Conceptual Piezoelectric-Based Energy Harvester from In Vivo Heartbeats’ Cyclic Kinetic Motion for Leadless Intracardiac Pacemakers
Abstract
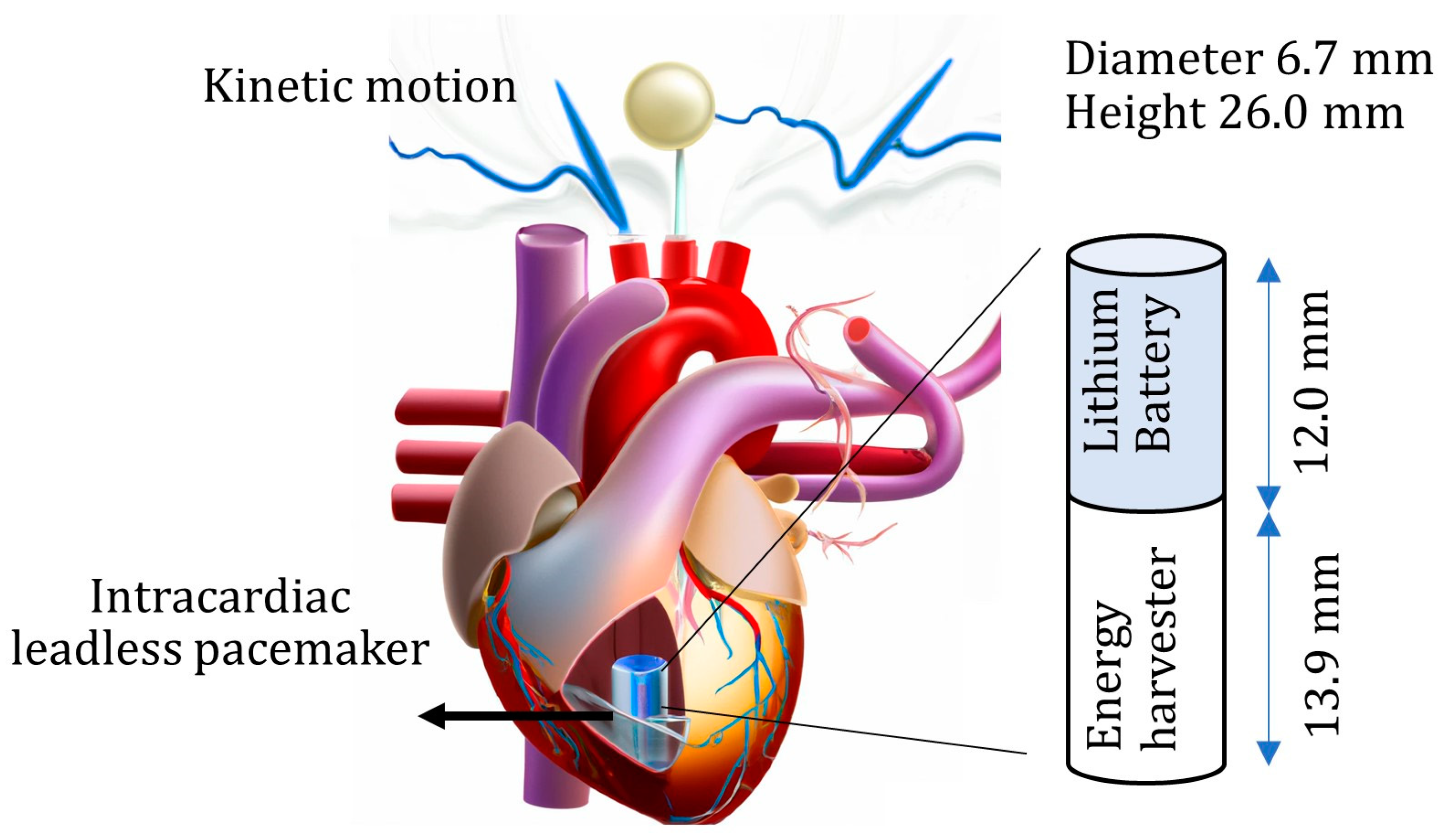
:1. Introduction
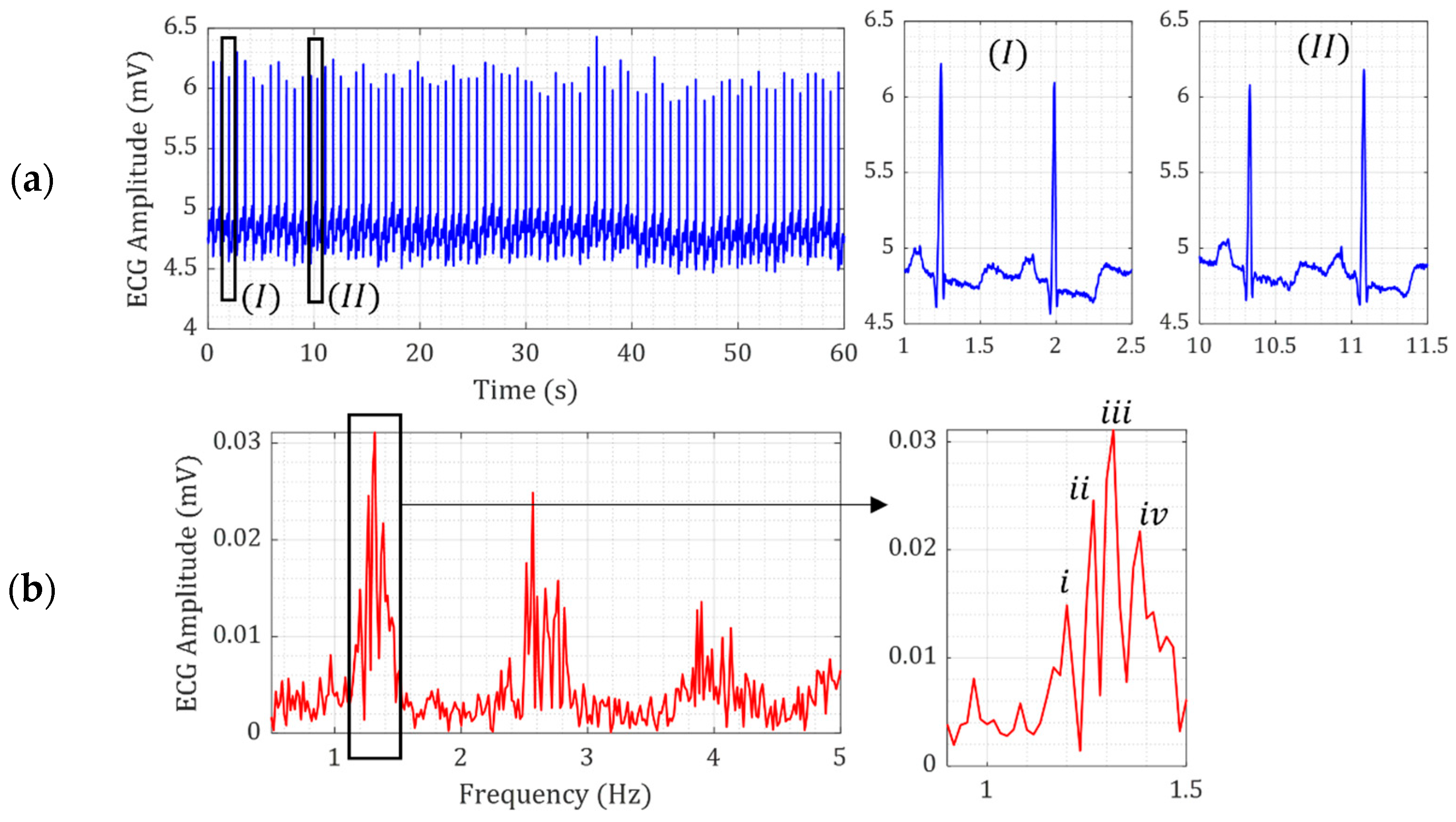
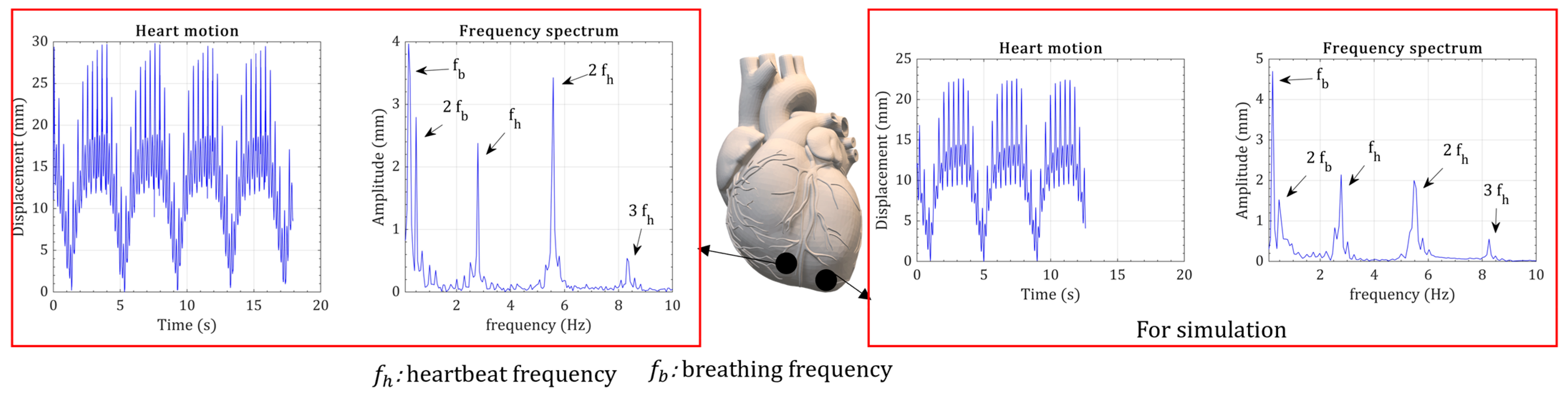
