Recent Advances in Polymer Science and Fabrication Processes for Enhanced Microfluidic Applications: An Overview
Abstract
1. Introduction
2. Fabrication Techniques
2.1. Photolithography
2.2. Soft Lithography
2.2.1. Replica Molding
2.2.2. Microcontact Printing (μCP)
2.2.3. Micromolding in Capillaries (MIMIC)
2.2.4. Microtransfer Molding (µTM)
2.3. Solvent-Assisted Molding
2.4. Injection Molding
2.5. 3D Printing
3. Materials Development
3.1. Flexible and Wearable Microfluidic Devices
3.2. Improved Mechanical Properties
3.3. Enhanced Optical Properties
3.4. Superior Chemical Resistance
3.5. Electrical Conductivity
4. Advanced Fabrication Techniques
4.1. Nanoimprint Lithography
4.2. Laser Micromachining
- Femtosecond laser micromachining: Utilizes ultrashort laser pulses to ablate material with minimal heat-affected zones, ideal for creating precise and intricate features in microfluidic devices. Femtosecond laser micromachining has been used for manufacturing micro- and nanofluidic devices, indicating its relevance in nanofluidics applications [314,315,316].
- Excimer laser micromachining: Uses ultraviolet lasers to achieve high-resolution patterning on polymers, suitable for microfluidic device fabrication with complex geometries [313].
- CO2 laser micromachining: Effective for cutting and engraving polymer substrates, often used in the initial stages of microfluidic device fabrication for rapid prototyping [317].
4.3. Hybrid Fabrication Techniques
4.4. Emerging Materials
5. Applications of Polymer-Based Microfluidic Devices
5.1. Biomedical Applications
5.1.1. Organ-on-a-Chip (OoC) Technology
5.1.2. Microfluidics in Personalized Medicine
5.1.3. Drug Screening
5.2. Environmental Monitoring
5.3. Industrial Applications
5.4. Point-of-Care Diagnostics
6. Future Challenges and Developments
6.1. Chemical Resistance and Gas Permeability
6.2. Advances in Materials Science
6.3. Integration of Multifunctional Systems
6.4. Multilayer and Modular Microfluidic Devices
6.5. Ensuring Biocompatibility and Preventing Biofouling
6.6. Scaling up Production
6.7. Overcoming Regulatory and Standardization Challenges
6.8. Ethical and Social Considerations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bragheri, F.; Vázquez, R.M.; Osellame, R. Microfluidics; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128178270. [Google Scholar]
- Novotný, J.; Foret, F. Fluid Manipulation on the Micro-Scale: Basics of Fluid Behavior in Microfluidics. J. Sep. Sci. 2017, 40, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Obeid, M.A.; Mishra, V.; El-Tanani, M.; Tambuwala, M.M. Customizable Microfluidic Devices: Progress, Constraints, and Future Advances. Curr. Drug Deliv. 2024, 21, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Ye, W.; Safdarin, F.; Baghaei, S. Microfluidics Devices for Sports: A Review on Technology for Biomedical Application Used in Fields Such as Biomedicine, Drug Encapsulation, Preparation of Nanoparticles, Cell Targeting, Analysis, Diagnosis, and Cell Culture. Tissue Cell 2024, 87, 102339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, X.; Qin, L. Recent Progress of Microfluidics in Translational Applications. Adv. Healthc. Mater. 2016, 5, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Malloggi, F. Microfluidics: From Basic Principles to Applications; Springer: Cham, Switzerland, 2016; Volume 917. [Google Scholar]
- Giri, B. Laboratory Methods in Microfluidics; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128132364. [Google Scholar]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Song, P.; Hu, R.; Tng, D.J.H.; Yong, K.-T. Moving towards Individualized Medicine with Microfluidics Technology. RSC Adv. 2014, 4, 11499–11511. [Google Scholar] [CrossRef]
- Burklund, A.; Tadimety, A.; Nie, Y.; Hao, N.; Zhang, J.X.J. Advances in Diagnostic Microfluidics. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 95, ISBN 9780128211656. [Google Scholar]
- Gharib, G.; Bütün, İ.; Muganlı, Z.; Kozalak, G.; Namlı, İ.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef]
- Yang, C.; Yu, Y.; Shang, L.; Zhao, Y. Flexible Hemline-Shaped Microfibers for Liquid Transport. Nat. Chem. Eng. 2024, 1, 87–96. [Google Scholar] [CrossRef]
- Yang, C.; Li, W.; Zhao, Y.; Shang, L. Flexible Liquid-Diode Microtubes from Multimodal Microfluidics. Proc. Natl. Acad. Sci. USA 2024, 121, e2402331121. [Google Scholar] [CrossRef]
- Dong, R.; Liu, Y.; Mou, L.; Deng, J.; Jiang, X. Microfluidics-Based Biomaterials and Biodevices. Adv. Mater. 2019, 31, 1805033. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials Synthesis through Microfluidic Methods: An Updated Overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.-Q.; Zhang, W.; Xu, Z.-R. Shape-Memory Microfluidic Chips for Fluid and Droplet Manipulation. Biomicrofluidics 2024, 18, 021301. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and Emerging Strategies for the Fabrication and Functionalization of PDMS-Based Microfluidic Devices. Lab. Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Fantino, E.; Chiadò, A.; Quaglio, M.; Vaghi, V.; Cocuzza, M.; Marasso, S.L.; Potrich, C.; Lunelli, L.; Pederzolli, C.; Pirri, C.F.; et al. Photofabrication of Polymeric Biomicrofluidics: New Insights into Material Selection. Mater. Sci. Eng. C 2020, 106, 110166. [Google Scholar] [CrossRef]
- Alvarez-Braña, Y.; Etxebarria-Elezgarai, J.; Ruiz de Larrinaga-Vicente, L.; Benito-Lopez, F.; Basabe-Desmonts, L. Modular Micropumps Fabricated by 3D Printed Technologies for Polymeric Microfluidic Device Applications. Sens. Actuators B Chem. 2021, 342, 129991. [Google Scholar] [CrossRef]
- Shakeri, A.; Jarad, N.A.; Khan, S.; Didar, T.F. Bio-Functionalization of Microfluidic Platforms Made of Thermoplastic Materials: A Review. Anal. Chim. Acta 2022, 1209, 339283. [Google Scholar] [CrossRef]
- Damiati, L.A.; El-Yaagoubi, M.; Damiati, S.A.; Kodzius, R.; Sefat, F.; Damiati, S. Role of Polymers in Microfluidic Devices. Polymers 2022, 14, 5132. [Google Scholar] [CrossRef]
- Kieviet, B.D.; Schön, P.M.; Vancso, G.J. Stimulus-Responsive Polymers and Other Functional Polymer Surfaces as Components in Glass Microfluidic Channels. Lab. Chip 2014, 14, 4159–4170. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, Y.S.; Santiago, G.T.-D.; Alvarez, M.M.; Ribas, J.; Jonas, S.J.; Weiss, P.S.; Andrews, A.M.; Aizenberg, J.; Khademhosseini, A. Interplay between Materials and Microfluidics. Nat. Rev. Mater. 2017, 2, 17016. [Google Scholar] [CrossRef]
- Aralekallu, S.; Boddula, R.; Singh, V. Development of Glass-Based Microfluidic Devices: A Review on Its Fabrication and Biologic Applications. Mater. Des. 2023, 225, 111517. [Google Scholar] [CrossRef]
- Yoon, S.; Kilicarslan You, D.; Jeong, U.; Lee, M.; Kim, E.; Jeon, T.-J.; Kim, S.M. Microfluidics in High-Throughput Drug Screening: Organ-on-a-Chip and C. Elegans-Based Innovations. Biosensors 2024, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Gohil, N.; Bhattacharjee, G.; Khambhati, K.; Alzahrani, K.J.; Ramakrishna, S.; Chu, D.-T.; Singh, V. Advances in Microfluidics Devices and Its Applications in Personalized Medicines. In Micro/Nanofluidics and Lab-on-Chip Based Emerging Technologies for Biomedical and Translational Research Applications—Part A; Academic Press: Cambridge, MA, USA, 2022; Volume 186, ISBN 9780323988995. [Google Scholar]
- Rukhiya, S.; Joseph, X.; Megha, K.B.; Mohanan, P.V. Lab-on-a-Chip for Functional Testing for Precision Medicine. In Microfluidics and Multi Organs on Chip; Springer Nature: Singapore, 2022; ISBN 9789811913792. [Google Scholar]
- Sun, Y.-S. Comparison of Chip Inlet Geometry in Microfluidic Devices for Cell Studies. Molecules 2016, 21, 778. [Google Scholar] [CrossRef]
- Aziz, A.U.R.; Geng, C.; Fu, M.; Yu, X.; Qin, K.; Liu, B. The Role of Microfluidics for Organ on Chip Simulations. Bioengineering 2017, 4, 39. [Google Scholar] [CrossRef]
- Gurkan, U.A.; Wood, D.K.; Carranza, D.; Herbertson, L.H.; Diamond, S.L.; Du, E.; Guha, S.; Di Paola, J.; Hines, P.C.; Papautsky, I.; et al. Next Generation Microfluidics: Fulfilling the Promise of Lab-on-a-Chip Technologies. Lab. Chip 2024, 24, 1867–1874. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Wang, H.; Wang, L. Application of Microfluidic Chips in the Detection of Airborne Microorganisms. Micromachines 2022, 13, 1576. [Google Scholar] [CrossRef]
- Rai, P.K.; Islam, M.; Gupta, A. Microfluidic Devices for the Detection of Contamination in Water Samples: A Review. Sens. Actuators A Phys. 2022, 347, 113926. [Google Scholar] [CrossRef]
- Aryal, P.; Hefner, C.; Martinez, B.; Henry, C.S. Microfluidics in Environmental Analysis: Advancements, Challenges, and Future Prospects for Rapid and Efficient Monitoring. Lab. Chip 2024, 24, 1175–1206. [Google Scholar] [CrossRef]
- Pouyanfar, N.; Harofte, S.Z.; Soltani, M.; Siavashy, S.; Asadian, E.; Ghorbani-Bidkorbeh, F.; Keçili, R.; Hussain, C.M. Artificial Intelligence-Based Microfluidic Platforms for the Sensitive Detection of Environmental Pollutants: Recent Advances and Prospects. Trends Environ. Anal. Chem. 2022, 34, e00160. [Google Scholar] [CrossRef]
- Ramya, K.; Amreen, K.; Pronin, I.; Karmanov, A.; Yakushova, N.; Goel, S. Emerging Trends in Microfluidic-Assisted Nanomaterial Synthesis for Their High-Resolution Gas Sensing Applications. Nano Futures 2023, 7, 032004. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Jiao, S.; Li, Y.; Zhou, Y.; Zhang, X.; Maryam, B.; Liu, X. Microfluidic Sensors for the Detection of Emerging Contaminants in Water: A Review. Sci. Total Environ. 2024, 929, 172734. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Pang, L.; Zhang, Y.; Yuan, M.; Wang, J.; Wang, D.; Liu, W.; Wang, J. Microfluidic Device: A Miniaturized Platform for Chemical Reactions. Chin. J. Chem. 2013, 31, 304–316. [Google Scholar] [CrossRef]
- Ling, F.W.M.; Abdulbari, H.A.; Chin, S.Y. Heterogeneous Microfluidic Reactors: A Review and an Insight of Enzymatic Reactions. ChemBioEng Rev. 2022, 9, 265–285. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Wang, P.; de Mello, A.; Feng, L.; Zhu, X.; Wen, W.; Kodzius, R.; Gong, X. Synthesis of Biomaterials Utilizing Microfluidic Technology. Genes. 2018, 9, 283. [Google Scholar] [CrossRef]
- Mea, H.; Wan, J. Microfluidics-Enabled Functional 3D Printing. Biomicrofluidics 2022, 16, 021501. [Google Scholar] [CrossRef]
- Parvatam, S.; Chavali, P.L. Organs-on-a-Chip in Preclinical Studies. In Microfluidics and Multi Organs on Chip; Springer Nature: Singapore, 2022; ISBN 9789811913792. [Google Scholar]
- Roberts, A.; Mahari, S.; Gandhi, S. Cells and Organs on a Chip in Biomedical Sciences. In Microfluidics and Multi Organs on Chip; Springer Nature: Singapore, 2022; ISBN 9789811913792. [Google Scholar]
- Salehi Moghaddam, A.; Salehi Moghaddam, Z.; Davachi, S.M.; Sarikhani, E.; Nemati Mahand, S.; Khonakdar, H.A.; Bagher, Z.; Ashammakhi, N. Recent Advances and Future Prospects of Functional Organ-on-a-Chip Systems. Mater. Chem. Front. 2022, 6, 3633–3661. [Google Scholar] [CrossRef]
- Caballero, D.; Reis, R.L.; Kundu, S.C. The Role of Organ-on-a-Chip Technology in Advancing Precision Medicine; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1–2, ISBN 9780128240106. [Google Scholar]
- Weisgrab, G.; Ovsianikov, A.; Costa, P.F. Functional 3D Printing for Microfluidic Chips. Adv. Mater. Technol. 2019, 4, 1900275. [Google Scholar] [CrossRef]
- Gong, L.; Lin, Y. Microfluidics in Smart Food Safety. Adv. Food Nutr. Res. 2024, 111, 305–354. [Google Scholar]
- Zhu, Z.; Chen, T.; Huang, F.; Wang, S.; Zhu, P.; Xu, R.X.; Si, T. Free-Boundary Microfluidic Platform for Advanced Materials Manufacturing and Applications. Adv. Mater. 2024, 36, 2304840. [Google Scholar] [CrossRef]
- Li, X.; Wu, N.; Rojanasakul, Y.; Liu, Y. Selective Stamp Bonding of PDMS Microfluidic Devices to Polymer Substrates for Biological Applications. Sens. Actuators A Phys. 2013, 193, 186–192. [Google Scholar] [CrossRef]
- Voicu, D.; Lestari, G.; Wang, Y.; DeBono, M.; Seo, M.; Cho, S.; Kumacheva, E. Thermoplastic Microfluidic Devices for Targeted Chemical and Biological Applications. RSC Adv. 2017, 7, 2884–2889. [Google Scholar] [CrossRef]
- Todd, D.; Krasnogor, N. Homebrew Photolithography for the Rapid and Low-Cost, “Do It Yourself” Prototyping of Microfluidic Devices. ACS Omega 2023, 8, 35393–35409. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Production of Hydrogel Microparticles in Microfluidic Devices: A Review. Microfluid. Nanofluidics 2021, 25, 10. [Google Scholar] [CrossRef]
