Exploring Electrochemical Direct Writing Machining of Patterned Microstructures on Zr702 with Polyacrylamide Polymer Electrolyte
Abstract
1. Introduction
2. Material and Methods
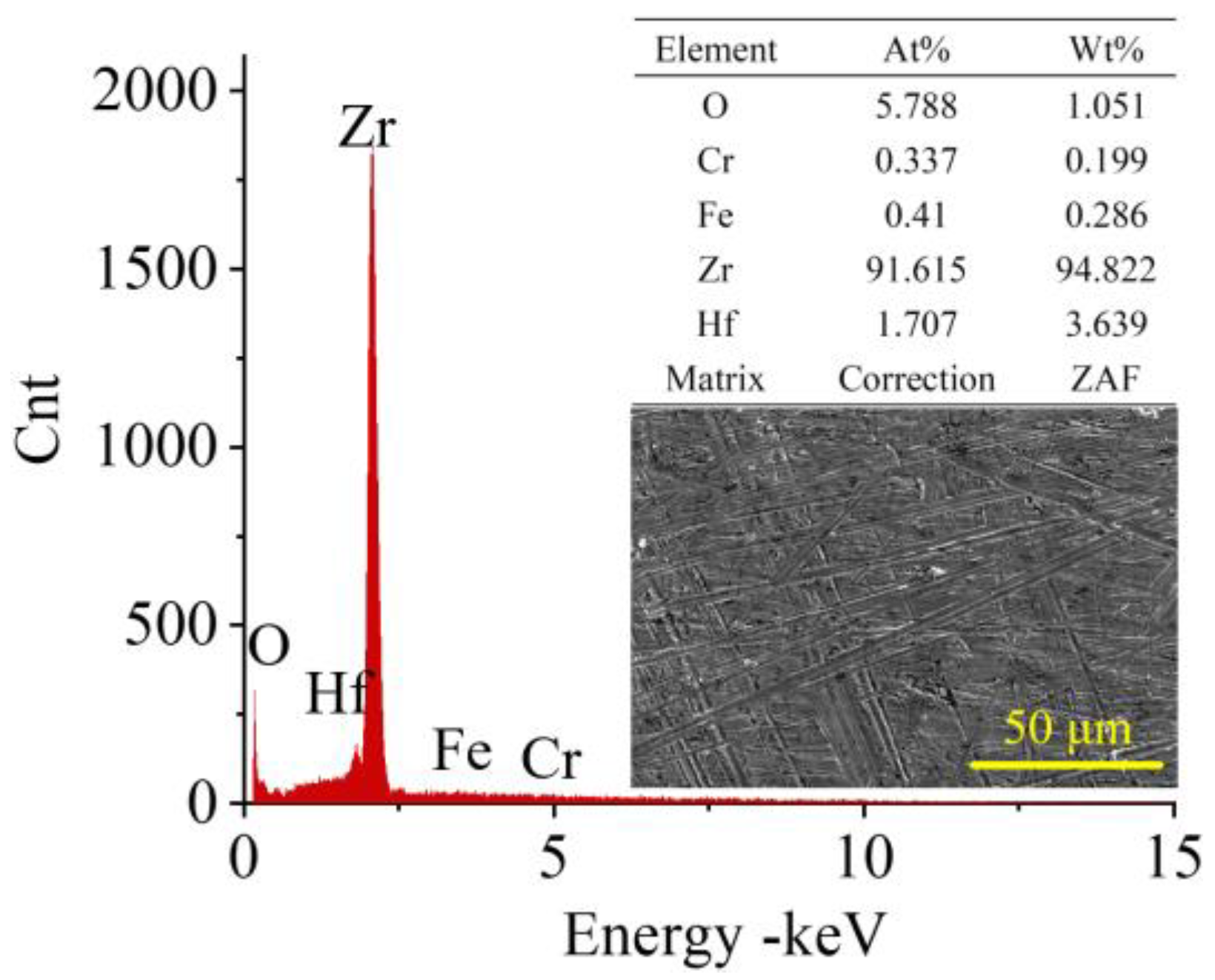
2.1. Experimental Materials
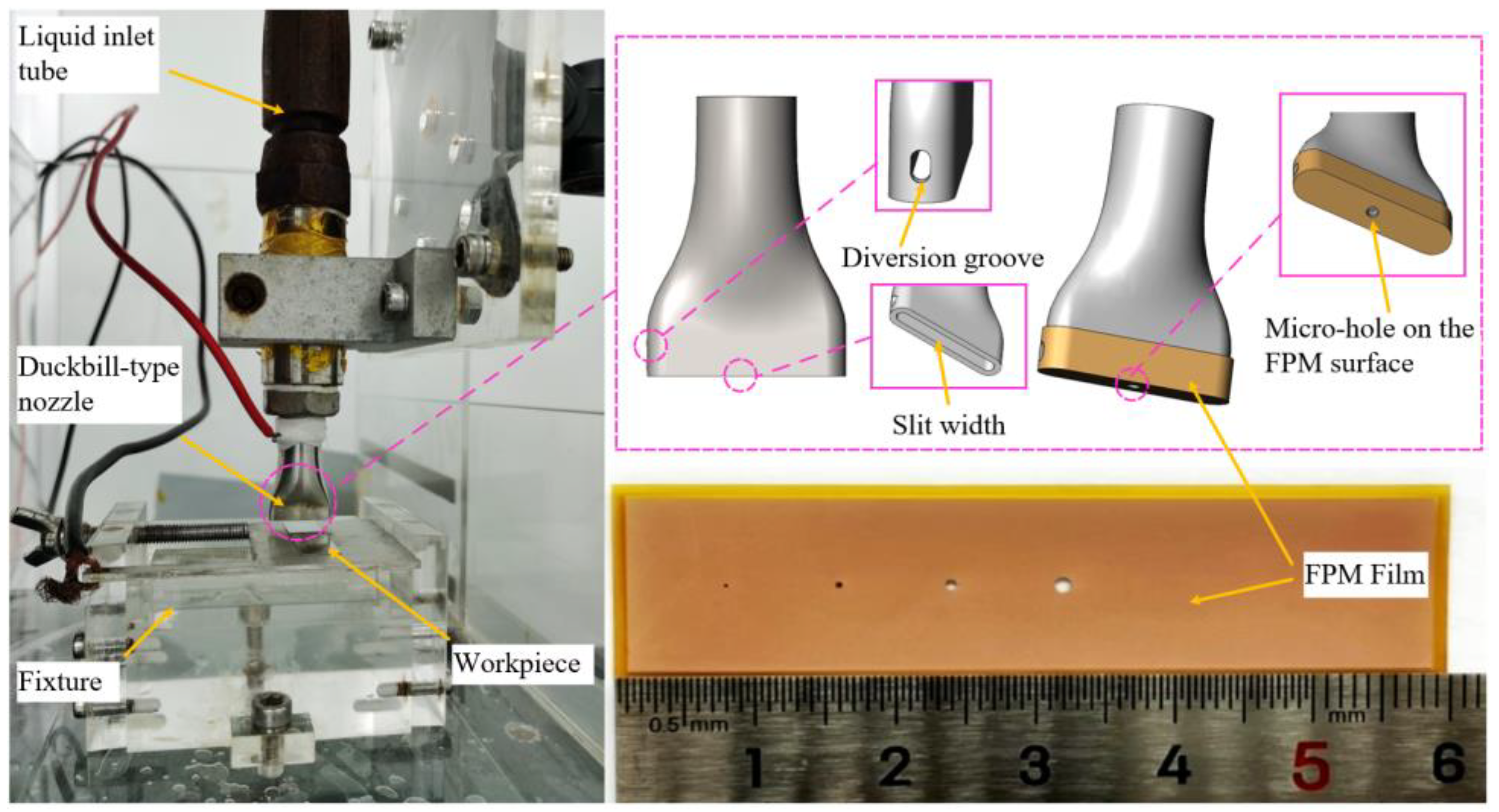
2.2. Experimental Device
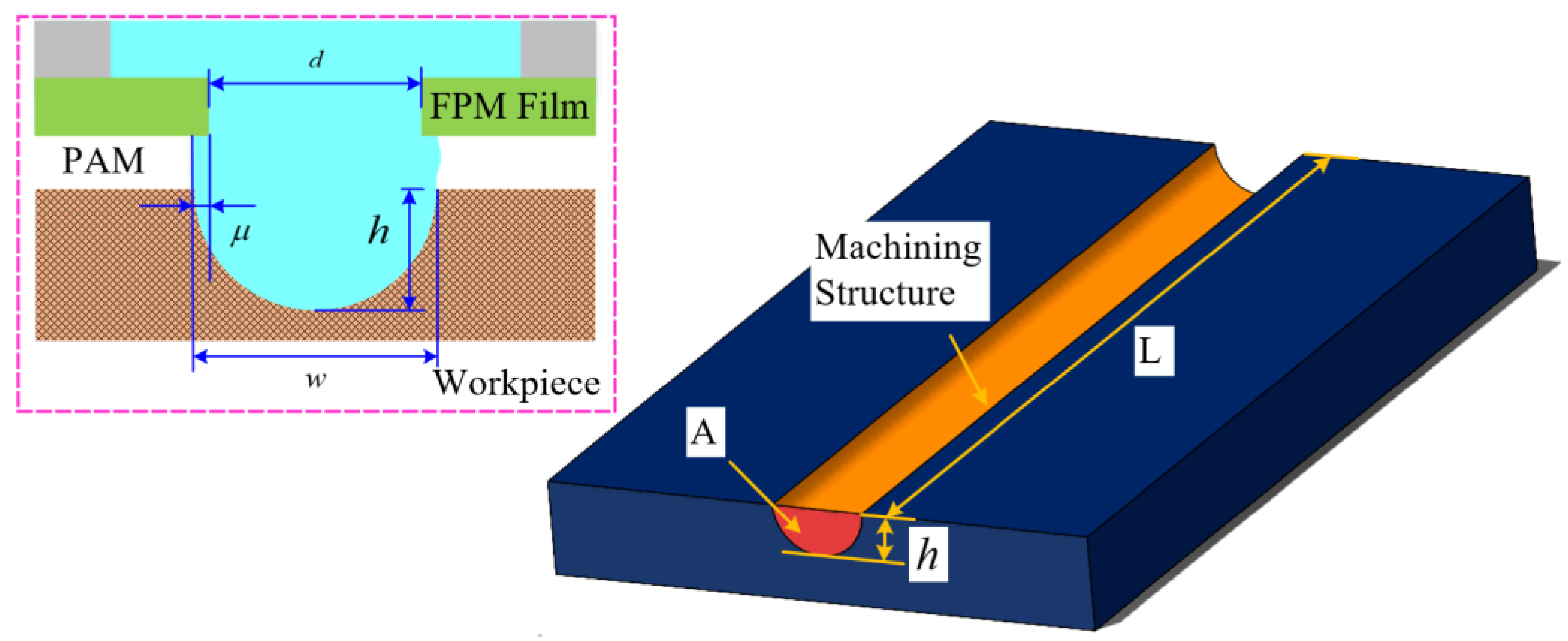
2.3. Experimental Design
3. Results and Discussion
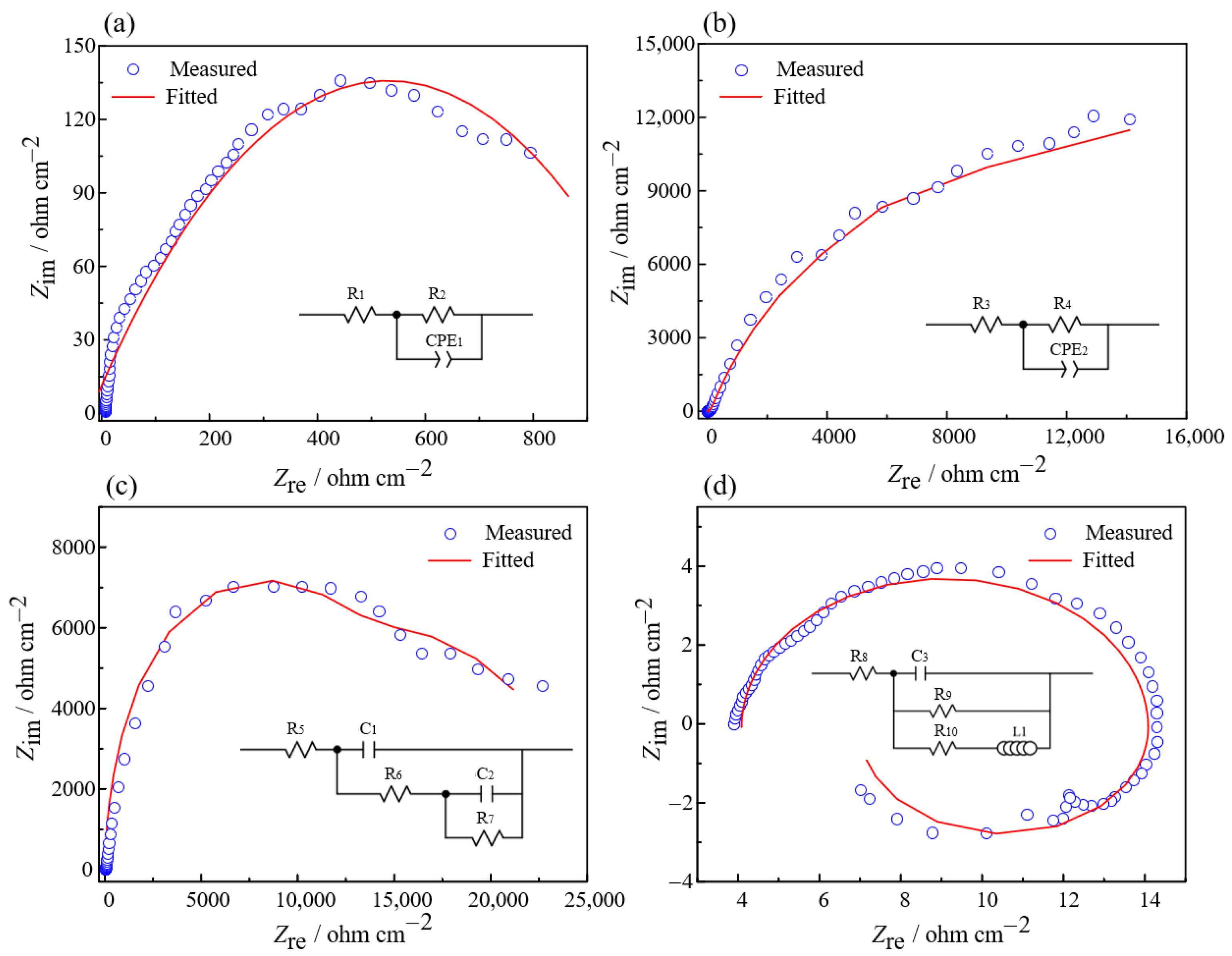
3.1. Electrochemical Properties of Zr702 with PAM
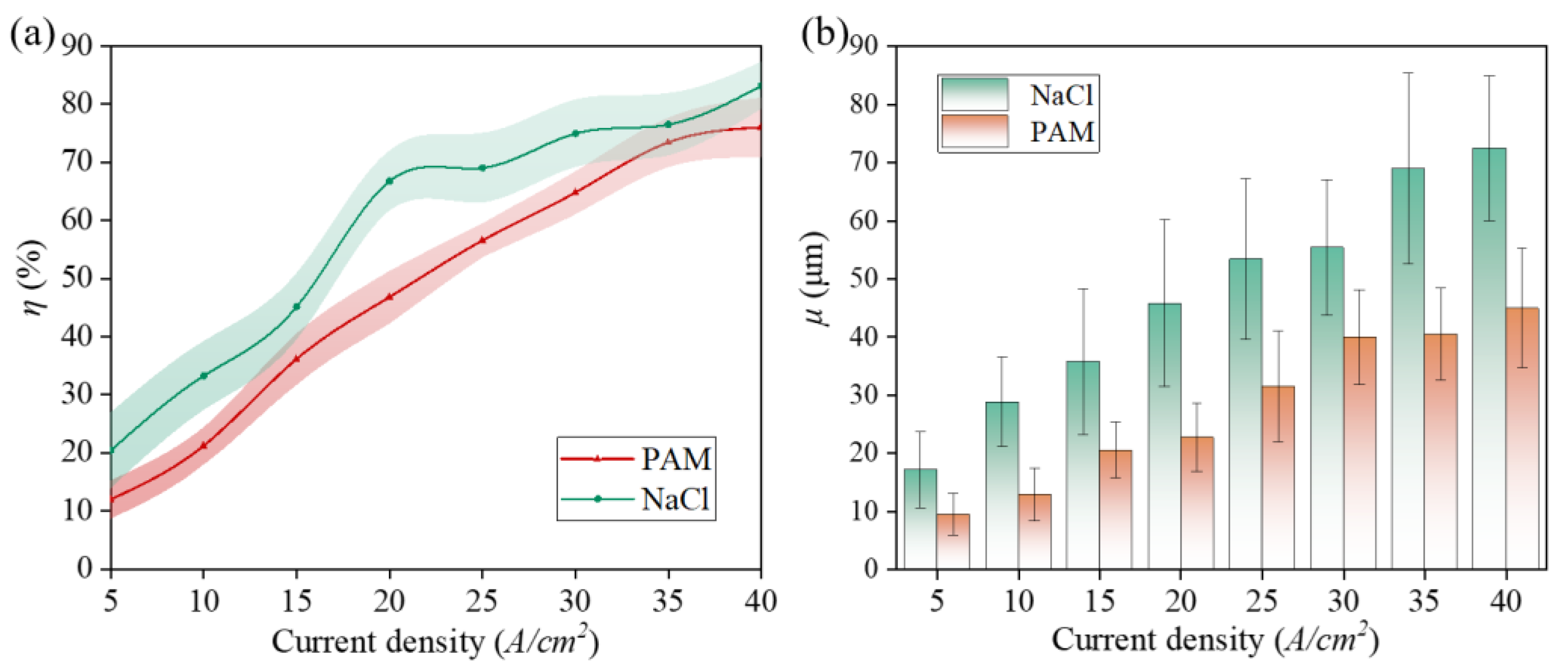
3.2. Analysis of Zr702 Electrochemical Dissolution Characteristics
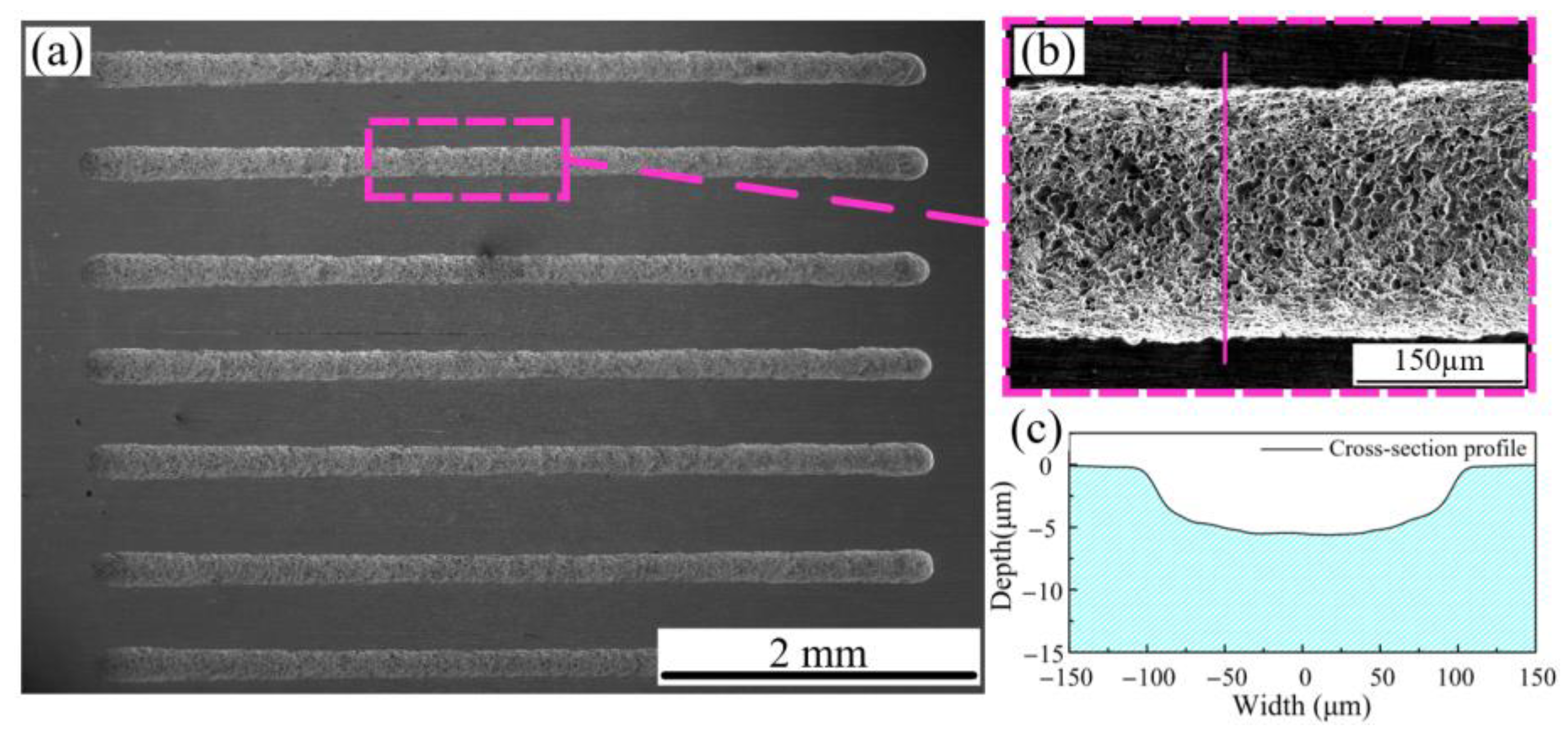
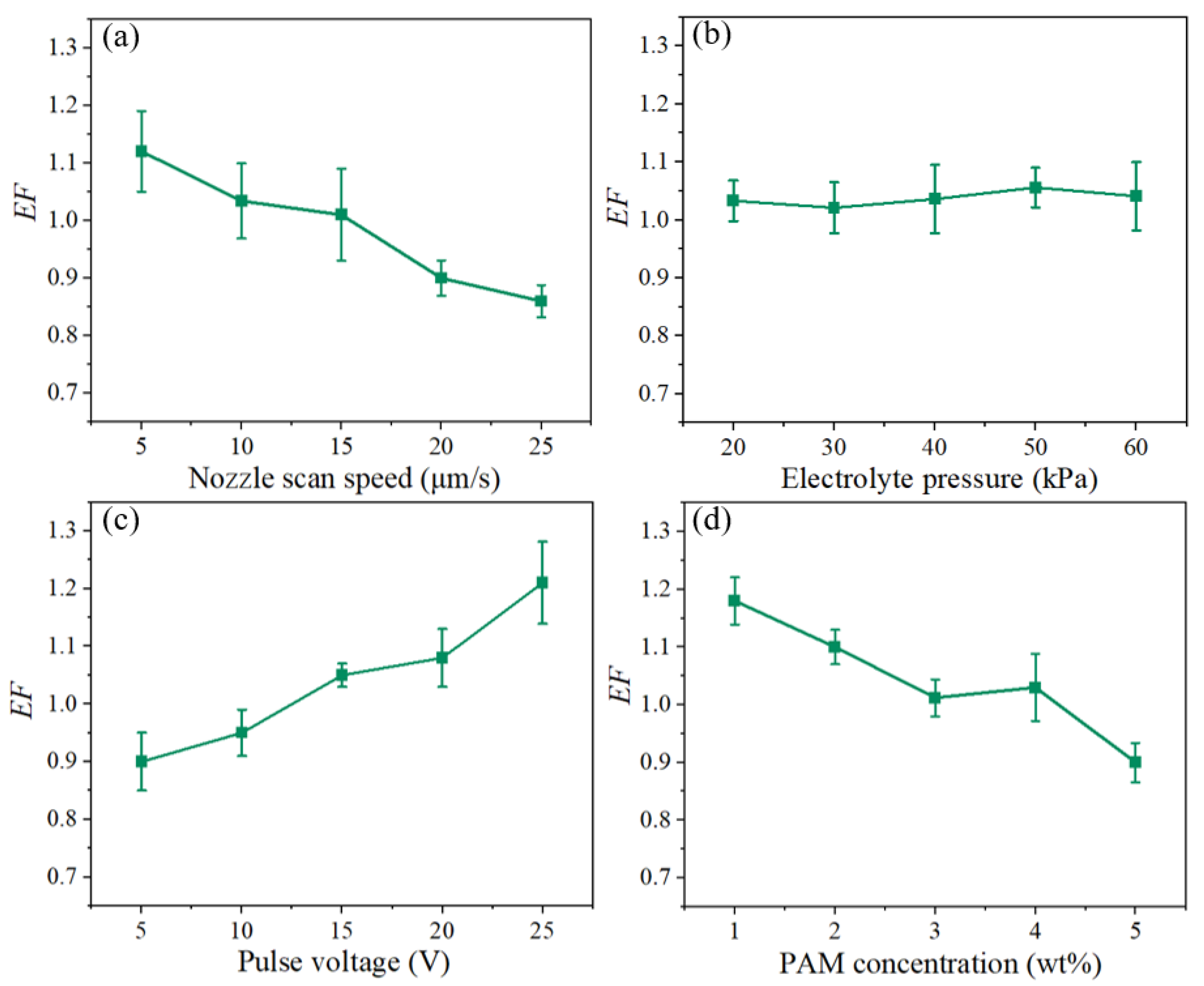
3.3. Experimental Machining Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Azevedo, C.R.F. Selection of fuel cladding material for nuclear fission reactors. Eng. Fail. Anal. 2011, 18, 1943–1962. [Google Scholar] [CrossRef]
