Determination of Arylcyclohexylamines in Biological Specimens: Sensors and Sample Pre-Treatment Approaches
Abstract
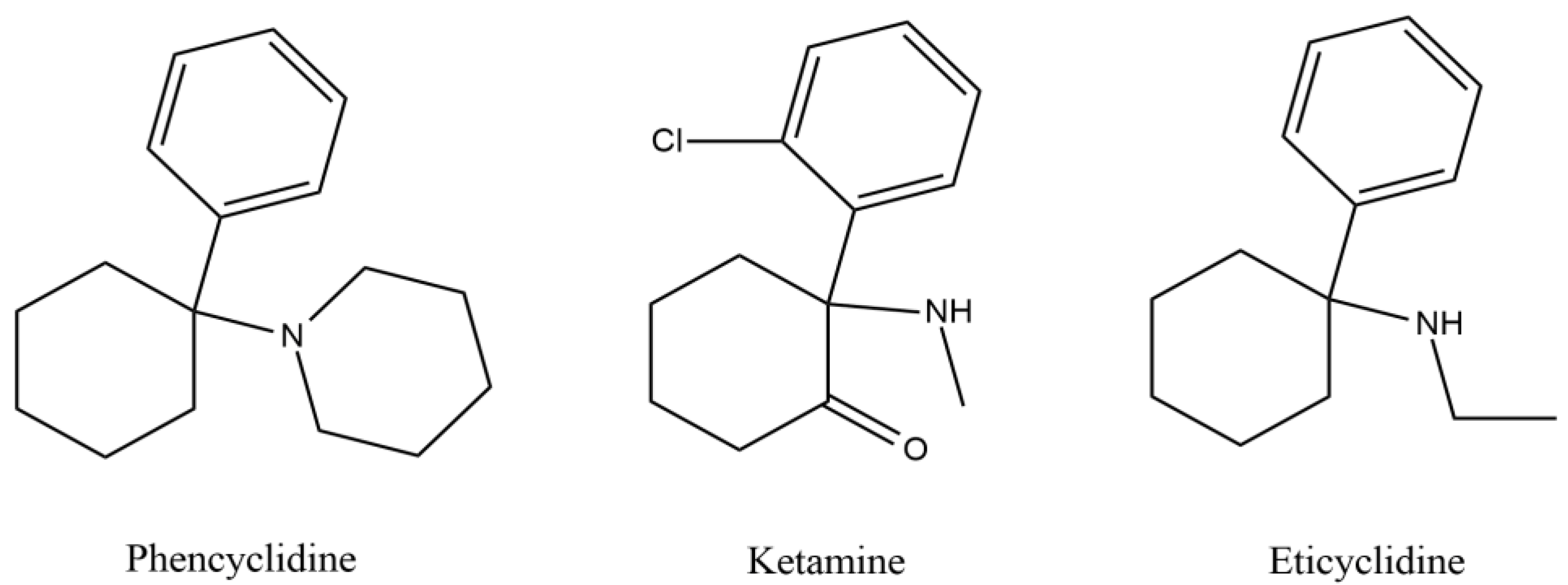
1. Introduction
2. Strategies to Determine Ketamine, Phencyclidine, and Eticyclidine Analogues in Biological Specimens
2.1. Blood and Derivates
| Matrix | Compounds | Sample Amount (μL) | Extraction Method | Screening | Quantification Method | LOD (ng/mL) | LOQ (ng/mL) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Serum | 3-MeO-PCE | 150 | SPE | LC-MS/MS | 0.4 | 5.0 | 59–79 | [15] | |
| 3-MeO-PCP | 0.4 | 61–64 | |||||||
| 4-MeO-PCP | 0.4 | 62–64 | |||||||
| KET | 0.8 | 74–83 | |||||||
| MXE | 0.4 | 76–86 | |||||||
| PCP | 4.0 | 57–74 | |||||||
| Blood | KET | 500 | DLLME | UHPLC-MS/MS | N.S. | 0.5 | N.S. | 55–86 | [18] |
| MXE | 0.2 | N.S. | 53–66 | ||||||
| Blood | KET | 2000 | DLLME | GC-MS | 10 | 10 | 92–115% | [19] | |
| NKET | 10 | 50 | |||||||
| MXE | 10 | 10 | |||||||
| Blood | 3-MeO-PCP | 200 | LLE | LC-MS/MS | 0.1 | 0.3 | 99 | [20] | |
| 4-MeO-PCP | 0.1 | 0.3 | 81 | ||||||
| KET | 0.3 | 0.5 | 95 | ||||||
| MXE | 0.3 | 0.5 | 93 | ||||||
| NKET | 0.3 | 0.5 | 95 | ||||||
| Plasma | NENK | 180 | m-SPE | LC-QTOF-MS | N.S. | 6 | N.S. | 82–91 | [21,22,23] |
| Blood | MXE | N.S. | DBS | N.S. | LC-MS/MS | N.S. | N.S. | N.S. | [24] |
| Postmortem Blood | 3-MeO-PCP | 200 | LLE | LC-TOF-MS | LC-MS/MS | N.S. | 1.0 | N.S. | [25] |
| Whole blood | 3-MeO-PCP | 0.5 g | LLE | LC-TOF-MS | LC-MS/MS | N.S. | 0.01 mg/g | N.S. | [16,26] |
| Postmortem Blood | |||||||||
| Postmortem Blood | 3-Meo-PCP | 1000 | SPE | GC-MS | 1 | 10 | N.S. | [27] | |
| Blood | 3-MeO-PCP | 100 | PPT & SPE | LC-QTOF-MS | UHPLC-MS/MS | N.S. | N.S. | N.S. | [28] |
| Blood | KET | 100 | DBS | UHPLC-MS/MS | 0.5 | 2 | 20–45 | [17] | |
| NKET | 0.5 | 2 | 18–41 | ||||||
| Postmortem Blood | KET | 100 | DBS | UHPLC-MS/MS | 0.5 | 2 | 47–58 | [17] | |
| NKET | 0.5 | 2 | 16–26 | ||||||
| Synthetic blood | 3-Meo-PCP | N.S. | N.S. | ELISA | N.S. | N.S. | N.S. | N.S. | [29] |
| 3-OH-PCP | |||||||||
| Femoral Blood | 3-MeO-PCP and metabolites | 1000 | LLE | GC-MS & LC-MS/MS & LC-HRMS | LC-MS/MS | 0.05 | 0.01 | 97 | [30,31] |
| Blood | KET | 500 | DLLME | N.S. | UHPLC-MS/MS | 0.5 | 2 | 87–110 | [32] |
| NKET | 0.05 | 0.5 | 72–89 | ||||||
| Blood | 2-oxo-PCE | 100 | LLE | LC-MS/MS | 0.22 | 0.62 | 76–99 | [33] | |
| DXE | 0.15 | 0.50 | 79–100 | ||||||
| Blood | PCP | 30 | N.S. | ELISA | GC-MS | N.S. | N.S. | N.S. | [34] |
| Blood | 3-MeO-PCP | 500 | LLE | GC -MS & HPLC-UV coupled MS/MS | LC-UV-MS/MS | 30 | 160 | >87 | [35] |
| 3-MeO-PCE | 50 | 160 | |||||||
| MXE | 30 | 160 | |||||||
| Blood | MXPr | N.S. | DLLME | GC-MS | LC-MS/MS | 0.5 | 2 | N.S. | [36] |
| 2F-DCK | 0.5 | 2 | |||||||
| DXE | 0.5 | 2 | |||||||
| MXPr | LC-HRMS | N.S. | 2 | N.S. | N.S. | ||||
| 2FDCK | 10 | ||||||||
| DXE | 10 | ||||||||
| Blood | 3-Meo-PCP | 250 and 500 | PPT & SPE | LC-HRAM-Orbitrap-MS & sd-GC-FTIR | N.S. | N.S. | N.S. | N.S. | [37] |
| Blood | KET | 1000 | SPE & LLE | GC-MS | N.S. | 25 | N.S. | [38] | |
| NET | N.S. | ||||||||
| DHNK | N.S. | ||||||||
| PCP | N.S. | ||||||||
| 2FDCK | N.S. | ||||||||
| Serum | 3-Meo-PCP | 1000 | SPE | GC-MS | 1 | 2 | >90 | [39] | |
| 4-Meo-PCP | N.S. | N.S. | N.S. | ||||||
| MXE | N.S. | N.S. | N.S. | ||||||
| Blood | KET | 50 | N.S. | CEDIA & DRI & EIA & LC-MS/MS | N.S. | N.S. | N.S. | N.S. | [40,41] |
| MXE | LC-MS/MS | 0.5 | 0.5 | N.S. | |||||
| Blood | KET | 1000 | SPE | LC-MS/MS | 1 | 5 | 86–92 | [42] | |
| NKET | 1 | 5 | 79–85 | ||||||
| Plasma | KET | 500 | MEPS | GC-MS/MS | 5 | 10 | 73–89 | [43] | |
| NKET | 5 | 10 | 63–75 | ||||||
2.2. Urine
| Compounds | Sample Amount (µL) | Extraction Method | Screening | Quantification Method | LOD (ng/mL) | LOQ (ng/mL) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| 2-oxo-PCE | 4000 | LLE | GC-MS & LC-MS/MS | N.S. | N.S. | N.S. | N.S. | [47,48] |
| 3-Meo-PCP | 250 and 500 | PPT & SPE | LC-HRAM-Orbitrap-MS & sd-GC-FTIR | N.S. | N.S. | N.S. | N.S. | [37] |
| KET | 1000 | SPE/LLE | GC-MS | N.S. | 25 | N.S. | [38] | |
| NKET | N.S. | N.S. | N.S. | |||||
| DHNK | N.S. | N.S. | N.S. | |||||
| PCP | N.S. | N.S. | N.S. | |||||
| 2FDCK | N.S. | N.S. | N.S. | |||||
| KET | N.S. | N.S. | LC-MS/MS | N.S. | N.S. | N.S. | [49] | |
| DXE | ||||||||
| KET | 1600 | SPE | Immunoassay & GC-MS/MS & LC-MS/MS | N.S. | 4 | N.S. | N.S. | [50,51] |
| DXE | N.S. | N.S. | N.S. | |||||
| 2-oxo-PCE | N.S. | N.S. | N.S. | |||||
| 3-Meo-PCP | N.S. | N.S. | N.S. | |||||
| 3-OH-PCP | N.S. | N.S. | N.S. | |||||
| KET | 200 | DME | LC-MS/MS | N.S. | 1 | N.S. | 48 | [45] |
| DXE | 1 | N.S. | 50 | |||||
| 2FDCK | 1 | N.S. | 51 | |||||
| 2-oxo-PCE | 1 | N.S. | 55 | |||||
