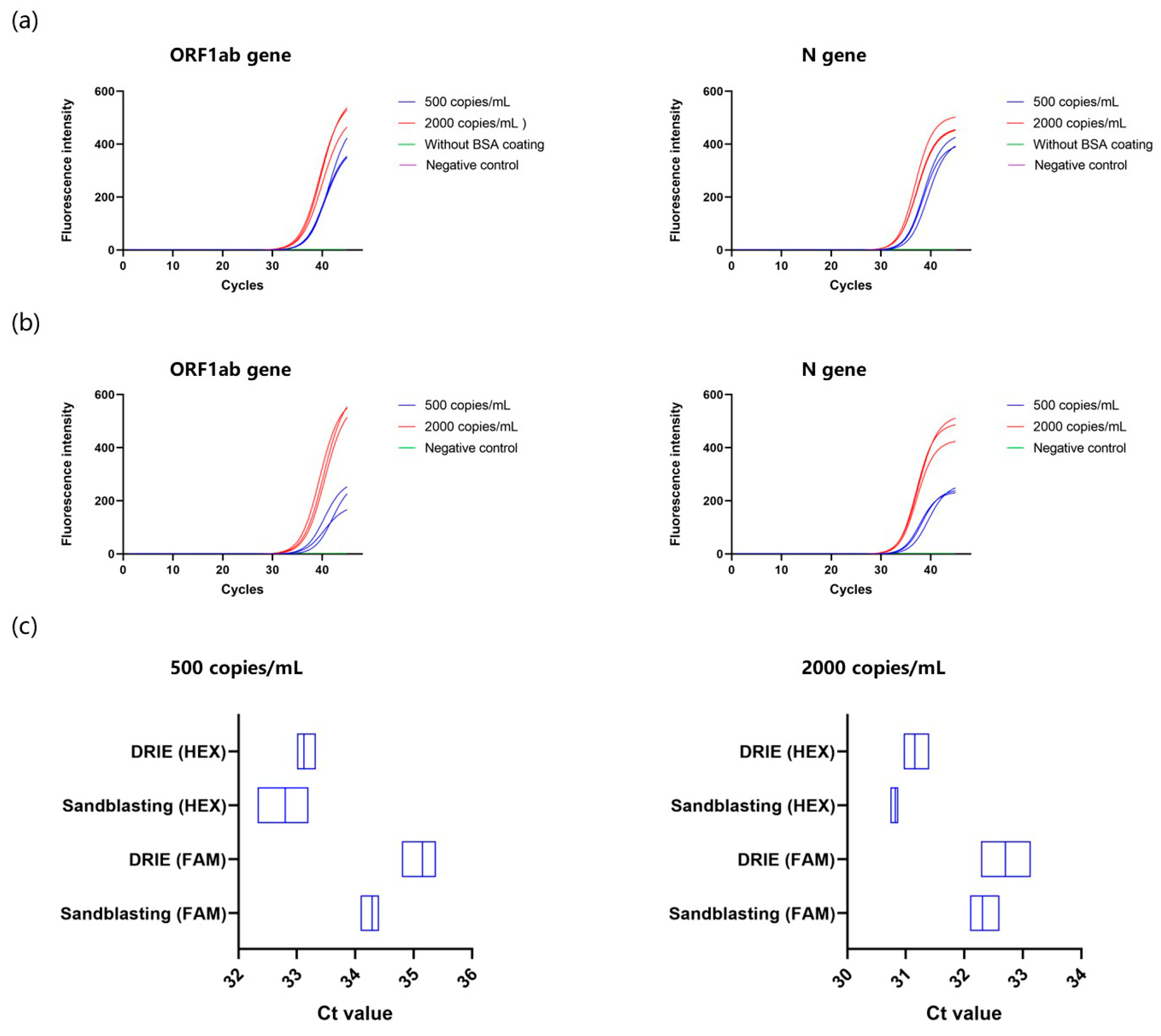
3.5. Comparison of COVID-19 PCR between DRIE and Sandblasted Microchips
3.6. Multiplex PCR Validation on the Sandblasted Microchip
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hui, D.S.; Zumla, A. Severe acute respiratory syndrome: Historical, epidemiologic, and clinical features. Infect. Dis. Clin. 2019, 33, 869–889. [Google Scholar]
- Goeijenbier, M.; Van Kampen, J.J.; Reusken, C.B.; Koopmans, M.P.; Van Gorp, E.C. Ebola virus disease: A review on epidemiology, symptoms, treatment and pathogenesis. Neth. J. Med. 2014, 72, 442–448. [Google Scholar]
- Baloch, S.; Baloch, M.A.; Zheng, T.; Pei, X. The coronavirus disease 2019 (COVID-19) pandemic. Tohoku J. Exp. Med. 2020, 250, 271–278. [Google Scholar] [CrossRef]
- Dutta, A.; Banerjee, N.; Chaudhuri, M.; Chatterjee, S. Nucleic Acid in Diagnostics. In Nucleic Acid Biology and its Application in Human Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 213–269. [Google Scholar]
- Narasimhan, V.; Kim, H.; Lee, S.H.; Kang, H.; Siddique, R.H.; Park, H.; Wang, Y.M.; Choo, H.; Kim, Y.; Kumar, S. Nucleic Acid Amplification-Based Technologies (NAAT)—Toward Accessible, Autonomous, and Mobile Diagnostics. Adv. Mater. Technol. 2023, 8, 2300230. [Google Scholar] [CrossRef]
- Hawkins, S.F.; Guest, P.C. Multiplex analyses using real-time quantitative PCR. In Multiplex Biomarker Techniques: Methods and Applications; Humana: New York, NY, USA, 2017; pp. 125–133. [Google Scholar]
- Clark, T.W.; Lindsley, K.; Wigmosta, T.B.; Bhagat, A.; Hemmert, R.B.; Uyei, J.; Timbrook, T.T. Rapid multiplex PCR for respiratory viruses reduces time to result and improves clinical care: Results of a systematic review and meta-analysis. J. Infect. 2023, 86, 462–475. [Google Scholar] [CrossRef]
- Ji, K.; Xu, Y.; Sun, J.; Huang, M.; Jia, X.; Jiang, C.; Feng, Y. Harnessing efficient multiplex PCR methods to detect the expanding Tet (X) family of tigecycline resistance genes. Virulence 2020, 11, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, H.; Xu, Y.; Laššáková, S.; Korabečná, M.; Neužil, P. PCR past, present and future. Biotechniques 2020, 69, 317–325. [Google Scholar] [CrossRef]
- Akbari Kenari, M.; Rezvani Ghomi, E.; Akbari Kenari, A.; Arabi, S.M.S.; Deylami, J.; Ramakrishna, S. Biomedical applications of microfluidic devices: Achievements and challenges. Polym. Adv. Technol. 2022, 33, 3920–3934. [Google Scholar] [CrossRef]
- Auroux, P.-A.; Iossifidis, D.; Reyes, D.R.; Manz, A. Micro total analysis systems. 2. Analytical standard operations and applications. Anal. Chem. 2002, 74, 2637–2652. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, H.; Pan, L.; Gu, X.; Xiao, C.; Liu, P.; Tang, Y.; Fang, J.; Li, X.; Lu, C. Rapid on-site nucleic acid testing: On-chip sample preparation, amplification, and detection, and their integration into all-in-one systems. Front. Bioeng. Biotechnol. 2023, 11, 1020430. