Design and Validation of a Monte Carlo Method for the Implementation of Noninvasive Wearable Devices for HbA1c Estimation Considering the Skin Effect
Abstract
1. Introduction
- -
- Intrinsic and extrinsic noise can be removed by the model (hardware design) itself. Hence, the model will have more accurate estimates of our target parameters.
- -
- Skin pigments (melanin and bilirubin) and the skin reflection coefficient are also considered in the epidermis of the wrist model.
- -
- A simple sensor (cylindrical type LED-PD pair) was added to compensate for the skin effect.
- -
- Calibration can be made simpler due to the Monte Carlo-based model considering different skin properties.
- -
- The performance of the proposed method was demonstrated by increasing the number of subjects to 50.
2. Methodology
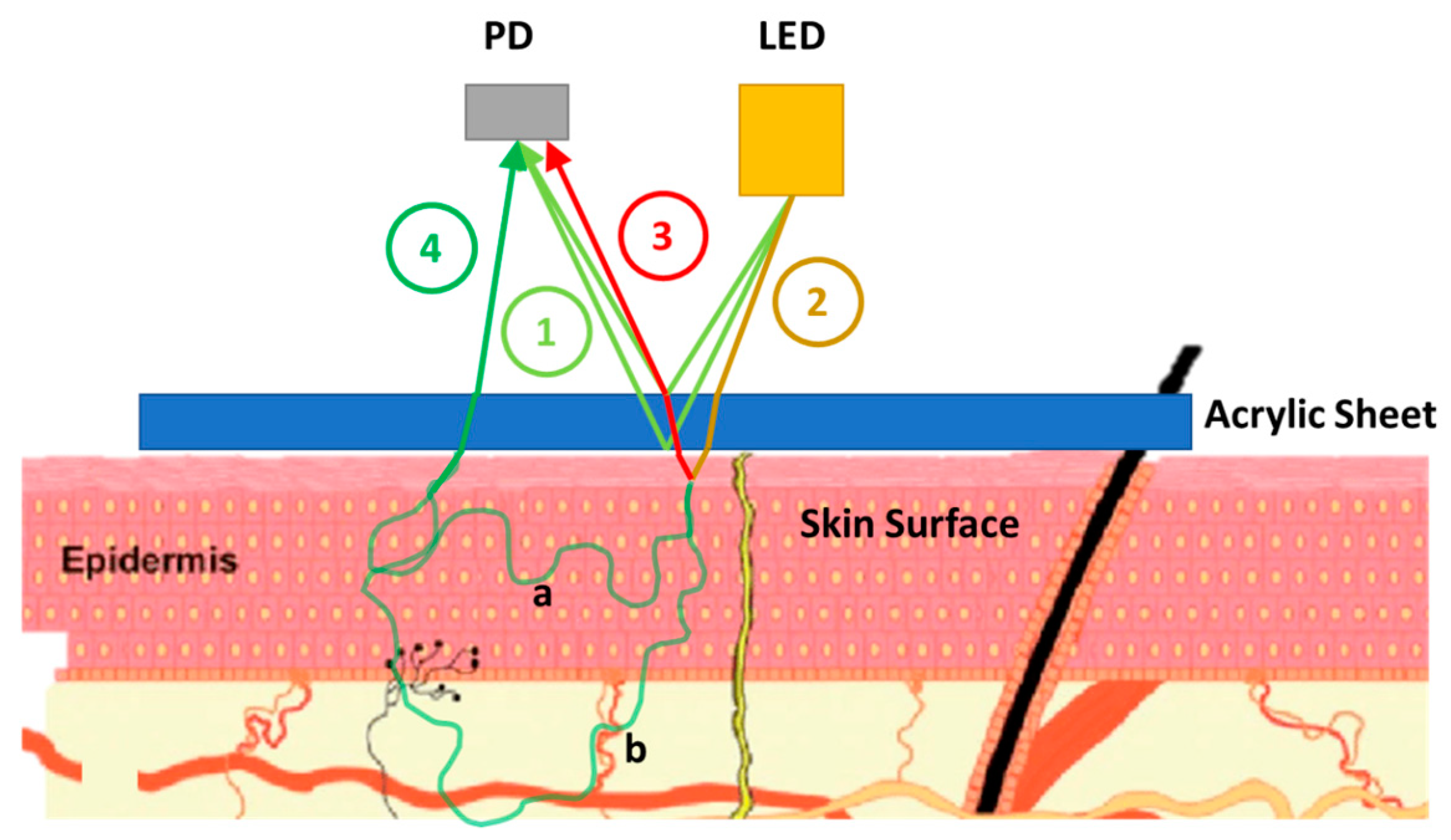
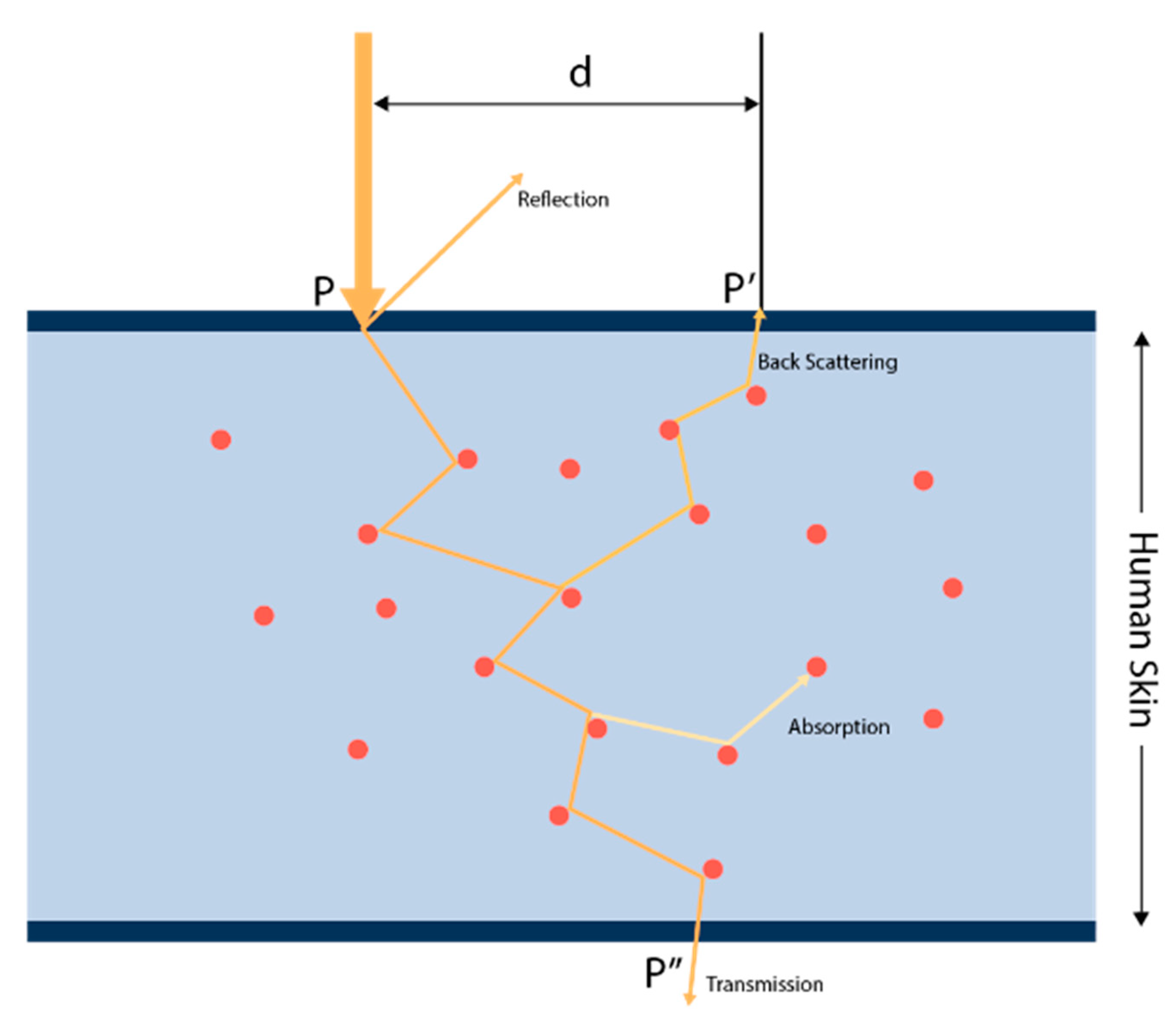
2.1. Skin Effect Analysis of a PPG Sensor (PPG Signal Impurities)
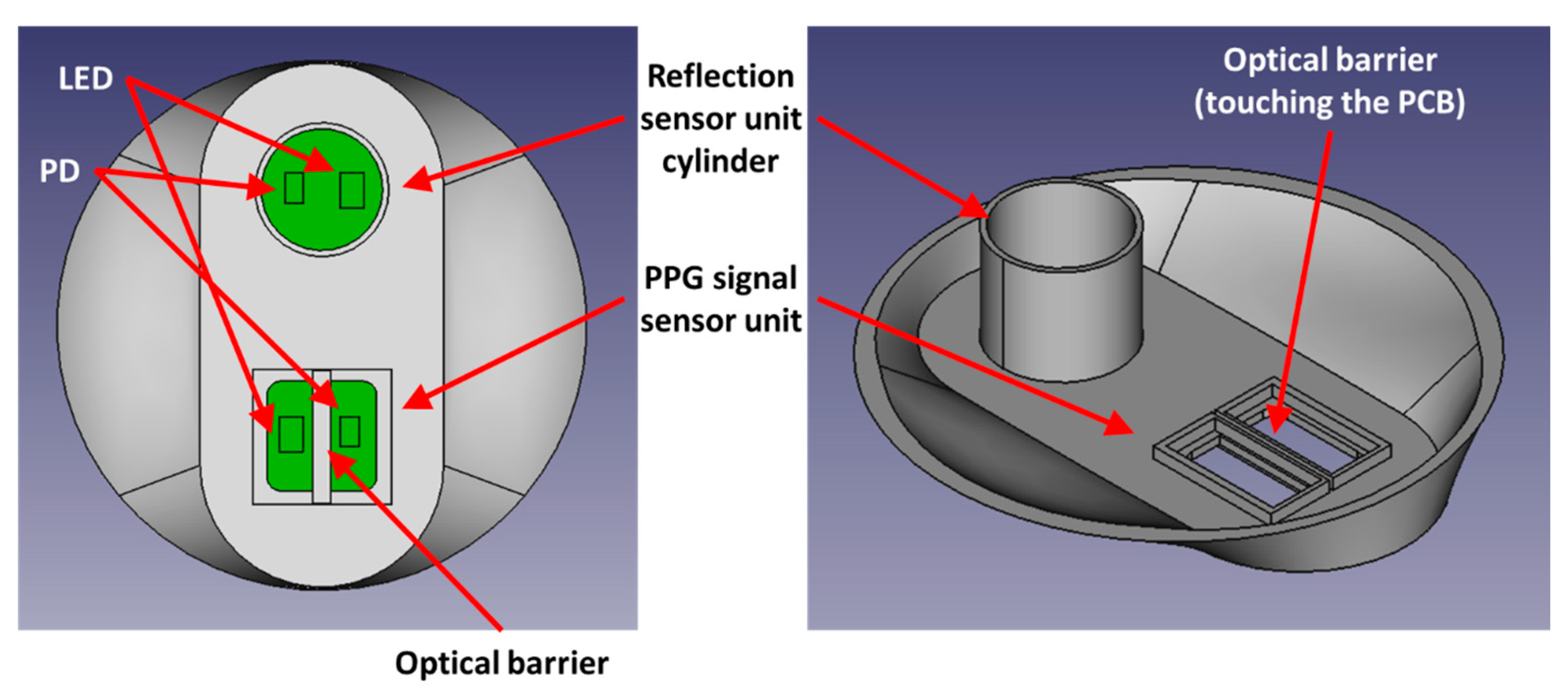