| 2FDCK and metabolites | 1000 | SPE | UHPLC-QTOF-MS & UHPLC-MS/MS | N.S. | N.S. | N.S. | N.S. | [48,52] |
| KET | 5 | N.S. | N.S. | |||||
| DXE | N.S. | N.S. | N.S. | |||||
| 2-oxo-PCE | N.S. | N.S. | N.S. | |||||
| Tiletamine | 5 | N.S. | N.S. | |||||
| 3-Meo-PCP | 1000 | SPE | Immunoassay’s & GC-MS | GC-MS | 1 | 2 | >90 | [39] |
| 4-Meo-PCP | N.S. | N.S. | N.S. | |||||
| MXE | N.S. | N.S. | N.S. | |||||
| KET | 50 | N.S. | CEDIA & DRI & EIA & LC-MS/MS | LC-MS/MS | N.S. | N.S. | N.S. | [40,41] |
| MXE | 0.5 | N.S. | N.S. | |||||
| KET | 1000 | SPE | LC-MS/MS | 1 | 5 | 86–92 | [42] | |
| NKET | 1 | 5 | 79–85 | |||||
| 2FDCNEK | 2000 | LLE | CG-MS & NMR & single-crystal X-ray diffraction & LC-HRMS | N.S. | N.S. | N.S. | N.S. | [46] |
| KET | 2000 | DLLME | GC-MS | 5 | 10 | 92–115 | [19] | |
| NKET | 10 | 50 | ||||||
| MXE | 10 | 10 | ||||||
| MXE | N.S. | N.S. | N.S. | LC-MS/MS | N.S. | N.S. | N.S. | [24] |
| 3-MeO-PCP and metabolites | 1000 | LLE | GC-MS & LC-MS/MS & LC-HRMS | LC-MS/MS | 0.05 | 0.01 | 97 | [30,31] |
| 3-MeO-PCP | 500 | LLE | GC-MS & HPLC-UV-MS/MS | HPLC-UV-MS/MS | 20 | 160 | >98% | [35] |
| 3-MeO-PCE | 10 | 160 | ||||||
| MXE | 40 | 160 | ||||||
| KET | 500 | MEPS | GC-MS/MS | 5 | 10 | 89–101 | [43] | |
| NKET | 5 | 10 | 73–77 | |||||
2.3. Other Specimens
| Matrix | Compounds | Sample Amount | Extraction Method | Screening | Method Quantification | LOD | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Hair | KET | 7 g | PLE & DLLME | HPLC-HRMS/MS | 2 pg/mg | 5 pg/mg | 60 | [58] | |
| MXE | 1 pg/mg | 25 pg/mg | 52 | ||||||
| PCP | 1 pg/mg | 25 pg/mg | 48 | ||||||
| Water to replicate the vitreous humour | 3-MeO-PCP | 500 μL | LLE | GC -MS & HPLC-UV coupled ESI-MS/MS | HPLC-UV coupled MS/MS | 50 ng/mL | 160 ng/mL | >98 | [35] |
| 3-MeO-PCE | 20 ng/mL | 160 ng/mL | |||||||
| MXE | 20 ng/mL | 160 ng/mL | |||||||
| Intraosseous fluid from tibia and humorous | PCP | 30 μL | N.S. | ELISA | GC-MS | N.S. | N.S. | N.S. | [34] |
| Hair | KET | 50 mg | LLE | UHPLC-MS/MS | N.S. | N.S. | N.S. | [59,60] | |
| Hair | KET | 30 mg | LLE | UHPLC-HRMS | N.S. | 5 pg/mg | N.S. | N.S. | [53] |
| MXE | N.S. | N.S. | N.S. | ||||||
| NKET | 10 pg/mg | N.S. | N.S. | ||||||
| Hair | KET | 21 and 50 mg | LLE | LC-HRMS | N.S. | 5 pg/mg | N.S. | N.S. | [61] |
| MXE | 5 pg/mg | N.S. | N.S. | ||||||
| Hair | MXPr | 30 mg | LLE | GC-MS | LC-MS/MS | 0.01 ng/mg | 0.05 ng/mg | N.S. | [36] |
| 2FDCK | 0.01 ng/mg | 0.05 ng/mg | N.S. | ||||||
| DXE | 0.01 ng/mg | 0.05 ng/mg | N.S. | ||||||
| MXPr | LC-HRMS | N.S. | 0.05 ng/mg | N.S. | N.S. | ||||
| 2FDCK | 0.05 ng/mg | N.S. | N.S. | ||||||
| DXE | 0.05 ng/mg | N.S. | N.S. | ||||||
| Hair | 2FDCK | 20 mg | LLE | Immunoassays & LC-HRMS & LC-MS/MS & HS-GC- FID | LC-HRMS | N.S. | N.S. | N.S. | [62,63] |
| 3-MeO-PCE | N.S. | N.S. | N.S. | ||||||
| Hair | KET | 50 mg | MEPS | GC-MS/MS | 0.01 ng/mg | 0.05 ng/mg | 39–60 | [64] | |
| NKET | 0.05 ng/mg | 0.05 ng/mg | 32–43 | ||||||
| Nasal swab | 2-oxo-PCE | N.S. | N.S. | HPLC-DAD & IT-TOF/MS | N.S. | N.S. | N.S. | N.S. | [47,65] |
| Oral fluid | KET | 400 μL | MEPS | GC-MS & DESI-HRMS | N.S. | 50 ng/mL | 99–120 | [55] | |
| Oral fluid | 3-OH-PCP | 1000 μL | MIP-SPE | IMS | 15 ng/mL | 50 ng/mL | 70 | [57] | |
2.4. Sensors
| Matrix | Compounds | Sample Amount | Type of Sensor | Transducing Mechanism | Sensing Mechanism | LOD | Time of Analysis | Reference |
|---|---|---|---|---|---|---|---|---|
| Solution | KET | N.S. | Optical | Fluorescence | Multi-colour fluorescent carbon dots | 1 ng/mL | 5 min | [72] |
| Oral fluid | KET | N.S. | Electrochemical | Potentiometry | Unmodified graphite screen printed electrodes | 1.1 µM | 10 min | [82] |
| Urine | KET | 0.1 mL | Optical | Colorimetry | DNA-AuNP’s | N.S. | N.S. | [83] |
| Solution | KET | N.S. | Optical | THz frequency | Porous core PCF | N.S. | N.S. | [84] |
| Aqueous solution | KET | 0.005 mL | Optical | Solid-state fluorescence | 8Ba-2f fluorescent probe on filter paper | 50 pg/cm2 | Real-time | [85] |
| Whiskey | KET | 20 mL | Electrochemical | Potentiometry | Polylactic acid electrodes | 0.14 mM | Real-time | [78] |
| Vodka | 0.11 mM | |||||||
| Gin | 0.13 mM | |||||||
| Beer | 0.18 mM | |||||||
| Water vapor | KET | N.S. | Optical | Fluorescence | PBI-CB | N.S. | 1 min and 10 s | [86] |
| Saliva | KET | N.S. | Optical | Reflected light | Antibody combined with magnetic nanobeads | 100 ng/mL | 120 s | [87] |
| Hair | KET | N.S. | Optical | Fluorescence | Up-converting nanoparticles | 1 ng/mL | 5 min | [88] |
| Saliva | KET | N.S. | Electrochemical | Potentiometry | Aptamer conjugated with gold electrodes | 10 nM | 30 s | [89] |
| Urine | ||||||||
| Oral fluid | KET | N.A. | Optical | Colorimetry | Competitive paper-based ELISA | 0.03 ng/mL | 6 min | [90] |