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hong, X.-Z.; Li, Y.-W.; Li, Y.; Wang, J.; Chen, P.; Liu, B.-F. Microfluidics-based strategies for molecular diagnostics of infectious diseases. Mil. Med. Res. 2022, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Wang, D.; Jiang, X. Recent advancements in nucleic acid detection with microfluidic chip for molecular diagnostics. TrAC Trends Anal. Chem. 2023, 158, 116871. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Guo, X.; Yang, Y.; Shi, H.; Hao, R.; Wang, S.; Li, Z.J.; Zhao, R.; Song, H. Microfluidic chip and isothermal amplification technologies for the detection of pathogenic nucleic acid. J. Biol. Eng. 2022, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, K.G.; Estrela, P.F.N.; de Melo Mendes, G.; Dos Santos, C.A.; de Paula Silveira-Lacerda, E.; Duarte, G.R.M. Rapid molecular diagnostics of COVID-19 by RT-LAMP in a centrifugal polystyrene-toner based microdevice with end-point visual detection. Analyst 2021, 146, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, R.; Yaseen, I.; Unrau, P.J.; Lowe, C.F.; Ritchie, G.; Romney, M.G.; Sin, D.D.; Gill, S.; Slyadnev, M. Microchip RT-PCR Detection of Nasopharyngeal SARS-CoV-2 Samples. J. Mol. Diagn. 2021, 23, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Carnazza, S.; De Plano, L.M.; Franco, D.; Nicolò, M.S.; Zammuto, V.; Petralia, S.; Calabrese, G.; Gugliandolo, C.; Conoci, S.; et al. Rapid detection of bacterial pathogens in blood through engineered phages-beads and integrated Real-Time PCR into MicroChip. Sens. Actuators B Chem. 2021, 329, 129227. [Google Scholar] [CrossRef]
- Manvi, M.; Swamy, K.M. Microelectronic materials, microfabrication processes, micromechanical structural configuration based stiffness evaluation in MEMS: A review. Microelectron. Eng. 2022, 263, 111854. [Google Scholar] [CrossRef]
- Guo, H.; Cao, S.; Li, L.; Zhang, X. A review on the mainstream through-silicon via etching methods. Mater. Sci. Semicond. Process. 2022, 137, 106182. [Google Scholar] [CrossRef]
- Juska, V.B.; Maxwell, G.; Estrela, P.; Pemble, M.E.; O’Riordan, A. Silicon microfabrication technologies for biology integrated advance devices and interfaces. Biosens. Bioelectron. 2023, 237, 115503. [Google Scholar] [CrossRef]
- Damodara, S.; Shahriari, S.; Wu, W.-I.; Rezai, P.; Hsu, H.-H.; Selvaganapathy, R. 1—Materials and methods for microfabrication of microfluidic devices. In Microfluidic Devices for Biomedical Applications, 2nd ed.; Li, X., Zhou, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 1–78. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Goswami, A. Recent developments in hot embossing–a review. Mater. Manuf. Process. 2021, 36, 501–543. [Google Scholar] [CrossRef]
- Marks, M.R.; Cheong, K.Y.; Hassan, Z. A review of laser ablation and dicing of Si wafers. Precis. Eng. 2022, 73, 377–408. [Google Scholar] [CrossRef]