- Hosseini, H.; Guo, F.; Ghahfarokhi, R.B.; Aryana, S.A. Microfluidic Fabrication Techniques for High-Pressure Testing of Microscale Supercritical CO2 Foam Transport in Fractured Unconventional Reservoirs. J. Vis. Exp. 2020, 2020, e61369. [Google Scholar] [CrossRef]
- Beck, A.; Obst, F.; Busek, M.; Grünzner, S.; Mehner, P.J.; Paschew, G.; Appelhans, D.; Voit, B.; Richter, A. Hydrogel Patterns in Microfluidic Devices by Do-It-Yourself UV-Photolithography Suitable for Very Large-Scale Integration. Micromachines 2020, 11, 479. [Google Scholar] [CrossRef]
- Emeigh, C.; Zhang, H.; Ryu, S. Fabrication of a Microfluidic Cell Compressor Using a 3D-Printed Mold. In Proceedings of the American Society of Mechanical Engineers, Fluids Engineering Division (Publication) FEDSM, Toronto, ON, Canada, 3–5 August 2022; Volume 2. [Google Scholar]
- Coluccio, M.L.; Gentile, F.; Barbani, N.; Cristallini, C. Surface Properties and Treatments. In Microfluidics for Cellular Applications; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 9780128224823. [Google Scholar]
- Akbari, Z.; Raoufi, M.A.; Mirjalali, S.; Aghajanloo, B. A Review on Inertial Microfluidic Fabrication Methods. Biomicrofluidics 2023, 17, 051504. [Google Scholar] [CrossRef]
- Lee, W.-K.; Whitener, K.E. Transferring Photolithography Patterns to Arbitrary Substrates with Graphene or Gelatin. MRS Commun. 2023, 13, 1423–1426. [Google Scholar] [CrossRef]
- Niu, X.-Z.; Pepel, R.D.; Paniego, R.; Abrell, L.; Field, J.A.; Chorover, J.; Sierra-Alvarez, R. Fate of Bis-(4-Tert-Butyl Phenyl)-Iodonium under Photolithography Relevant Irradiation and the Environmental Risk Properties of the Formed Photoproducts. Environ. Sci. Pollut. Res. 2022, 29, 25988–25994. [Google Scholar] [CrossRef]
- Fujimori, T. Recent Status of the Stochastic Issues of Photoresist Materials in EUV Lithography. J. Photopolym. Sci. Technol. 2022, 35, 35–40. [Google Scholar] [CrossRef]
- Dinh, D.-H.; Chien, H.-L.; Lee, Y.-C. Maskless Lithography Based on Digital Micromirror Device (DMD) and Double Sided Microlens and Spatial Filter Array. Opt. Laser Technol. 2019, 113, 407–415. [Google Scholar] [CrossRef]
- Lee, S.H.; Seo, S.E.; Kim, K.H.; Lee, J.; Park, C.S.; Jun, B.-H.; Park, S.J.; Kwon, O.S. Single Photomask Lithography for Shape Modulation of Micropatterns. J. Ind. Eng. Chem. 2020, 84, 196–201. [Google Scholar] [CrossRef]
- Cho, Y.; Ouyang, C.Y.; Krysak, M.; Sun, W.; Gamez, V.; Sierra-Alvarez, R.; Ober, C.K. Environmentally Friendly Natural Materials Based Photoacid Generators for next Generation Photolithography. In Proceedings of the SPIE—The International Society for Optical Engineering, San Jose, CA, USA, 24–25 January 2011; Volume 7972. [Google Scholar]
- Nguyen, T.; Sarkar, T.; Tran, T.; Moinuddin, S.M.; Saha, D.; Ahsan, F. Multilayer Soft Photolithography Fabrication of Microfluidic Devices Using a Custom-Built Wafer-Scale PDMS Slab Aligner and Cost-Efficient Equipment. Micromachines 2022, 13, 1357. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shi, Z.Z. Microfluidic Paper-Based Analytical Devices Fabricated by Low-Cost Photolithography and Embossing of Parafilm®. Lab. Chip 2015, 15, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Rühe, J. And There Was Light: Prospects for the Creation of Micro- and Nanostructures through Maskless Photolithography. ACS Nano 2017, 11, 8537–8541. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, B.; Chen, L.; Fan, F.; Ji, Z.; Duan, H. Wafer-Recyclable, Eco-Friendly, and Multiscale Dry Transfer Printing by Transferable Photoresist for Flexible Epidermal Electronics. ACS Appl. Mater. Interfaces 2024, 16, 13525–13533. [Google Scholar] [CrossRef]
- Hachikubo, Y.; Miura, S.; Yamagishi, R.; Ando, M.; Kobayashi, M.; Ota, T.; Amano, T.; Takei, S. Amylopectin-Based Eco-Friendly Photoresist Material in Water-Developable Lithography Processes for Surface Micropatterns on Polymer Substrates. J. Photopolym. Sci. Technol. 2023, 36, 197–204. [Google Scholar] [CrossRef]
- Greant, C.; Van Durme, B.; Van Hoorick, J.; Van Vlierberghe, S. Multiphoton Lithography as a Promising Tool for Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2212641. [Google Scholar] [CrossRef]
- Guan, L.; Cao, C.; Liu, X.; Liu, Q.; Qiu, Y.; Wang, X.; Yang, Z.; Lai, H.; Sun, Q.; Ding, C.; et al. Light and Matter Co-Confined Multi-Photon Lithography. Nat. Commun. 2024, 15, 2387. [Google Scholar] [CrossRef]
- Alvankarian, J.; Majlis, B.Y. A New UV-Curing Elastomeric Substrate for Rapid Prototyping of Microfluidic Devices. J. Micromechanics Microeng. 2012, 22, 035006. [Google Scholar] [CrossRef]
- Mokkapati, V.R.S.S.; Bethge, O.; Hainberger, R.; Brueckl, H. Microfluidic Chips Fabrication from UV Curable Adhesives for Heterogeneous Integration. In Proceedings of the Electronic Components and Technology Conference, San Diego, CA, USA, 29 May—1 June 2012; pp. 1965–1969. [Google Scholar]
- Alvankarian, J.; Majlis, B.Y. Low Cost Prototyping of Microfluidic Structure. In Proceedings of the IEEE International Conference on Semiconductor Electronics, ICSE, Malacca, Malaysia, 28–30 June 2010; pp. 317–320. [Google Scholar]
- Gómez-Varela, A.I.; Viña, A.; Bao-Varela, C.; Flores-Arias, M.T.; Carnero, B.; González-Peteiro, M.; González-Juanatey, J.R.; Álvarez, E. Biocompatibility Testing of UV-Curable Polydimethylsiloxane for Human Umbilical Vein Endothelial Cell Culture on-a-Chip. ACS Omega 2024, 9, 30281–30293. [Google Scholar] [CrossRef]
- Rapp, B.E. Low Refractive PTFE Based Polymers—A Suitable Material for Combined Microfluidics and Optics. In Proceedings of the 2011 IEEE Winter Topicals, WTM 2011, Keystone, CO, USA, 10–12 January 2011; pp. 77–78. [Google Scholar]
- Gómez, M.; Lazzari, M. PFPE-Based Materials for the Fabrication of Micro- and Nano-Optical Components. Microelectron. Eng. 2012, 97, 208–211. [Google Scholar] [CrossRef]
- Park, S.; Mondal, K.; Treadway, R.M.; Kumar, V.; Ma, S.; Holbery, J.D.; Dickey, M.D. Silicones for Stretchable and Durable Soft Devices: Beyond Sylgard-184. ACS Appl. Mater. Interfaces 2018, 10, 11261–11268. [Google Scholar] [CrossRef]
- Jiménez-Díaz, E.; Cano-Jorge, M.; Zamarrón-Hernández, D.; Cabriales, L.; Páez-Larios, F.; Cruz-Ramírez, A.; Vázquez-Victorio, G.; Fiordelisio, T.; Hautefeuille, M. Micro-Macro: Selective Integration of Microfeatures inside Low-Cost Macromolds for PDMS Microfluidics Fabrication. Micromachines 2019, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Trantidou, T.; Friddin, M.S.; Gan, K.B.; Han, L.; Bolognesi, G.; Brooks, N.J.; Ces, O. Mask-Free Laser Lithography for Rapid and Low-Cost Microfluidic Device Fabrication. Anal. Chem. 2018, 90, 13915–13921. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gao, C.; Gritsenko, D.; Zhou, R.; Xu, J. Soft Lithography Based on Photolithography and Two-Photon Polymerization. Microfluid. Nanofluidics 2018, 22, 97. [Google Scholar] [CrossRef]
- Azarsa, E.; Jeyhani, M.; Ibrahim, A.; Tsai, S.S.H.; Papini, M. A Novel Abrasive Water Jet Machining Technique for Rapid Fabrication of Three-Dimensional Microfluidic Components. Biomicrofluidics 2020, 14, 044103. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.; Rosenthal, K. Validation of Easy Fabrication Methods for PDMS-Based Microfluidic (Bio)Reactors. Sci. 2022, 4, 36. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical Microfluidic Devices by Using Low-Cost Fabrication Techniques: A Review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef]
- Lin, L.; Chung, C.-K. PDMS Microfabrication and Design for Microfluidics and Sustainable Energy Application: Review. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef]
- Wu, J.; Issadore, D.A.; Lee, D. Patterning Wettability on Solvent-Resistant Elastomers with High Spatial Resolution for Replica Mold Fabrication of Droplet Microfluidics. ACS Appl. Mater. Interfaces 2022, 15, 10212–10218. [Google Scholar] [CrossRef]
- Crisóstomo-Rodríguez, T.J.; Alonso-Santacruz, V.D.; Villa-Vargas, L.A.; Ramírez-Salinas, M.A.; Alemán-Arce, M.Á.; Solís-Tinoco, V.I. Low-Cost Microfabrication Methodology for Microfluidic Chips Using 3D Printer and Replica Molding Techniques for Biosensors. Microfluid. Nanofluidics 2024, 28, 53. [Google Scholar] [CrossRef]
- Cho, I.H.; Ji, M.G.; Kim, J. Analytical Investigation of Replica-Molding-Enabled Nanopatterned Tribocharging Process on Soft-Material Surfaces. Micromachines 2024, 15, 417. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ng, H.W.; Gates, B.D.; Menon, C. Material Versatility Using Replica Molding for Large-Scale Fabrication of High Aspect-Ratio, High Density Arrays of Nano-Pillars. Nanotechnology 2014, 25, 285303. [Google Scholar] [CrossRef]
- Sticker, D.; Rothbauer, M.; Lechner, S.; Hehenberger, M.-T.; Ertl, P. Multi-Layered, Membrane-Integrated Microfluidics Based on Replica Molding of a Thiol-Ene Epoxy Thermoset for Organ-on-a-Chip Applications. Lab. Chip 2015, 15, 4542–4554. [Google Scholar] [CrossRef]
- Wang, C.-K.; Liao, W.-H.; Wu, H.-M.; Tung, Y.-C. One-Step Approach to Fabricating Polydimethylsiloxane Microfluidic Channels of Different Geometric Sections by Sequential Wet Etching Processes. J. Vis. Exp. 2018, 2018, 57868. [Google Scholar] [CrossRef]
- Mustin, B.; Stoeber, B. Low Cost Integration of 3D-Electrode Structures into Microfluidic Devices by Replica Molding. Lab. Chip 2012, 12, 4702–4708. [Google Scholar] [CrossRef]
- Amadeo, F.; Mukherjee, P.; Gao, H.; Zhou, J.; Papautsky, I. Polycarbonate Masters for Soft Lithography. Micromachines 2021, 12, 1392. [Google Scholar] [CrossRef]
- Guan, Y.; Xu, F.; Wang, X.; Hui, Y.; Sha, J.; Tian, Y.; Wang, Z.; Zhang, S.; Chen, D.; Yang, L. Implementation of Hybrid PDMS-Graphite/Ag Conductive Material for Flexible Electronic Devices and Microfluidic Applications. Microelectron. Eng. 2021, 235, 111455. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with Designer Functionalities—Properties, Modifications Strategies, and Applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Deshpande, A.; Karkhanis, M.U.; Banerjee, A.; Ghosh, C.; Pourshaban, E.; Kim, H.; Mastrangelo, C.H. Integration of PDMS microfluidic channels with electronic systems using SIO2 mediated bonding of PDMS and polyimide. In Proceedings of the MicroTAS 2021—25th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Palm Spring, CA, USA, 10–14 October 2021; pp. 1231–1232. [Google Scholar]
- Pu, Z.; Ma, J.; Li, W.; Lai, X.; Su, X.; Yu, H.; Li, D. A Flexible Precise Volume Sensor Based on Metal-on-Polyimide Electrodes Sandwiched by PDMS Channel for Microfluidic Systems. Microfluid. Nanofluidics 2019, 23, 132. [Google Scholar] [CrossRef]
- Wang, S.; Yu, S.; Lu, M.; Zuo, L. Microfabrication of Plastic-PDMS Microfluidic Devices Using Polyimide Release Layer and Selective Adhesive Bonding. J. Micromechanics Microeng. 2017, 27, 055015. [Google Scholar] [CrossRef]
- Chai, H.; Chen, F.; Song, Z.; Xiong, L.; Xiao, G.; Lu, Z.; Yu, L. A Versatile Wax Assisted Double Replica Molding and Its Application in Flexible Electronic Skin. Sens. Actuators B Chem. 2021, 343, 130132. [Google Scholar] [CrossRef]
- Zhang, J.M.; Ji, Q.; Liu, Y.; Huang, J.; Duan, H. An Integrated Micro-Millifluidic Processing System. Lab. Chip 2018, 18, 3393–3404. [Google Scholar] [CrossRef]
- Kipper, S.; Frolov, L.; Guy, O.; Pellach, M.; Glick, Y.; Malichi, A.; Knisbacher, B.A.; Barbiro-Michaely, E.; Avrahami, D.; Yavets-Chen, Y.; et al. Control and Automation of Multilayered Integrated Microfluidic Device Fabrication. Lab. Chip 2017, 17, 557–566. [Google Scholar] [CrossRef]
- Temiz, Y.; Lovchik, R.D.; Delamarche, E. Capillary-Driven Microfluidic Chips for Miniaturized Immunoassays: Patterning Capture Antibodies Using Microcontact Printing and Dry-Film Resists. In Microchip Diagnostics. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1547. [Google Scholar]
- Hamon, C.; Henriksen-Lacey, M.; La Porta, A.; Rosique, M.; Langer, J.; Scarabelli, L.; Montes, A.B.S.; González-Rubio, G.; de Pancorbo, M.M.; Liz-Marzán, L.M.; et al. Tunable Nanoparticle and Cell Assembly Using Combined Self-Powered Microfluidics and Microcontact Printing. Adv. Funct. Mater. 2016, 26, 8053–8061. [Google Scholar] [CrossRef]
- Foncy, J.; Estève, A.; Degache, A.; Colin, C.; Dollat, X.; Cau, J.-C.; Vieu, C.; Trévisiol, E.; Malaquin, L. Dynamic Inking of Large-Scale Stamps for Multiplexed Microcontact Printing and Fabrication of Cell Microarrays. PLoS ONE 2018, 13, e0202531. [Google Scholar] [CrossRef]