2. Heart Kinetic Motion
3. Results and Discussion
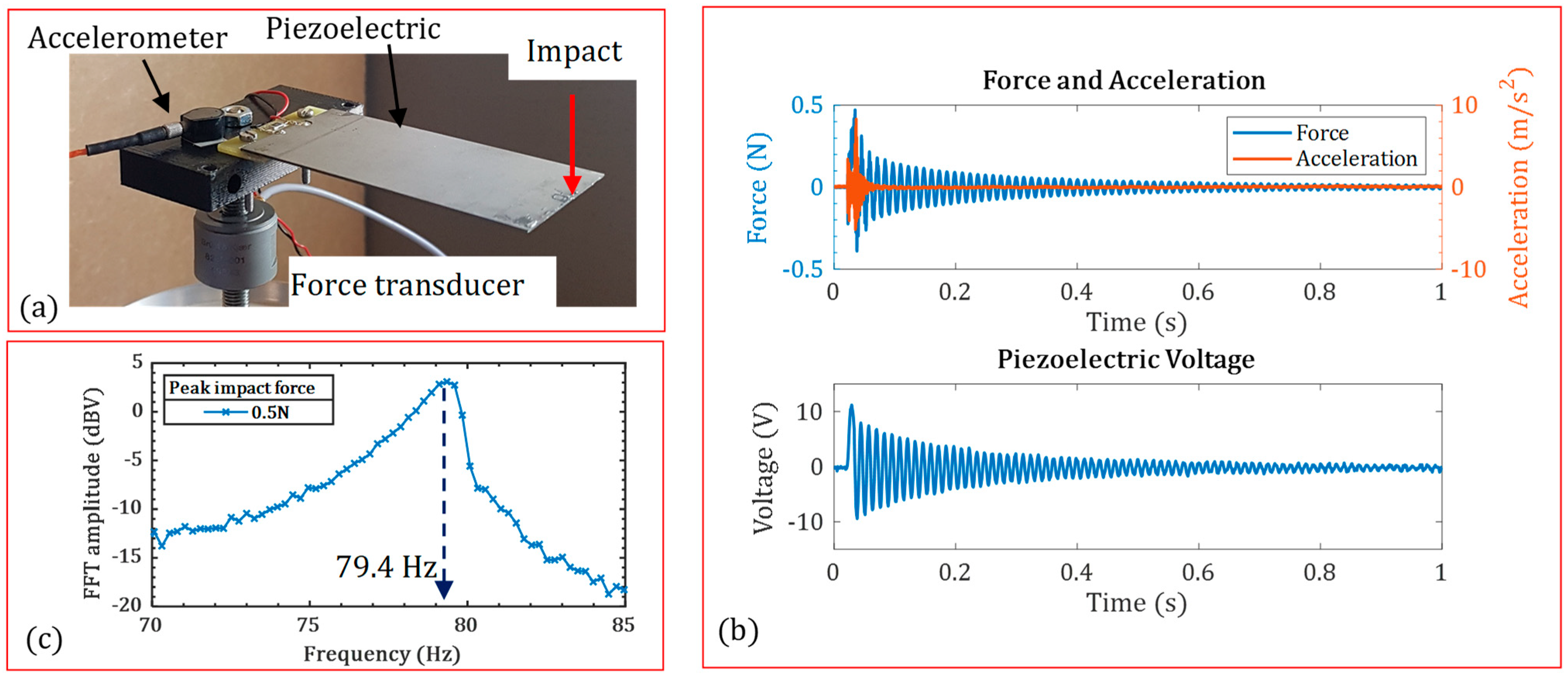
3.1. Verification of Model
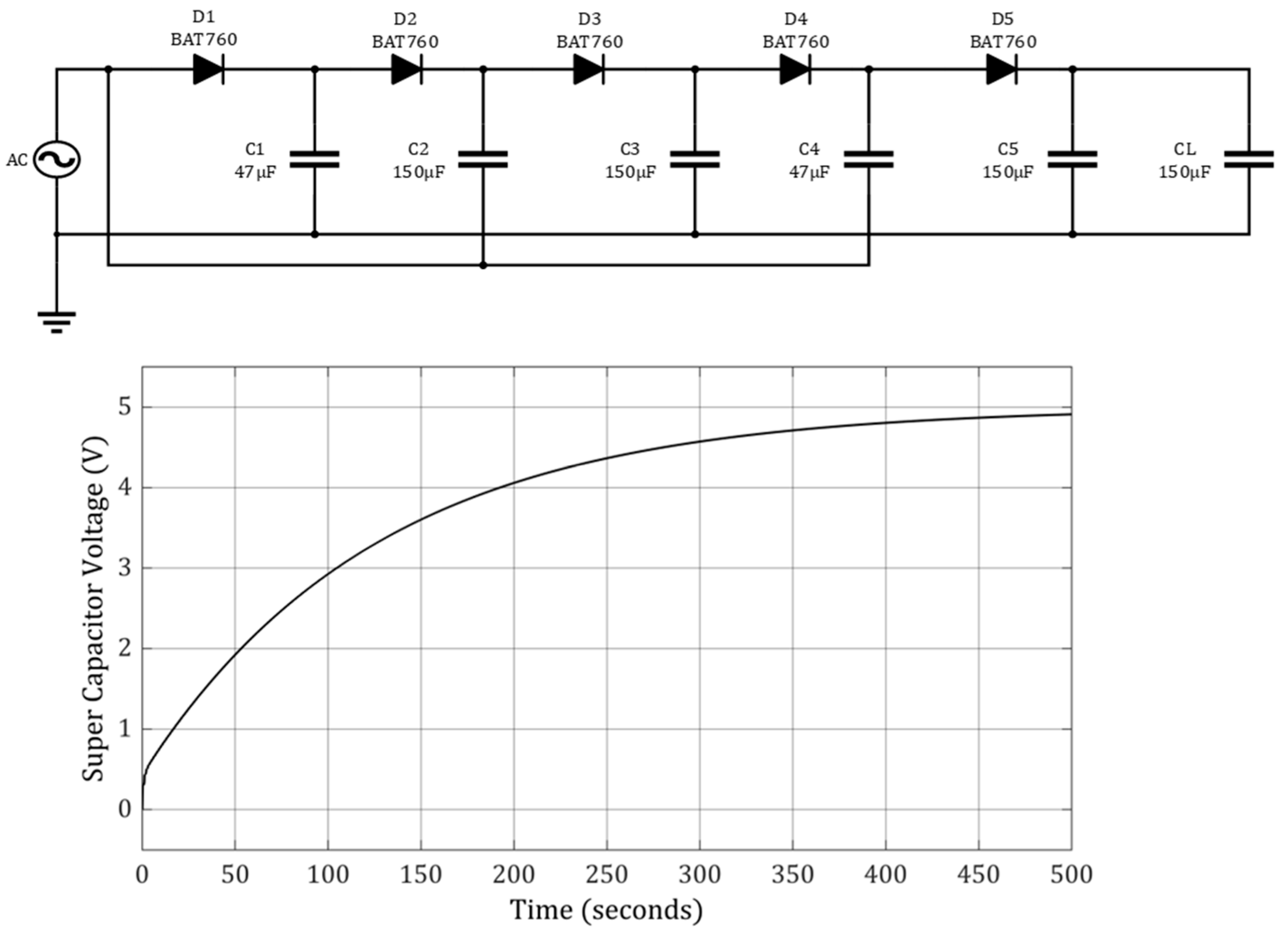
3.2. Energy Harvesters for Leadless Pacemakers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, C.U.; Murthy, A.S.D.; Prasanna, B.L.; Reddy, M.P.P.; Panigrahy, A.K. An Automated Detection of Heart Arrhythmias Using Machine Learning Technique: SVM. Mater. Today Proc. 2021, 45, 1393–1398. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 8 July 2024).
