Customization of Computed Tomography Radio-Opacity in 3D-Printed Contrast-Injectable Tumor Phantoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Phantom Construction
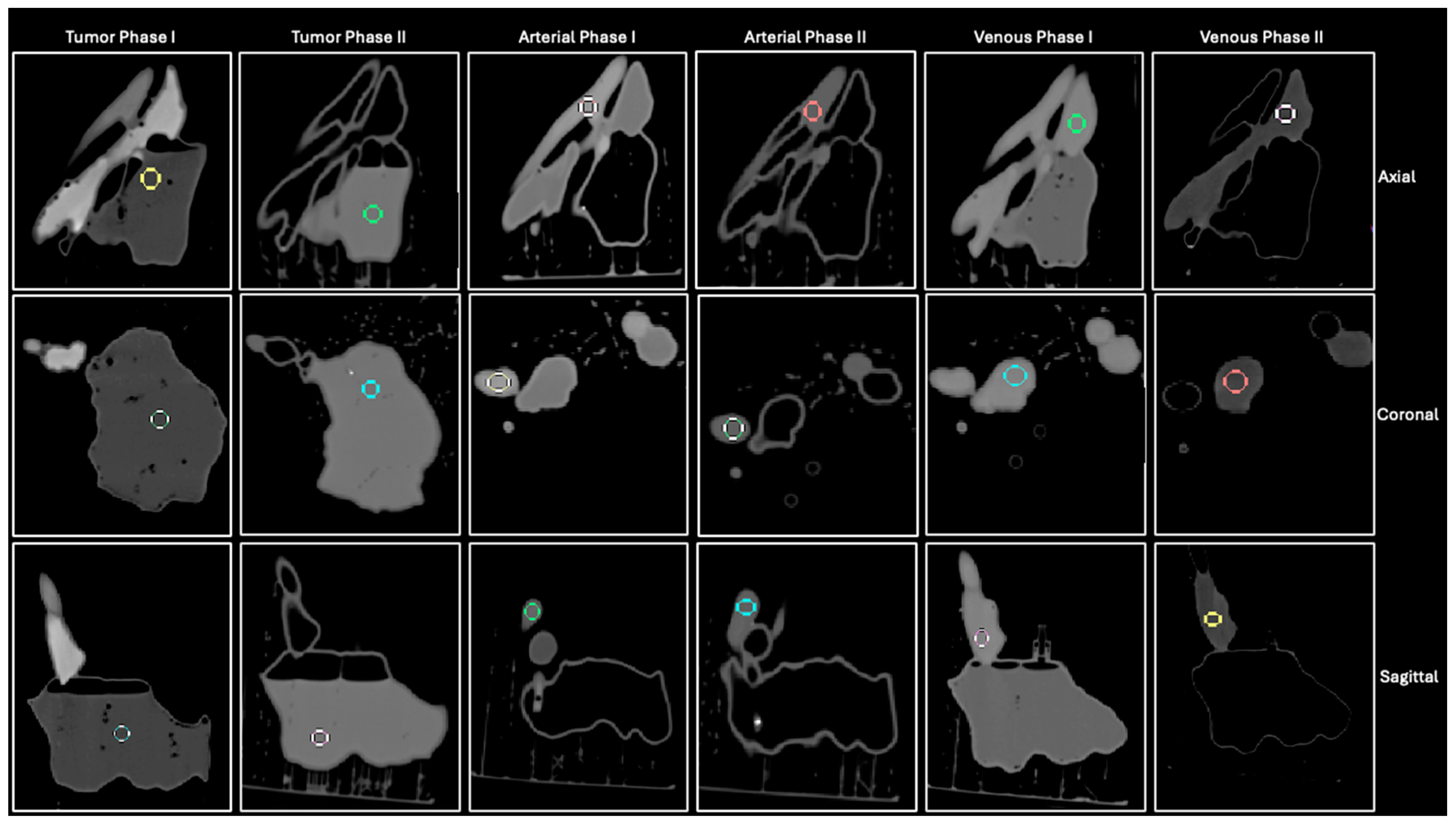
2.2. Tumor Phantom Contrast Injection
3. Results
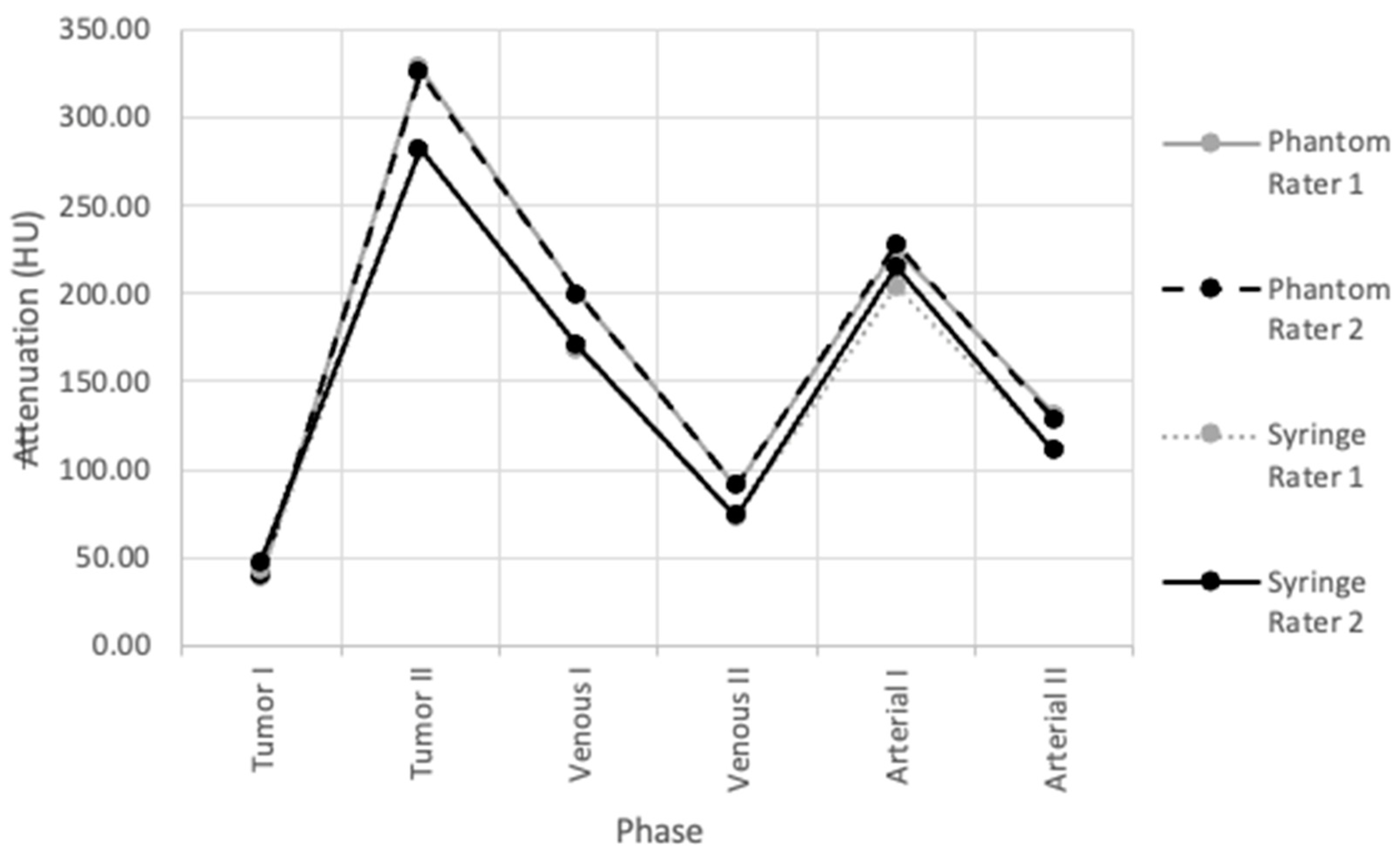
3.1. Contrast Injection and Phantom Attenuation
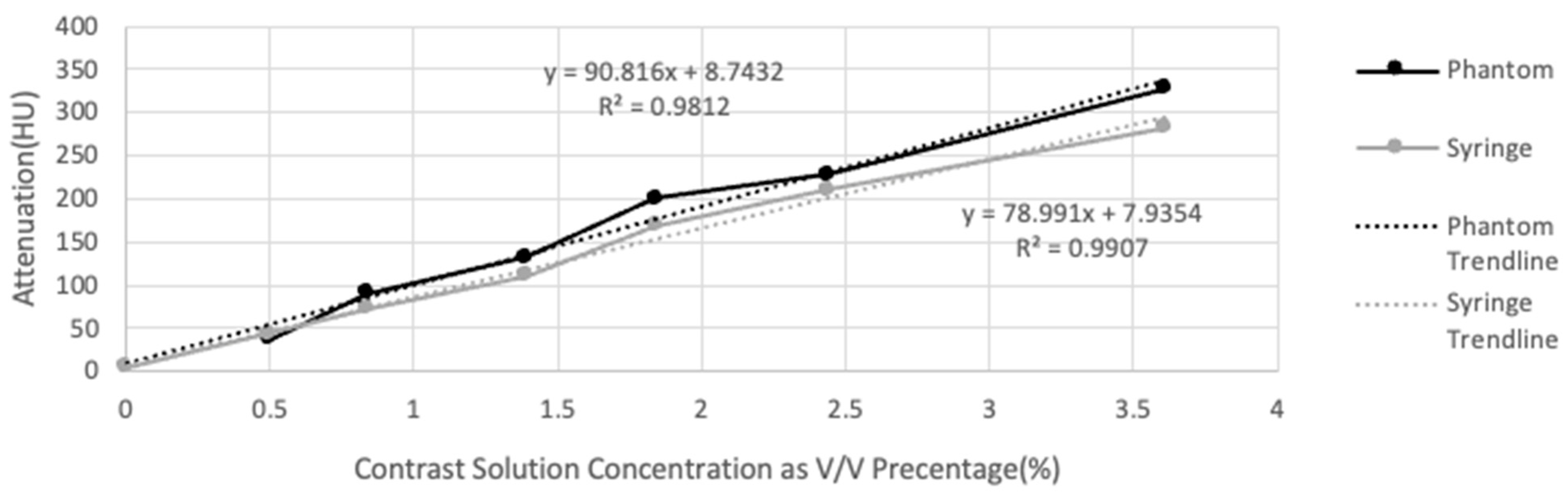
3.2. Attenuation versus Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Ho, C.-C.; Zhang, C.; Wang, B. A Review on the 3D Printing of Functional Structures for Medical Phantoms and Regenerated Tissue and Organ Applications. Engineering 2017, 3, 653–662. [Google Scholar] [CrossRef]
- Leary, M.; Kron, T.; Keller, C.; Franich, R.; Lonski, P.; Subic, A.; Brandt, M. Additive manufacture of custom radiation dosimetry phantoms: An automated method compatible with commercial polymer 3D printers. Mater. Des. 2015, 86, 487–499. [Google Scholar] [CrossRef]
- Bücking, T.M.; Hill, E.R.; Robertson, J.L.; Maneas, E.; Plumb, A.A.; Nikitichev, D.I. From medical imaging data to 3D printed anatomical models. PLoS ONE 2017, 12, e0178540. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Zhang, S.; Guo, X.; Fu, T.; Zhao, L.; He, C. Clinical Application of Individualized 3D-Printed Chest Wall Conformal Device in IMRT for Post-Mastectomy Breast Cancer. Curr. Med. Imaging 2024, 20, 9. [Google Scholar] [CrossRef]
- Burleson, S.; Baker, J.; Hsia, A.T.; Xu, Z. Use of 3D printers to create a patient-specific 3D bolus for external beam therapy. J. Appl. Clin. Med. Phys. 2015, 16, 5247. [Google Scholar] [CrossRef]