- Hsiao, T.W.; Swarup, V.P.; Eichinger, C.D.; Hlady, V. Cell Substrate Patterning with Glycosaminoglycans to Study Their Biological Roles in the Central Nervous System. In Glycosaminoglycans. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1229. [Google Scholar]
- Leva, V.; Chatzipetrou, M.; Alexopoulos, L.; Tzeranis, D.S.; Zergioti, I. Direct Laser Printing of Liver Cells on Porous Collagen Scaffolds. J. Laser Micro Nanoeng. 2018, 13, 234–237. [Google Scholar] [CrossRef]
- García, J.R.; Singh, A.; García, A.J. High Fidelity Nanopatterning of Proteins onto Well-Defined Surfaces through Subtractive Contact Printing. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 119. [Google Scholar]
- Foncy, J.; Colin, C.; Degache, A.; Estève, A.; Cau, J.-C.; Berteloite, B.; Trévisiol, E.; Vieu, C.; Malaquin, L. Microfluidic Inking Processes for Large-Scale and Multiplexed Micro-Contact Printing. In Proceedings of the 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2016, Dublin, Ireland, 9–13 October 2016; pp. 1037–1038. [Google Scholar]
- Shakeri, A.; Imani, S.M.; Yousefi, H.; Shabbir, R.; Didar, T. A Rapid and Simple Technique for Covalent Micro Patterning of Biomolecules inside Microfluidic Channels. In Proceedings of the 22nd International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2018, Kaohsiung, Taiwan, 11–15 November 2018; Volume 4, pp. 2250–2253. [Google Scholar]
- Yamagishi, K.; Ching, T.; Chian, N.; Tan, M.; Zhou, W.; Huang, S.Y.; Hashimoto, M. Flexible and Stretchable Liquid-Metal Microfluidic Electronics Using Directly Printed 3D Microchannel Networks. Adv. Funct. Mater. 2024, 34, 2311219. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Y.; Cai, L.; Wang, Y.; Shi, K.; Shang, L.; Pan, J.; Zhao, Y. Microfluidics for Flexible Electronics. Mater. Today 2021, 44, 105–135. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, H.; Cheng, J.; Zhao, N.; Chen, S.-C. Flexure-Based Roll-to-Roll Platform: A Practical Solution for Realizing Large-Area Microcontact Printing. Sci. Rep. 2015, 5, 10402. [Google Scholar] [CrossRef]
- Wang, C.; Linghu, C.; Nie, S.; Li, C.; Lei, Q.; Tao, X.; Zeng, Y.; Du, Y.; Zhang, S.; Yu, K.; et al. Programmable and Scalable Transfer Printing with High Reliability and Efficiency for Flexible Inorganic Electronics. Sci. Adv. 2020, 6, eabb2393. [Google Scholar] [CrossRef]
- Nagel, R.D.; Haeberle, T.; Schmidt, M.; Lugli, P.; Scarpa, G. Large Area Nano-Transfer Printing of Sub-50-Nm Metal Nanostructures Using Low-Cost Semi-Flexible Hybrid Templates. Nanoscale Res. Lett. 2016, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Rêche, J.; Argoud, M.; De Lehelle D’Affroux, A.; Benotmane, K.; Haumann, S.; Khan, J.; Eibelhuber, M. Integration of Sub 50 Nm Features Based on EVG SmartNIL for 8-Inch Substrates. In Proceedings of the SPIE—The International Society for Optical Engineering, Online, 22–27 February 2021; Volume 11610. [Google Scholar]
- Li, F.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. Increasing the Functionalities of 3D Printed Microchemical Devices by Single Material, Multimaterial, and Print-Pause-Print 3D Printing. Lab. Chip 2019, 19, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Tong, C. Current Trends and Prospects in Advanced Manufacturing for Printed Electronics. In Advanced Materials for Printed Flexible Electronics; Springer: Cham, Switzerland, 2022; Volume 317. [Google Scholar]
- Hizir, F.E.; Hale, M.R.; Hardt, D.E. Manufacturing Conductive Patterns on Polymeric Substrates: Development of a Microcontact Printing Process. J. Micromechanics Microeng. 2020, 30, 115008. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Tan, Z.; Tang, J.; Yao, C.; Hao, B. Research Progress of Microtransfer Printing Technology for Flexible Electronic Integrated Manufacturing. Micromachines 2021, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Delamarche, E.; Temiz, Y.; Lovchik, R.D.; Christiansen, M.G.; Schuerle, S. Capillary Microfluidics for Monitoring Medication Adherence. Angew. Chem.-Int. Ed. 2021, 60, 17784–17796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xie, Y.; Liang, K.; Zhang, Y.; Fan, Y. Progress of Capillary Microfluidic Chip Devices. Gaofenzi Cailiao Kexue Yu Gongcheng/Polym. Mater. Sci. Eng. 2023, 39, 182–190. [Google Scholar] [CrossRef]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse Double Emulsions Generated from a Microcapillary Device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef]
- Kanda, Y.; Takehara, H.; Ichiki, T. High Aspect Ratio Microneedles of Bioabsorbable Polymer Fabricated by Micromolding. In Proceedings of the 22nd International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2018, Kaohsiung, Taiwan, 11–15 November 2018; Volume 1, pp. 533–534. [Google Scholar]
- Li, K.; Hernandez-Castro, J.A.; Veres, T. Fabrication of Large-Area Polymeric Membranes with Micro and Nano Aperatures. In Advanced Materials: TechConnect Briefs 2017; TechConnect: Washington, DC, USA, 2017; Volume 1, pp. 383–386. [Google Scholar]
- Watanabe, S.; Asanuma, T.; Sasahara, T.; Hyodo, H.; Matsumoto, M.; Soga, K. 3D Micromolding of Arrayed Waveguide Gratings on Upconversion Luminescent Layers for Flexible Transparent Displays without Mirrors, Electrodes, and Electric Circuits. Adv. Funct. Mater. 2015, 25, 4390–4396. [Google Scholar] [CrossRef]
- Ye, F.; Jiang, J.; Chang, H.; Xie, L.; Deng, J.; Ma, Z.; Yuan, W. Improved Single-Cell Culture Achieved Using Micromolding in Capillaries Technology Coupled with Poly (HEMA). Biomicrofluidics 2015, 9, 044106. [Google Scholar] [CrossRef]
- Zhang, D.; Xing, W.; Li, W.; Liu, S.; Dong, Y.; Zhang, L.; Zhao, F.; Wang, J.; Xu, Z. Fabrication of Multiple Parallel Microchannels in a Single Microgroove via the Heating Assisted MIMIC Technique. Micromachines 2022, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Lau, W.M.; Abdelghany, T.M.; Vukajlovic, D.; Novakovic, K.; Ng, K.W. Vac-and-Fill: A Micromoulding Technique for Fabricating Microneedle Arrays with Vacuum-Activated, Hands-Free Mould-Filling. Int. J. Pharm. 2024, 650, 123706. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, H.; Wang, C.; Li, X.; Shao, J.; Ding, Y.; Wang, L. Electrowetting Assisted Air Detrapping in Transfer Micromolding for Difficult-to-Mold Microstructures. ACS Appl. Mater. Interfaces 2014, 6, 12737–12743. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yu, J.; Hu, J.; Tang, K.; Xu, B.; Wang, F. Effects of Uniform/Nonuniform Interface Friction on Mold-Filling Behavior of Glass Microarray: A Numerical-Experimental Study. Tribol. Lett. 2022, 70, 20. [Google Scholar] [CrossRef]
- Du, Y.; Xu, J.; Sakizadeh, J.D.; Weiblen, D.G.; McCormick, A.V.; Francis, L.F. Modulus- and Surface-Energy-Tunable Thiol-Ene for UV Micromolding of Coatings. ACS Appl. Mater. Interfaces 2017, 9, 24976–24986. [Google Scholar] [CrossRef]
- Müller, E.; Pompe, T.; Freudenberg, U.; Werner, C. Solvent-Assisted Micromolding of Biohybrid Hydrogels to Maintain Human Hematopoietic Stem and Progenitor Cells Ex Vivo. Adv. Mater. 2017, 29, 1703489. [Google Scholar] [CrossRef]
- Amer, M.; Chen, R.K. Self-Adhesive Microneedles with Interlocking Features for Sustained Ocular Drug Delivery. Macromol. Biosci. 2020, 20, 2000089. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, B.; Luan, X.; Jiang, L.; Lu, C.; Wu, C.; Pan, X.; Peng, T. State of the Art in Constructing Gas-Propelled Dissolving Microneedles for Significantly Enhanced Drug-Loading and Delivery Efficiency. Pharmaceutics 2023, 15, 1059. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, H.; Ding, Y. Inherent Constraint for Three-Dimensional Patterning by Microtransfer Molding. Mater. Manuf. Process. 2014, 29, 59–63. [Google Scholar] [CrossRef]
- Matsuda, A.; Kawamura, G. Sol-Gel Nano-/Micropatterning Process. In Handbook of Sol-Gel Science and Technology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 9783319321011. [Google Scholar]
- Paul, M.T.Y.; Kim, D.; Saha, M.S.; Stumper, J.; Gates, B.D. Patterning Catalyst Layers with Microscale Features by Soft Lithography Techniques for Proton Exchange Membrane Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 478–486. [Google Scholar] [CrossRef]
- Li, M.; Su, B.; Zhou, B.; Wang, H.; Meng, J. Soft Lithographic Fabrication of Free-Standing Ceramic Microcomponents Using Poly(N-Isopropylacrylamide) Brushes Grafted Poly(Dimethylsiloxane) Micromolds. J. Micromechanics Microeng. 2020, 30, 085009. [Google Scholar] [CrossRef]
- Cordero-Guerrero, J.; Jiménez-Thuel, G.; Paniagua, S.A. Sub-Micron Patterning of Metal Oxide Surfaces via Microcontact Printing and Microtransfer Molding of Amphiphilic Molecules and Antifouling Application. J. Mater. Res. 2023, 38, 1573–1582. [Google Scholar] [CrossRef]
- Peer, A.; Dhakal, R.; Biswas, R.; Kim, J. Nanoscale Patterning of Biopolymers for Functional Biosurfaces and Controlled Drug Release. Nanoscale 2016, 8, 18654–18664. [Google Scholar] [CrossRef]
- Biggemann, J.; Müller, P.; Köllner, D.; Simon, S.; Hoffmann, P.; Heik, P.; Lee, J.H.; Fey, T. Hierarchical Surface Texturing of Hydroxyapatite Ceramics: Influence on the Adhesive Bonding Strength of Polymeric Polycaprolactone. J. Funct. Biomater. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Su, B.; Zhou, B.; Wu, Y.; Meng, J. Soft Lithographic Fabrication of Free-Standing Ceramic Microparts Using Moisture-Sensitive PDMS Molds. J. Micromechanics Microeng. 2019, 29, 035002. [Google Scholar] [CrossRef]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-Care Microfluidic Devices for Pathogen Detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef]
- Guo, J.; Liu, K.; Wang, Z.; Tnay, G.L. Magnetic Field-Assisted Finishing of a Mold Insert with Curved Microstructures for Injection Molding of Microfluidic Chips. Tribol. Int. 2017, 114, 306–314. [Google Scholar] [CrossRef]
- Asif, M.; Tait, R.N.; Berini, P. Hot Embossing of Microfluidics in Cyclic-Olefin Co-Polymer Using a Wafer Aligner-Bonder. Microsyst. Technol. 2021, 27, 3899–3906. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, B.; Li, X.; Zhou, M. Investigation of Solvent-Assisted In-Mold Bonding of Cyclic Olefin Copolymer (COC) Microfluidic Chips. Micromachines 2022, 13, 965. [Google Scholar] [CrossRef]
- Novak, R.; Ng, C.F.; Ingber, D.E. Rapid Prototyping of Thermoplastic Microfluidic Devices. In Cell-Based Microarrays. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1771. [Google Scholar]
- Lu, Y.; Liu, B.; Zhang, Z.; Guo, M.; Wang, J.; Wang, C. Process Chain for the Mass Production of Polymeric Microfluidic Chips. Int. J. Adv. Manuf. Technol. 2023, 127, 3665–3680. [Google Scholar] [CrossRef]
- Rein, C.; Toner, M.; Sevenler, D. Rapid Prototyping for High-Pressure Microfluidics. Sci. Rep. 2023, 13, 1232. [Google Scholar] [CrossRef]
- Dutra, C.M.B.; Amico, S.C.; Souza, J.A. Evaluation of Flow-Mesh Influence in Resin Injection Processes. Appl. Compos. Mater. 2021, 28, 369–380. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, N.; Gilchrist, M.D. The Use of Variotherm Systems for Microinjection Molding. J. Appl. Polym. Sci. 2016, 133, 42962. [Google Scholar] [CrossRef]
- Hubert, P.; Demaria, C.; Keulen, C.; Mobuchon, C.; Poursartip, A. Development of a Workflow for the Design of Liquid Composite Moulding Processes. In Proceedings of the American Society for Composites—29th Technical Conference, ASC 2014, 16th US-Japan Conference on Composite Materials, ASTM-D30 Meeting, San Diego, CA, USA, 8–10 September 2014. [Google Scholar]
- Bell, B.; Barnes, N.; Ede, A.; Read, T.C. Casting Non-Repetitive Geometries with Digitally Reconfigurable Surfaces. In Proceedings of the ACADIA 2014—Design Agency: 34th Annual Conference of the Association for Computer Aided Design in Architecture, Los Angeles, CA, USA, 23–25 October 2014; Volume 2014, pp. 453–462. [Google Scholar]
- Wu, S.-Y.; Hulme, J.P. Post Modification of Injection Molded Polystyrene Components Using Green Solvents and Flexible Masks. Sens. Actuators B Chem. 2015, 211, 187–197. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Xu, J.; Ma, B. One-Step Liquid Molding Based Modular Microfluidic Circuits. Analyst 2020, 145, 6813–6820. [Google Scholar] [CrossRef]
- Glick, C.C.; Srimongkol, M.T.; Schwartz, A.; Zhuang, W.; Lin, J.; Warren, R.; Tekell, D.; Satimalee, P.; Kim, J.; Su, C.; et al. Fabrication of Double-Sided Microfluidic Structures via 3D Printed Transfer Molding. In Proceedings of the 2016 Solid-State Sensors, Actuators and Microsystems Workshop, Hilton Head Island, SC, USA, 5–9 June 2016; pp. 153–156. [Google Scholar]