- Bencardino, G.; Scacciavillani, R.; Narducci, M.L. Leadless Pacemaker Technology: Clinical Evidence of New Paradigm of Pacing. Rev. Cardiovasc. Med. 2022, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Spickler, J.W.; Rasor, N.S.; Kezdi, P.; Misra, S.; Robins, K.; LeBoeuf, C. Totally Self-Contained Intracardiac Pacemaker. J. Electrocardiol. 1970, 3, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mulpuru, S.K.; Madhavan, M.; McLeod, C.J.; Cha, Y.-M.; Friedman, P.A. Cardiac Pacemakers: Function, Troubleshooting, and Management: Part 1 of a 2-Part Series. J. Am. Coll. Cardiol. 2017, 69, 189–210. [Google Scholar] [CrossRef]
- Sideris, S.; Archontakis, S.; Dilaveris, P.; Gatzoulis, K.A.; Trachanas, K.; Sotiropoulos, I.; Arsenos, P.; Tousoulis, D.; Kallikazaros, I. Leadless Cardiac Pacemakers: Current Status of a Modern Approach in Pacing. Hell. J. Cardiol. 2017, 58, 403–410. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Miller, M.A.; Knops, R.E.; Neuzil, P.; Defaye, P.; Jung, W.; Doshi, R.; Castellani, M.; Strickberger, A.; Mead, R.H.; et al. Retrieval of the Leadless Cardiac Pacemaker: A Multicenter Experience. Circ. Arrhythmia Electrophysiol. 2016, 9, e004626. [Google Scholar] [CrossRef]
- Kypta, A.; Blessberger, H.; Lichtenauer, M.; Steinwender, C. Complete Encapsulation of a Leadless Cardiac Pacemaker. Clin. Res. Cardiol. 2016, 105, 94. [Google Scholar] [CrossRef]
- Beurskens, N.E.G.; Tjong, F.V.Y.; Knops, R.E. End-of-Life Management of Leadless Cardiac Pacemaker Therapy. Arrhythmia Electrophysiol. Rev. 2017, 6, 129–133. [Google Scholar] [CrossRef]
- Fernandez, S.V.; Cai, F.; Chen, S.; Suh, E.; Tiepelt, J.; McIntosh, R.; Marcus, C.; Acosta, D.; Mejorado, D.; Dagdeviren, C. On-Body Piezoelectric Energy Harvesters through Innovative Designs and Conformable Structures. ACS Biomater. Sci. Eng. 2021, 9, 2070–2086. [Google Scholar] [CrossRef]
- Tomiyoshi, T. UC Davis Doctor First in the World to Implant a Retrievable Leadless Pacemaker in a Child. UC Davis Health, 27 January 2023. [Google Scholar]
- MicraTM. MC1VR01 Clinical Manual; Medtronic: Minneapolis, MN, USA, 2021. [Google Scholar]
- Jackson, N.; Olszewski, O.; O’Murchu, C.; Mathewson, A. Powering a Leadless Pacemaker Using a PiezoMEMS Energy Harvester. Smart Sens. Actuators MEMS VIII 2017, 10246, 102460V. [Google Scholar] [CrossRef]
- Park, S.; Heo, S.W.; Lee, W.; Inoue, D.; Jiang, Z.; Yu, K.; Jinno, H.; Hashizume, D.; Sekino, M.; Yokota, T.; et al. Self-Powered Ultra-Flexible Electronics via Nano-Grating-Patterned Organic Photovoltaics. Nature 2018, 561, 516–521. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Haeberlin, A.; Bereuter, L.; Wagner, J.; Pfenniger, A.; Omari, S.; Schaerer, J.; Jutzi, F.; Huber, C.; Fuhrer, J.; et al. The Swiss Approach for a Heartbeat-Driven Lead- and Batteryless Pacemaker. Heart Rhythm 2017, 14, 294–299. [Google Scholar] [CrossRef]