- Alssabbagh, M.; Tajuddin, A.A.; Abdulmanap, M.; Zainon, R. Evaluation of 3D printing materials for fabrication of a novel multi-functional 3D thyroid phantom for medical dosimetry and image quality. Radiat. Phys. Chem. 2017, 135, 106–112. [Google Scholar] [CrossRef]
- Ardila Pardo, G.L.; Conzelmann, J.; Genske, U.; Hamm, B.; Scheel, M.; Jahnke, P. 3D printing of anatomically realistic phantoms with detection tasks to assess the diagnostic performance of CT images. Eur. Radiol. 2020, 30, 4557–4563. [Google Scholar] [CrossRef]
- Green, S.; Grice, J. Technical note: 3D-printed phantom for dedicated cardiac protocols and geometries in nuclear medicine. Med. Phys. 2022, 49, 943–951. [Google Scholar] [CrossRef]
- Driscoll, B.; Keller, H.; Coolens, C. Development of a dynamic flow imaging phantom for dynamic contrast-enhanced CT. Med. Phys. 2011, 38, 4866–4880. [Google Scholar] [CrossRef]
- Arconada-Alvarez, S.J.; Lemaster, J.E.; Wang, J.; Jokerst, J.V. The development and characterization of a novel yet simple 3D printed tool to facilitate phantom imaging of photoacoustic contrast agents. Photoacoustics 2017, 5, 17–24. [Google Scholar] [CrossRef]
- Qiu, J.; Hou, K.; Dyer, B.A.; Chen, J.-C.; Shi, L.; Sun, Y.; Xu, L.; Zhao, H.; Li, Z.; Chen, T.; et al. Constructing Customized Multimodal Phantoms Through 3D Printing: A Preliminary Evaluation. Front. Phys. 2021, 9, 605630. [Google Scholar] [CrossRef]
- Ng, W.L.; An, J.; Chua, C.K. Process, Material, and Regulatory Considerations for 3D Printed Medical Devices and Tissue Constructs. Engineering 2024, 36, 146–166. [Google Scholar] [CrossRef]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef]