- Shiri, F.; Choi, J.; Vietz, C.; Rathnayaka, C.; Manoharan, A.; Shivanka, S.; Li, G.; Yu, C.; Murphy, M.C.; Soper, S.A.; et al. Nano-Injection Molding with Resin Mold Inserts for Prototyping of Nanofluidic Devices for Single Molecular Detection. Lab. Chip 2023, 23, 4876–4887. [Google Scholar] [CrossRef]
- Witzleben, M.; Moritz, T. Ceramic Injection Molding; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2021; Volume 1, ISBN 9780128185421. [Google Scholar]
- Schiffers, R.; Topic, N.; Moser, S.; Drummer, D. A Method for Controlling the Mold Filling Volume for BMC Injection Molding. In Proceedings of the Annual Technical Conference—ANTEC, Anaheim, CA, USA, 8–10 May 2017; Volume 2017, pp. 1537–1542. [Google Scholar]
- Kauffer, P.H. Injection Molding: Process, Design and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2021; ISBN 9781617614200. [Google Scholar]
- Maristany, E.; Cordero, Z.C.; Boyer, J.; Grant, L.O. Economics of 3D Printing Ceramic Cores for Gas Turbine Investment Castings. Addit. Manuf. Lett. 2024, 10, 100223. [Google Scholar] [CrossRef]
- Guevara-Morales, A.; Figueroa-López, U. Residual Stresses in Injection Molded Products. J. Mater. Sci. 2014, 49, 4399–4415. [Google Scholar] [CrossRef]
- Stricker, M.; Jasser, F.; Lake, S. Optimization of Heat Transfer in Injection Molds and Its Impact on Process Efficiency and Part Quality. AIP Conf. Proc. 2024, 3012, 020002. [Google Scholar]
- Sepe, M. Plastics Technology; CRC Press: Boca Raton, FL, USA, 2020; pp. 24–26. [Google Scholar]
- Lee, U.N.; Berthier, E.; Su, X.; Guckenberger, D.J.; Dostie, A.M.; Zhang, T.; Theberge, A.B. Fundamentals of Injection Molding for Microfluidic Cell-Based Assays. In Proceedings of the 21st International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2017, Savannah, GA, USA, 22–26 October 2017; pp. 397–398. [Google Scholar]
- Menezes, P.D.; Hunter, A.; Dickson, T.; Hecht, S.; Kumar, C.; Busek, M.; Krauss, S.; Gadegaard, N. Scalable, Transparent, and Micro: 3D-Printed Rapid Tooling for Injection Molded Microfluidics. Adv. Eng. Mater. 2024, 2024, 2400276. [Google Scholar] [CrossRef]
- Wan, A.M.D.; Moore, T.A.; Young, E.W.K. Solvent Bonding for Fabrication of PMMA and COP Microfluidic Devices. J. Vis. Exp. 2017, 2017, 55175. [Google Scholar] [CrossRef]
- Sameoto, D.; Wasay, A. Materials Selection and Manufacturing of Thermoplastic Elastomer Microfluidics. In Proceedings of the Progress in Biomedical Optics and Imaging (SPIE), San Franciso, CA, USA, 7–12 February 2015; Volume 9320. [Google Scholar]
- Wu, W.; Lei, Y.; Shan, Z.; Jiang, B. Experimental Study on Multi-Objective Optimization of PMMA Microfluidic Chip Injection Molding. Zhongnan Daxue Xuebao (Ziran Kexue Ban)/J. Cent. South Univ. 2023, 54, 2630–2641. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, F.; Gilchrist, M.D.; Zhang, N. Filling of High Aspect Ratio Micro Features of a Microfluidic Flow Cytometer Chip Using Micro Injection Moulding. J. Micromechanics Microeng. 2018, 28, 075005. [Google Scholar] [CrossRef]
- Lucchetta, G.; Sorgato, M.; Carmignato, S.; Savio, E. Investigating the Technological Limits of Micro-Injection Molding in Replicating High Aspect Ratio Micro-Structured Surfaces. CIRP Ann. Manuf. Technol. 2014, 63, 521–524. [Google Scholar] [CrossRef]
- Shankles, P.G.; Millet, L.J.; Aufrecht, J.A.; Retterer, S.T. Accessing Microfluidics through Feature-Based Design Software for 3D Printing. PLoS ONE 2019, 13, e0192752. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Raj, R. Role of 3D Printing in Microfluidics and Applications; Elsevier: Amsterdam, The Netherlands, 2024; ISBN 9780323988056. [Google Scholar]
- Heuer, C.; Preuß, J.-A.; Habib, T.; Enders, A.; Bahnemann, J. 3D Printing in Biotechnology—An Insight into Miniaturized and Microfluidic Systems for Applications from Cell Culture to Bioanalytics. Eng. Life Sci. 2022, 22, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Anciaux, S.K.; Geiger, M.; Bowser, M.T. 3D Printed Micro Free-Flow Electrophoresis Device. Anal. Chem. 2016, 88, 7675–7682. [Google Scholar] [CrossRef]
- Manzanares Palenzuela, C.L.; Pumera, M. (Bio)Analytical Chemistry Enabled by 3D Printing: Sensors and Biosensors. TrAC-Trends Anal. Chem. 2018, 103, 110–118. [Google Scholar] [CrossRef]
- Hiniduma, K.; Bhalerao, K.S.; De Silva, P.I.T.; Chen, T.; Rusling, J.F. Design and Fabrication of a 3D-Printed Microfluidic Immunoarray for Ultrasensitive Multiplexed Protein Detection. Micromachines 2023, 14, 2187. [Google Scholar] [CrossRef]
- Skoog, S.A.; Narayan, R.J. Stereolithography in Medical Device Fabrication. Adv. Mater. Process. 2013, 171, 32–34. [Google Scholar] [CrossRef]
- Zhakeyev, A.; Zhang, L.; Xuan, J. Photoactive Resin Formulations and Composites for Optical 3D and 4D Printing of Functional Materials and Devices. In 3D and 4D Printing of Polymer Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128168059. [Google Scholar]
- Masood, S.H. Advances in Fused Deposition Modeling; Elsevier: Amsterdam, The Netherlands, 2014; Volume 10, ISBN 9780080965338. [Google Scholar]
- Moritzer, E.; Hecker, F.; Wächter, J.; Knaup, F. Investigation of the Deposition Velocity Related Temperature Deviations for High Temperature Materials in the FDM Process. AIP Conf. Proc. 2023, 2884, 170001. [Google Scholar]
- Taniguchi, H.; Ishida, N.; Oi, J. High Temperature (500C) Hotend for FDM 3D Printer. In Proceedings of the International Conference on Digital Printing Technologies, Denver, CO, USA, 5–9 November 2017; Volume 2017, pp. 165–169. [Google Scholar]
- Luo, Z.; Zhang, H.; Chen, R.; Li, H.; Cheng, F.; Zhang, L.; Liu, J.; Kong, T.; Zhang, Y.; Wang, H. Digital Light Processing 3D Printing for Microfluidic Chips with Enhanced Resolution via Dosing- and Zoning-Controlled Vat Photopolymerization. Microsyst. Nanoeng. 2023, 9, 103. [Google Scholar] [CrossRef]
- Gong, H.; Woolley, A.T.; Nordin, G.P. 3D Printed Microfluidic Pumps and Multiplexers. In Proceedings of the 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2016, Dublin, Ireland, 9–13 October 2016; pp. 928–929. [Google Scholar]
- Nordin, G.P.; Sanchez Noriega, J.L.; Valdoz, J.C.; Chartrand, N.A.; Viglione, M.S.; Woolley, A.T.; Van Ry, P.M.; Christensen, K.A. Reenvisioned 3D printing as an enabler for extreme microfluidic component miniaturization and integration. In Proceedings of the MicroTAS 2021—25th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Palm Springs, CA, USA, 10–14 October 2021; pp. 75–76. [Google Scholar]
- Zhu, Z.; Chen, T.; Zhu, Y.; Huang, F.; Mu, K.; Si, T.; Xu, R.X. Programmable Pulsed Aerodynamic Printing for Multi-Interface Composite Manufacturing. Matter 2023, 6, 2034–2051. [Google Scholar] [CrossRef]
- Duarte, L.C.; Figueredo, F.; Chagas, C.L.S.; Cortón, E.; Coltro, W.K.T. A Review of the Recent Achievements and Future Trends on 3D Printed Microfluidic Devices for Bioanalytical Applications. Anal. Chim. Acta 2024, 1299, 342429. [Google Scholar] [CrossRef]
- Mehta, V.; Rath, S.N. 3D Printed Microfluidic Devices: A Review Focused on Four Fundamental Manufacturing Approaches and Implications on the Field of Healthcare. Biodes Manuf. 2021, 4, 311–343. [Google Scholar] [CrossRef]
- Lepowsky, E.; Tasoglu, S. Emerging Anti-Fouling Methods: Towards Reusability of 3d-Printed Devices for Biomedical Applications. Micromachines 2018, 9, 196. [Google Scholar] [CrossRef]
- Prabhakar, P.; Sen, R.K.; Dwivedi, N.; Khan, R.; Solanki, P.R.; Srivastava, A.K.; Dhand, C. 3D-Printed Microfluidics and Potential Biomedical Applications. Front. Nanotechnol. 2021, 3, 609355. [Google Scholar] [CrossRef]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Puttegowda, M.; Rangappa, S.M.; Alexey, K.; Gorbatyuk, S.; Khan, A.; Doddamani, M.; Siengchin, S. A Comprehensive Review on 3D Printing Advancements in Polymer Composites: Technologies, Materials, and Applications. Int. J. Adv. Manuf. Technol. 2022, 121, 127–169. [Google Scholar] [CrossRef]
- Song, K.; Li, G.; Zu, X.; Du, Z.; Liu, L.; Hu, Z. The Fabrication and Application Mechanism of Microfluidic Systems for High Throughput Biomedical Screening: A Review. Micromachines 2020, 11, 297. [Google Scholar] [CrossRef]
- Park, S.; Hong, S.; Kim, J.; Son, S.Y.; Lee, H.; Kim, S.J. Eco Friendly Nanofluidic Platforms Using Biodegradable Nanoporous Materials. Sci. Rep. 2021, 11, 3804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; He, X.; Luo, Q.; Fan, Y. Biodegradable PLA Nonwoven Fabric-Based Microfluidic Devices. Appl. Phys. A Mater. Sci. Process 2023, 129, 572. [Google Scholar] [CrossRef]
- Bishop, G.W. 3D Printed Microfluidic Devices. In Microfluidics for Biologists: Fundamentals and Applications; Springer Nature: Berlin/Heidelberg, Germany, 2016; ISBN 9783319400365. [Google Scholar]
- He, Y.; Wu, Y.; Fu, J.-Z.; Gao, Q.; Qiu, J.-J. Developments of 3D Printing Microfluidics and Applications in Chemistry and Biology: A Review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Gray, B.L. Smart and Functional Polymer Materials for Smart and Functional Microfluidic Instruments. In Proceedings of the SPIE—The International Society for Optical Engineering, San Diego, CA, USA, 17–21 August 2014; Volume 9060. [Google Scholar]
- Zhu, P.; Wang, L. Microfluidics-Enabled Soft Manufacture of Materials with Tailorable Wettability. Chem. Rev. 2022, 122, 7010–7060. [Google Scholar] [CrossRef]
- Li, S.-C.; Chiang, C.-C.; Tsai, Y.-S.; Chen, C.-J.; Lee, T.-H. Fabrication of a Three-Dimensional Microfluidic System from Poly(Methyl Methacrylate) (PMMA) Using an Intermiscibility Vacuum Bonding Technique. Micromachines 2024, 15, 454. [Google Scholar] [CrossRef]
- Ahmed, M.A.M.; Jurczak, K.M.; Lynn, N.S.; Mulder, J.-P.S.H.; Verpoorte, E.M.J.; Nagelkerke, A. Rapid Prototyping of PMMA-Based Microfluidic Spheroid-on-a-Chip Models Using Micromilling and Vapour-Assisted Thermal Bonding. Sci. Rep. 2024, 14, 2831. [Google Scholar] [CrossRef]
- Emadzadeh, K.; Ghafarinia, V. Development of a Direct PMMA-PCB Bonding Method for Low Cost and Rapid Prototyping of Microfluidic-Based Gas Analysers. RSC Adv. 2024, 14, 22598–22605. [Google Scholar] [CrossRef]
- Rodríguez, C.F.; Báez-Suárez, M.; Muñoz-Camargo, C.; Reyes, L.H.; Osma, J.F.; Cruz, J.C. Zweifach–Fung Microfluidic Device for Efficient Microparticle Separation: Cost-Effective Fabrication Using CO2 Laser-Ablated PMMA. Micromachines 2024, 15, 932. [Google Scholar] [CrossRef]
- Yan, Y.; Mao, Y.; Li, B.; Zhou, P. Machinability of the Thermoplastic Polymers: Peek, Pi, and Pmma. Polymers 2021, 13, 69. [Google Scholar] [CrossRef]
- Agha, A.; Abu-Nada, E.; Alazzam, A. Integration of Acoustic Micromixing with Cyclic Olefin Copolymer Microfluidics for Enhanced Lab-on-a-Chip Applications in Nanoscale Liposome Synthesis. Biofabrication 2024, 16, 045004. [Google Scholar] [CrossRef]
- Agha, A.; Waheed, W.; Alamoodi, N.; Mathew, B.; Alnaimat, F.; Abu-Nada, E.; Abderrahmane, A.; Alazzam, A. A Review of Cyclic Olefin Copolymer Applications in Microfluidics and Microdevices. Macromol. Mater. Eng. 2022, 307, 2200053. [Google Scholar] [CrossRef]
- Jagannath, A.; Yu, M.; Li, J.; Zhang, N.; Gilchrist, M.D. Improving Assay Feasibility and Biocompatibility of 3D Cyclic Olefin Copolymer Microwells by Superhydrophilic Modification via Ultrasonic Spray Deposition of Polyvinyl Alcohol. Biomater. Adv. 2024, 163, 213934. [Google Scholar] [CrossRef]
- Su, S.; Jing, G.; Zhang, M.; Liu, B.; Zhu, X.; Wang, B.; Fu, M.; Zhu, L.; Cheng, J.; Guo, Y. One-Step Bonding and Hydrophobic Surface Modification Method for Rapid Fabrication of Polycarbonate-Based Droplet Microfluidic Chips. Sens. Actuators B Chem. 2019, 282, 60–68. [Google Scholar] [CrossRef]
- Hashimoto, Y. Surface Modification of Polymers by Vacuum Ultraviolet Illumination Containing Low Wavelength below 160 Nm and Microfluidic Applications of Irradiated Polycarbonate. Polymer 2023, 287, 126439. [Google Scholar] [CrossRef]
- Baldo, T.A.; Ataide, V.N.; Park, J.; Panraksa, Y.; Martinez, B.; Anderson, L.B.R.; Malsick, L.E.; Gallichotte, E.N.; Ebel, G.D.; Geiss, B.J.; et al. Automated Enzyme-Linked Immunosorbent Assay for Point-of-Care COVID-19 Testing. Electrochim. Acta 2024, 497, 144525. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.; Ling, Y.; Zhang, Z. Fabrication of Flexible Au Microsensors by a Simple Stamping Method for the Point-of-Care Direct Detection of Trace Leucomalachite Green. Microchem. J. 2024, 205, 111299. [Google Scholar] [CrossRef]