- Gebert, A.; Gostin, P.F.; Sueptitz, R.; Oswald, S.; Abdi, S.; Uhlemann, M.; Eckert, J. Polarization studies of Zr-based bulk metallic glasses for electrochemical machining. J. Electrochem. Soc. 2014, 161, E66–E73. [Google Scholar] [CrossRef]
- Chen, B.; Chai, L.; Wang, S.; Guo, N.; Song, B.; Zhou, Z.; Huang, C. Investigation of microstructures of laser surface-treated Zr702 sheet using electron channeling contrast imaging and electron backscatter diffraction techniques. Surf. Coat. Technol. 2016, 296, 13–19. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, H.Y.; Liu, Y.D.; Qi, M.; Ren, Q.; Xu, J.; Shan, D.; Guo, B. Improvement of multi-functional properties by fabricating micro-pillar arrays structures on zirconium alloy surface. Sci. China Technol. Sci. 2022, 65, 1243–1252. [Google Scholar] [CrossRef]
- Hood, R.; Soo, S.L.; Sage, C.; Carcass, P. High speed end milling of a zirconium alloy. CIRP Ann. 2015, 64, 105–108. [Google Scholar] [CrossRef]
- Chen, B.; Xiong, F.; Tang, H.; He, L.; Hu, S. Effect of cooling method on small diameter blind-hole drilling of new β-type dental Ti-Zr-Nb alloy. J. Manuf. Process. 2020, 59, 421–431. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, B.; Ding, W.; Xin, L.; Nian, Z.; Peng, J.; He, N.; Xu, J. Nontraditional energy-assisted mechanical machining of difficult-to-cut materials and components in aerospace community: A comparative analysis. Int. J. Extreme Manuf. 2024, 6, 022007. [Google Scholar] [CrossRef]
- Mu, X.; Zhou, M.; Zhang, J.; Lu, N.; Ye, Q. Intelligent electrical discharge machining (EDM) molybdenum-titanium-zirconium alloy by an extended adaptive control system. J. Manuf. Process. 2022, 77, 207–218. [Google Scholar] [CrossRef]
- Kumar, J.; Soota, T.; Rajput, S.; Saxena, K. Machining and optimization of Zircaloy-2 using different tool electrodes. Mater. Manuf. Process. 2021, 36, 1513–1523. [Google Scholar] [CrossRef]
- Lei, J.; Wu, X.; Zhou, Z.; Xu, B.; Zhu, L.; Tang, Y. Sustainable mass production of blind multi-microgrooves by EDM with a long-laminated electrode. J. Clean. Product. 2021, 279, 123492. [Google Scholar] [CrossRef]