- Mamo, H.B.; Adamiak, M.; Kunwar, A. 3D printed biomedical devices and their applications: A review on state-of-the-art technologies, existing challenges, and future perspectives. J. Mech. Behav. Biomed. Mater. 2023, 143, 105930. [Google Scholar] [CrossRef]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of Polymers: Fused Deposition Modelling (FDM), Selective Laser Sintering (SLS), and Stereolithography (SLA). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.F.; Thieringer, F.M. Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef]
- Szczęch, M.; Sikora, W. The Influence of Printing Parameters on Leakage and Strength of Fused Deposition Modelling 3D Printed Parts. Adv. Sci. Technol. Res. J. 2024, 18, 195–201. [Google Scholar] [CrossRef]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Ahmed, I.; Sullivan, K.; Priye, A. Multi-Resin Masked Stereolithography (MSLA) 3D Printing for Rapid and Inexpensive Prototyping of Microfluidic Chips with Integrated Functional Components. Biosensors 2022, 12, 652. [Google Scholar] [CrossRef]
- Patmonoaji, A.; Mahardika, M.A.; Nasir, M.; She, Y.; Wang, W.; Muflikhun, M.A.; Suekane, T. Stereolithography 3D Printer for Micromodel Fabrications with Comprehensive Accuracy Evaluation by Using Microtomography. Geosciences 2022, 12, 183. [Google Scholar] [CrossRef]
- Formlabs Inc. Tough 1500 Resin Technical Data Sheet (FLTO1501). 2020. Available online: https://formlabs.com/store/materials/tough-1500-resin/ (accessed on 22 February 2024).