- Song, Q.; Hamza, A.; Li, C.; Sedeky, A.S.; Chen, Y.; Zhu, M.; Goralczyk, A.; Mayoussi, F.; Zhu, P.; Hou, P.; et al. 3D Printed Elastic Fluoropolymer with High Stretchability and Enhanced Chemical Resistance for Microfluidic Applications. Addit. Manuf. 2024, 81, 103991. [Google Scholar] [CrossRef]
- Montalbo, R.C.K.; Wu, M.-J.; Tu, H.-L. One-Step Flow Synthesis of Size-Controlled Polymer Nanogels in a Fluorocarbon Microfluidic Chip. RSC Adv. 2024, 14, 11258–11265. [Google Scholar] [CrossRef]
- Finny, A.S. 3D Bioprinting in Bioremediation: A Comprehensive Review of Principles, Applications, and Future Directions. PeerJ 2024, 12, 16897. [Google Scholar] [CrossRef]
- Sölle, B.; Reisinger, D.; Heupl, S.; Jelinek, A.; Schlögl, S.; Rossegger, E. Reshapable Bio-Based Thiol-Ene Vitrimers for Nanoimprint Lithography: Advanced Covalent Adaptability for Tunable Surface Properties. React. Funct. Polym. 2024, 202, 105972. [Google Scholar] [CrossRef]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel Fabrication on Glass Materials for Microfluidic Devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Rad, M.A.; Ibrahim, K.; Mohamed, K.; Najimudin, N. Surface Modification of Polydimethylsiloxane Microchannel Using Air Plasma for DNA Capillary Migration in Polydimethylsiloxane-Glass Microfluidic Devices. Micro Nano Lett. 2013, 8, 305–307. [Google Scholar] [CrossRef]
- Lakhera, P.; Chaudhary, V.; Bhardwaj, B.; Kumar, P.; Kumar, S. Development and Recent Advancement in Microfluidics for Point of Care Biosensor Applications: A Review. Biosens. Bioelectron. X 2022, 11, 100218. [Google Scholar] [CrossRef]
- Ota, N.; Tanaka, N.; Sato, A.; Shen, Y.; Yalikun, Y.; Tanaka, Y. Microenvironmental Analysis and Control for Local Cells under Confluent Conditions via a Capillary-Based Microfluidic Device. Anal. Chem. 2022, 94, 16299–16307. [Google Scholar] [CrossRef] [PubMed]
- Takakura, N.; Kurashina, Y.; Onoe, H. Glass-Capillary-Embedded 3D Coaxial Microfluidic Device with Pneumatic Microvalve Control for Producing Patterned Functional Materials. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Tokyo, Japan, 9–13 January 2022; Volume 2022, pp. 267–270. [Google Scholar]
- Shi, Z.; Dai, C.; Fang, F.; Shuai, Y.; Xiong, C.; Liu, Q. Wearable Sensors for In Situ Biofluid Analysis. In Portable and Wearable Sensing Systems: Techniques, Fabrication, and Biochemical Detection; Wiley: Hoboken, NJ, USA, 2024; ISBN 9783527841080. [Google Scholar]
- Apoorva, S.; Nguyen, N.-T.; Sreejith, K.R. Recent Developments and Future Perspectives of Microfluidics and Smart Technologies in Wearable Devices. Lab. Chip 2024, 24, 1833–1866. [Google Scholar] [CrossRef]
- Li, S.; Ma, Z.; Cao, Z.; Pan, L.; Shi, Y. Advanced Wearable Microfluidic Sensors for Healthcare Monitoring. Small 2020, 16, 1903822. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, J. Wearable Biosensors for Non-Invasive Sweat Diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef]
- Yeo, J.C.; Kenry; Lim, C.T. Emergence of Microfluidic Wearable Technologies. Lab. Chip 2016, 16, 4082–4090. [Google Scholar] [CrossRef]
- Digiglio, P.; Li, R.; Wang, W.; Pan, T. Microflotronic Arterial Tonometry for Continuous Wearable Non-Invasive Hemodynamic Monitoring. Ann. Biomed. Eng. 2014, 42, 2278–2288. [Google Scholar] [CrossRef]
- Heo, J.S.; Shishavan, H.H.; Soleymanpour, R.; Kim, J.; Kim, I. Textile-Based Stretchable and Flexible Glove Sensor for Monitoring Upper Extremity Prosthesis Functions. IEEE Sens. J. 2020, 20, 1754–1760. [Google Scholar] [CrossRef]
- Gao, W.; Yao, J.; Zhu, K.; Zhao, P.; Chen, X. Highly Sensitive, Wide-Range Pressure Sensor Based on Negative Poisson’s Ratio for Human Motion Detection. IEEE Sens. J. 2023, 23, 12618–12625. [Google Scholar] [CrossRef]
- Vo, T.S.; Nguyen, T.S.; Lee, S.-H.; Vo, D.C.T.; Kim, D.; Kim, K. Realization of Motion Sensing Composites Prepared from the Incorporation of Three-Dimensional Porous Conductive Foams and Polydimethylsiloxane. J. Sci. Adv. Mater. Devices 2023, 8, 100554. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.; Bermak, A. Recent Developments in Printing Flexible and Wearable Sensing Electronics for Healthcare Applications. Sensors 2019, 19, 1230. [Google Scholar] [CrossRef]
- Yang, G.; Hong, J.; Park, S.-B. Wearable Device for Continuous Sweat Lactate Monitoring in Sports: A Narrative Review. Front. Physiol. 2024, 15, 1376801. [Google Scholar] [CrossRef]
- Luo, T.; Zheng, L.; Chen, D.; Zhang, C.; Liu, S.; Jiang, C.; Xie, Y.; Du, D.; Zhou, W. Implantable Microfluidics: Methods and Applications. Analyst 2023, 148, 4637–4654. [Google Scholar] [CrossRef]
- Fallahi, H.; Zhang, J.; Phan, H.-P.; Nguyen, N.-T. Flexible Microfluidics: Fundamentals, Recent Developments, and Applications. Micromachines 2019, 10, 830. [Google Scholar] [CrossRef]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable Flexible Sweat Sensors for Healthcare Monitoring: A Review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef]
- Yoon, S.; Yoon, H.; Zahed, M.A.; Park, C.; Kim, D.; Park, J.Y. Multifunctional Hybrid Skin Patch for Wearable Smart Healthcare Applications. Biosens. Bioelectron. 2022, 196, 113685. [Google Scholar] [CrossRef]
- Honda, S.; Tanaka, R.; Matsumura, G.; Seimiya, N.; Takei, K. Wireless, Flexible, Ionic, Perspiration-Rate Sensor System with Long-Time and High Sweat Volume Functions Toward Early-Stage, Real-Time Detection of Dehydration. Adv. Funct. Mater. 2023, 33, 2306516. [Google Scholar] [CrossRef]
- Güngör, S.; Kahraman, E.; Erdal, M.S.; Özsoy, Y. Recent Advances in Biopolymer-Based Transdermal Patches. In Biopolymer Membranes and Films; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128181348. [Google Scholar]
- McKenna, P.E.; Abbate, M.T.A.; Vora, L.K.; Sabri, A.H.; Peng, K.; Volpe-Zanutto, F.; Tekko, I.A.; Permana, A.D.; Maguire, C.; Dineen, D.; et al. Polymeric Microarray Patches for Enhanced Transdermal Delivery of the Poorly Soluble Drug Olanzapine. ACS Appl. Mater. Interfaces 2023, 15, 31300–31319. [Google Scholar] [CrossRef]
- Evanghelidis, A.; Beregoi, M.; Diculescu, V.C.; Galatanu, A.; Ganea, P.; Enculescu, I. Flexible Delivery Patch Systems Based on Thermoresponsive Hydrogels and Submicronic Fiber Heaters. Sci. Rep. 2018, 8, 17555. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, I.; Elwood, J.; Li, X.; Beker, L.; Sweet, E.; Cai, W.; Lin, L. Membraneless Microfluidic Redox Battery for Wearable Electronics Applications. In Proceedings of the TRANSDUCERS 2017—19th International Conference on Solid-State Sensors, Actuators and Microsystems, Kaohsiung, Taiwan, 18–22 June 2017; pp. 1820–1823. [Google Scholar]
- Jeong, S.H.; Hjort, K.; Wu, Z. Tape Transfer Printing of a Liquid Metal Alloy for Stretchable RF Electronics. Sensors 2014, 14, 16311–16321. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, C.; Lapiere, M.; Ciatti, J.L.; Yang, D.S.; Berkovich, J.; Model, J.B.; Banks, A.; Ghaffari, R.; Chang, J.-K.; et al. Thermoplastic Elastomers for Wireless, Skin-Interfaced Electronic, and Microfluidic Devices. Adv. Mater. Technol. 2023, 8, 2300732. [Google Scholar] [CrossRef]
- Yang, Q.; Nguyen, E.P.; Silva, C.C.C.; Rosati, G.; Merkoçi, A. Signal Enhancement Strategies. In Wearable Physical, Chemical and Biological Sensors; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780128216613. [Google Scholar]
- Mejía-Salazar, J.R.; Cruz, K.R.; Vásques, E.M.M.; de Oliveira, O.N. Microfluidic Point-of-Care Devices: New Trends and Future Prospects for Ehealth Diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef]
- Do Nascimento, D.F.; Avendaño, J.A.; Mehl, A.; Moura, M.J.B.; Carvalho, M.S.; Duncanson, W.J. Flow of Tunable Elastic Microcapsules through Constrictions. Sci. Rep. 2017, 7, 11898. [Google Scholar] [CrossRef]
- Gu, P.; Nishida, T.; Fan, Z.H. The Use of Polyurethane as an Elastomer in Thermoplastic Microfluidic Devices and the Study of Its Creep Properties. Electrophoresis 2014, 35, 289–297. [Google Scholar] [CrossRef]
- Nellepalli, P.; Patel, T.; Oh, J.K. Dynamic Covalent Polyurethane Network Materials: Synthesis and Self-Healability. Macromol. Rapid Commun. 2021, 42, 2100391. [Google Scholar] [CrossRef]
- Rollins, D.; Drzal, L.T. Multifunctional Polymer Nanocomposite Foams for Space Applications: The Effect of Edge Functionalized Nano-Reinforcers on Nanocomposite Polyurethane/Polyisocyanurate Rigid Foam. In Proceedings of the Society of Plastics Engineers—12th International Conference on Foam Materials and Technology, FOAMS 2014, Iselin, NJ, USA, 8–11 September 2014; pp. 31–37. [Google Scholar]
- Roth, M.R.; Pisani, W.A.; Wedgeworth, D.N.; Newman, J.K.; Shukla, M.K. Computational Analysis on Mechanical Property Reinforcement of Nylon 6 Polymer and Nanofiller Dispersion through Addition of CNT/Graphene/CNT-Graphene Nanofillers. J. Polym. Res. 2022, 29, 294. [Google Scholar] [CrossRef]
- Depan, D. Biodegradable Polymeric Nanocomposites: Advances in Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781482260526. [Google Scholar]
- Mondal, P.; Purkait, M.K. Bio-Based Polymeric Nanocomposites for Stimuli-Responsive Membranes. In Handbook of Polymer and Ceramic Nanotechnology; Springer: Cham, Switzerland, 2021; Volume 1, ISBN 9783030405137. [Google Scholar]
- Kim, S.; Jeon, H.; Koo, J.M.; Oh, D.X.; Park, J. Practical Applications of Self-Healing Polymers Beyond Mechanical and Electrical Recovery. Adv. Sci. 2024, 11, 2302463. [Google Scholar] [CrossRef]
- El Choufi, N.; Mustapha, S.; Tehrani, B.A.; Grady, B.P. An Overview of Self-Healable Polymers and Recent Advances in the Field. Macromol. Rapid Commun. 2022, 43, 2200164. [Google Scholar] [CrossRef]
- Saikia, B.J.; Das, D.; Gogoi, P.; Dolui, S.K. Designing Self-Healing Polymers by Atom Transfer Radical Polymerization and Click Chemistry. In Industrial Applications for Intelligent Polymers and Coatings; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319268934. [Google Scholar]
- Parihar, S.; Gaur, B. Self Healing Approaches in Polymeric Materials-an Overview. J. Polym. Res. 2023, 30, 217. [Google Scholar] [CrossRef]
- Salehuddin, S.M.F.; Hawaji, M.H.; Khan, A.S.M.B.; Man, S.H.C.; Ali, W.K.W.; Baharulrazi, N. A Review of Recent Developments: Self-Healing Approaches for Polymeric Materials. Chem. Eng. Trans. 2019, 72, 433–438. [Google Scholar] [CrossRef]
- Cutroneo, M.; Silipigni, L.; Mackova, A.; Malinsky, P.; Miksova, R.; Holy, V.; Maly, J.; Stofik, M.; Aubrecht, P.; Fajstavr, D.; et al. Mask-Assisted Deposition of Ti on Cyclic Olefin Copolymer Foil by Pulsed Laser Deposition. Micromachines 2023, 14, 1298. [Google Scholar] [CrossRef] [PubMed]
- Cutroneo, M.; Torrisi, L.; Silipigni, L.; Havranek, V.; Mackova, A.; Malinsky, P.; Miksova, R.; Maly, J.; Stofik, M.; Aubrecht, P.; et al. Laminated Cyclic Olefin Copolymer Foil by Pulsed Laser Deposition. Coatings 2023, 13, 596. [Google Scholar] [CrossRef]
- Polanco, E.R.; Griffin, J.; Zangle, T.A. Fabrication and Bonding of Refractive Index Matched Microfluidics for Precise Measurements of Cell Mass. Polymers 2021, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Eyer, K.; Root, K.; Robinson, T.; Dittrich, P.S. A Simple Low-Cost Method to Enhance Luminescence and Fluorescence Signals in PDMS-Based Microfluidic Devices. RSC Adv. 2015, 5, 12511–12516. [Google Scholar] [CrossRef]
- Bou, S.J.M.C.; Ellis, A.V. Microfluidic Devices Using Thiol-Ene Polymers. In Proceedings of the SPIE—The International Society for Optical Engineering, Melbourne, VIC, Australia; 2013; Volume 8923. [Google Scholar]
- Pope, B.L.; Zhang, M.; Jo, S.; Dragnea, B.; Jacobson, S.C. Microscale Diffractive Lenses Integrated into Microfluidic Devices for Size-Selective Optical Trapping of Particles. Anal. Chem. 2024, 96, 11845–11852. [Google Scholar] [CrossRef]
- Roghani-Mamaqani, H.; Tajmoradi, Z. Photoresponsive Polymers. In Smart Stimuli-Responsive Polymers, Films, and Gels; Wiley: Hoboken, NJ, USA, 2022; ISBN 9783527832385. [Google Scholar]
- Di Martino, M.; Sessa, L.; Diana, R.; Piotto, S.; Concilio, S. Recent Progress in Photoresponsive Biomaterials. Molecules 2023, 28, 3712. [Google Scholar] [CrossRef]
- Noguchi, T.; Akioka, N.; Kojima, Y.; Kawamura, A.; Miyata, T. Photoresponsive Polymer Films with Directly Micropatternable Surfaces Based on the Change in Free Volume by Photo-Crosslinking. Adv. Mater. Interfaces 2022, 9, 2101965. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.; You, S.; Cai, B.; Liu, H.; Zhang, L.; Rao, L.; Liu, W.; Guo, S.-S.; Zhao, X.-Z. Ultraviolet-Assisted Microfluidic Generation of Ferroelectric Composite Particles. Biomicrofluidics 2016, 10, 024106. [Google Scholar] [CrossRef]