| Soda drinks | KET | N.S. | Optical | Colorimetry | AuNP’s | 2.70 × 10−5 M | N.S. | [73] |
| Optical | Fluorescence | Fluorescent CDs | 1.33 × 10−5 M | N.S. | ||||
| Serum | KET | 2 mL | Optical | Fluorescence | Fluorescein tagged with Y-shape DNA and AuNPs | 3 pg/mL | 1.5 h | [91] |
| Hair | KET | 10 mg | Electrochemical | Electrochemiluminescence | AuNP’s/Indium tin oxide via antibody recognition | 5.73 pg/g | 30 min | [92] |
| Soft beverages | KET | Single aliquot | Electrochemical | Potentiometry | Polyaniline nano-dispersion | 3.2 × 10−6 M | 20 s | [93] |
| Optical | Fluorimetry | CD/AuNP’s | 2 × 10−4 M | 1 min | ||||
| Colorimetry | Co(SCN)2 | 10 mg/mL | Instant | |||||
| Urine | KET | N.S. | Electrochemical | Resonance frequency | KET antibody | 0.86 pg/mL | 10 min | [94] |
| Pepsi | KET | 0.1 mL | Optical | Colorimetry | Bromocresol green | N.S. | 5 s | [95] |
| Rum | N.S. | N.S. | ||||||
| Whiskey | N.S. | N.S. | ||||||
| Urine | KET | 1 mL | Electrochemical | Chemiluminescence | Lu + NBS | N.S. | N.S. | [96] |
| Lu+; K3Fe[CN]6 | N.S. | N.S. | ||||||
| Cal + NBS | N.S. | N.S. | ||||||
| Cal + KMnO4 | N.S. | N.S. | ||||||
| Artificial urine | KET | N.S. | Optical | Fluorescence | Palmatine within the cavity of Q8 | 5.0 ng/mL | 30 s | [74] |
| Solution | KET | 10 mL | Electrochemical | Potentiometry | PM-CE | 2.6 × 10−6 M | 60–10 s | [77] |
| PM–CE/KTpClPB | 7.9 × 10−7 M | 50–10 s | ||||||
| PM-KT | 1.3 × 10−5 M | 60–20 s | ||||||
| CE | 4.5 × 10−5 M | 90–40 s | ||||||
| Urine | KET | N.S. | Electrochemical | Potentiometry | Carbon paste electrode | 7.3 × 10−6 M | 8–18 s | [97] |
| Ampoules | ||||||||
| Urine | KET | 5 mL | Electrochemical | Potentiometry | PM-KT, Na-TPB, and DBP electrode | 1.2 × 10−7 M | 7 s | [98] |
| Whiskey | KET | µL range | Electrochemical | Potentiometry | Zeo-GO | 0.001 nm/mL | 2 s | [99] |
| Real Juice | ||||||||
| Urine | ||||||||
| Blood | KET | 1.5 mL | Optical | Fluorescence | DNA-templated silver nanoclusters probes | 0.06 ng/mL | 5 min | [100] |
| Serum | KET | N.S. | Electrochemical | Potentiometry | Au electrode modified by 3-MPA, EDC and NHS with KET antibody | 0.41 × 10−6 µM | N.S. | [101] |
| Whiskey | KET | 0.01 mL | Electrochemical | Admittance | Au interdigitated electrodes | 0.16 ng/μL | N.S. | [102] |
| Capacitance | 0.73 ng/μL | |||||||
| Plasma | KET | 2 mL | Electrochemical | Potentiometry | PTY, sol-gel, f-MWCNTS@AuNPS on a PGE | 0.7 nM | 420 s | [79] |
| Urine | KET | N.S. | Electrochemical | Potentiometry | MAA and EGDMA on a MOFS@G modified screen-printed electrode | 4.0 × 10−11 M | 5 min | [103] |
| Powdered illicit drugs | KET | 20–50 mg | Optical | Colorimetric | Co(SCN)2 into PDMS | 100 µg/ml | 10 min | [104] |
| Cigarette gas | KET | 1000 mL | Electronic nose | Resistance variation rate | Figaro TGS metal oxide | N.S. | 3 min 20 s | [81] |
| Gas | ||||||||
| Hair | KET | 20 mg | Electrochemical | Potentiometry | Poly-L-cysteine molecular imprinted membrane | 1.6 × 10−7 M | 10 min | [76] |
| Urine | 9 mL | |||||||
| Blood | KET | N.S. | Optical | Thz frequency | Hexagonal photonic crystal fibre | N.S. | N.S. | [75] |
| Powder | KET | 5–10 mg | Electrochemical | Photoluminescence | TA-Ag NCs | 0.16 mM | N.S. | [105] |
| Sewage | KET | 10 mL | Electrochemical | Potentiometry | Fe3O4 @MIPs/magnetic glassy carbon electrode | 8.0 × 10−13 M | 4 min | [106] |
| Controlled drugs | KET | N.S. | Optical | Visible and infrared frequency | PCF | N.S. | N.S. | [107] |
| Plasma | KET | 1 mL | Electrochemical | Potentiometry | TMSPMA modified Si-NPs MIP on polyaniline modified carbon screen-printed electrode | 9.29 × 10−7 M | 3–5 s | [108] |
| Urine | ||||||||
| Beverages | ||||||||
| Solution | KET | N.S. | Electrochemical | SPR | PANI- rgo-Fe3O4 nanocomposite | 0.1 ppm | 220 s | [80] |
| Powder | KET | 0.05 mL | Electrochemical | Potentiometry | Carbon-based screen-printed electrodes | N.S. | 60 s | [109] |
| Crystals |
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| 2FDCK | 2-fluorodeschloroketamine |
| 2FDCNEK | 2-fluorodeschloro-n-ethyl-ketamine |
| 2-oxo-PCE | n-ethyl-deschloroketamine |
| 3-MeO-PCE | 3-methoxyeticyclidine |
| 3-MeO-PCP | 3-methoxyphencyclidine |
| 3-MPA | 3-mercaptopropionic |
| 3-OH-PCP | 3-hydroxyphencyclidine |
| 4-MeO-PCP | 4-methoxyphencyclidine |
| 8BA-2F | ((((Hexane-1,6-diylbis(2,7-bis(4-(hydroxymethyl)-phenyl)-9H-fluorine-9,9-diyl))bis(hexane-6,1-diyl))bis-(9H-carbazole-9,3,6-triyl))tetrakis(benzene-4,1-diyl))tetramethanol |
| ACHs | Arylcyclohexylamines |
| Cal | Calcein |
| CD | carbon dots |
| CEDIA | cloned enzyme donor immunoassay |
| CE | 18-crown-6 |
| CNS | central nervous system |
| DAD | diode array detector |