2.2. Proposed Method and Hardware Design for Purifying Impurities
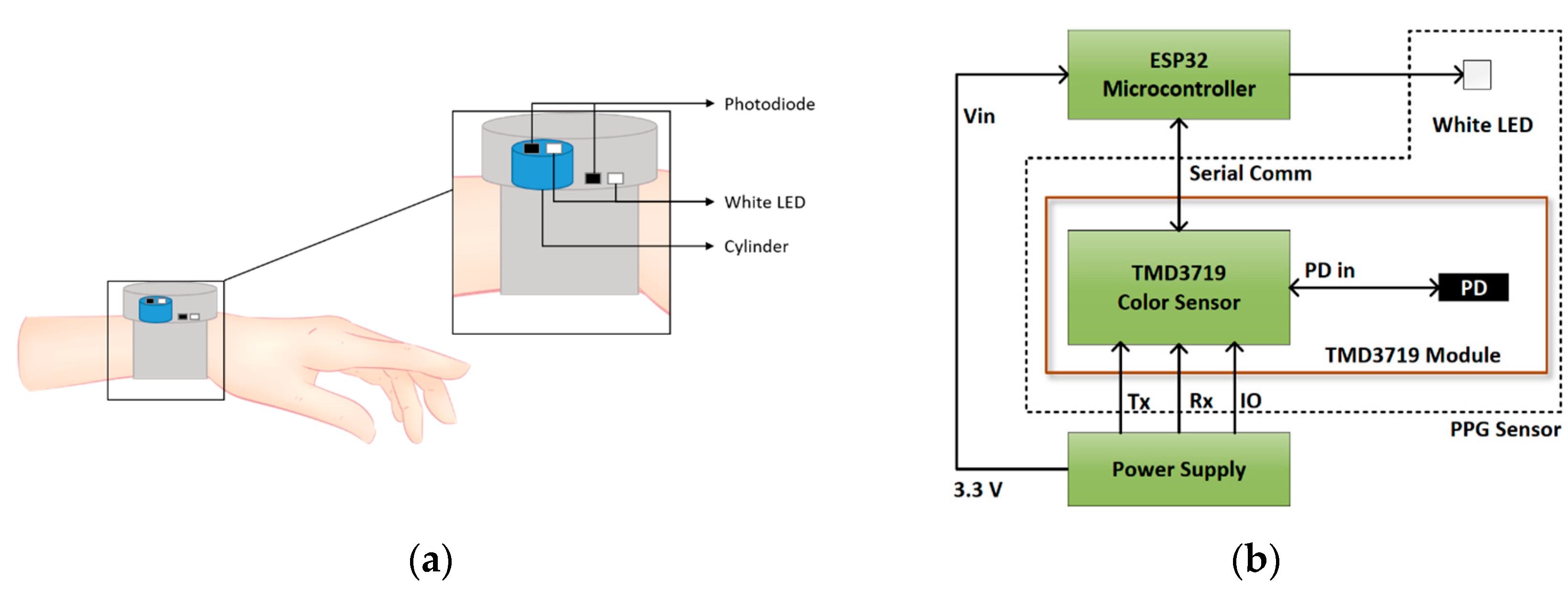
2.3. Data Acquisition System
2.3.1. PPG Data Acquisition System Description
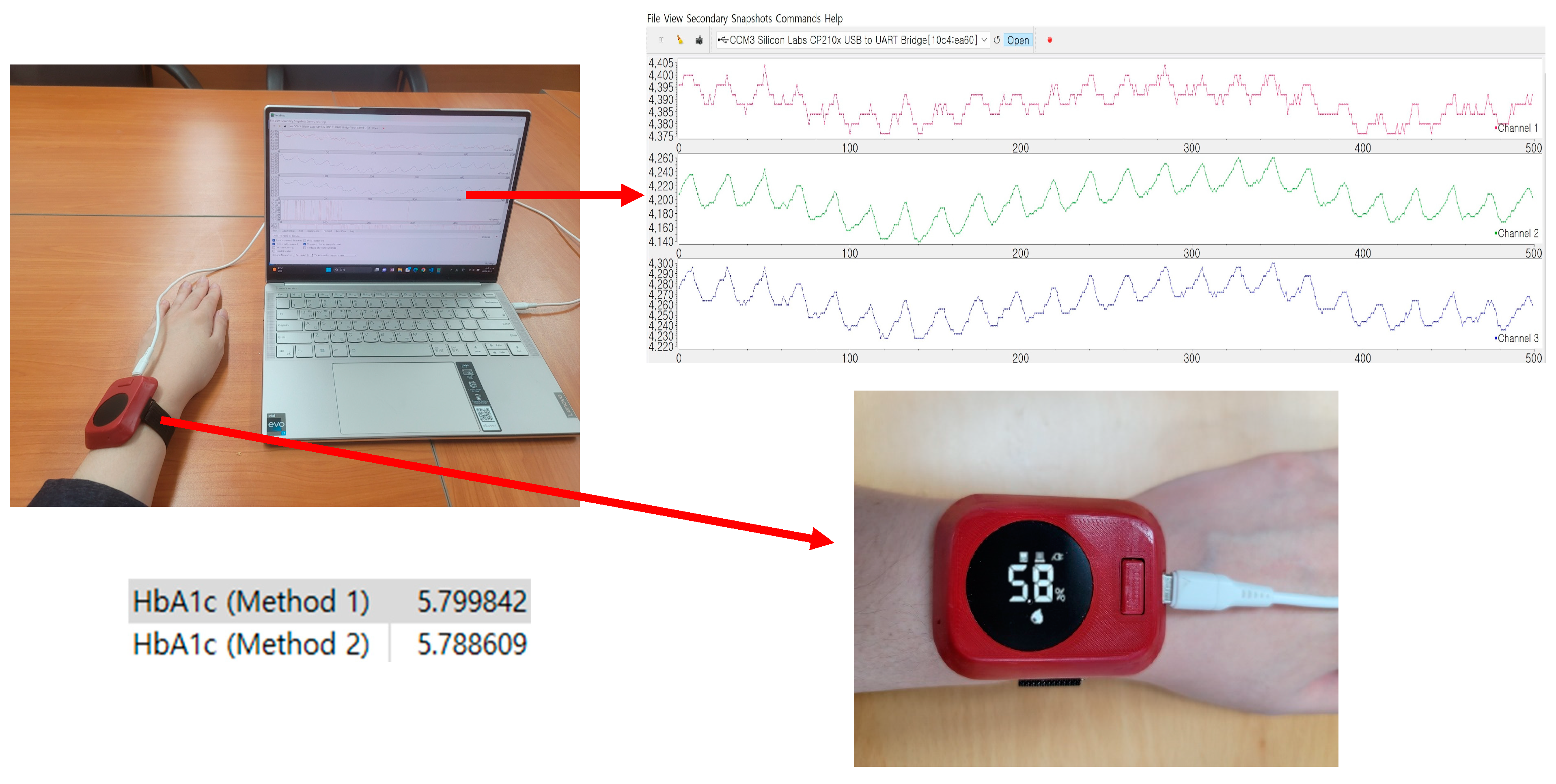
2.3.2. PPG Data Source and Analysis
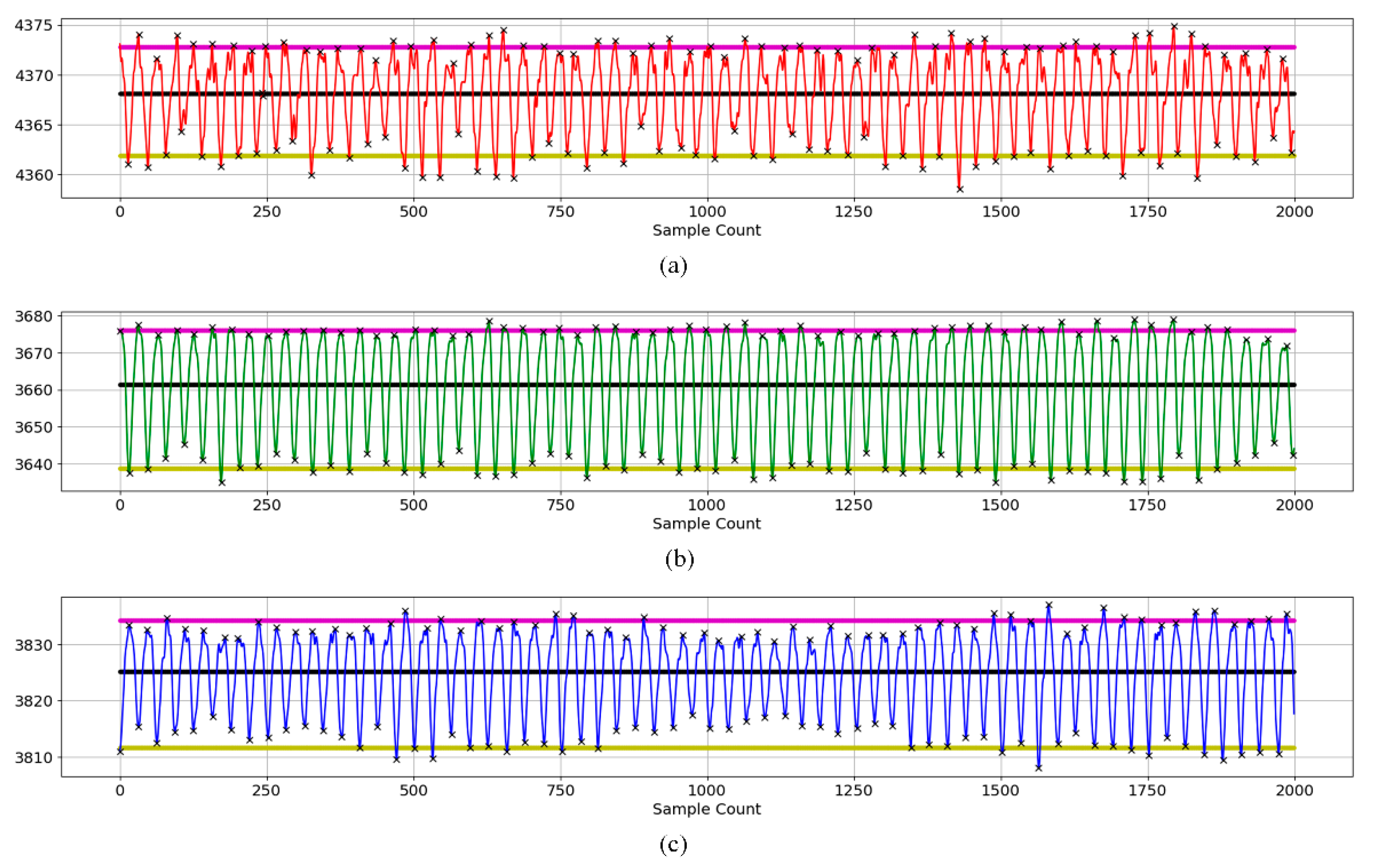
2.3.3. PPG Data Analysis
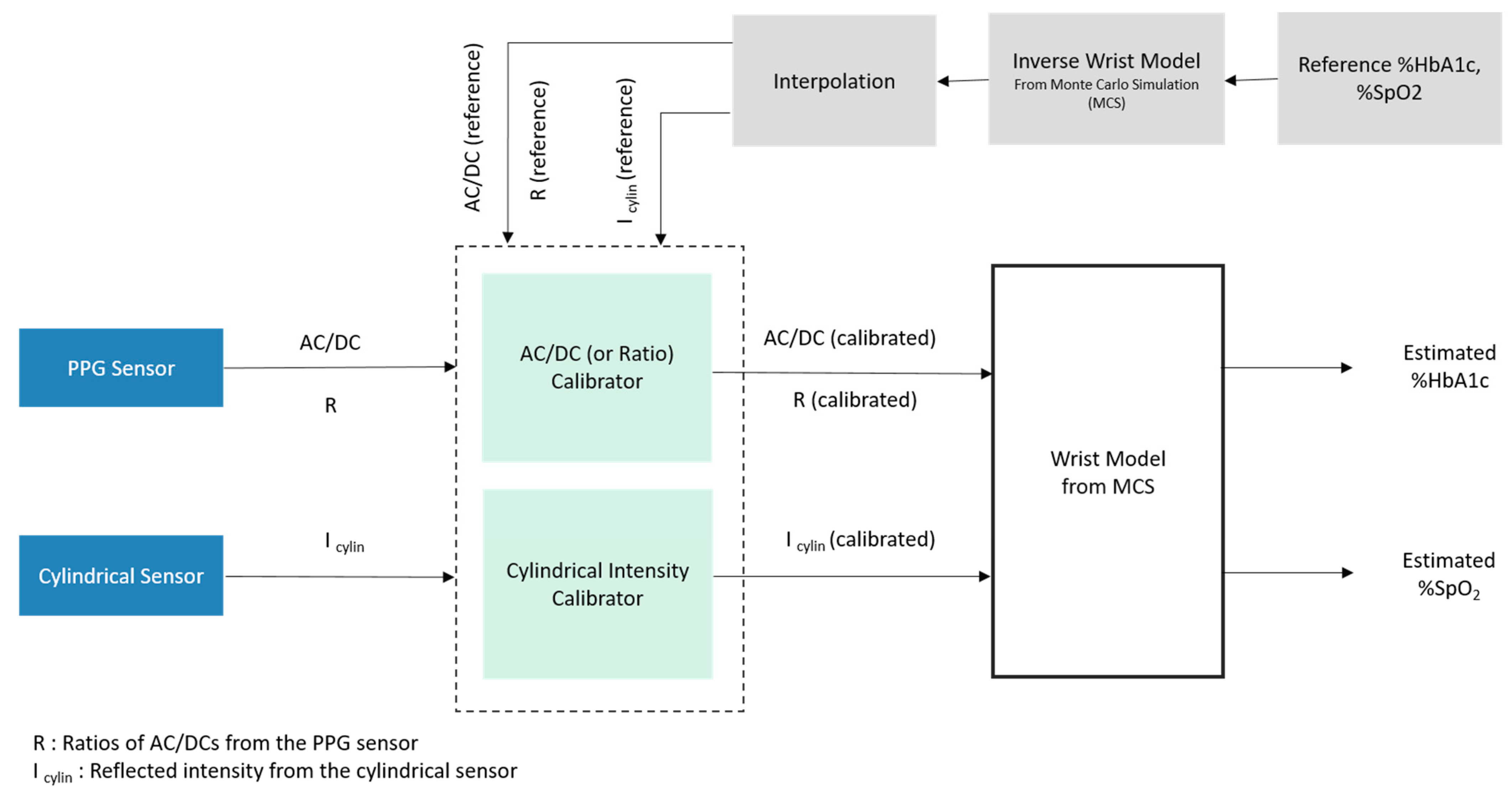
2.4. Estimation Process with Monte Carlo Simulation
2.4.1. Overall Estimation Process
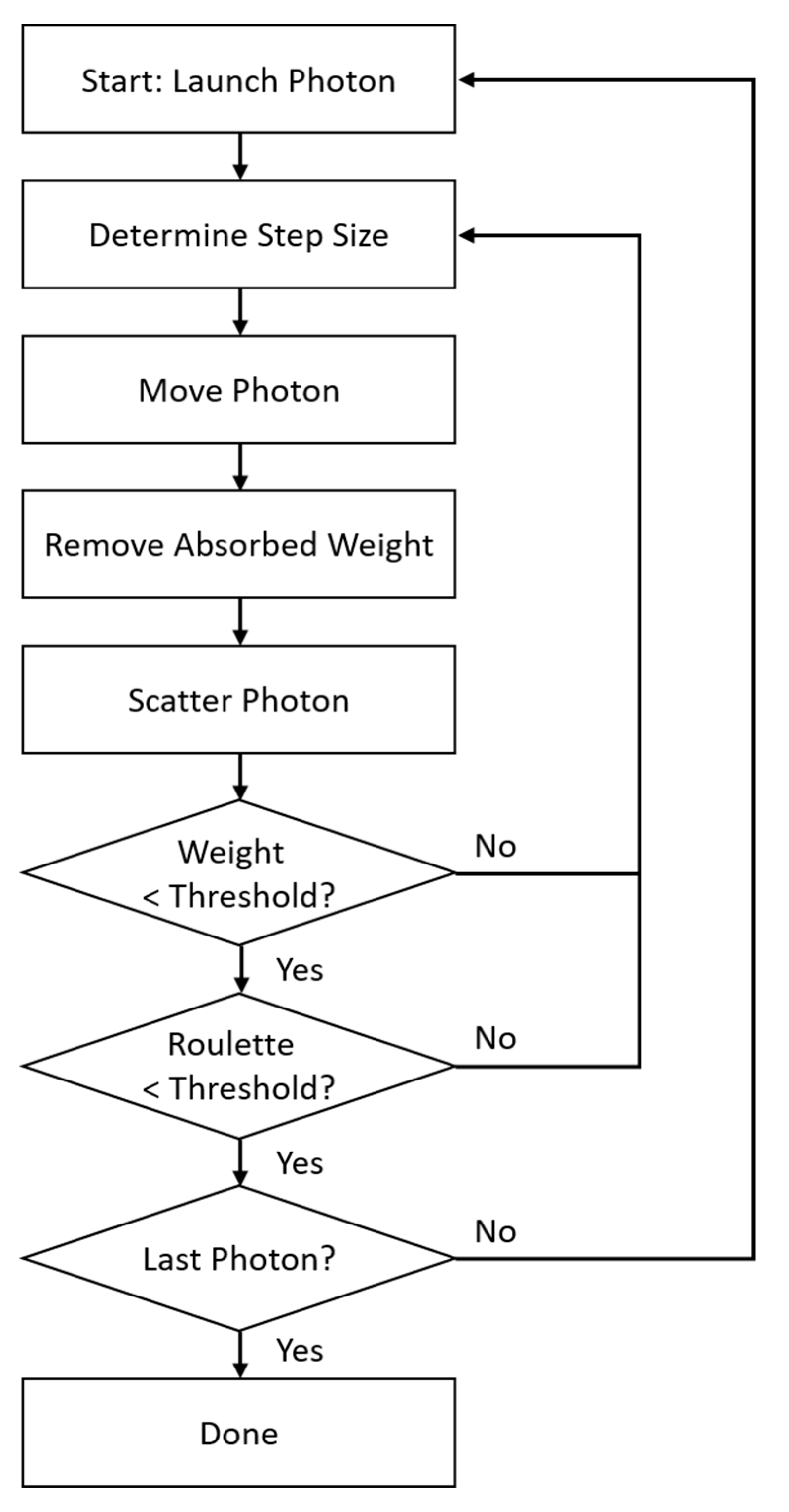
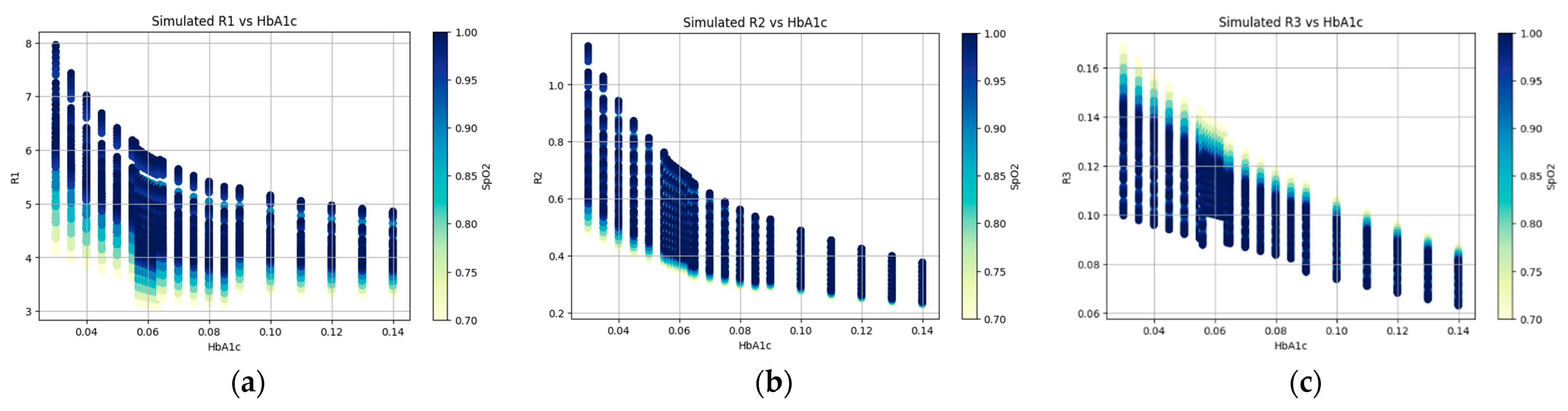
2.4.2. Monte Carlo Photon Propagation Simulation
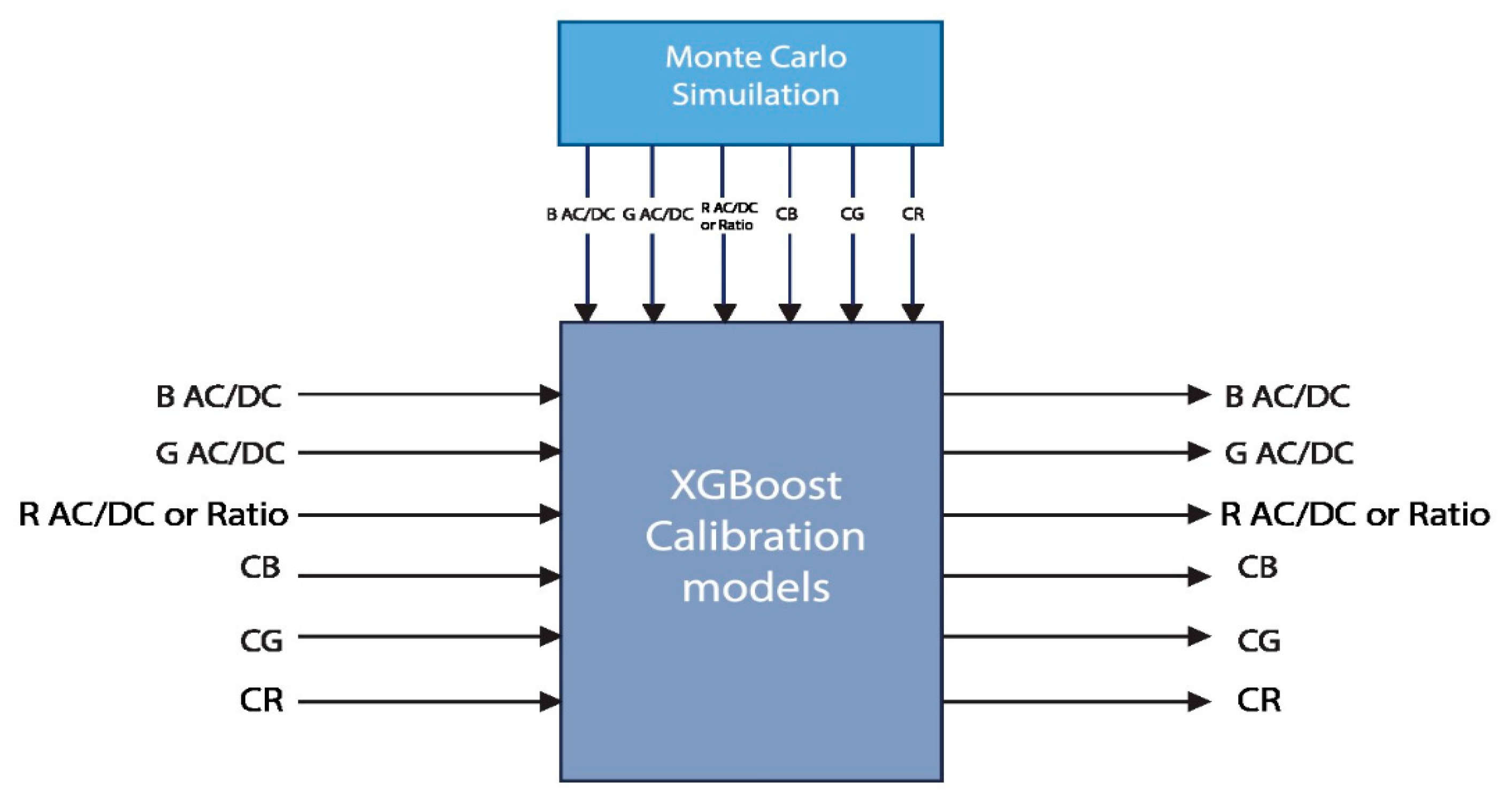
2.5. Calibration Using XGBoost Regression
3. Results and Discussion
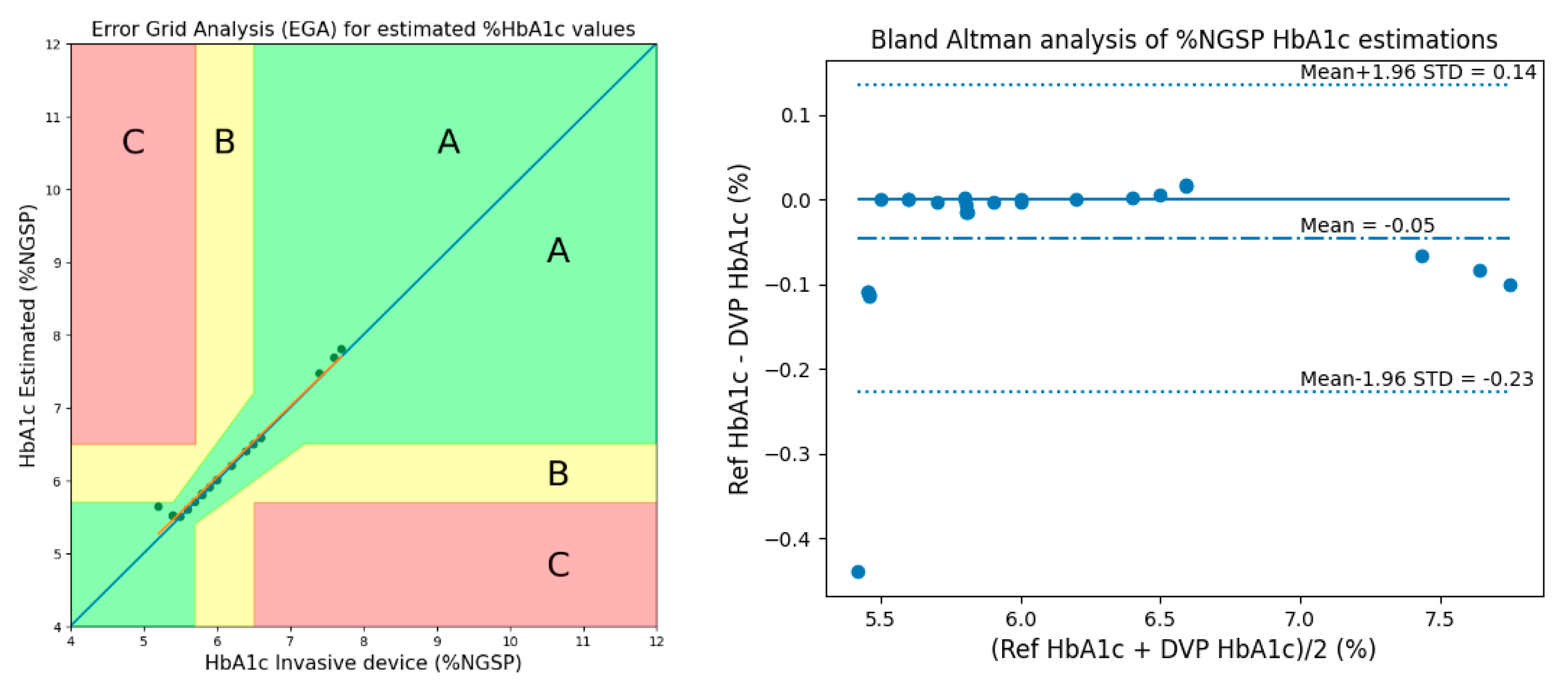
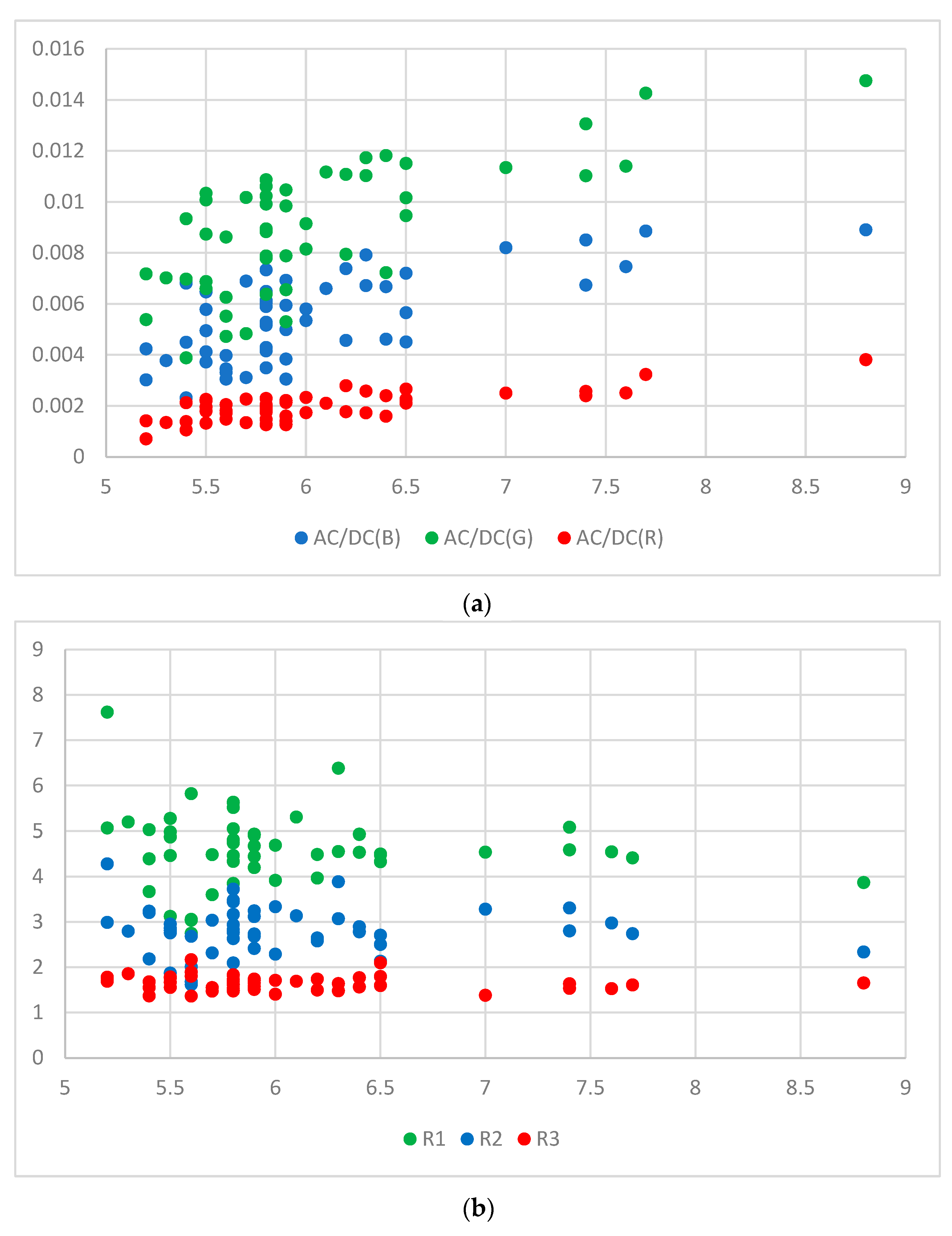
3.1. Method 1: Using 3 AC/DC and 3 Cylindrical Values
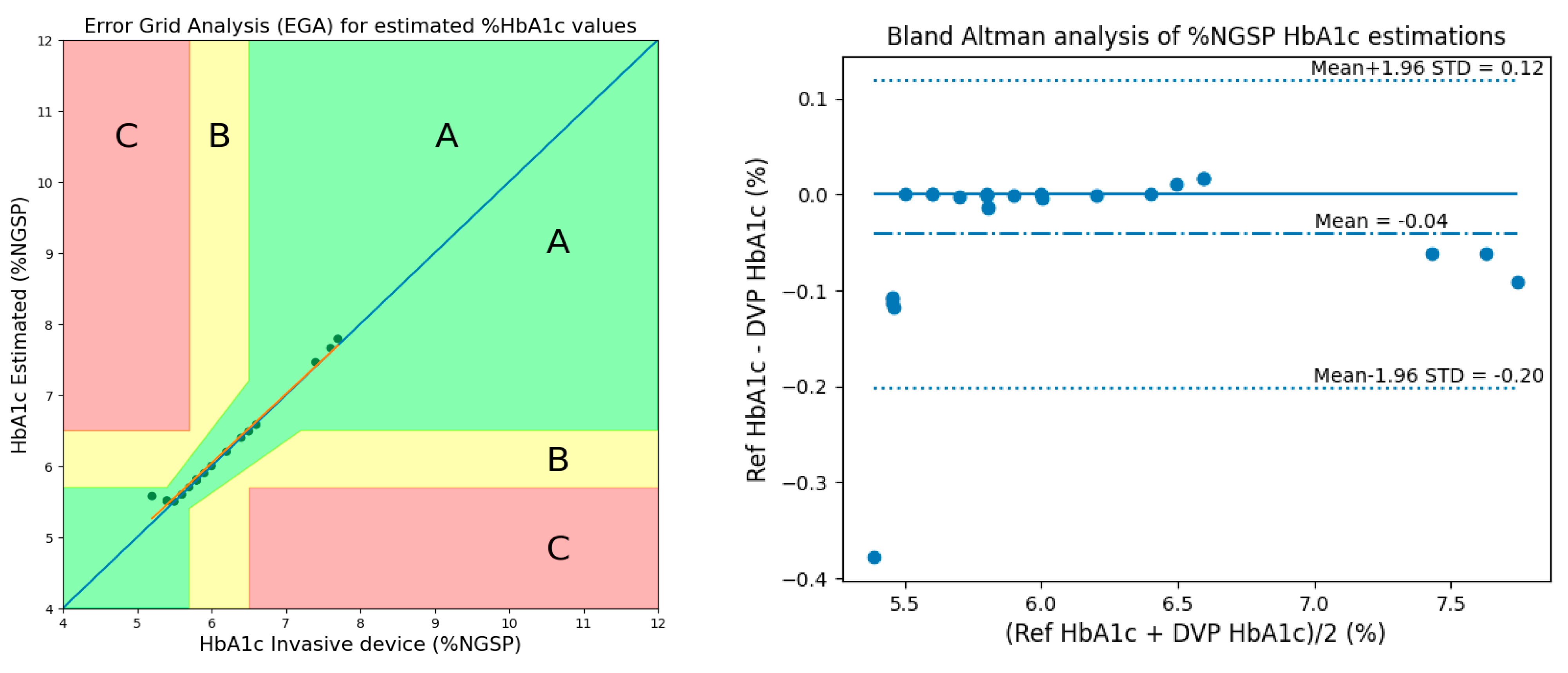
3.2. Method 2: Using 2 AC/DCs, 1 Ratio, and 3 Cylindrical Values
3.3. Comparison with Previous Studies
3.4. Result Using 50 Subjects’ Data