- Wu, J.; Lee, N.Y. Imprint Molding of a Microfluidic Optical Cell on Thermoplastics with Reduced Surface Roughness for the Detection of Copper Ions. Anal. Sci. 2016, 32, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Feringa, B.L. Photoresponsive Supramolecular Polymers: From Light-Controlled Small Molecules to Smart Materials. Adv. Mater. 2023, 35, 2204413. [Google Scholar] [CrossRef]
- Lu, J.; Litster, J.D.; Nagy, Z.K. Nucleation Studies of Active Pharmaceutical Ingredients in an Air-Segmented Microfluidic Drop-Based Crystallizer. Cryst. Growth Des. 2015, 15, 3645–3651. [Google Scholar] [CrossRef]
- Alfihed, S.; Bergen, M.H.; Ciocoiu, A.; Holzman, J.F.; Foulds, I.G. Characterization and Integration of Terahertz Technology within Microfluidic Platforms. Micromachines 2018, 9, 453. [Google Scholar] [CrossRef]
- Min, K.-I. Fabrication of 3D Multilayered Microfluidic Channel Using Fluorinated Ethylene Propylene Nanoparticle Dispersion | (Received 12 July 2021; Received in Revised from 2 August. Korean Chem. Eng. Res. 2021, 59, 639–643. [Google Scholar] [CrossRef]
- Mays, R.L.; Dickey, M.D.; Genzer, J. Microfluidic Channels Fabricated from Poly(Vinylmethylsiloxane) Networks That Resist Swelling by Organic Solvents. Lab. Chip 2013, 13, 4317–4320. [Google Scholar] [CrossRef]
- Sun, P.; Horton, J.H. Perfluorinated Poly(Dimethylsiloxane) via the Covalent Attachment of Perfluoroalkylsilanes on the Oxidized Surface: Effects on Zeta-Potential Values. Appl. Surf. Sci. 2013, 271, 344–351. [Google Scholar] [CrossRef]
- Geczy, R.; Sticker, D.; Bovet, N.; Häfeli, U.O.; Kutter, J.P. Chloroform Compatible, Thiol-Ene Based Replica Molded Micro Chemical Devices as an Alternative to Glass Microfluidic Chips. Lab. Chip 2019, 19, 798–806. [Google Scholar] [CrossRef]
- Farnese, J.; Zhao, P.; Ren, C.L. Effect of Surface Roughness on Bond Strength between PCTE Membranes and PDMS towards Microfluidic Applications. Int. J. Adhes. Adhes. 2021, 106, 102800. [Google Scholar] [CrossRef]
- Männel, M.J.; Hauck, N.; Thiele, J. Solvent-Resistant Microfluidic Devices Made from PFHDA Resins by Micro-Stereolithography. In Proceedings of the Progress in Biomedical Optics and Imaging, SPIE, San Francisco, CA, USA; 2020; Volume 11235. [Google Scholar]
- Inoue, A.; Yuk, H.; Lu, B.; Zhao, X. Strong Adhesion of Wet Conducting Polymers on Diverse Substrates. Sci. Adv. 2020, 6, eaay5394. [Google Scholar] [CrossRef]
- Kulandaivalu, S.; Zainal, Z.; Sulaiman, Y. A New Approach for Electrodeposition of Poly (3, 4-Ethylenedioxythiophene)/Polyaniline (PEDOT/PANI)Copolymer. Int. J. Electrochem. Sci. 2015, 10, 8926–8940. [Google Scholar] [CrossRef]
- Ishak, N.; Afiq Husin, M.; Mohd, Y.; Sajidah Abd Aziz, A.; Mohd Zain, Z. Polyaniline on Poly (3,4-Ethylenedioxythiophene) Screen Printed Electrode as Dissolved Ammonia Sensor. Proc. Mater. Today Proc. 2019, 19, 1682–1686. [Google Scholar] [CrossRef]
- Kousseff, C.J.; Taifakou, F.E.; Neal, W.G.; Palma, M.; Nielsen, C.B. Controlling Morphology, Adhesion, and Electrochromic Behavior of PEDOT Films through Molecular Design and Processing. J. Polym. Sci. 2022, 60, 504–516. [Google Scholar] [CrossRef]
- Sezen-Edmonds, M.; Loo, Y.-L. Processing-Structure-Function Relationships of Polymer-Acid-Templated Conducting Polymers for Solid-State Devices. In Conjugated Polymers; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781315159294. [Google Scholar]
- Hui, Y.; Bian, C.; Xia, S.; Tong, J.; Wang, J. Synthesis and Electrochemical Sensing Application of Poly(3,4-Ethylenedioxythiophene)-Based Materials: A Review. Anal. Chim. Acta 2018, 1022, 1–19. [Google Scholar] [CrossRef]
- Puttaswamy, S.V.; Xue, P.; Kang, Y.; Ai, Y. Simple and Low Cost Integration of Highly Conductive Three-Dimensional Electrodes in Microfluidic Devices. Biomed. Microdevices 2015, 17, 4. [Google Scholar] [CrossRef]
- McIntyre, D.; Lashkaripour, A.; Densmore, D. Rapid and Inexpensive Microfluidic Electrode Integration with Conductive Ink. Lab. Chip 2020, 20, 3690–3695. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Q.; Korman, C.E.; Li, Z.; Zaghloul, M.E. Flexible Packaging of Solid-State Integrated Circuit Chips with Elastomeric Microfluidics. Sci. Rep. 2013, 3, 1098. [Google Scholar] [CrossRef]
- Hilbich, D.; Shannon, L.; Gray, B.L. Stretchable Electronics for Wearable and High-Current Applications. In Proceedings of the SPIE—The International Society for Optical Engineering, Las Vegas, NV, USA; 2016; Volume 9802. [Google Scholar]
- Paszkiewicz, S. Functional Properties of PTT-Based Composites and Nanocomposites. In Poly Trimethylene Terephthalate: Based Blends, IPNs, Composites and Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Payandehpeyman, J.; Mazaheri, M. Geometrical and Physical Effects of Nanofillers on Percolation and Electrical Conductivity of Polymer Carbon-Based Nanocomposites: A General Micro-Mechanical Model. Soft Matter 2022, 19, 530–539. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, G.J. Electrical Conductivity of Carbon Nanotubeand Graphene-Based Nanocomposites. In Micromechanics and Nanomechanics of Composite Solids; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319527949. [Google Scholar]
- Lin, H.; Jian, Q.; Bai, X.; Li, D.; Huang, Z.; Huang, W.; Feng, S.; Cheng, Z. Recent Advances in Thermal Conductivity and Thermal Applications of Graphene and Its Derivatives Nanofluids. Appl. Therm. Eng. 2023, 218, 119176. [Google Scholar] [CrossRef]
- Pech-Pisté, R.; Cen-Puc, M.; Balam, A.; May-Pat, A.; Avilés, F. Multifunctional Sensing Properties of Polymer Nanocomposites Based on Hybrid Carbon Nanostructures. Mater. Today Commun. 2020, 25, 101472. [Google Scholar] [CrossRef]
- Liu, H.; Thostenson, E.T. Conductive Nanocomposites for Multifunctional Sensing Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780081005330. [Google Scholar]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with Carbon Nanotubes and Graphene: An Outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Zelikman, E.; Suckeveriene, R.Y. Ultrasonically Induced Polymerization and Polymer Grafting in the Presence of Carbonaceous Nanoparticles. Processes 2020, 8, 1680. [Google Scholar] [CrossRef]
- Martin, A.; Vilela, D.; Escarpa, A. Chapter 6: Carbon Nanomaterials for Advanced Analytical Micro- and Nanotechnologies. In Carbon-Based Nanomaterials in Analytical Chemistry; Royal Society of Chemistry: London, UK, 2019; Volume 2019, ISBN 9781788011020. [Google Scholar]
- Civelekoglu, O.; Liu, R.; Asmare, N.; Arifuzzman, A.K.M.; Sarioglu, A.F. Wrap-around Sensors for Electrical Detection of Particles in Microfluidic Channels. Sens. Actuators B Chem. 2023, 375, 132874. [Google Scholar] [CrossRef]
- Akbari Kenari, M.; Rezvani Ghomi, E.; Akbari Kenari, A.; Arabi, S.M.S.; Deylami, J.; Ramakrishna, S. Biomedical Applications of Microfluidic Devices: Achievements and Challenges. Polym. Adv. Technol. 2022, 33, 3920–3934. [Google Scholar] [CrossRef]
- Fernández-la-Villa, A.; Pozo-Ayuso, D.F.; Castaño-Álvarez, M. Microfluidics and Electrochemistry: An Emerging Tandem for next-Generation Analytical Microsystems. Curr. Opin. Electrochem. 2019, 15, 175–185. [Google Scholar] [CrossRef]
- Peroz, C.; Reboud, V.; Torres, C.M.S. Nanoimprint Technologies. In Nanofabrication; Springer: Berlin/Heidelberg, Germany, 2014; Volume 9783709104, ISBN 9783709104248. [Google Scholar]
- Xiong, W.; Zhou, Y.; Hou, W.; Jiang, L.; Mahjouri-Samani, M.; Park, J.; He, X.; Gao, Y.; Fan, L.; Baldacchini, T.; et al. Laser-Based Micro/Nanofabrication in One, Two and Three Dimensions. Front. Optoelectron. 2015, 8, 351–378. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Borenstein, J.T.; Langer, R. Microfabrication Techniques in Scaffold Development. In Nanotechnology and Regenerative Engineering; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781466585386. [Google Scholar]
- Gao, X.; Chen, Y.; Zheng, M.; Duan, H. Large-Area Nanoimprint Lithography: Processes and Device Applications. Guangxue Jingmi Gongcheng/Opt. Precis. Eng. 2022, 30, 555–573. [Google Scholar] [CrossRef]
- Li, K.; Morton, K.; Veres, T.; Cui, B. Nanoimprint Lithography and Its Application in Tissue Engineering and Biosensing. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444640475. [Google Scholar]
- Sugioka, K. Nanofluidics Fabricated By 3D Femtosecond Laser Processing. In Ultrafast Laser Nanostructuring: The Pursuit of Extreme Scales; Springer: Berlin/Heidelberg, Germany, 2023; Volume 239. [Google Scholar]
- Lim, H.; Ryu, J.; Kim, G.; Choi, K.-B.; Lee, S.; Lee, J. Nanoimprint Lithography with a Focused Laser Beam for the Fabrication of Nanopatterned Microchannel Molds. Lab. Chip 2013, 13, 3188–3191. [Google Scholar] [CrossRef]
- Barcelo, S.; Li, Z. Nanoimprint Lithography for Nanodevice Fabrication. Nano Converg. 2016, 3, 21. [Google Scholar] [CrossRef]
- Beck, M.; Lee, K.D.; Heidari, B.; Eriksson, T. Nanoimprint Lithography: Technology for High Volume Manufacturing. Nanotechnol. Res. J. 2014, 7, 265. [Google Scholar]
- Ogusu, M.; Tamura, M.; Nomura, Y.; Saito, T.; Kunugi, H.; Yamaji, T.; Tanaka, F.; Abe, T. Optimization of NIL and Associated Pattern Transfer Processes for the Fabrication of Advanced Devices. In Proceedings of the SPIE—The International Society for Optical Engineering, San Jose, CA, USA, 30 January–1 February 2024; Volume 12956. [Google Scholar]
- Yamamoto, K.; Wada, H.; Suzaki, Y.; Sato, K.; Iino, S.; Jimbo, S.; Morimoto, O.; Hiura, M.; Roy, N.; Cherala, A.; et al. Nanoimprint Lithography Methods for Achieving Sub-3nm Overlay. In Photomask Technology; SPIE: Bellingham, WA, USA, 2021; Volume 11855. [Google Scholar]
- Wu, D.; Rajput, N.S.; Luo, X. Nanoimprint Lithography—The Past, the Present and the Future. Curr. Nanosci. 2016, 12, 712–724. [Google Scholar] [CrossRef]
- Srikantaprasad, G.; Mathew, N.T.; Nagar, S.V. Laser Micromachining on PMMA: An Efficient Fabrication of Microchannels for Sustainable Microfluidic Devices. J. Braz. Soc. Mech. Sci. Eng. 2024, 46, 325. [Google Scholar] [CrossRef]
- Konari, P.R.; Clayton, Y.-D.; Vaughan, M.B.; Khandaker, M.; Hossan, M.R. Article Experimental Analysis of Laser Micromachining of Microchannels in Common Microfluidic Substrates. Micromachines 2021, 12, 138. [Google Scholar] [CrossRef]
- Guo, J.-K.; Zhao, Z.-J.; Ling, J.-Z.; Yuan, Y.; Wang, X.-R. Laser Micro/Nanomachining Technology for Soft Matter. Wuli Xuebao/Acta Phys. Sin. 2022, 71, 220625. [Google Scholar] [CrossRef]
- Johari, S.; Ting, Z.K.; Mazalan, M.; Wahab, Y.; Noor, A.M.; Ahmad, M.F.; Ramli, M.M. Characterization of Excimer Laser Micromachining Parameters to Derive Optimal Performance for the Production of Polydimethylsiloxane (PDMS)-Based Microfluidic Devices. Lasers Eng. 2024, 57, 257–273. [Google Scholar]
- Sugioka, K.; Cheng, Y. Summary and Outlook. In Femtosecond Laser 3D Micromachining for Microfluidic and Optofluidic Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Ngomegni, F.G.M.; Ouambo, S.A.T.; Petmegni, D.S.M.; Zobo, B.E. Laser Dynamics and Stability of Continuous-Waves in Nonlinear Optical Transparent Medium with Saturable Absorber: Competing Effects between Kerr Nonlinearity, Saturable Absorber, and Electron–Hole Radiative Recombination Processes. J. Mater. Sci. Mater. Electron. 2022, 33, 11475–11486. [Google Scholar] [CrossRef]
- Tanvir Ahmmed, K.M.; Grambow, C.; Kietzig, A.-M. Fabrication of Micro/Nano Structures on Metals by Femtosecond Laser Micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- Imran, H.J.; Hubeatir, K.A.; Al-Khafaji, M.M. CO2 Laser Micro-Engraving of PMMA Complemented by Taguchi and ANOVA Methods. J. Phys. Conf. Ser. 2021, 1795, 012062. [Google Scholar] [CrossRef]
- Jagadeesha, T.; Kunar, S. Hybrid Laser Electrochemical Micromachining. In Laser-Assisted Machining: Processes and Applications; Wiley: Hoboken, NJ, USA, 2024; ISBN 9781394214655. [Google Scholar]
- Matellan, C.; Del Río Hernández, A.E. Cost-Effective Rapid Prototyping and Assembly of Poly(Methyl Methacrylate) Microfluidic Devices. Sci. Rep. 2018, 8, 6971. [Google Scholar] [CrossRef]
- Ali, A.; Sundaram, M. Drilling of Crack Free Micro Holes in Glass by Chemo-Thermal Micromachining Process. Precis. Eng. 2018, 54, 33–38. [Google Scholar] [CrossRef]