- Wang, C.; Shi, Q.; Lee, C. Advanced Implantable Biomedical Devices Enabled by Triboelectric Nanogenerators. Nanomaterials 2022, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Li, Z.; Wang, Z.L. Energy Harvesting from the Animal/Human Body for Self-Powered Electronics. Annu. Rev. Biomed. Eng. 2017, 19, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Qing, S.; Rezania, A.; Rosendahl, L.A.; Gou, X. Design of Flexible Thermoelectric Generator as Human Body Sensor. Mater. Today Proc. 2018, 5, 10338–10346. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Yang, B.D.; Su, Y.; Tran, P.L.; Joe, P.; Anderson, E.; Xia, J.; Doraiswamy, V.; Dehdashti, B.; Feng, X.; et al. Conformal Piezoelectric Energy Harvesting and Storage from Motions of the Heart, Lung, and Diaphragm. Proc. Natl. Acad. Sci. USA 2014, 111, 1927–1932. [Google Scholar] [CrossRef]
- O’Keeffe, R. Piezoelectric Energy Harvesting Using Blood-Flow-like Excitation for Implantable Devices. Ph.D. Thesis, University College Cork, Cork, Ireland, 2013. [Google Scholar]
- Zhang, H.; Zhang, X.S.; Cheng, X.; Liu, Y.; Han, M.; Xue, X.; Wang, S.; Yang, F.; Smitha, A.S.; Zhang, H.; et al. A Flexible and Implantable Piezoelectric Generator Harvesting Energy from the Pulsation of Ascending Aorta: In Vitro and in Vivo Studies. Nano Energy 2015, 12, 296–304. [Google Scholar] [CrossRef]
- Hwang, G.T.; Park, H.; Lee, J.H.; Oh, S.; Park, K., II; Byun, M.; Park, H.; Ahn, G.; Jeong, C.K.; No, K.; et al. Self-Powered Cardiac Pacemaker Enabled by Flexible Single Crystalline PMN-PT Piezoelectric Energy Harvester. Adv. Mater. 2014, 26, 4880–4887. [Google Scholar] [CrossRef]
- Jackson, N.; Olszewski, O.Z.; O’Murchu, C.; Mathewson, A. Shock-Induced Aluminum Nitride Based MEMS Energy Harvester to Power a Leadless Pacemaker. Sens. Actuators A Phys. 2017, 264, 212–218. [Google Scholar] [CrossRef]
- Karami, M.A.; Inman, D.J. Powering Pacemakers from Heartbeat Vibrations Using Linear and Nonlinear Energy Harvesters. Appl. Phys. Lett. 2012, 100, 42901. [Google Scholar] [CrossRef]
- Deterre, M.; Lefeuvre, E.; Zhu, Y.; Woytasik, M.; Boutaud, B.; Molin, R.D. Micro Blood Pressure Energy Harvester for Intracardiac Pacemaker. J. Microelectromech. Syst. 2014, 23, 651–660. [Google Scholar] [CrossRef]
- Jackson, N.; Olszewski, O.; Mathewson, A.; Murchu, C.O. Ultra-Low Frequency Piezomems Energy Harvester for a Leadless Pacemaker. In Proceedings of the 2018 IEEE Micro Electro Mechanical Systems (MEMS), Belfast, UK, 21–25 January 2018; pp. 642–645. [Google Scholar] [CrossRef]
- Ansari, M.H.; Karami, M.A. Piezoelectric Energy Harvesting from Heartbeat Vibrations for Leadless Pacemakers. J. Phys. Conf. Ser. 2015, 660, 12121. [Google Scholar] [CrossRef]
- Roy, A.; Dwari, D.; Ram, M.K.; Datta, P. Piezoelectric Nanomaterials for Biomedical Applications. In Food, Medical, and Environmental Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 355–377. [Google Scholar] [CrossRef]