| DBP | dibutyl phthalate |
| DBS | dried blood spots |
| DEA | Drug Enforcement Administration |
| DESI | desorption electrospray ionization |
| DHNK | Dehydronorketamine |
| DLLME | dispersive liquid/liquid microextraction |
| DME | dual mode extraction |
| DNA | deoxyribonucleic acid |
| DXE | deschloroketamine |
| EDC | 1-ethyl-3-(3-dimethylaminoprophyl) carbodiimide |
| EGDMA | ethylene glycol dimethacrylate |
| EI/CI | ion trap electron and chemical ionization |
| EIA | enzyme immunoassay |
| EIS | electrochemical impedance spectroscopy |
| ELISA | enzyme-linked immunosorbent assays |
| EMCDDA | European Monitoring Centre for Drugs and Drug Addiction |
| ESI | electrospray ionization |
| EU | European Union |
| FID | flame ionization detector |
| GC | gas chromatography |
| HPLC | high pressure liquid chromatography |
| HRAM | high resolution accurate mass |
| HRMS | high resolution mass spectrometry |
| HS | headspace |
| IMS | ion mobility spectrometry |
| IT | ion trap |
| KET | ketamine |
| KTpClPB | potassium tetrakis(p-chlorophenyl)borate |
| LC | liquid chromatography |
| LLE | liquid/liquid extraction |
| LOD | limit of detection |
| Lu | luminol |
| MAA | methacrylic acid |
| MEPS | microextraction by packed sorbent |
| MIP | molecularly imprinted polymer |
| MOFs@G | metal-organic framework/graphene nanocomposite |
| MS/MS | tandem mass spectrometry |
| MXE | methoxetamine |
| MXPr | methoxpropamine |
| Na-TPB | lipophilic anionic additive |
| NBS | n-bromosuccinimide |
| NENK | n-ethyl-norketamine |
| NHS | n-hydroxysulfsuccinimide |
| NKET | norketamine |
| NMDAr | N-methyl-D-aspartate receptor |
| NMR | nuclear magnetic resonance |
| NP | nanoparticles |
| N.S. | non-specified |
| N.A. | non-applicable |
| PANI-rGO | polyaniline-reduced graphene oxide |
| PBI-CB | o-carborane derivative of perylene bisimide |
| PCE | eticyclidine |
| PCP | phencyclidine |
| PCF | photonic crystal fibres |
| PDMS | polydimethylsiloxane |
| PGE | pencil graphite electrode |
| PLE | pressurized liquid extraction |
| PM-CE | a-keggin polyoxomolybdophosphate:18-crown-6 |
| PM-KT | phosphomolybdic–ketamine |
| PPT | protein precipitation |
| PTY | polytyramine |
| Q8 | cucurbit[8]uril |
| QTOF | quantitative time-of-flight |
| sd-GC-FTIR | solid deposition gas chromatography Fourier transform infrared spectrometry |
| SMART | Global Synthetics Monitoring: Analyses, Reporting and Trends |
| SPE | solid-phase extraction |
| SPR | surface plasmonic resonance |
| TA-Ag NCs | thiosalicylic acid-stabilized silver nanoclusters |
| THz | terahertz |
| TMSPMA | 3-(trimethoxysilyl)propyl methacrylate |
| TOF | time-of-flight |
| UHPLC | Ultra-high pressure liquid chromatography |
| UNODC | United Nations Office on Drugs and Crime |
| UV | ultra-violet |
| Zeo–GO | zeolites nanoflakes and graphene-oxide nanocrystal |
References
- Carroll, F.I.; Lewin, A.H.; Mascarella, S.W.; Seltzman, H.H.; Reddy, P.A. Designer drugs: A medicinal chemistry perspective. Ann. N. Y. Acad. Sci. 2012, 1248, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Garey, R.E.; Weisberg, L.A.; Heath, R.G. Phencyclidine: An overview. J. Psychoact. Drugs 1977, 9, 280–285. [Google Scholar] [CrossRef]
- Diversion Control Division, PHENCYCLIDINE (Street Names: PCP, Angel Dust, Supergrass, Boat, Tic Tac, Zoom, Shermans). 2023. Available online: https://www.deadiversion.usdoj.gov/drug_chem_info/pcp.pdf (accessed on 1 April 2024).
- Petersen, R.C.; Stillman, R.C. Phencyclidine (PCP) Abuse: An Appraisal; Petersen: RC, Stillman RC, USA, 1978. [Google Scholar]
- Pelletier, R.; Le Daré, B.; Le Bouëdec, D.; Kernalléguen, A.; Ferron, P.J.; Morel, I.; Gicquel, T. Arylcyclohexylamine Derivatives: Pharmacodynamic, Clinical and Forensic Aspects. Int. J. Mol. Sci. 2022, 23, 15574. [Google Scholar] [CrossRef] [PubMed]
- Zarantonello, P.; Bettini, E.; Paio, A.; Simoncelli, C.; Terreni, S.; Cardullo, F. Novel analogues of ketamine and phencyclidine as NMDA receptor antagonists. Bioorg. Med. Chem. Lett. 2011, 21, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.S.; Caramelo, D.; Simão, A.; Soares, S.P.S.; Gonçalves, J.; Rosado, T.; Antunes, M.; Barroso, M.; Gallardo, E. Ketamine and Other Phencyclidine Analogues: A Review of Their Use as Drugs of Abuse, Toxicological Aspects and Bioanalytical Approaches. In Ketamine: History, Uses and Health Effects; McBride, L.A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2019; pp. 1–111. [Google Scholar]
- New Psychoactive Substances-the Current Situation in Europe (European Drug Report 2023), n.d. Available online: https://www.emcdda.europa.eu/publications/european-drug-report/2023/new-psychoactive-substances_en (accessed on 1 April 2024).