- Liu, H.; Lin, W.; Hong, M. Hybrid Laser Precision Engineering of Transparent Hard Materials: Challenges, Solutions and Applications. Light Sci. Appl. 2021, 10, 162. [Google Scholar] [CrossRef]
- Sahu, A.K.; Malhotra, J.; Jha, S. Laser-Based Hybrid Micromachining Processes: A Review. Opt. Laser Technol. 2022, 146, 107554. [Google Scholar] [CrossRef]
- Benton, M.; Hossan, M.R.; Konari, P.R.; Gamagedara, S. Effect of Process Parameters and Material Properties on Laser Micromachining of Microchannels. Micromachines 2019, 10, 123. [Google Scholar] [CrossRef]
- Tsuji, T.; Doi, K.; Kawano, S. Optical Trapping in Micro- and Nanoconfinement Systems: Role of Thermo-Fluid Dynamics and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2022, 52, 100533. [Google Scholar] [CrossRef]
- Hossan, M.R.; Konari, P.R. Laser Micromachining of Glass Substrates for Microfluidics Devices. AIP Conf. Proc. 2021, 2324, 060002. [Google Scholar]
- Du, Y.; Reitemeier, J.; Jiang, Q.; Bappy, M.O.; Bohn, P.W.; Zhang, Y. Hybrid Printing of Fully Integrated Microfluidic Devices for Biosensing. Small 2024, 20, 2304966. [Google Scholar] [CrossRef]
- Kojić, S.; Birgermajer, S.; Radonić, V.; Podunavac, I.; Jevremov, J.; Petrović, B.; Marković, E.; Stojanović, G.M. Optimization of Hybrid Microfluidic Chip Fabrication Methods for Biomedical Application. Microfluid. Nanofluidics 2020, 24, 66. [Google Scholar] [CrossRef]
- Alapan, Y.; Hasan, M.N.; Shen, R.; Gurkan, U.A. Three-Dimensional Printing Based Hybrid Manufacturing of Microfluidic Devices. J. Nanotechnol. Eng. Med. 2015, 6, 021007. [Google Scholar] [CrossRef]
- Cao, Y.; Bontrager-Singer, J.; Zhu, L. A 3D Microfluidic Device Fabrication Method Using Thermopress Bonding with Multiple Layers of Polystyrene Film. J. Micromechanics Microeng. 2015, 25, 065005. [Google Scholar] [CrossRef]
- Etxebarria-Elezgarai, J.; Garcia-Hernando, M.; Basabe-Desmonts, L.; Benito-Lopez, F. Precise Integration of Polymeric Sensing Functional Materials within 3D Printed Microfluidic Devices. Chemosensors 2023, 11, 253. [Google Scholar] [CrossRef]
- Zips, S.; Wenzel, O.J.; Rinklin, P.; Grob, L.; Terkan, K.; Adly, N.Y.; Weiß, L.; Wolfrum, B. Direct Stereolithographic 3D Printing of Microfluidic Structures on Polymer Substrates for Printed Electronics. Adv. Mater. Technol. 2019, 4, 1800455. [Google Scholar] [CrossRef]
- Gong, H.; Woolley, A.T.; Nordin, G.P. High Density 3D Printed Microfluidic Valves, Pumps, and Multiplexers. Lab. Chip 2016, 16, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Wen, J.; Su, Q.; Wiederoder, M.S.; Koester, S.J.; Uzarski, J.R.; McAlpine, M.C. 3D Printed Self-Supporting Elastomeric Structures for Multifunctional Microfluidics. Sci. Adv. 2020, 6, eabc9846. [Google Scholar] [CrossRef]
- Supekar, O.D.; Brown, J.J.; Eigenfeld, N.T.; Gertsch, J.C.; Bright, V.M. Atomic Layer Deposition Ultrathin Film Origami Using Focused Ion Beams. Nanotechnology 2016, 27, 49LT02. [Google Scholar] [CrossRef]
- Shahar, S.F.M.; Jaafar, I.H.; Ali, M.Y. Parametric Study of Sputtering Microchannels via Focused Ion Beam (FIB). ARPN J. Eng. Appl. Sci. 2015, 10, 17397–17401. [Google Scholar]
- Nurbaya, Z.; Rahmah, E.S.; Fairuz, A.M.; Kamariana, Y.; Hashim, M.; Akmam, A.S.; Bazura, A.R. Study on Isolation Formation on Si Wafer Using Focused Ion Beam Technique. In Proceedings of the 8th IEEE International Conference on Control System, Computing and Engineering, ICCSCE 2018, Penang, Malaysia, 23–25 November 2018; pp. 142–145. [Google Scholar]
- Kamaliya, B.; Mote, R.G. Nanofabrication Using Focused Ion Beam. In Advanced Machining Science; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9781482211108. [Google Scholar]
- Singh, K.; Rout, S.S.; Krywka, C.; Davydok, A. Local Structural Modifications in Metallic Micropillars Induced by Plasma Focused Ion Beam Processing. Materials 2023, 16, 7220. [Google Scholar] [CrossRef]
- Chen, Y.; An, L.B.; Yang, X.X. Recent Development of Focused Ion Beam System and Application. In Advanced Materials Research; Trans Tech Publications: Stafa-Zurich, Switzerland, 2013; Volume 753–755, ISBN 9783037857649. [Google Scholar]
- Guo, D.; Fan, S.; Yang, Y.; Chen, Z.; Huang, H.; Wen, P.; Lin, J.; Liu, Y.; Xu, J.; Wang, X. Large-Area and High-Precision Milling of Focused Ion Beam Based on the Integration of Nanoscale Machine Vision and Compensation Control. Microsc. Microanal. 2023, 29, 43–49. [Google Scholar] [CrossRef]
- Dadi, S.S.O.; Sharma, V.; Patel, D.S. Electrochemical Micromachining. In Miniaturized Electrochemical Devices: Advanced Concepts, Fabrication, and Applications; CRC Press: Boca Raton, FL, USA, 2023; ISBN 9781000917000. [Google Scholar]
- Pooranachandran, K.; Deepak, J.; Hariharan, P.; Mouliprasanth, B. Effect of Flushing on Electrochemical Micromachining of Copper and Inconel 718 Alloy. In Advances in Manufacturing Processes: Select Proceedings of ICEMMM; Springer: Singapore, 2018. [Google Scholar]
- Jain, V.K.; Chauhan, A.S.; Thakur, A.; Sidpara, A. Fabrications of Micro Tools and Micro Patterns by Electrochemical Micromachining and Some Investigation into Overpotential. J. Adv. Manuf. Syst. 2013, 12, 85–106. [Google Scholar] [CrossRef]
- Perla, V.R.; Suraparaju, S.K.; RathanRaj, K.J.; Sreenivasulu Reddy, A. Effect of Electrochemical Micromachining Process Parameters on Surface Roughness and Dimensional Deviation of Ti6Al4V by Tungsten Electrode. In Recent Advances in Mechanical Engineering; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9789811510700. [Google Scholar]
- Khot, S.; Rawal, S.U.; Patel, M.M. Dissolvable-Soluble or Biodegradable Polymers. In Drug Delivery Devices and Therapeutic Systems; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128198384. [Google Scholar]
- López-López, L.I. Tendencies in Development of Biodegradable Polymers. In Biodegradable Polymers; CRC Press: Boca Raton, FL, USA, 2023; ISBN 9781000886962. [Google Scholar]
- De Oliveira Sousa Neto, V.; Do Nascimento, R.F. Recent Progress in Biocomposite of Biodegradable Polymer. In Handbook of Composites from Renewable Materials; Wiley: Hoboken, NJ, USA, 2017; Volume 1–8, ISBN 9781119441632. [Google Scholar]
- Alaswad, S.O.; Mahmoud, A.S.; Arunachalam, P. Recent Advances in Biodegradable Polymers and Their Biological Applications: A Brief Review. Polymers 2022, 14, 4924. [Google Scholar] [CrossRef]
- Parthasarathy, M.; John, A.A. Tribology of Biodegradable Polymeric Systems. In Tribology of Polymers, Polymer Composites, and Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323907484. [Google Scholar]
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector-Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Coleman, S.; Azouz, A.B.; Schiphorst, J.T.; Saez, J.; Whyte, J.; McCluskey, P.; Kent, N.; Benito-Lopez, F.; Schenning, A.; Diamond, D. Next Generation, in-Situ Microfluidic Flow Control Using Stimuli Responsive Materials for Biomemetic Microfluicic Platforms. In Proceedings of the 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2016, Dublin, Ireland, 9–13 October 2016; pp. 1126–1127. [Google Scholar]
- Dunne, A.; Francis, W.; Delaney, C.; Florea, L.; Diamond, D.; Ramadan, M. Stimuli-Controlled Fluid Control and Microvehicle Movement in Microfluidic Channels; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128157336. [Google Scholar]
- Biswas, P.; Mukunthan Sulochana, G.N.; Banuprasad, T.N.; Goyal, P.; Modak, D.; Ghosh, A.K.; Chakraborty, S. All-Serotype Dengue Virus Detection through Multilayered Origami-Based Paper/Polymer Microfluidics. ACS Sens. 2022, 7, 3720–3729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Y.; Lu, X. An Integrated Paper Microfluidic Device Based on Isothermal Amplification for Simple Sample-to-Answer Detection of Campylobacter Jejuni. Appl. Environ. Microbiol. 2023, 89, e00695-23. [Google Scholar] [CrossRef] [PubMed]
- Kyatanwar, A.; Nagarsenker, M.; Prabhakar, B. Biodegradable Polymer-Based Microspheres for Depot Injection-Industry Perception. Recent. Adv. Drug Deliv. Formul. 2023, 17, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Fabozzi, A.; Della Sala, F.; di Gennaro, M.; Barretta, M.; Longobardo, G.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. Design of Functional Nanoparticles by Microfluidic Platforms as Advanced Drug Delivery Systems for Cancer Therapy. Lab. Chip 2023, 23, 1389–1409. [Google Scholar] [CrossRef]
- Fabozzi, A.; Barretta, M.; Valente, T.; Borzacchiello, A. Preparation and Optimization of Hyaluronic Acid Decorated Irinotecan-Loaded Poly(Lactic-Co-Glycolic Acid) Nanoparticles by Microfluidics for Cancer Therapy Applications. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131790. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Q.; Lin, J.; Cai, Z.; Liao, G.; Wang, K.; Bai, L.; Zhao, P.; Yu, Z. Recent Advance in Polymer Based Microspheric Systems for Controlled Protein and Peptide Delivery. Curr. Med. Chem. 2019, 26, 2285–2296. [Google Scholar] [CrossRef]
- Petta, D.; D’Amora, U.; D’Arrigo, D.; Tomasini, M.; Candrian, C.; Ambrosio, L.; Moretti, M. Musculoskeletal Tissues-on-a-Chip: Role of Natural Polymers in Reproducing Tissue-Specific Microenvironments. Biofabrication 2022, 14, 042001. [Google Scholar] [CrossRef]
- Rosellini, E.; Cascone, M.G. Microfluidic Fabrication of Natural Polymer-Based Scaffolds for Tissue Engineering Applications: A Review. Biomimetics 2023, 8, 74. [Google Scholar] [CrossRef]
- Sun, G.; Wang, P.; Ge, S.; Ge, L.; Yu, J.; Yan, M. Photoelectrochemical Sensor for Pentachlorophenol on Microfluidic Paper-Based Analytical Device Based on the Molecular Imprinting Technique. Biosens. Bioelectron. 2014, 56, 97–103. [Google Scholar] [CrossRef]
- Anthony, B.W.; Hardt, D.E.; Hale, M.; Zarrouati, N. A Research Factory for Polymer Microdevices: MuFac. In Proceedings of the Progress in Biomedical Optics and Imaging, SPIE, San Francisco, CA, USA, 23–25 January 2010; Volume 7593. [Google Scholar]
- Sardar, M.; Arun, R.K.; Ige, E.O.; Singh, P.; Kumar, G.; Chanda, N.; Biswas, G. Sustainable Polymer-Based Microfluidic Fuel Cells for Low-Power Applications. In Advances in Sustainable Polymers; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Nguyen, T.; Vinayaka, A.C.; Bang, D.D.; Wolff, A. A Complete Protocol for Rapid and Low-Cost Fabrication of Polymer Microfluidic Chips Containing Three-Dimensional Microstructures Used in Point-of-Care Devices. Micromachines 2019, 10, 624. [Google Scholar] [CrossRef]
- Hu, J.; Bai, H.; Wang, L.; Li, J.; Shen, Y.; Zhang, L.; Tang, J.; Wang, M.; Liu, Q.; Zhou, J.; et al. Hermetic Microfluidic Device for Point-of-Care Viral Nucleic Acid Testing. Sens. Actuators B Chem. 2024, 411, 135740. [Google Scholar] [CrossRef]
- Erice, E.; Mitxelena-Iribarren, O.; Arana, S.; Lawrie, C.H.; Mujika, M. Efficient Enrichment of Free Target Sequences in an Integrated Microfluidic Device for Point-of-Care Detection Systems. Nanomedicine 2024, 61, 102771. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shou, X.; Sheng, B.; Mei, J.; Shi, K.; Shang, L.; Zhu, X. Cell Membranes from Tumor-Tropic MSCs Screened by a Microfluidic Chip for Drug Nanoparticles Encapsulation and Cancer Targeted Therapy. Adv. Health Healthc. Mater. 2023, 12, 2202904. [Google Scholar] [CrossRef]
- Zhang, Q.; Kuang, G.; Wang, L.; Fan, L.; Zhao, Y. Tailoring Drug Delivery Systems by Microfluidics for Tumor Therapy. Mater. Today 2024, 73, 151–178. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, S.; Bharadwaj, A.; Rastogi, A. Microfluidic Systems: Recent Advances in Chronic Disease Diagnosis and Their Therapeutic Management. Indian J. Microbiol. 2024; 1–15. [Google Scholar] [CrossRef]
- Hrynevich, A.; Li, Y.; Cedillo-Servin, G.; Malda, J.; Castilho, M. (Bio)Fabrication of Microfluidic Devices and Organs-on-a-Chip; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323898317. [Google Scholar]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Su, H.; Zhao, P.; Deng, X.; Fan, Y.; Liu, X. The Research Progress of Microfluidic Organ-on-Chips. Yiyong Shengwu Lixue/J. Med. Biomech. 2019, 34, 320–326. [Google Scholar] [CrossRef]
- Zheng, F.; Fu, F.; Cheng, Y.; Wang, C.; Zhao, Y.; Gu, Z. Organ-on-a-Chip Systems: Microengineering to Biomimic Living Systems. Small 2016, 12, 2253–2282. [Google Scholar] [CrossRef]
- Li, Z.; Hui, J.; Yang, P.; Mao, H. Microfluidic Organ-on-a-Chip System for Disease Modeling and Drug Development. Biosensors 2022, 12, 370. [Google Scholar] [CrossRef]
- Han, C.; Labuz, J.M.; Takayama, S.; Park, J. Organs-on-a-Chip; 2014; ISBN 9780124201453.