- Liu, M.; Qian, F.; Mi, J.; Zuo, L. Biomechanical Energy Harvesting for Wearable and Mobile Devices: State-of-the-Art and Future Directions. Appl. Energy 2022, 321, 119379. [Google Scholar] [CrossRef]
- Yue, R.; Ramaraj, S.G.; Liu, H.; Elamaran, D.; Elamaran, V.; Gupta, V.; Arya, S.; Verma, S.; Satapathi, S.; Hayawaka, Y.; et al. A Review of Flexible Lead-Free Piezoelectric Energy Harvester. J. Alloys Compd. 2022, 918, 165653. [Google Scholar] [CrossRef]
- Gu, L.; Liu, J.; Cui, N.; Xu, Q.; Du, T.; Zhang, L.; Wang, Z.; Long, C.; Qin, Y. Enhancing the Current Density of a Piezoelectric Nanogenerator Using a Three-Dimensional Intercalation Electrode. Nat. Commun. 2020, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hansen, B.J.; Wang, Z.L. Piezoelectric-Nanowire-Enabled Power Source for Driving Wireless Microelectronics. Nat. Commun. 2010, 1, 93. [Google Scholar] [CrossRef]
- Su, Y.; Li, W.; Cheng, X.; Zhou, Y.; Yang, S.; Zhang, X.; Chen, C.; Yang, T.; Pan, H.; Xie, G.; et al. High-Performance Piezoelectric Composites via β Phase Programming. Nat. Commun. 2022, 13, 4867. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Choi, C.; Hardwick, J.; Bagchi, B.; Tiwari, M.K.; Subramanian, S. Transmissive Labyrinthine Acoustic Metamaterial-Based Holography for Extraordinary Energy Harvesting. Adv. Eng. Mater. 2022, 25, 2201117. [Google Scholar] [CrossRef]
- Guo, T.M.; Gong, Y.J.; Li, Z.G.; Liu, Y.M.; Li, W.; Li, Z.Y.; Bu, X.H. A New Hybrid Lead-Free Metal Halide Piezoelectric for Energy Harvesting and Human Motion Sensing. Small 2022, 18, 2103829. [Google Scholar] [CrossRef]
- Jiang, F.; Zhou, X.; Lv, J.; Chen, J.; Chen, J.; Kongcharoen, H.; Zhang, Y.; Lee, P.S. Stretchable, Breathable, and Stable Lead-Free Perovskite/Polymer Nanofiber Composite for Hybrid Triboelectric and Piezoelectric Energy Harvesting. Adv. Mater. 2022, 34, e2200042. [Google Scholar] [CrossRef]
- Pei, H.; Shi, S.; Chen, Y.; Xiong, Y.; Lv, Q. Combining Solid-State Shear Milling and FFF 3D-Printing Strategy to Fabricate High-Performance Biomimetic Wearable Fish-Scale PVDF-Based Piezoelectric Energy Harvesters. ACS Appl. Mater. Interfaces 2022, 14, 15346–15359. [Google Scholar] [CrossRef]
- Lv, P.; Qian, J.; Yang, C.; Liu, T.; Wang, Y.; Wang, D.; Huang, S.; Cheng, X.; Cheng, Z. Flexible All-Inorganic Sm-Doped PMN-PT Film with Ultrahigh Piezoelectric Coefficient for Mechanical Energy Harvesting, Motion Sensing, and Human-Machine Interaction. Nano Energy 2022, 97, 107182. [Google Scholar] [CrossRef]
- Mitra, R.; Sheetal Priyadarshini, B.; Ramadoss, A.; Manju, U. Stretchable Polymer-Modulated PVDF-HFP/TiO2 Nanoparticles-Based Piezoelectric Nanogenerators for Energy Harvesting and Sensing Applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2022, 286, 116029. [Google Scholar] [CrossRef]