- Other Drugs—The Current Situation in Europe (European Drug Report 2023). Available online: https://www.emcdda.europa.eu/publications/european-drug-report/2023/other-drugs_en (accessed on 1 April 2024).
- European Web Survey on Drugs: Patterns of Use. Available online: https://www.euda.europa.eu/activities/european-web-survey-on-drugs_en (accessed on 1 April 2024).
- Controlled Substances—Alphabetical Order. Available online: https://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf (accessed on 1 April 2024).
- Evropska unija. Evropska komisija. Evropski Center za Spremljanje Drog in Zasvojenosti z Drogami., New Psychoactive Substances in Europe: Legislation and Prosecution—Current Challenges and Solutions; Office for Official Publication of the European Communities: Luxembourg, 2016. [Google Scholar]
- Bertron, J.L.; Seto, M.; Lindsley, C.W. DARK Classics in Chemical Neuroscience: Phencyclidine (PCP). ACS Chem. Neurosci. 2018, 9, 2459–2474. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol 2018, 70, 621–660. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Kieliba, T.; Beike, J.; Thevis, M.; Mercer-Chalmers-Bender, K. Determination of 74 new psychoactive substances in serum using automated in-line solid-phase extraction-liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1064, 124–138. [Google Scholar] [CrossRef]
- Johansson, A.; Lindstedt, D.; Roman, M.; Thelander, G.; Nielsen, E.I.; Lennborn, U.; Sandler, H.; Rubertsson, S.; Ahlner, J.; Kronstrand, R.; et al. A non-fatal intoxication and seven deaths involving the dissociative drug 3-MeO-PCP. Forensic Sci. Int. 2017, 275, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Odoardi, S.; Anzillotti, L.; Strano-Rossi, S. Simplifying sample pretreatment: Application of dried blood spot (DBS) method to blood samples, including postmortem, for UHPLC-MS/MS analysis of drugs of abuse. Forensic Sci. Int. 2014, 243, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Odoardi, S.; Fisichella, M.; Romolo, F.S.; Strano-Rossi, S. High-throughput screening for new psychoactive substances (NPS) in whole blood by DLLME extraction and UHPLC-MS/MS analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1000, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Mercieca, G.; Odoardi, S.; Cassar, M.; Rossi, S.S. Rapid and simple procedure for the determination of cathinones, amphetamine-like stimulants and other new psychoactive substances in blood and urine by GC–MS. J. Pharm. Biomed. Anal. 2018, 149, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, F.; Busardò, F.P.; Palumbo, D.; Kyriakou, C.; Fioravanti, A.; Catalani, V.; Mari, F.; Bertol, E. A novel screening method for 64 new psychoactive substances and 5 amphetamines in blood by LC–MS/MS and application to real cases. J. Pharm. Biomed. Anal. 2016, 129, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Han, M.; An, S.; Moon, J.H.; Shim, G.; Chung, H. Screening of new psychoactive substances in human plasma by magnetic solid phase extraction and LC-QTOF-MS. Forensic Sci. Int. 2022, 332, 111176. [Google Scholar] [CrossRef] [PubMed]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Gonzáez-Díez, M.; Cela, R. Screening and selective quantification of illicit drugs in wastewater by mixed-mode solid-phase extraction and quadrupole-time-of-flight liquid chromatography-mass spectrometry. Anal. Chem. 2012, 84, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Park, J.; Hwang, J.; Chung, H. Magnetic Solid-Phase Extraction of Drugs and Pesticides from Human Plasma Using COOH-mMWCNTs. J. Anal. Toxicol. 2020, 44, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, P.; Zuba, D. Fatal Intoxication with Methoxetamine. J. Forensic Sci. 2015, 60, S264–S268. [Google Scholar] [CrossRef] [PubMed]
- Bakota, E.; Arndt, C.; Romoser, A.A.; Wilson, S.K. Fatal intoxication involving 3-MeO-PCP: A case report and validated method. J. Anal. Toxicol. 2016, 40, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Ström, L.; Tell, H.; Josefsson, M. Liquid chromatography/time-of-flight mass spectrometry analysis of postmortem blood samples for targeted toxicological screening. Anal. Bioanal. Chem. 2013, 405, 4107–4125. [Google Scholar] [CrossRef] [PubMed]
- Mitchell-Mata, C.; Thomas, B.; Peterson, B.; Couper, F. Two fatal intoxications involving 3-methoxyphencyclidine. J. Anal. Toxicol. 2017, 41, 503–507. [Google Scholar] [CrossRef] [PubMed]
- de Jong, L.A.A.; Olyslager, E.J.H.; Duijst, W.L.J.M. The risk of emerging new psychoactive substances: The first fatal 3-MeO-PCP intoxication in The Netherlands. J. Forensic Leg. Med. 2019, 65, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Mastrovito, R.; Trail, C.; Lino, M.; Cervantes, A.; Chan-Hosokawa, A.; Strathmann, F.; Logan, B. Determination of Cross-Reactivity of Novel Psychoactive Substances with Drug Screen Immunoassays Kits in Whole Blood. J. Anal. Toxicol. 2022, 46, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Arbouche, N.; Kintz, P.; Zagdoun, C.; Gheddar, L.; Raul, J.S.; Ameline, A. Determination of 3-MeO-PCP in human blood and urine in a fatal intoxication case, with a specific focus on metabolites identification. Forensic Sci. Res. 2021, 6, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Bertol, E.; Pascali, J.; Palumbo, D.; Catalani, V.; Di Milia, M.G.; Fioravanti, A.; Mari, F.; Vaiano, F. 3-MeO-PCP intoxication in two young men: First in vivo detection in Italy. Forensic Sci. Int. 2017, 274, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Fisichella, M.; Odoardi, S.; Strano-Rossi, S. High-throughput dispersive liquid/liquid microextraction (DLLME) method for the rapid determination of drugs of abuse, benzodiazepines and other psychotropic medications in blood samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and application to forensic cases. Microchem. J. 2015, 123, 33–41. [Google Scholar] [CrossRef]
- Cheng, W.C.; Dao, K.L. The emergence of deschloro-N-ethyl-ketamine, a ketamine analog, in drug seizures and drug driving cases in Hong Kong. J. Anal. Toxicol. 2020, 44, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Rodda, L.N.; Volk, J.A.; Moffat, E.; Williams, C.M.; Lynch, K.L.; Wu, A.H.B. Evaluation of intraosseous fluid as an alternative biological specimen in postmortem toxicology. J. Anal. Toxicol. 2018, 42, 163–169. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, G.; Brandt, S.D.; Wallach, J.; Archer, R.P.; Pounder, D.J. From the street to the laboratory: Analytical profiles of methoxetamine, 3-methoxyeticyclidine and 3-methoxyphencyclidine and their determination in three biological matrices. J. Anal. Toxicol. 2013, 37, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Mestria, S.; Odoardi, S.; Biosa, G.; Valentini, V.; Di Masi, G.; Cittadini, F.; Strano-Rossi, S. Method development for the identification of methoxpropamine, 2-fluoro-deschloroketamine and deschloroketamine and their main metabolites in blood and hair and forensic application. Forensic Sci. Int. 2021, 323, 110817. [Google Scholar] [CrossRef] [PubMed]