- Kalidindi, Y.; Ganapathy, A.K.; Nayak, Y.; Elumalai, A.; Chen, D.Z.; Bishop, G.; Sanchez, A.; Albers, B.; Shetty, A.S.; Ballard, D.H. Computed Tomography Attenuation of Three-Dimensional (3D) Printing Materials-Depository to Aid in Constructing 3D-Printed Phantoms. Micromachines 2023, 14, 928. [Google Scholar] [CrossRef]
- Lee, K.H.; O’Malley, M.E.; Haider, M.A.; Hanbidge, A. Triple-phase MDCT of hepatocellular carcinoma. AJR Am. J. Roentgenol. 2004, 182, 643–649. [Google Scholar] [CrossRef]
- Subhawong, T.K.; Fishman, E.K.; Swart, J.E.; Carrino, J.A.; Attar, S.; Fayad, L.M. Soft-tissue masses and masslike conditions: What does CT add to diagnosis and management? AJR Am. J. Roentgenol. 2010, 194, 1559–1567. [Google Scholar] [CrossRef]
- Garner, H.W.; Paturzo, M.M.; Gaudier, G.; Pickhardt, P.J.; Wessell, D.E. Variation in Attenuation in L1 Trabecular Bone at Different Tube Voltages: Caution Is Warranted When Screening for Osteoporosis With the Use of Opportunistic CT. Am. J. Roentgenol. 2016, 208, 165–170. [Google Scholar] [CrossRef]