- Cui, H.; Hensleigh, R.; Yao, D.; Maurya, D.; Kumar, P.; Kang, M.G.; Priya, S.; Zheng, X. (Rayne) Three-Dimensional Printing of Piezoelectric Materials with Designed Anisotropy and Directional Response. Nat. Mater. 2019, 18, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Hong, S.; Hwang, B.; Ahn, S.H.; Song, J.H. Improved Performance of Stretchable Piezoelectric Energy Harvester Based on Stress Rearrangement. Sci. Rep. 2022, 12, 19149. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Parida, K.; Zhang, H.; Wang, X.; Li, Y.; Zhou, X.; Morris, S.A.; Liew, W.H.; Wang, H.; Li, T.; et al. Bond Engineering of Molecular Ferroelectrics Renders Soft and High-Performance Piezoelectric Energy Harvesting Materials. Nat. Commun. 2022, 13, 5607. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, C.; Kwon, S.H.; Jiang, Z.; Dong, L. Flexible Hybrid Piezoelectric-Electrostatic Device for Energy Harvesting and Sensing Applications. Adv. Mater. Interfaces 2023, 10, 2202173. [Google Scholar] [CrossRef]
- Bae, J.; Song, J.; Jeong, W.; Nandanapalli, K.R.; Son, N.; Zulkifli, N.A.B.; Gwon, G.; Kim, M.; Yoo, S.; Lee, H.; et al. Multi-Deformable Piezoelectric Energy Nano-Generator with High Conversion Efficiency for Subtle Body Movements. Nano Energy 2022, 97, 107223. [Google Scholar] [CrossRef]
- Li, H.; Tian, C.; Deng, Z.D. Energy Harvesting from Low Frequency Applications Using Piezoelectric Materials. Appl. Phys. Rev. 2014, 1, 41301. [Google Scholar] [CrossRef]
- Khazaee, M.; Hasani, M.; Riahi, S.; Rosendahl, L.; Rezania, A. Harnessing Cardiac Power: Heart Kinetic Motion Analysis for Energy Harvesters. Biomed. Signal Process. Control 2024, 95, 106421. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Lü, C. Optimal Patching Locations and Orientations for Maximum Energy Harvesting Efficiency of Ultrathin Flexible Piezoelectric Devices Mounted on Heart Surface. Theor. Appl. Mech. Lett. 2022, 12, 100341. [Google Scholar] [CrossRef]
- Pławiak, P. Novel Methodology of Cardiac Health Recognition Based on ECG Signals and Evolutionary-Neural System. Expert Syst. Appl. 2018, 92, 334–349. [Google Scholar] [CrossRef]
- Moosavian, A.; Khazaee, M.; Najafi, G.; Khazaee, M.; Sakhaei, B.; Mohammad Jafari, S. Wavelet Denoising Using Different Mother Wavelets for Fault Diagnosis of Engine Spark Plug. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2017, 231, 359–370. [Google Scholar] [CrossRef]
- Sauvée, M.; Poignet, P.; Triboulet, J.; Dombre, E.; Malis, E.; Demaria, R. 3D Heart Motion Estimation Using Endoscopic Monocular Vision System. IFAC Proc. Vol. 2006, 39, 141–146. [Google Scholar] [CrossRef]
- Nakamura, K. Ultrasonic Transducers: Materials and Design for Sensors, Actuators and Medical Applications; Woodhead Publishing Limited: Sawston, UK, 2012; ISBN 9781845695347. [Google Scholar]