- Frison, G.; Zancanaro, F.; Frasson, S.; Quadretti, L.; Agnati, M.; Vlassich, F.; Gagliardi, G.; Salerno, T.M.G.; Donato, P.; Mondello, L. Analytical Characterization of 3-MeO-PCP and 3-MMC in Seized Products and Biosamples: The Role of LC-HRAM-Orbitrap-MS and Solid Deposition GC-FTIR. Front. Chem. 2021, 8, 618339. [Google Scholar] [CrossRef] [PubMed]
- Arango, E.; Toriello, A.; Rosario, Z.; Cooper, G. Increasing Prevalence of Ketamine in Drivers in New York City including the Identification of 2-Fluoro-Deschloroketamine. J. Anal. Toxicol. 2021, 45, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Gomila, I.; Leciñena, M.Á.; Elorza, M.Á.; Pastor, Y.; Sahuquillo, L.; Servera, M.; Puiguriguer, J.; Barcelo, B. Detectability of Dissociative Psychoactive Substances in Urine by Five Commercial Phencyclidine Immunoassays. J. Anal. Toxicol. 2019, 43, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Helander, A.; Bäckberg, M.; Hultén, P.; Al-Saffar, Y.; Beck, O. Detection of new psychoactive substance use among emergency room patients: Results from the Swedish STRIDA project. Forensic Sci. Int. 2014, 243, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Al-Saffar, Y.; Stephanson, N.N.; Beck, O. Multicomponent LC-MS/MS screening method for detection of new psychoactive drugs, legal highs, in urine-Experience from the Swedish population. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 930, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.A.; Liu, H.C.; Lin, D.L.; Liu, R.H.; Hsieh, Y.Z.; Wu, S.P. Simultaneous quantitation of methamphetamine, ketamine, opiates and their metabolites in urine by SPE and LC-MS-MS. J. Anal. Toxicol. 2017, 41, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Barroso, M.; Martinho, A.; Cruz, A.; Gallardo, E. Determination of ketamine and its major metabolite, norketamine, in urine and plasma samples using microextraction by packed sorbent and gas chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1004, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Extraction of Illicit and Prescribed Drugs from Enzyme-Hydrolyzed Urine Using ISOLUTE ® HYDRO DME+ Prior to UPLC-MS/MS Analysis. 2019. Available online: https://www.biotage.com/documents/extraction-illicit-prescribed-drugs-enzyme-hydrolyzed-urine-isolute-hydro-dme (accessed on 15 March 2024).
- Wong, G.F.; Lee, W.M.; Li, C.K. Qualitative Screening of Amphetamine-and Ketamine-Type Abuse Drugs in Urine Employing Dual Mode Extraction Column by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS-MS). J. Anal. Toxicol. 2023, 46, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.T.; Tsai, Y.S.; Su, W.L.; Huang, D.Y.; Wu, H.H.; Tseng, S.H.; Wang, H.H.; Chiu, C.Y.; Wang, C.F.; Liu, C.Y.; et al. New ketamine analogue: 2-fluorodeschloro-N-ethyl-ketamine and its suggested metabolites. Forensic Sci. Int. 2022, 341, 111501. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.H.Y.; Chong, Y.K.; Chan, C.Y.; Ching, C.K.; Lai, C.K.; Li, Y.K.; Mak, T.W.L. Cluster of acute poisonings associated with an emerging ketamine analogue, 2-oxo-PCE. Forensic Sci. Int. 2018, 290, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.H.Y.; Ching, C.K.; Lee, C.Y.W.; Lam, Y.H.; Mak, T.W.L. Simultaneous detection of 93 conventional and emerging drugs of abuse and their metabolites in urine by UHPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 969, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Weng, T.I.; Ng, C.J.; Shih, C.P.; Hsu, J.; Liao, Y.C.; Yang, C.C.; Fang, C.C. Emergency department visits due to new psychoactive substances and other illicit drugs in Taiwan: Preliminary results of the Taiwan Emergency Department Drug Abuse Surveillance (TEDAS) project. Clin. Toxicol. 2022, 60, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.C.; Dao, K.L. Prevalence of drugs of abuse found in forensic testing of illicit drug seizures and urine samples from offenders/probationers in Hong Kong: A 3-year update. Forensic Sci. Int. 2020, 317, 110535. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.C.; Yau, T.S.; Wong, M.K.; Chan, L.P.; Mok, V.K.K. A high-throughput urinalysis of abused drugs based on a SPE-LC-MS/MS method coupled with an in-house developed post-analysis data treatment system. Forensic Sci. Int. 2006, 162, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.H.Y.; Li, T.C.; Lai, C.K.; Chong, Y.K.; Ching, C.K.; Mak, T.W.L. Emergence of new psychoactive substance 2-fluorodeschloroketamine: Toxicology and urinary analysis in a cluster of patients exposed to ketamine and multiple analogues. Forensic Sci. Int. 2020, 312, 110327. [Google Scholar] [CrossRef] [PubMed]
- Odoardi, S.; Valentini, V.; De Giovanni, N.; Pascali, V.L.; Strano-Rossi, S. High-throughput screening for drugs of abuse and pharmaceutical drugs in hair by liquid-chromatography-high resolution mass spectrometry (LC-HRMS). Microchem. J. 2017, 133, 302–310. [Google Scholar] [CrossRef]
- Favretto, D.; Cooper, G.; Andraus, M.; Sporkert, F.; Agius, R.; Appenzeller, B.; Baumgartner, M.; Binz, T.; Cirimele, V.; Kronstrand, R.; et al. The Society of Hair Testing consensus on general recommendations for hair testing and drugs of abuse testing in hair. Drug Test. Anal. 2023, 15, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Agazzi, S.; Riboni, N.; Erdal, N.; Hakkarainen, M.; Ilag, L.L.; Anzillotti, L.; Andreoli, R.; Marezza, F.; Moroni, F.; et al. Novel sample-substrates for the determination of new psychoactive substances in oral fluid by desorption electrospray ionization-high resolution mass spectrometry. Talanta 2019, 202, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Health and Human Services Department; Substance Abuse and Mental Health Services Administration (SAMHSA), Mandatory Guidelines for Federal Workplace Drug Testing Programs. 2023. Available online: https://www.federalregister.gov/documents/2023/10/12/2023-21735/mandatory-guidelines-for-federal-workplace-drug-testing-programs (accessed on 27 June 2024).