- Marques, M.L.; da Silva, N.P.; van der Heijde, D.; Reijnierse, M.; Baraliakos, X.; Braun, J.; van Gaalen, F.; Ramiro, S. Hounsfield Units measured in low dose CT reliably assess vertebral trabecular bone density changes over two years in axial spondyloarthritis. Semin. Arthritis Rheum. 2023, 58, 152144. [Google Scholar] [CrossRef]
- Formlabs Inc. Form Wash and Form Wash L Time Settings. Available online: https://support.formlabs.com/s/article/Form-Wash-Time-Settings?language=en_US (accessed on 8 July 2024).
- Formlabs Inc. Form Cure Time and Temperature Settings. 2024. Available online: https://support.formlabs.com/s/article/Form-Cure-Time-and-Temperature-Settings?language=en_US (accessed on 22 February 2024).
- DenOtter, T.D.; Schubert, J. Hounsfield Unit. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lamba, R.; McGahan, J.P.; Corwin, M.T.; Li, C.S.; Tran, T.; Seibert, J.A.; Boone, J.M. CT Hounsfield numbers of soft tissues on unenhanced abdominal CT scans: Variability between two different manufacturers’ MDCT scanners. AJR Am. J. Roentgenol. 2014, 203, 1013–1020. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chiou, Y.Y.; Wu, M.H.; Huang, S.S.; Shen, S.H. Attenuation values of renal parenchyma in virtual noncontrast images acquired from multiphase renal dual-energy CT: Comparison with standard noncontrast CT. Eur. J. Radiol. 2018, 101, 103–110. [Google Scholar] [CrossRef]