- Kumar, S.S.; Varol, T.; Canakci, A.; Kumaran, S.T.; Uthayakumar, M. A review on the performance of the materials by surface modification through EDM. Int. J. Lightweight Mater. Manuf. 2021, 4, 127–144. [Google Scholar] [CrossRef]
- Yue, M.; Liu, Y.; He, G.; Lian, L. Microstructure and mechanical performance of zirconium, manufactured by selective laser melting. Mater. Sci. Eng. A 2022, 840, 142900. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Wang, Y. Effect of femtosecond laser ablate ultra-fine microgrooves on surface properties of dental zirconia materials. J. Mech. Behav. Biomed. Mater. 2022, 134, 105361. [Google Scholar] [CrossRef]
- Wang, J.; Fang, F.; An, H.; Wu, S.; Qi, H.; Cai, Y.; Guo, G. Laser machining fundamentals: Micro, nano, atomic and close-to-atomic scales. Int. J. Extreme Manuf. 2023, 5, 012005. [Google Scholar] [CrossRef]
- He, J.; Wang, Z.; Wang, J.; Liang, H.; Lian, H. Investigation of the microhole arrays generated by masked jet electrochemical machining with polyaluminum chloride electrolyte. Precis. Eng. 2023, 82, 370–382. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Liang, H.; Zhou, L.; Jian, Y.; Zhou, W. Experimental study on the evolution process of Zr702 microdimple array generated with masked jet electrochemical machining. Int. J. Adv. Manuf. Technol. 2023, 124, 973–987. [Google Scholar] [CrossRef]
- Wu, M.; Liu, J.; He, J.; Chen, X.; Guo, Z. Fabrication of surface microstructures by mask electrolyte jet machining. Int. J. Mach. Tool. Manuf. 2020, 148, 103471. [Google Scholar] [CrossRef]
- Fang, M.; Yu, L.; Chu, X.F.; Hou, L.L.; Cheng, X.; Wang, J.L. Research on simulation method of multiple time scales dissolution process in tube electrode pulse electrochemical machining. J. Manuf. Process. 2024, 110, 318–330. [Google Scholar] [CrossRef]