- Khazaee, M.; Rezaniakolaei, A.; Rosendahl, L. A Broadband Macro-Fiber-Composite Piezoelectric Energy Harvester for Higher Energy Conversion from Practical Wideband Vibrations. Nano Energy 2020, 76, 104978. [Google Scholar] [CrossRef]
- Khazaee, M.; Rezaniakolaei, A.; Rosendahl, L. A Comprehensive Electromechanically Coupled Model for Non-Uniform Piezoelectric Energy Harvesting Composite Laminates. Mech. Syst. Signal Process. 2020, 145, 106927. [Google Scholar] [CrossRef]
- Khazaee, M.; Enkeshafi, A.A.; Riahi, S.; Rosendahl, L.; Kjærgaard, B.; Rezania, A. Contact-Based Impact Energy Harvesting from the Ultra-Low Frequency Intracardiac Kinetic Energy. ACS Lett. 2024, 9, 2944–2952. [Google Scholar]
- Khazaee, M.; Rezaniakolaie, A.; Moosavian, A.; Rosendahl, L. A Novel Method for Autonomous Remote Condition Monitoring of Rotating Machines Using Piezoelectric Energy Harvesting Approach. Sens. Actuators A Phys. 2019, 295, 37–50. [Google Scholar] [CrossRef]

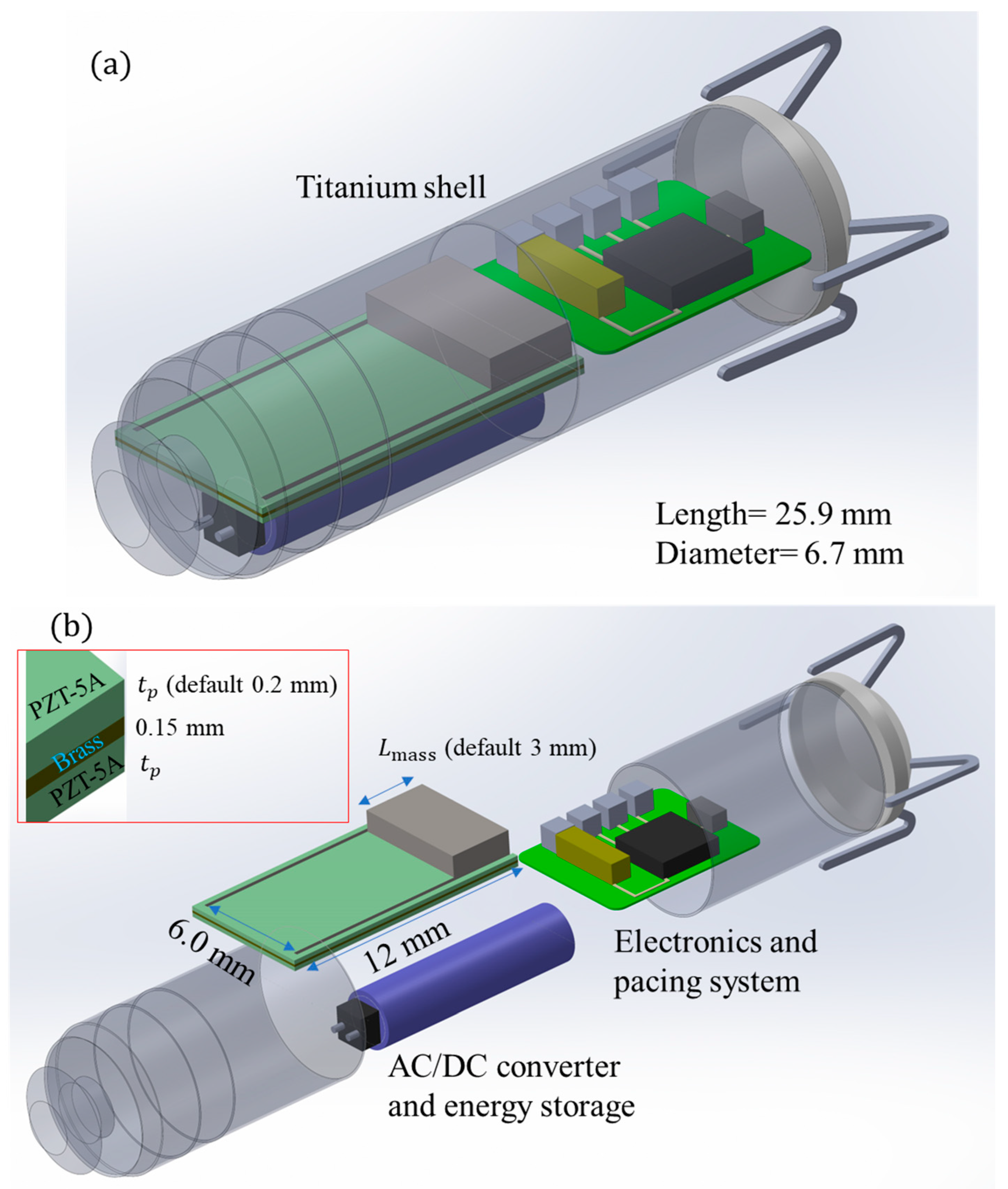

| Description | Piezoelectric Sheets | Supporting Substrate | Bonding Layers |
|---|---|---|---|
| Material | PZT-5A | Brass | Epoxy |
| ) | |||
| ) | |||
| ) | (each layer) | (each layer) | |
| ) | |||
| ) | 1s=100 | 1b=1.05 | |
| Poisson’s ratio | 0.32 | 0.32 | 0.3 |
| ) | - | - | |
| Relative permittivity | - | - | |
| Impact Force | The Open-Circuit First Natural Frequency (Hz) | Difference | |
|---|---|---|---|
| Experiment | COMSOL FE Model | ||
| 0.5 N | 79.4 | 81.6 | 2.7% |
| Variable Parameter Description | Variable Parameter [Best] | Fixed Parameters | Open Circuit Voltage | Difference with Default Configuration (%) |
|---|---|---|---|---|
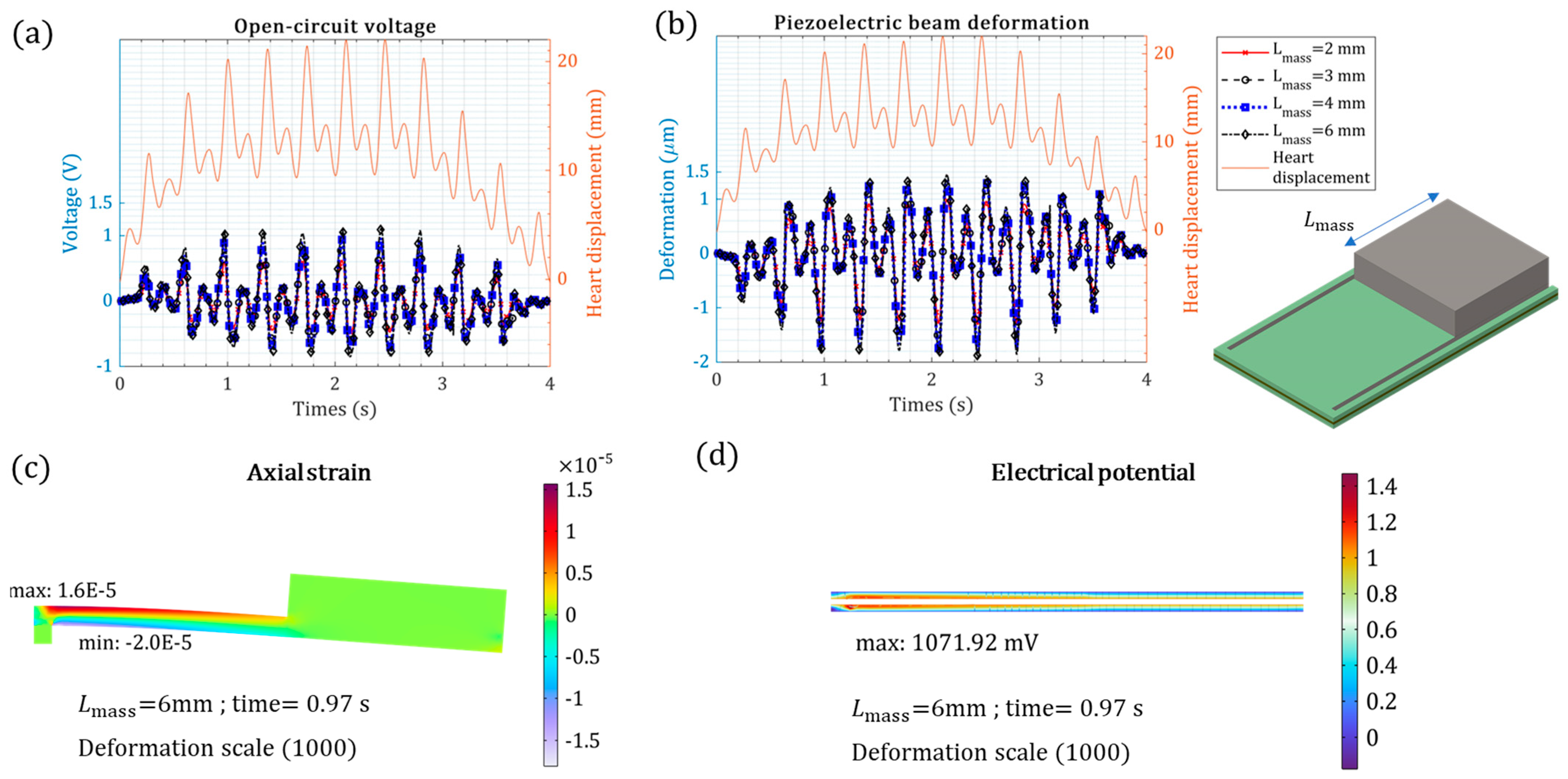
| Tip mass weight | [= 6 mm] | ) | 1.12 V | +36.6% |
| Piezoelectric layer thickness(default configuration) | [= 0.2 mm] | ) | 0.82 V | - |
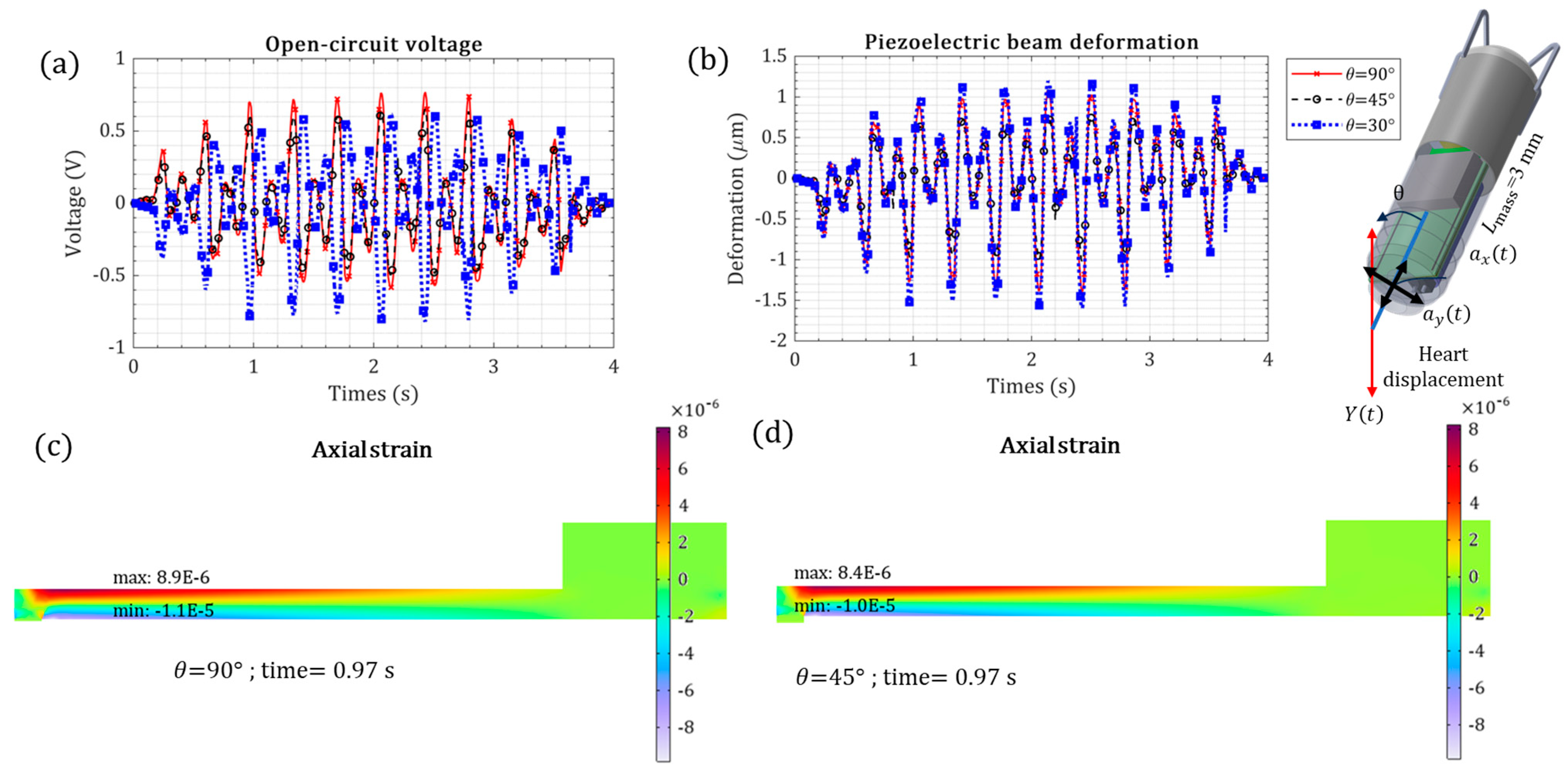
| Orientation of energy harvester inside the heart | ) | → 0.76 V | → −7.3% | |
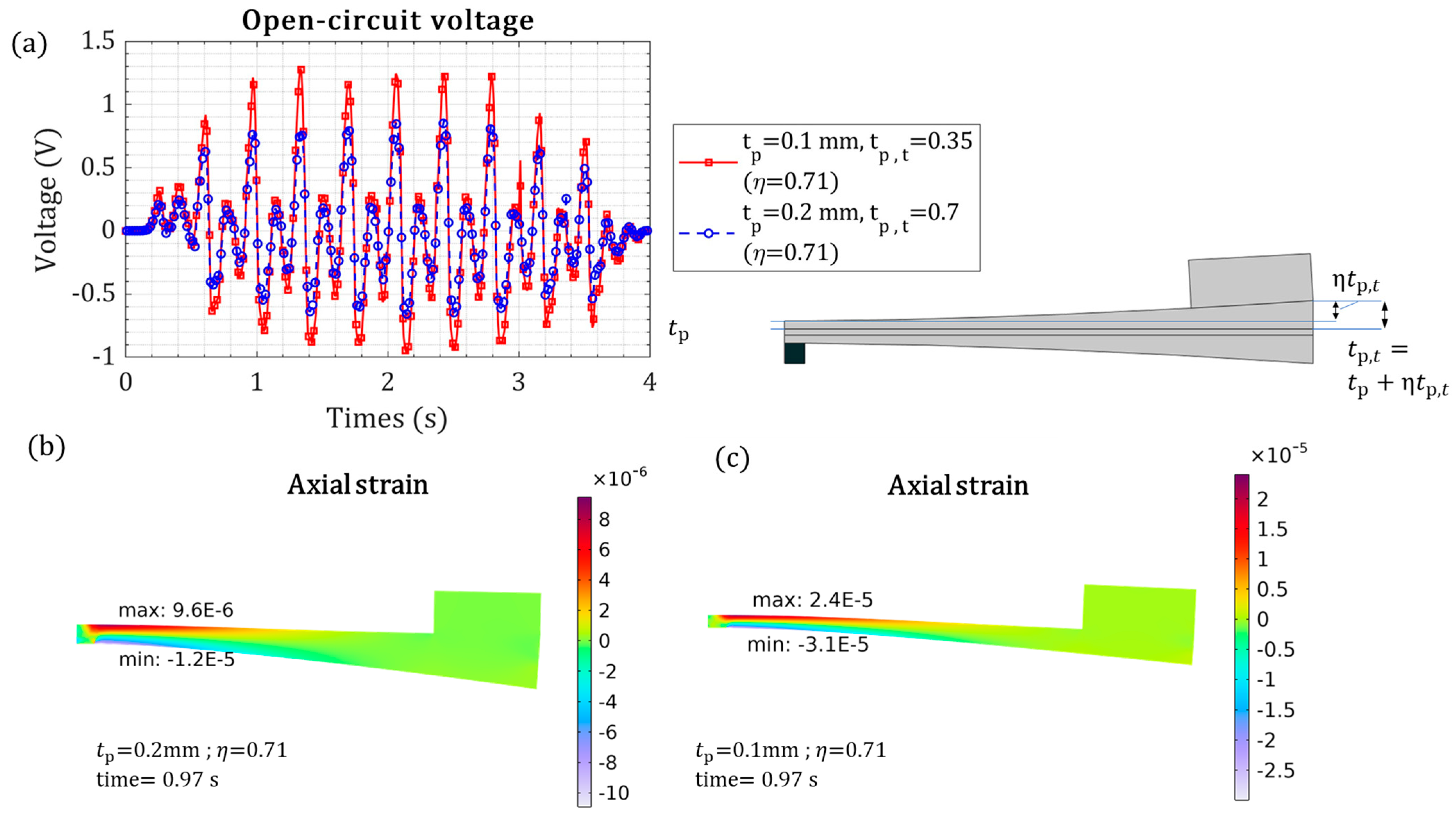
| Variable piezoelectric layer thickness | ] | 1.28 V | 56.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khazaee, M.; Riahi, S.; Rezania, A. Conceptual Piezoelectric-Based Energy Harvester from In Vivo Heartbeats’ Cyclic Kinetic Motion for Leadless Intracardiac Pacemakers. Micromachines 2024, 15, 1133. https://doi.org/10.3390/mi15091133
Khazaee M, Riahi S, Rezania A. Conceptual Piezoelectric-Based Energy Harvester from In Vivo Heartbeats’ Cyclic Kinetic Motion for Leadless Intracardiac Pacemakers. Micromachines. 2024; 15(9):1133. https://doi.org/10.3390/mi15091133
Chicago/Turabian StyleKhazaee, Majid, Sam Riahi, and Alireza Rezania. 2024. "Conceptual Piezoelectric-Based Energy Harvester from In Vivo Heartbeats’ Cyclic Kinetic Motion for Leadless Intracardiac Pacemakers" Micromachines 15, no. 9: 1133. https://doi.org/10.3390/mi15091133