- Holcombe, S.A.; Horbal, S.R.; Ross, B.E.; Brown, E.; Derstine, B.A.; Wang, S.C. Variation in aorta attenuation in contrast-enhanced CT and its implications for calcification thresholds. PLoS ONE 2022, 17, e0277111. [Google Scholar] [CrossRef]
- Almeida, R.R.; Lo, G.C.; Patino, M.; Bizzo, B.; Canellas, R.; Sahani, D.V. Advances in Pancreatic CT Imaging. AJR Am. J. Roentgenol. 2018, 211, 52–66. [Google Scholar] [CrossRef]
- Furlan, A.; Marin, D.; Vanzulli, A.; Patera, G.P.; Ronzoni, A.; Midiri, M.; Bazzocchi, M.; Lagalla, R.; Brancatelli, G. Hepatocellular carcinoma in cirrhotic patients at multidetector CT: Hepatic venous phase versus delayed phase for the detection of tumour washout. Br. J. Radiol. 2011, 84, 403–412. [Google Scholar] [CrossRef]
- Chougule, V.; Mulay, A.; Ahuja, B. Clinical Case Study: Spine Modeling for Minimum Invasive Spine Surgeries (MISS) using Rapid Prototyping. Bone 2018, 26, 3071. [Google Scholar]
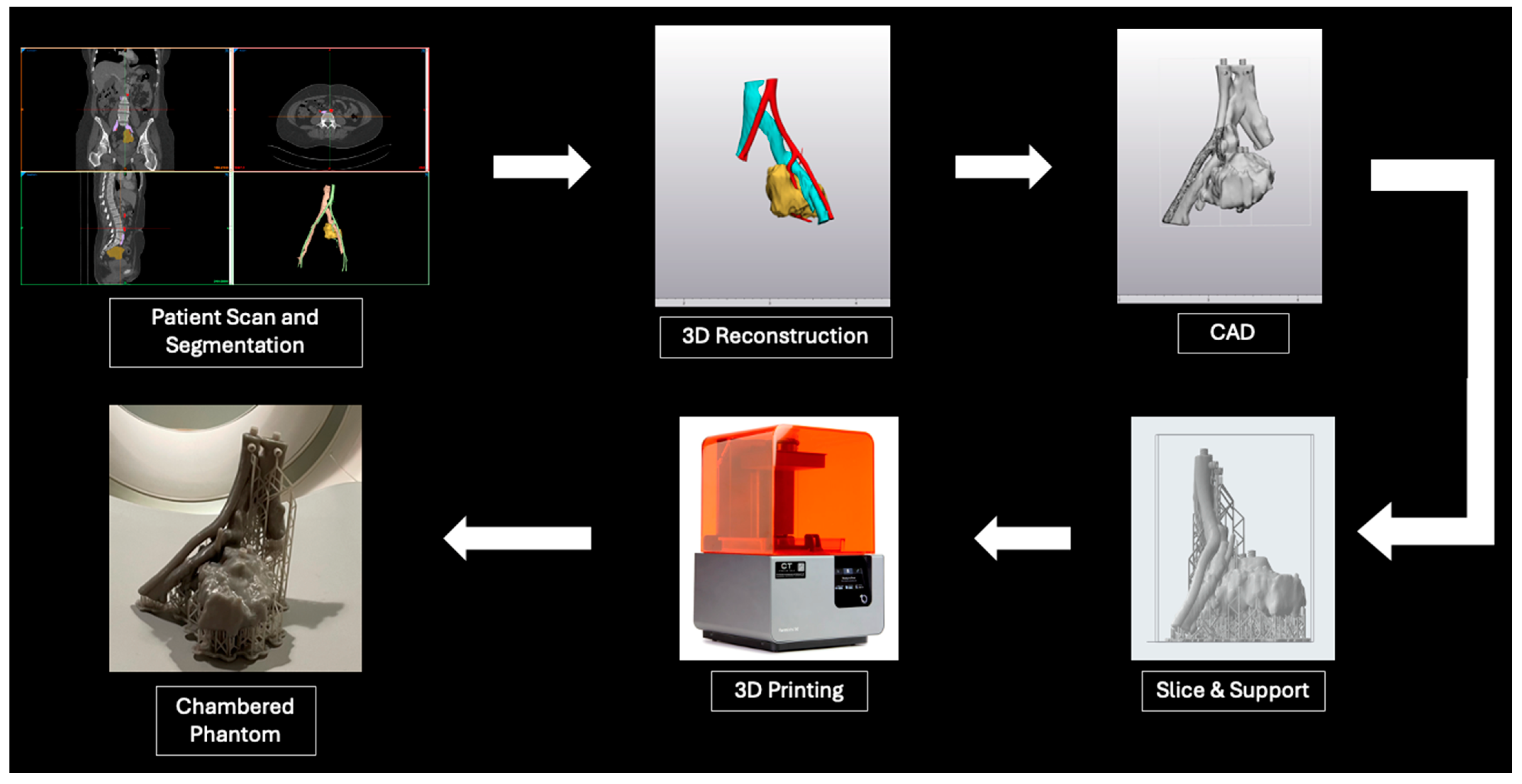
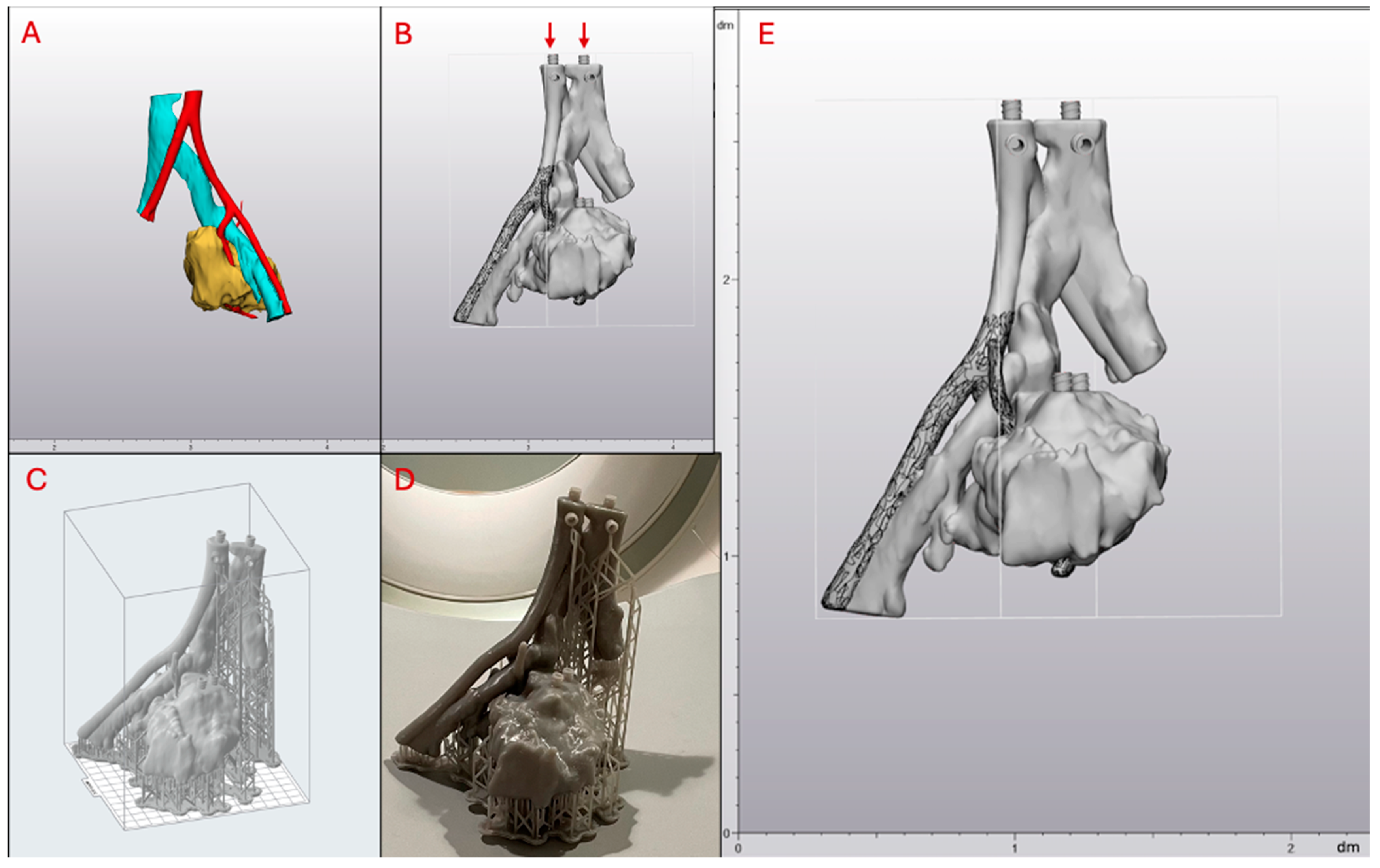
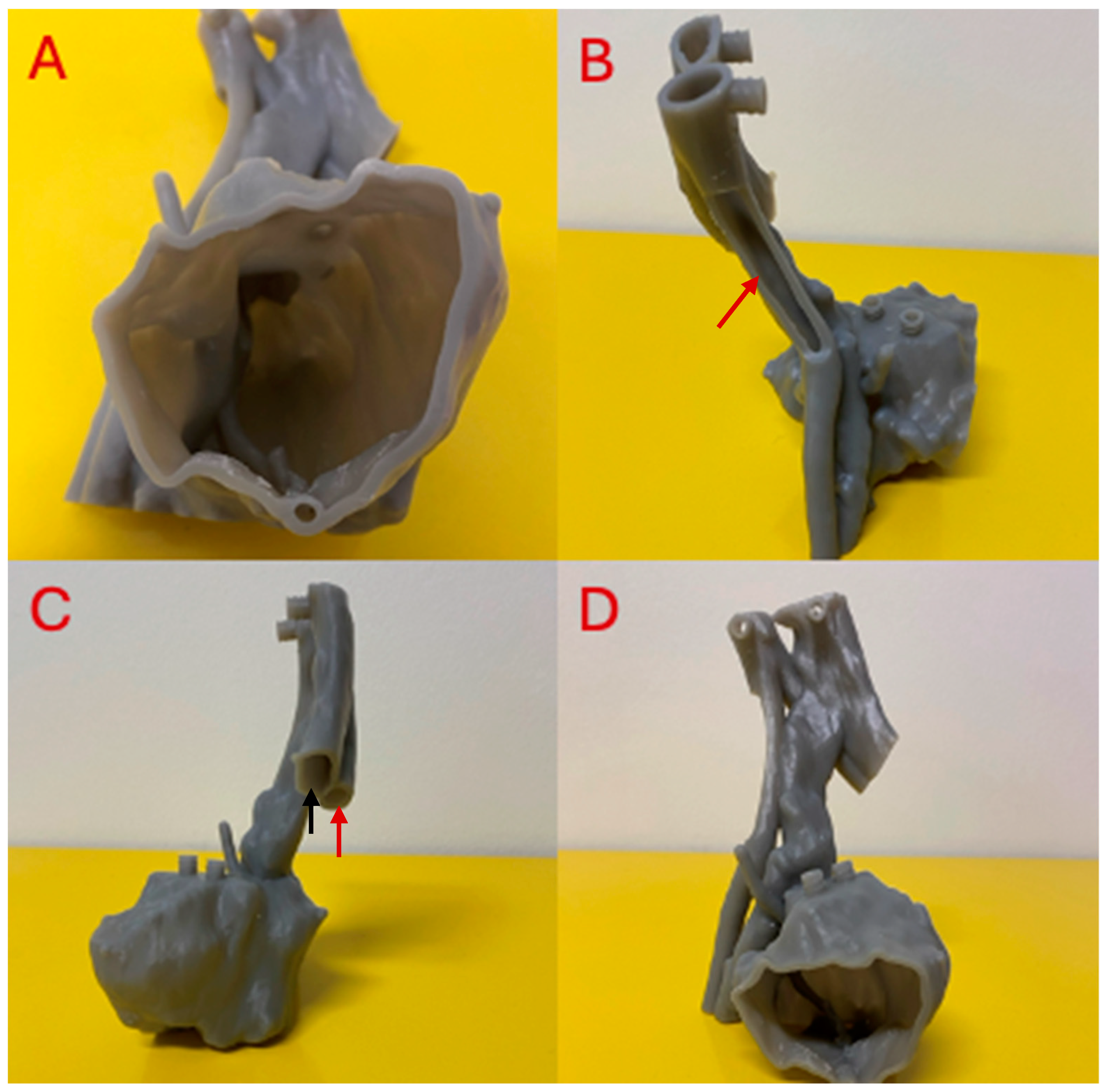
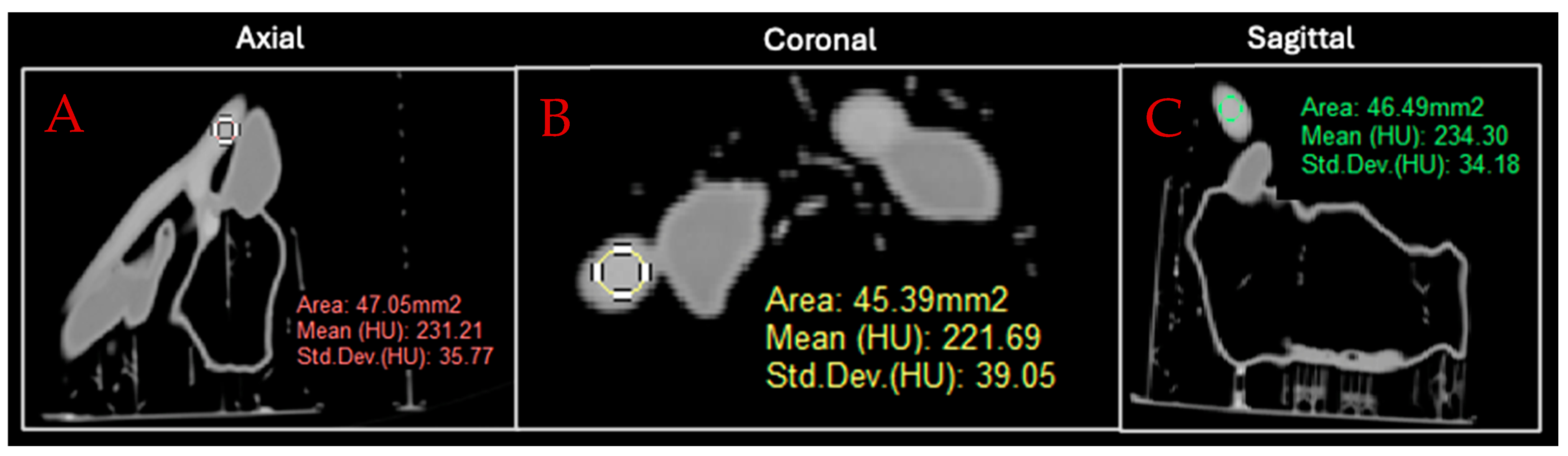
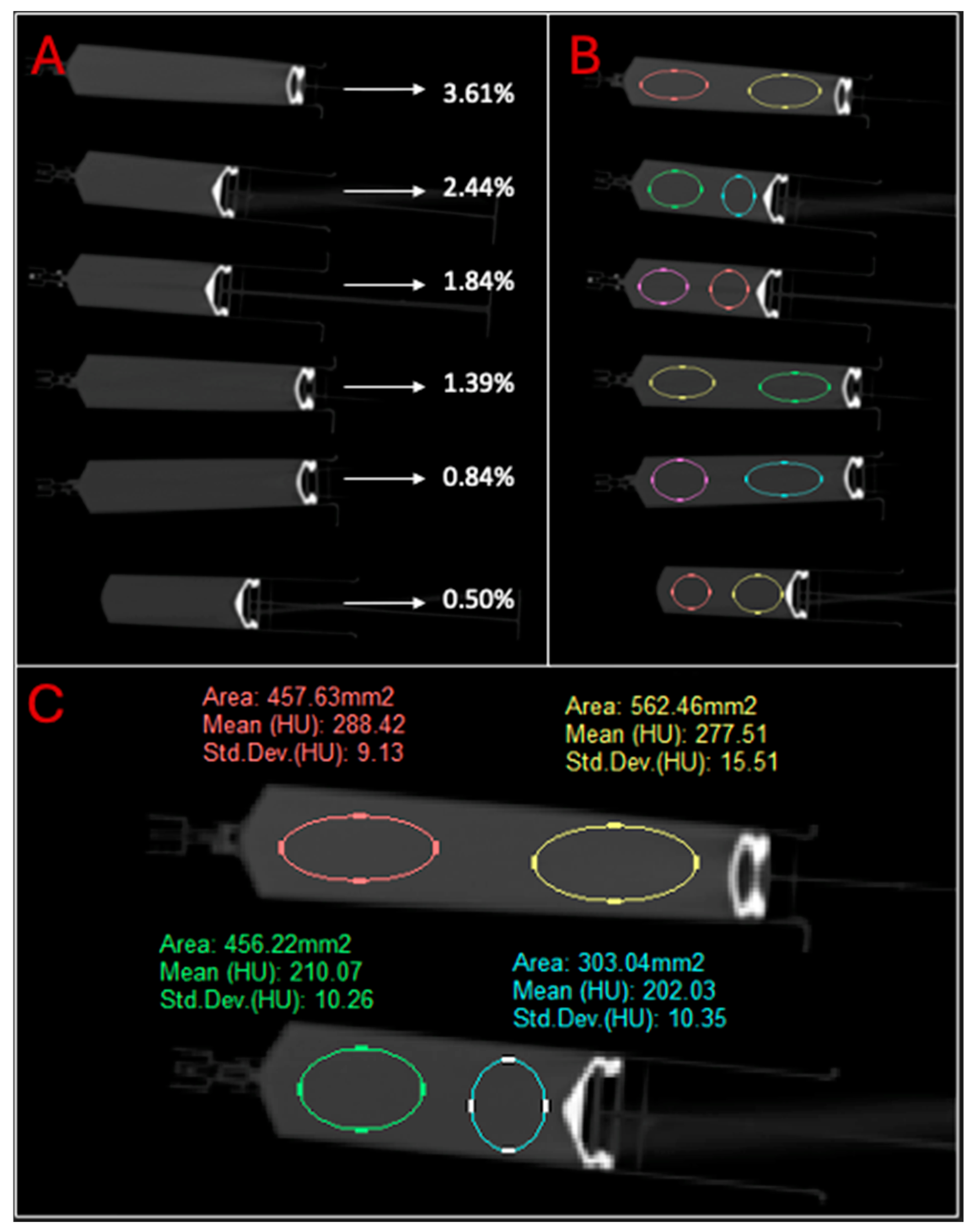