3.5. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, BMI-S38440. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Joshi, A.M.; Maddila, R.K.; Vipparthi, S.K. A Review of Non-Invasive HbA1c and Blood Glucose Measurement Methods. In Proceedings of the 2021 IEEE International Symposium on Smart Electronic Systems (iSES), Jaipur, India, 18–22 December 2021; IEEE: Jaipur, India, 2021; pp. 339–342. [Google Scholar]
- Li, N.; Zang, H.; Sun, H.; Jiao, X.; Wang, K.; Liu, T.C.-Y.; Meng, Y. A Noninvasive Accurate Measurement of Blood Glucose Levels with Raman Spectroscopy of Blood in Microvessels. Molecules 2019, 24, 1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, S.; Jin, H.; Luo, Y.; Zheng, Z.; Gao, F.; Zheng, Y. Noninvasive Electromagnetic Wave Sensing of Glucose. Sensors 2019, 19, 1151. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.M.C.; Forlenza, G.P.; Prioleau, T.O.; Zhou, X. Noninvasive Glucose Sensing In Vivo. Sensors 2023, 23, 7057. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, M.M.; Hosseini, M.S.; Sheini, A.; Safaei, E.; Halabian, R.; Daryanavard, S.M.; Samadinia, H.; Bagheri, H. Monitoring Saliva Compositions for Non-invasive Detection of Diabetes using a Colorimetric-based Multiple Sensor. Sci. Rep. 2023, 13, 16174. [Google Scholar] [CrossRef] [PubMed]
- Saraoğlu, H.M.; Selvi, A.O. Determination of glucose and Hba1c values in blood from human breath by using Radial Basis Function Neural Network via electronic nose. In Proceedings of the 2014 18th National Biomedical Engineering Meeting, Istanbul, Turkey, 16–17 October 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Mandal, S.; Manasreh, M.O. An In-Vitro Optical Sensor Designed to Estimate Glycated Hemoglobin Levels. Sensors 2018, 18, 1084. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Kim, K.-D. Non-Invasive In Vivo Estimation of HbA1c Using Monte Carlo Photon Propagation Simulation: Application of Tissue-Segmented 3D MRI Stacks of the Fingertip and Wrist for Wearable Systems. Sensors 2023, 23, 540. [Google Scholar] [CrossRef] [PubMed]