- Elvira, K.S.; Gielen, F.; Tsai, S.S.; Nightingale, A.M. Materials and methods for droplet microfluidic device fabrication. Lab Chip 2022, 22, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Dou, M.; Timilsina, S.S.; Xu, F.; Li, X. Recent innovations in cost-effective polymer and paper hybrid microfluidic devices. Lab Chip 2021, 21, 2658–2683. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jiang, L.; Pan, X.; Ma, H.; Lin, B.; Qin, J. A simple photolithography method for microfluidic device fabrication using sunlight as UV source. Microfluid. Nanofluidics 2010, 9, 1247–1252. [Google Scholar] [CrossRef]
- Martin, A.; Teychené, S.; Camy, S.; Aubin, J. Fast and inexpensive method for the fabrication of transparent pressure-resistant microfluidic chips. Microfluid. Nanofluidics 2016, 20, 92. [Google Scholar] [CrossRef]
- Sreejith, K.P.; Sharma, A.K.; Basu, P.K.; Kottantharayil, A. Etching methods for texturing industrial multi-crystalline silicon wafers: A comprehensive review. Sol. Energy Mater. Sol. Cells 2022, 238, 111531. [Google Scholar] [CrossRef]
- Eun, D.-S.; Kong, D.-Y.; Kong, S.H.; Choi, P.; Shin, J.-K.; Lee, J.-H. Fabrication of a based fluidic chip equipped with porous silicon filter and micro-channels. Jpn. J. Appl. Phys. 2008, 47, 5236. [Google Scholar] [CrossRef]
- Elias, M.; Dutoya, A.; Laborde, A.; Lecestre, A.; Montis, C.; Caselli, L.; Berti, D.; Lonetti, B.; Roux, C.; Joseph, P. Microfluidic characterization of biomimetic membrane mechanics with an on-chip micropipette. Micro Nano Eng. 2020, 8, 100064. [Google Scholar] [CrossRef]
- Tong, R.; Zhang, L.J.; Song, Q.; Hu, C.D.; Chen, X.E.; Lou, K.; Gong, X.Q.; Gao, Y.B.; Wen, W.J. A fully portable microchip real-time polymerase chain reaction for rapid detection of pathogen. Electrophoresis 2019, 40, 1699–1707. [Google Scholar] [CrossRef]
- Chen, X.E.; Song, Q.; Zhang, B.N.; Gao, Y.B.; Lou, K.; Liu, Y.T.; Wen, W.J. A Rapid Digital PCR System with a Pressurized Thermal Cycler. Micromachines 2021, 12, 1562. [Google Scholar] [CrossRef]
- Kodzius, R.; Xiao, K.; Wu, J.B.; Yi, X.; Gong, X.Q.; Foulds, I.G.; Wen, W.J. Inhibitory effect of common microfluidic materials on PCR outcome. Sens. Actuators B-Chem. 2012, 161, 349–358. [Google Scholar] [CrossRef]
- Christensen, T.B.; Pedersen, C.M.; Gröndahl, K.; Jensen, T.G.; Sekulovic, A.; Bang, D.D.; Wolff, A. PCR biocompatibility of lab-on-a-chip and MEMS materials. J. Micromech. Microeng. 2007, 17, 1527. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, E.; Qi, H.; Ji, S.; Chen, Z. A novel polishing method for single-crystal silicon using the cavitation rotary abrasive flow. Precis. Eng. 2020, 61, 72–81. [Google Scholar] [CrossRef]
- Pal, P.; Swarnalatha, V.; Rao, A.V.N.; Pandey, A.K.; Tanaka, H.; Sato, K. High speed silicon wet anisotropic etching for applications in bulk micromachining: A review. Micro Nano Syst. Lett. 2021, 9, 4. [Google Scholar] [CrossRef]