- Sorribes-Soriano, A.; Armenta, S.; Turrillas, F.A.E.; Herrero-Martínez, J.M. Tuning the selectivity of molecularly imprinted polymer extraction of arylcyclohexylamines: From class-selective to specific. Anal. Chim. Acta 2020, 1124, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Montesano, C.; Cellucci, L.; Gregori, A.; Fanti, F.; Compagnone, D.; Curini, R.; Sergi, M. Combination of pressurized liquid extraction with dispersive liquid liquid micro extraction for the determination of sixty drugs of abuse in hair. J. Chromatogr. A 2019, 1605, 360348. [Google Scholar] [CrossRef] [PubMed]
- Salomone, A.; Galletto, M.; Massano, M.; Di, D.; Palamar, J.J.; Vincenti, M. Detection of fentanyl; synthetic opioids, and ketamine in hair specimens from purposive samples of American and Italian populations. J. Forensic Sci. 2023, 68, 1698–1707. [Google Scholar] [CrossRef]
- Di Corcia, D.; Salomone, A.; Gerace, E. Analysis of Drugs of Abuse in Hair Samples by Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry (UHPLC-MS/MS). In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; pp. 107–114. [Google Scholar] [CrossRef]
- Fabresse, N.; Larabi, I.A.; Stratton, T.; Mistrik, R.; Pfau, G.; Lorin de la Grandmaison, G.; Etting, I.; Grassin Delyle, S.; Alvarez, J.C. Development of a sensitive untargeted liquid chromatography–high resolution mass spectrometry screening devoted to hair analysis through a shared MS2 spectra database: A step toward early detection of new psychoactive substances. Drug Test. Anal. 2019, 11, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Gicquel, T.; Richeval, C.; Mesli, V.; Gish, A.; Hakim, F.; Pelletier, R.; Cornez, R.; Balgairies, A.; Allorge, D.; Gaulier, J.M. Fatal intoxication related to two new arylcyclohexylamine derivatives (2F-DCK and 3-MeO-PCE). Forensic Sci. Int. 2021, 324, 110852. [Google Scholar] [CrossRef] [PubMed]
- Richeval, C.; Gaulier, J.M.; Romeuf, L.; Allorge, D.; Gaillard, Y. Case report: Relevance of metabolite identification to detect new synthetic opioid intoxications illustrated by U-47700. Int. J. Legal. Med. 2019, 133, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.Y.; Oliveira, P.; Rosendo, L.M.; Rosado, T.; Andraus, M.; Barroso, M.; Gallardo, E. Microextraction by Packed Sorbent as a Clean-up Approach for the Determination of Ketamine and Norketamine in Hair by Gas Chromatography—Tandem Mass Spectrometry. J. Anal. Toxicol. 2023, 47, 227–235. [Google Scholar] [CrossRef]
- Lam, R.P.K.; Tang, M.H.Y.; Leung, S.C.; Chong, Y.K.; Tsui, M.S.H.; Mak, T.W.L. Supraventricular tachycardia and acute confusion following ingestion of e-cigarette fluid containing AB-FUBINACA and ADB-FUBINACA: A case report with quantitative analysis of serum drug concentrations. Clin. Toxicol. 2017, 55, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Queiroz, J.A. The role of alternative specimens in toxicological analysis. Biomed. Chromatogr. 2008, 22, 795–821. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Gallardo, E. Hair analysis for forensic applications: Is the future bright? Bioanalysis 2014, 6, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Barroso, M.; Queiroz, J.A. Current technologies and considerations for drug bioanalysis in oral fluid. Bioanalysis 2009, 1, 637–667. [Google Scholar] [CrossRef] [PubMed]
- Margalho, C.; Castanheira, A.; Real, F.C.; Gallardo, E.; López-Rivadulla, M. Determination of “new psychoactive substances” in postmortem matrices using microwave derivatization and gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1020, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Moreno, I.; Da Fonseca, B.; Queiroz, J.A.; Gallardo, E. Role of microextraction sampling procedures in forensic toxicology. Bioanalysis 2012, 4, 1805–1826. [Google Scholar] [CrossRef] [PubMed]
- Simao, A.Y.; Antunes, M.; Marques, H.; Rosado, T.; Soares, S.; Goncalves, J.; Barroso, M.; Andraus, M.; Gallardo, E. Recent bionalytical methods for the determination of new psychoactive substances in biological specimens. Bioanalysis 2020, 12, 1557–1595. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, Y.; Hu, J.; Peng, A.; Hu, W. Deep learning-assisted multicolor fluorescent probes for image and spectral dual-modal identification of illicit drugs. Sens. Actuators B Chem. 2023, 394, 134348. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Farag, M.A.; Yehia, A.M. A gold-carbon dots nanoprobe for dual mode detection of ketamine HCl in soda drinks. New J. Chem. 2020, 44, 7058–7064. [Google Scholar] [CrossRef]
- Yan, K.; Wang, L.; Zhou, H.; Hua, Z.; Xu, P.; Xu, H.; Wang, Y.; Di, B.; Hu, C. Cucurbituril-mediated AIE: An unconventional indicator displacement assay for ketamine detection. Dye. Pigment. 2022, 197, 109875. [Google Scholar] [CrossRef]
- Monir, M.K.; Uddin, M.S.; Sen, S. Design of a novel photonic crystal fiber and numerical analysis of sensitivity for the detection of illegal drugs in terahertz regime. Sens. Biosensing Res. 2023, 39, 100551. [Google Scholar] [CrossRef]
- Jin, C.; Li, M.; Duan, S.; Zhang, Q.; Zhang, G.; Liu, Q.; Zhang, R.; Bai, H. An electrochemical sensor for direct and sensitive detection of ketamine. Biosens. Bioelectron. 2023, 226, 115134. [Google Scholar] [CrossRef] [PubMed]
- El-Naby, E.H. Selective ketamine recognition based on membrane potential changes induced by a hybrid organic/inorganic supramolecular assembly. Anal. Methods 2014, 6, 900–906. [Google Scholar] [CrossRef]
- Poulladofonou, G.; Freris, C.; Economou, A.; Kokkinos, C. Wearable Electronic Finger for Date Rape Drugs Screening: From “do-It-Yourself” Fabrication to Self-Testing. Anal. Chem. 2022, 94, 4087–4094. [Google Scholar] [CrossRef] [PubMed]
- Deiminiat, B.; Rounaghi, G.H. Fabrication of a new electrochemical imprinted sensor for determination of ketamine based on modified polytyramine/sol-gel/f-MWCNTs@AuNPs nanocomposite/pencil graphite electrode. Sens. Actuators B Chem. 2018, 259, 133–141. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Hamidi, S.M.; Kazemzad, M.; Rafiei, A.; Amouyan, F.; Sadeghi, S. Ketamine plasmonic sensor using polyaniline-rGO-Fe3O4 nanocomposite thin layer. Sens. Actuators A Phys. 2022, 347, 113896. [Google Scholar] [CrossRef]
- Wu, C.C.; Chiu, S.W.; Tang, K.T. An Electronic Nose System for Rapid Detection of Ketamine Smoke. IEEE Sens. Lett. 2019, 3, 6001604. [Google Scholar] [CrossRef]
- Parrilla, M.; Joosten, F.; De Wael, K. Enhanced electrochemical detection of illicit drugs in oral fluid by the use of surfactant-mediated solution. Sens. Actuators B Chem. 2021, 348, 130659. [Google Scholar] [CrossRef]
- Huang, L.; Li, T.; Zhang, Y.; Sun, X.; Wang, Y.; Nie, Z. Discrimination of narcotic drugs in human urine based on nanoplasmonics combined with chemometric method. J. Pharm. Biomed. Anal. 2020, 186, 113174. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Khaleque, A.; Ali, M.Y.; Rahman, M.T. THz spectroscopic sensing of liquid chemicals using a photonic crystal fiber. OSA Contin. 2020, 3, 2982. [Google Scholar] [CrossRef]