- Duteil, L.; Roussel, K.; Bahadoran, P. Skin Color and Pigmentation. In Agache’s Measuring the Skin; Humbert, P., Maibach, H., Fanian, F., Agache, P., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Satter, S.; Kwon, T.-H.; Kim, K.-D. A Comparative Analysis of Various Machine Learning Algorithms to Improve the Accuracy of HbA1c Estimation Using Wrist PPG Data. Sensors 2023, 23, 7231. [Google Scholar] [CrossRef] [PubMed]
- Bagal, T.; Bhole, K. Calibration of an Optical Sensor for in Vivo Blood Glucose Measurement. In Proceedings of the 2019 2nd International Conference on Intelligent Computing, Instrumentation and Control Technologies (ICICICT), Kannur, India, 5–6 July 2019; IEEE: Kannur, India, 2019; pp. 1029–1032. [Google Scholar]
- Singha, S.K.; Ahmad, M.; Islam, M.R. Multiple Regression Analysis Based Non-Invasive Blood Glucose Level Estimation. In Proceedings of the 2021 International Conference on Automation, Control and Mechatronics for Industry 4.0 (ACMI), Rajshahi, Bangladesh, 8–9 July 2021. [Google Scholar]
- Clarke, W.L. The Original Clarke Error Grid Analysis (EGA). Diabetes Technol. Ther. 2005, 7, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Shchelkanova, E.; Shchelkanov, A.; Shchapova, L.; Shibata, T. An Exploration of Blue PPG Signal Using a Novel Color Sensor-Based PPG System. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar]




| Min | Max | Mean | Median | SD | 25th Percentile | 75th Percentile | |
|---|---|---|---|---|---|---|---|
| %HbA1c | 5.20 | 7.70 | 6.04 | 5.80 | 0.678 | 5.60 | 6.40 |
| MSE | ME | MAD | RMSE | Pearson’s r | %Accuracy | |
|---|---|---|---|---|---|---|
| Proposed (Method 1, 28 subjects) | 0.0106 | −0.0456 | 0.0490 | 0.1030 | 0.9907 | 99.14 |
| MSE | ME | MAD | RMSE | Pearson’s r | %Accuracy | |
|---|---|---|---|---|---|---|
| Proposed (Method 2, 28 subjects) | 0.0084 | −0.0416 | 0.0451 | 0.0917 | 0.9928 | 99.21 |
| Metric | MSE | ME | MAD | RMSE | Pearson’s r | |