| Anatomical Structure | Volume (mL) |
|---|---|
| Artery | 12.96 |
| Vein | 29.18 |
| Tumor | 160.44 |
| Phase | Goal Attenuation (HU) | Volume of Contrast in 80 mL Water (mL) | Volume/Volume Percentage |
|---|---|---|---|
| Tumor I | 40 | 0.4 | 0.50% |
| Venous I | 150 | 1.5 | 1.84% |
| Arterial I | 200 | 2 | 2.44% |
| Phase | Goal Attenuation (HU) | Observed Mean Attenuation (HU) | Percent Difference |
|---|---|---|---|
| Tumor I | 40 | 37.49 ± 3.95 | 6.48% |
| Tumor II | 400 | 326.20 ± 2.70 | 20.32% |
| Venous I | 150 | 200.50 ± 4.03 | 28.82% |
| Venous II | 90 | 91.32 ± 1.90 | 1.45% |
| Arterial I | 200 | 227.92 ± 5.21 | 13.05% |
| Arterial II | 150 | 132.08 ± 5.43 | 12.71% |
| Phase | Goal Attenuation (HU) | Volume of Contrast in 80 mL Water (mL) | Volume/Volume Percentage |
|---|---|---|---|
| Tumor II | 400 | 3 | 3.61% |
| Venous II | 90 | 0.68 | 0.84% |
| Arterial II | 150 | 1.13 | 2.44% |
| v/v Percentage (%) | Mean Attenuation (HU) |
|---|---|
| 0.5 | 44.69 ± 4.26 |
| 0.84 | 73.01 ± 5.00 |
| 1.39 | 110.99 ± 3.81 |
| 1.84 | 169.19 ± 3.47 |
| 2.44 | 210.12 ± 8.24 |
| 3.61 | 281.87 ± 6.02 |
| Attenuation Type | ICC Value |
|---|---|
| Tumor phantom | 0.9998415 |