- Vajpayee, K.; Dash, H.R.; Parekh, P.B.; Shukla, R.K. PCR Inhibitors and Facilitators-Their Role in Forensic DNA Analysis. Forensic Sci. Int. 2023, 349, 111773. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, R.G. Sigmoidal curve-fitting redefines quantitative real-time PCR with the prospective of developing automated high-throughput applications. Nucleic Acids Res. 2004, 32, e178. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-g.; Zheng, H.-y.; Gu, J.; Li, F.; Lv, R.-l.; Deng, Y.-y.; Xu, W.-z.; Tong, Y.-q. Effects of different temperature and time durations of virus inactivation on results of real-time fluorescence PCR testing of COVID-19 viruses. Curr. Med. Sci. 2020, 40, 614–617. [Google Scholar] [CrossRef]
- Madadelahi, M.; Agarwal, R.; Martinez-Chapa, S.O.; Madou, M.J. A roadmap to high-speed polymerase chain reaction (PCR): COVID-19 as a technology accelerator. Biosens. Bioelectron. 2024, 246, 115830. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wereley, S.T.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: Norwood, MA, USA, 2019. [Google Scholar]
- ABI. Applied Biosystems 7500 Fast and 7500 Real-Time PCR Systems. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/brochures/cms_072493.pdf (accessed on 6 June 2024).
- Roche. LightCycler® 2.0 System. Available online: https://diagnostics.roche.com/global/en/products/instruments/lightcycler-2-0-ins-409.html#productSpecs (accessed on 6 June 2024).
- Labx. Price Information of the Commercial PCR Instruments. Available online: https://www.labx.com/product/applied-biosystems-7500 (accessed on 15 June 2024).
- Jahed, M.; Gustavsson, J.S.; Larsson, A. Precise setting of micro-cavity resonance wavelength by dry etching. J. Vac. Sci. Technol. B 2019, 37, 031217. [Google Scholar] [CrossRef]
- Chen, J.J.; Qiu, X.C. The effect of the surface passivation on polymerase chain reaction inside a continuous flow microfluidic chip. Microsyst. Technol. 2024, 1–19. [Google Scholar] [CrossRef]
- Marshall, P.L.; King, J.L.; Budowle, B. Utility of amplification enhancers in low copy number DNA analysis. Int. J. Leg. Med. 2015, 129, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wu, J.; Li, Y.; Li, J.; Ouyang, Y.; He, Z.; Zhao, S. Enhancement of ginsenoside biosynthesis and secretion by Tween 80 in Panax ginseng hairy roots. Biotechnol. Appl. Biochem. 2015, 62, 193–199. [Google Scholar] [CrossRef]
- Su, Y.; Chu, H.; Tian, J.; Du, Z.; Xu, W. Insight into the nanomaterials enhancement mechanism of nucleic acid amplification reactions. TrAC Trends Anal. Chem. 2021, 137, 116221. [Google Scholar] [CrossRef]
- Karunanathie, H.; Kee, P.S.; Ng, S.F.; Kennedy, M.A.; Chua, E.W. PCR enhancers: Types, mechanisms, and applications in long-range PCR. Biochimie 2022, 197, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Nanosystem Fabrication Facility (CWB), H. The NFF (CWB) Laboratory Charging Scheme. Available online: https://nff.hkust.edu.hk/en/our-services/charging-scheme.html (accessed on 15 June 2024).