- Fan, T.; Xu, W.; Yao, J.; Jiao, Z.; Fu, Y.; Zhu, D.; He, Q.; Cao, H.; Cheng, J. Naked-Eye Visible Solid Illicit Drug Detection at Picogram Level via a Multiple-Anchored Fluorescent Probe. ACS Sens. 2016, 1, 312–317. [Google Scholar] [CrossRef]
- Liu, K.; Shang, C.; Wang, Z.; Qi, Y.; Miao, R.; Liu, K.; Liu, T.; Fang, Y. Non-contact identification and differentiation of illicit drugs using fluorescent films. Nat. Commun. 2018, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, Z.; Jiang, H.; Wang, W.; Wu, Y. Drugs of abuse detection in saliva based on actuated optical method. In International Symposium on Optoelectronic Technology and Application 2014: Laser and Optical Measurement Technology and Fiber Optic Sensors; SPIE: Paris, France, 2014; p. 92971K. [Google Scholar] [CrossRef]
- Guo, J.; Tian, S.; Liu, K.; Guo, J. IoT-Enabled Fluorescence Sensor for Quantitative KET Detection and Anti-Drug Situational Awareness. IEEE Trans. Nanobiosci. 2021, 20, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lin, J.H.; Chen, L.Y.; Feng, L.; Chen, Z.M.; Zheng, J.X.; Qin, S.N.; Li, G.W.; Salminen, K.; Sun, J.J. Rapid nanomolar detection of ketamine in biofluids based on electrochemical aptamer-based sensor for drugged driving screening within 30 s. Sens. Actuators B Chem. 2023, 390, 133903. [Google Scholar] [CrossRef]
- Chen, C.A.; Wang, P.W.; Yen, Y.C.; Lin, H.L.; Fan, Y.C.; Wu, S.M.; Chen, C.F. Fast analysis of ketamine using a colorimetric immunosorbent assay on a paper-based analytical device. Sens. Actuators B Chem. 2019, 282, 251–258. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Jiang, X.; Cao, F.; Liu, W. An enzyme-free FRET nanoprobe for ultrasensitive ketamine detection based on ATP-fueled target recycling. RSC Adv. 2019, 9, 36884–36889. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhai, S.; Liu, C.; Wang, X.; Tu, Y. Disposable Immunosensor Based on Electrochemiluminescence for Ultrasensitive Detection of Ketamine in Human Hair. ACS Omega 2019, 4, 801–809. [Google Scholar] [CrossRef]
- Yehia, A.M.; Farag, M.A.; Tantawy, M.A. A novel trimodal system on a paper-based microfluidic device for on-site detection of the date rape drug “ketamine”. Anal. Chim. Acta 2020, 1104, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tu, Y.; Wang, X.; Pan, J.; Ding, Y. A label-free immunosensor for ultrasensitive detection of ketamine based on quartz crystal microbalance. Sensors 2015, 15, 8540–8549. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Singhal, C.; Mathur, A.; Dubey, A.K.; PN, A.K.; Anil, A.; Pundir, C.S. Naked-eye quantitative assay on paper device for date rape drug sensing via smart phone APP. Vacuum 2018, 153, 300–305. [Google Scholar] [CrossRef]
- Zhou, Z.; Gong, Y.; Zhang, C.; Niu, W. A chemiluminescence sensor array for discrimination of seven toxicants. Luminescence 2021, 36, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Shawish, H.M.A.; Saadeh, S.M.; Tamos, H.; Abed-Almonem, K.I.; Al Khalili, O. A new potentiometric sensor for the determination of ketamine hydrochloride in ampoules and urine. Anal. Methods 2015, 7, 301–308. [Google Scholar] [CrossRef]
- Shawish, H.M.A.; Tamous, H.; Saadeh, S.M.; Abed-Almonem, K.I.; Al Khalili, O. A new approach for decreasing the detection limit for a ketamine(I) ion-selective electrode. Mater. Sci. Eng. C 2015, 49, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Malhotra, N.; Singhal, C.; Mathur, A.; Chakraborty, D.; Anil, A.; Ingle, A.; Pundir, C.S. Point of care with micro fluidic paper based device integrated with nano zeolite–graphene oxide nanoflakes for electrochemical sensing of ketamine. Biosens. Bioelectron. 2017, 88, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, X.; Guo, Y.; Yan, J.; Ling, J.; Li, W.; Lan, L.; Chang, Y.; Cai, J.; Zha, L. Rapid and sensitive detection of ketamine in blood using novel fluorescence genosensor. Anal. Bioanal. Chem. 2017, 409, 7027–7034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Tu, Y. An electrochemical impedimetric immunosensor for ultrasensitive determination of ketamine hydrochloride. Sens. Actuators B Chem. 2013, 183, 150–156. [Google Scholar] [CrossRef]
- Phan, T.L.; Van Hieu, N.; Lin, J.J.; Wu, H.J.; Chu, P.Y.; Hsiao, C.S.; Yao, F.Y.D.; Lin, Y.K.; Ching, C.T.S. An enzyme-free interdigitated electrode for ketamine detection. J. Chin. Inst. Eng. 2023, 46, 441–452. [Google Scholar] [CrossRef]
- Fu, K.; Zhang, R.; He, J.; Bai, H.; Zhang, G. Sensitive detection of ketamine with an electrochemical sensor based on UV-induced polymerized molecularly imprinted membranes at graphene and MOFs modified electrode. Biosens. Bioelectron. 2019, 143, 111636. [Google Scholar] [CrossRef] [PubMed]
- Argente-García, A.; Jornet-Martínez, N.; Herráez-Hernández, R.; Campíns-Falcó, P. A passive solid sensor for in-situ colorimetric estimation of the presence of ketamine in illicit drug samples. Sens. Actuators B Chem. 2017, 253, 1137–1144. [Google Scholar] [CrossRef]
- Yen, Y.T.; Lin, Y.S.; Chang, Y.J.; Li, M.T.; Chyueh, S.C.; Chang, H.T. Nanomaterial-Based Sensor Arrays With Deep Learning for Screening of Illicit Drugs. Adv. Mater. Technol. 2022, 7, 2200243. [Google Scholar] [CrossRef]
- Zou, F.; Fu, K.; Jin, C.; Li, M.; Zhang, G.; Zhang, R.; Bai, H. Microwave-prepared surface imprinted magnetic nanoparticles based electrochemical sensor for adsorption and determination of ketamine in sewage. Anal. Chim. Acta 2022, 1217, 340025. [Google Scholar] [CrossRef] [PubMed]
- Maidi, A.M.; Salam, R.; Zou, N.; Begum, F. Performance evaluation of photonic crystal fibre sensor for controlled drugs detection: A simulation approach. Phys. Scr. 2023, 98, 115511. [Google Scholar] [CrossRef]
- Soliman, S.S.; Mahmoud, A.M.; Elghobashy, M.R.; Zaazaa, H.E.; Sedik, G.A. Point-of-care electrochemical sensor for selective determination of date rape drug “ketamine” based on core-shell molecularly imprinted polymer. Talanta 2023, 254, 124151. [Google Scholar] [CrossRef] [PubMed]
- Van Echelpoel, R.; Schram, J.; Parrilla, M.; Daems, D.; Slosse, A.; Van Durme, F.; De Wael, K. Electrochemical methods for on-site multidrug detection at festivals. Sens. Diagn. 2022, 1, 793–802. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelixo, R.; Barroso, M.; Gallardo, E.; Rosado, T. Determination of Arylcyclohexylamines in Biological Specimens: Sensors and Sample Pre-Treatment Approaches. Micromachines 2024, 15, 984. https://doi.org/10.3390/mi15080984
Pelixo R, Barroso M, Gallardo E, Rosado T. Determination of Arylcyclohexylamines in Biological Specimens: Sensors and Sample Pre-Treatment Approaches. Micromachines. 2024; 15(8):984. https://doi.org/10.3390/mi15080984
Chicago/Turabian StylePelixo, Rodrigo, Mário Barroso, Eugenia Gallardo, and Tiago Rosado. 2024. "Determination of Arylcyclohexylamines in Biological Specimens: Sensors and Sample Pre-Treatment Approaches" Micromachines 15, no. 8: 984. https://doi.org/10.3390/mi15080984
APA StylePelixo, R., Barroso, M., Gallardo, E., & Rosado, T. (2024). Determination of Arylcyclohexylamines in Biological Specimens: Sensors and Sample Pre-Treatment Approaches. Micromachines, 15(8), 984. https://doi.org/10.3390/mi15080984