| Syringe | 0.9987372 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalidindi, Y.; Ganapathy, A.K.; Cunningham, L.; Lovato, A.; Albers, B.; Shetty, A.S.; Ballard, D.H. Customization of Computed Tomography Radio-Opacity in 3D-Printed Contrast-Injectable Tumor Phantoms. Micromachines 2024, 15, 992. https://doi.org/10.3390/mi15080992
Kalidindi Y, Ganapathy AK, Cunningham L, Lovato A, Albers B, Shetty AS, Ballard DH. Customization of Computed Tomography Radio-Opacity in 3D-Printed Contrast-Injectable Tumor Phantoms. Micromachines. 2024; 15(8):992. https://doi.org/10.3390/mi15080992
Chicago/Turabian StyleKalidindi, Yuktesh, Aravinda Krishna Ganapathy, Liam Cunningham, Adriene Lovato, Brian Albers, Anup S. Shetty, and David H. Ballard. 2024. "Customization of Computed Tomography Radio-Opacity in 3D-Printed Contrast-Injectable Tumor Phantoms" Micromachines 15, no. 8: 992. https://doi.org/10.3390/mi15080992
APA StyleKalidindi, Y., Ganapathy, A. K., Cunningham, L., Lovato, A., Albers, B., Shetty, A. S., & Ballard, D. H. (2024). Customization of Computed Tomography Radio-Opacity in 3D-Printed Contrast-Injectable Tumor Phantoms. Micromachines, 15(8), 992. https://doi.org/10.3390/mi15080992