- Chen, X.L.; Dong, B.Y.; Zhang, C.Y.; Luo, H.P.; Liu, J.W.; Zhang, Y.J.; Guo, Z.N. Electrochemical direct-writing machining of micro-channel array. J. Mater. Process. Technol. 2019, 265, 138–149. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, Y.; He, X.; Wang, Z.; Chi, G.; Chen, X.; Song, M. Micro electrochemical machining of array micro-grooves using in-situ disk electrode fabricated by micro-WEDM. Micromachines 2020, 11, 66. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, J.; Xu, Z.; Su, G. Modeling and experimental research on the evolution process of micro through-slit array generated with masked jet electrochemical machining. J. Mater. Process. Technol. 2021, 298, 117304. [Google Scholar] [CrossRef]
- Chen, X.L.; Fan, G.C.; Lin, C.H.; Dong, B.Y.; Guo, Z.N.; Fang, X.L.; Qu, N.S. Investigation on the electrochemical machining of micro groove using masked porous cathode. J. Mater. Process. Technol. 2020, 276, 116406. [Google Scholar] [CrossRef]






| Parameter | Value |
|---|---|
| PAM (wt%) | 1, 2, 3, 4, 5 |
| Shear rate (rpm) | 120, 180, 240, 300, 360 |
| Time (s) | 30 |
| Pressure (kPa) | 101.325 |
| Temperature (°C) | 25 |
| Parameter | Value |
|---|---|
| PAM concentration (wt%) | 1, 2, 3, 4, 5 |
| Measuring potential (V) | −2 to 4 |
| Scan rate (mV/s) | 1 |
| Temperature (°C) | 25 |
| Parameter | Value |
|---|---|
| Nozzle scan speed (μm/s) | 5, 10, 15, 20, 25 |
| Electrolyte pressure (kPa) | 20, 30, 40, 50, 60 |
| Pulse voltage (V) | 5, 10, 15, 20, 25 |
| PAM concentration (wt%) | 1, 2, 3, 4, 5 |
| pulse frequency (Hz) | 1000 |
| FPM aperture (μm) | 150 |
| Duty ratio (%) | 50 |
| Category | Original Zr702 | Self-Corrosion Potential | Activation Potential | Stable Passivation Potential | Threshold Passivation Potential |
|---|---|---|---|---|---|
| Stage | 0 | / | 1 | 2 | 3 |
| PAM | / | −0.124 V | −0.085 V | 0.241 V | 0.737 V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Chen, W.; Wang, J.; Wu, M.; Zhou, L.; Chen, R.; Liang, H. Exploring Electrochemical Direct Writing Machining of Patterned Microstructures on Zr702 with Polyacrylamide Polymer Electrolyte. Micromachines 2024, 15, 1074. https://doi.org/10.3390/mi15091074
He J, Chen W, Wang J, Wu M, Zhou L, Chen R, Liang H. Exploring Electrochemical Direct Writing Machining of Patterned Microstructures on Zr702 with Polyacrylamide Polymer Electrolyte. Micromachines. 2024; 15(9):1074. https://doi.org/10.3390/mi15091074
Chicago/Turabian StyleHe, Junfeng, Wenjie Chen, Junjie Wang, Ming Wu, Li Zhou, Ri Chen, and Huazhuo Liang. 2024. "Exploring Electrochemical Direct Writing Machining of Patterned Microstructures on Zr702 with Polyacrylamide Polymer Electrolyte" Micromachines 15, no. 9: 1074. https://doi.org/10.3390/mi15091074
APA StyleHe, J., Chen, W., Wang, J., Wu, M., Zhou, L., Chen, R., & Liang, H. (2024). Exploring Electrochemical Direct Writing Machining of Patterned Microstructures on Zr702 with Polyacrylamide Polymer Electrolyte. Micromachines, 15(9), 1074. https://doi.org/10.3390/mi15091074