- Matsarskaia, O.; Bühl, L.; Beck, C.; Grimaldo, M.; Schweins, R.; Zhang, F.; Seydel, T.; Schreiber, F.; Roosen-Runge, F. Evolution of the structure and dynamics of bovine serum albumin induced by thermal denaturation. Phys. Chem. Chem. Phys. 2020, 22, 18507–18517. [Google Scholar] [CrossRef]
- Hossain, M.W.; Hossain, M.; Arafath, K.; Ety, S.S.; Shetu, M.M.H.; Kabir, M.; Noor, F.A.; Mannoor, K. Real-Time fast PCR amplification using designated and conventional real time thermal cycler systems: COVID-19 perspective. PLoS ONE 2022, 17, e0276464. [Google Scholar] [CrossRef]
- Choi, J.A.; Bae, S.M.; Kim, J.W.; Lee, K.J. Development of a Two Triplex Real-Time Polymerase Chain Reaction for Rapid Detection of Six Carbapenemase Genes in Enterobacteriaceae. Osong Public Health Res. Perspect. 2020, 11, 53–59. [Google Scholar] [CrossRef]





| Device | Size/mm | Weight/kg |
|---|---|---|
| Sandblasted microdevice | 380 × 245 × 245 | 6.8 |
| ABI 7500 fast | 450 × 340 × 490 | 34 |
| LightCycler® 2.0 System | 505 × 280 × 385 | 22 |
| Chip Type | Average Roughness (Ra)/nm | Root Mean Square Roughness (Rq)/nm | Image Rmax/nm |
|---|---|---|---|
| DRIE | 2.41 | 3.04 | 25.4 |
| Sandblasting | 344 | 416 | 2211 |
| Sandblasting (BSA coating) | 196 | 256 | 1855 |
| Sandblasting (BSA coating) after 45 thermal cycles | 211 | 260 | 1527 |
| No. | Sandblasted Microchip | Cost | DRIE Microchip | Cost |
|---|---|---|---|---|
| 1 | RapidMask handling | USD 10.8 | Mask preparation | USD 128 |
| 2 | Sandblasting | USD 50 | Lithography | USD 145 |
| 3 | Thermal oxidation | USD 145 | DRIE | USD 145 |
| 4 | Anodic bonding | USD 145 | Thermal oxidation | USD 145 |
| 5 | Dicing | USD 35 | Anodic bonding | USD 145 |
| 6 | - | - | Dicing | USD 35 |
| Total (6-inch wafer) | USD 385.8 | USD 743 | ||
| Total cost per chip | USD 8.03 | USD 15.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hu, Z.; Yang, S.; Xu, N.; Song, Q.; Gao, Y.; Wen, W. Fabrication of Cost-Effective Microchip-Based Device Using Sandblasting Technique for Real-Time Multiplex PCR Detection. Micromachines 2024, 15, 944. https://doi.org/10.3390/mi15080944
Liu Y, Hu Z, Yang S, Xu N, Song Q, Gao Y, Wen W. Fabrication of Cost-Effective Microchip-Based Device Using Sandblasting Technique for Real-Time Multiplex PCR Detection. Micromachines. 2024; 15(8):944. https://doi.org/10.3390/mi15080944
Chicago/Turabian StyleLiu, Yiteng, Zhiyang Hu, Siyu Yang, Na Xu, Qi Song, Yibo Gao, and Weijia Wen. 2024. "Fabrication of Cost-Effective Microchip-Based Device Using Sandblasting Technique for Real-Time Multiplex PCR Detection" Micromachines 15, no. 8: 944. https://doi.org/10.3390/mi15080944
APA StyleLiu, Y., Hu, Z., Yang, S., Xu, N., Song, Q., Gao, Y., & Wen, W. (2024). Fabrication of Cost-Effective Microchip-Based Device Using Sandblasting Technique for Real-Time Multiplex PCR Detection. Micromachines, 15(8), 944. https://doi.org/10.3390/mi15080944