|---|---|---|---|---|---|---|
| Method | ||||||
| Shifat et al. [9] (28 subjects) | 0.0500 | 0.0100 | 0.1300 | 0.2100 | 0.9700 | |
| Proposed (Method 1, 28 subjects) | 0.0106 | 0.0456 | 0.0490 | 0.1030 | 0.9907 | |
| Proposed (Method 2, 28 subjects) | 0.0084 | 0.0416 | 0.0451 | 0.0917 | 0.9928 | |
| Metric | MSE | ME | MAD | RMSE | Pearson’s r | |
|---|---|---|---|---|---|---|
| Method | ||||||
| Shifat et al. [9] (28 subjects) | 0.0500 | 0.0100 | 0.1300 | 0.2100 | 0.9700 | |
| Proposed (Method 1, 28 subjects) | 0.0106 | 0.0456 | 0.0490 | 0.1030 | 0.9907 | |
| Proposed (Method 2, 28 subjects) | 0.0084 | 0.0416 | 0.0451 | 0.0917 | 0.9928 | |
| Proposed (Method 1, 50 subjects) | 0.0116 | 0.0402 | 0.0877 | 0.1075 | 0.9894 | |
| Proposed (Method 2, 50 subjects) | 0.0121 | 0.0451 | 0.0887 | 0.110 | 0.9894 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, T.-H.; Hossain, S.; Turja, M.S.; Kim, K.-D. Design and Validation of a Monte Carlo Method for the Implementation of Noninvasive Wearable Devices for HbA1c Estimation Considering the Skin Effect. Micromachines 2024, 15, 1067. https://doi.org/10.3390/mi15091067
Kwon T-H, Hossain S, Turja MS, Kim K-D. Design and Validation of a Monte Carlo Method for the Implementation of Noninvasive Wearable Devices for HbA1c Estimation Considering the Skin Effect. Micromachines. 2024; 15(9):1067. https://doi.org/10.3390/mi15091067
Chicago/Turabian StyleKwon, Tae-Ho, Shifat Hossain, Mrinmoy Sarker Turja, and Ki-Doo Kim. 2024. "Design and Validation of a Monte Carlo Method for the Implementation of Noninvasive Wearable Devices for HbA1c Estimation Considering the Skin Effect" Micromachines 15, no. 9: 1067. https://doi.org/10.3390/mi15091067
APA StyleKwon, T.-H., Hossain, S., Turja, M. S., & Kim, K.-D. (2024). Design and Validation of a Monte Carlo Method for the Implementation of Noninvasive Wearable Devices for HbA1c Estimation Considering the Skin Effect. Micromachines, 15(9), 1067. https://doi.org/10.3390/mi15091067