- Lombello, C.B.; Rezende, L.R.; Martins, A.F.; Lameu, J. Organs-on-a-Chip: Principles and Applications. In Current Trends in Biomedical Engineering; Springer: Cham, Switzerland, 2023; ISBN 9783031387432. [Google Scholar]
- Huang, Y.; Liu, T.; Huang, Q.; Wang, Y. From Organ-on-a-Chip to Human-on-a-Chip: A Review of Research Progress and Latest Applications. ACS Sens. 2024, 9, 3466–3488. [Google Scholar] [CrossRef]
- Fu, J.; Qiu, H.; Tan, C.S. Microfluidic Liver-on-a-Chip for Preclinical Drug Discovery. Pharmaceutics 2023, 15, 1300. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, Y.; Li, Y.; Shen, C. Hydrogel Microfluidic-Based Liver-on-a-Chip: Mimicking the Mass Transfer and Structural Features of Liver. Biotechnol. Bioeng. 2021, 118, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Kulsharova, G.; Kurmangaliyeva, A.; Darbayeva, E.; Rojas-Solórzano, L.; Toxeitova, G. Development of a Hybrid Polymer-Based Microfluidic Platform for Culturing Hepatocytes towards Liver-on-a-Chip Applications. Polymers 2021, 13, 3215. [Google Scholar] [CrossRef]
- Rothbauer, M.; Bachmann, B.E.M.; Eilenberger, C.; Kratz, S.R.A.; Spitz, S.; Höll, G.; Ertl, P. A Decade of Organs-on-a-Chip Emulating Human Physiology at the Microscale: A Critical Status Report on Progress in Toxicology and Pharmacology. Micromachines 2021, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Marsano, A.; Conficconi, C.; Lemme, M.; Occhetta, P.; Gaudiello, E.; Votta, E.; Cerino, G.; Redaelli, A.; Rasponi, M. Beating Heart on a Chip: A Novel Microfluidic Platform to Generate Functional 3D Cardiac Microtissues. Lab. Chip 2016, 16, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Jayne, R.K.; Karakan, M.Ç.; Zhang, K.; Pierce, N.; Michas, C.; Bishop, D.J.; Chen, C.S.; Ekinci, K.L.; White, A.E. Direct Laser Writing for Cardiac Tissue Engineering: A Microfluidic Heart on a Chip with Integrated Transducers. Lab. Chip 2021, 21, 1724–1737. [Google Scholar] [CrossRef]
- Kato, Y.; Hirai, Y.; Kamei, K.; Tsuchiya, T.; Tabata, O. Microfluidic Device to Interconnect Multiple Organs via Fluidic Circulation: Towards Body-on-a-Chip. In Proceedings of the 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems, TRANSDUCERS 2015, Anchorage, AK, USA, 21–25 June 2015; pp. 1549–1552. [Google Scholar]
- Shuler, M.L.; Esch, M.B. Body-on-a Chip: Using Microfluidic Systems to Predict Human Responses to Drugs. Pure Appl. Chem. 2010, 82, 1635–1645. [Google Scholar] [CrossRef]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/Body-on-a-Chip Based on Microfluidic Technology for Drug Discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef]
- Venkatesalu, S.; Dilliyappan, S.; Satish Kumar, A.; Palaniyandi, T.; Baskar, G.; Ravi, M.; Sivaji, A. Prospectives and Retrospectives of Microfluidics Devices and Lab-on-A-Chip Emphasis on Cancer. Clin. Chim. Acta 2024, 552, 117646. [Google Scholar] [CrossRef]
- Ji, W.; Ho, T.-Y.; Wang, J.; Yao, H. Microfluidic Design for Concentration Gradient Generation Using Artificial Neural Network. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2020, 39, 2544–2557. [Google Scholar] [CrossRef]
- Zec, H.; Shin, D.J.; Wang, T.-H. Novel Droplet Platforms for the Detection of Disease Biomarkers. Expert. Rev. Mol. Diagn. 2014, 14, 787–801. [Google Scholar] [CrossRef]
- Gray, B.L. New Opportunities for Polymer Nanocomposites in Microfluidics and Biomedical MEMS: An Introduction to Cutting-Edge Composite Polymer Materials for Use in Microfluidics and Biomedical MEMS. IEEE Nanotechnol. Mag. 2014, 8, 6–16. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and Active Droplet Generation with Microfluidics: A Review. Lab. Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Jiang, L.; Liu, K.; Lin Bingcheng, B.; Qin Jianhua, J. A Microfluidic Device Integrated with Multichamber Polymerase Chain Reaction and Multichannel Separation for Genetic Analysis. Anal. Chim. Acta 2010, 674, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, Y.; Xu, G.; Liu, B.; Wang, Y.; Reboud, J.; Jajesniak, P.; Yan, S.; Ma, P.; Liu, F.; et al. Genetic and Phenotypic Profiling of Single Living Circulating Tumor Cells from Patients with Microfluidics. Proc. Natl. Acad. Sci. USA 2024, 121, e2315168121. [Google Scholar] [CrossRef]
- Qiao, Z.; Teng, X.; Liu, A.; Yang, W. Novel Isolating Approaches to Circulating Tumor Cell Enrichment Based on Microfluidics: A Review. Micromachines 2024, 15, 706. [Google Scholar] [CrossRef]
- Cinque, L.; Yamada, A.; Ghomchi, Y.; Baigl, D.; Chen, Y. Cell Trapping, DNA Extraction and Molecular Combing in a Microfluidic Device for High Throughput Genetic Analysis of Human DNA. Microelectron. Eng. 2011, 88, 1733–1736. [Google Scholar] [CrossRef]
- Kumar Thimmaraju, M.; Trivedi, R.; Hemalatha, G.; Thirupathy, B.; Mohathasim Billah, A. Microfluidic Revolution and Its Impact on Pharmaceutical Materials: A Review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Verma, A.; Bhattacharyya, S. Microfluidics—The State-of-the-Art Technology for Pharmaceutical Application. Adv. Pharm. Bull. 2022, 12, 700–711. [Google Scholar] [CrossRef]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes. 2018, 9, 103. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Jiang, X. Cell-Based Assays on Microfluidics for Drug Screening. ACS Sens. 2019, 4, 1465–1475. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Qi, H.; Jiao, Q.; Wu, L.; Wang, Y.; Liang, Q. The Latest Advances in High Content Screening in Microfluidic Devices. Expert. Opin. Drug Discov. 2023, 18, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, G.S.; Cruz-Moreira, D.; Visone, R.; Redaelli, A.; Rasponi, M. Microfabricated Physiological Models for in Vitro Drug Screening Applications. Micromachines 2016, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-W.; Ahmed, A.R.; Dereli-Korkut, Z.; Wang, S. Microfluidic Cell Chips for High-Throughput Drug Screening. Bioanalysis 2016, 8, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, W.; Choi, G.; Yang, X.; Miao, J.; Cui, L.; Guan, W. Arbitrarily Accessible 3D Microfluidic Device for Combinatorial High-Throughput Drug Screening. Sensors 2016, 16, 1616. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Pan, C.-W.; Lee, W.-C.; Li, K.-M. An Experimental Study of Micromilling Parameters to Manufacture Microchannels on a PMMA Substrate. Int. J. Adv. Manuf. Technol. 2014, 71, 1623–1630. [Google Scholar] [CrossRef]
- Alhalaili, B.; Popescu, I.N.; Rusanescu, C.O.; Vidu, R. Microfluidic Devices and Microfluidics-Integrated Electrochemical and Optical (Bio)Sensors for Pollution Analysis: A Review. Sustainability 2022, 14, 12844. [Google Scholar] [CrossRef]
- Campos, C.D.M.; Da Silva, J.A.F. Applications of Autonomous Microfluidic Systems in Environmental Monitoring. RSC Adv. 2013, 3, 18216–18227. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Guo, C.L.; Li, Y.; Zhao, B.; Cui, X. Research Progress of Paper-Based Microfluidic Devices. In Applied Mechanics and Materials; Trans Tech Publications: Stafa-Zurich, Switzerland, 2013; Volume 421, ISBN 9783037858783. [Google Scholar]
- Damodara, S.; Zhu, Y.; Selvaganapathy, P.R. Patterned Threads as Solid-State Reagent Storage and Delivery Medium for Automated Periodic Colorimetric Monitoring of the Environment. Microfluid. Nanofluidics 2021, 25, 93. [Google Scholar] [CrossRef]
- Keriel, N.-A.; Delezoide, C.; Chauvin, D.; Korri-Youssoufi, H.; Lai, N.D.; Ledoux-Rak, I.; Nguyen, C.-T. Optofluidic Sensor Based on Polymer Optical Microresonators for the Specific, Sensitive and Fast Detection of Chemical and Biochemical Species. Sensors 2023, 23, 7373. [Google Scholar] [CrossRef]
- Su, X.; Harynuk, A.; Alan Hatton, T.; Nazemifard, N. An Organometallic Polymer-Based Microfluidic Platform for Redox-Mediated Electrochemical Sensing. In Proceedings of the 21st International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2017, Online, 4–9 October 2020; pp. 507–508. [Google Scholar]
- Arif, A.; Zubair, A.; Khaliq, H.S.; Zubair, M.; Riaz, K.; Mehmood, M.Q. EBG-Based Sensor for Dielectric Characterization in Liquids. In Proceedings of the 2020 17th International Bhurban Conference on Applied Sciences and Technology, IBCAST 2020, Islamabad, Pakistan, 14–18 January 2020; pp. 633–636. [Google Scholar]
- Zhu, L.; Meier, D.; Montgomery, C.; Semancik, S.; DeVoe, D.L. A Water-Based Chemical Monitoring System Using Integrated Silicon-in-Plastic Microfabrication. In Proceedings of the Micro Total Analysis Systems, MicroTAS 2005 Conference: 9th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Boston, MA, USA, 9–13 October 2005; Volume 1, pp. 482–484. [Google Scholar]
- Hossein-Babaei, F.; Hooshyar Zare, A. The Selective Flow of Volatile Organic Compounds in Conductive Polymer-Coated Microchannels. Sci. Rep. 2017, 7, 42299. [Google Scholar] [CrossRef]
- Meredith, N.A.; Quinn, C.; Cate, D.M.; Reilly, T.H.; Volckens, J.; Henry, C.S. Paper-Based Analytical Devices for Environmental Analysis. Analyst 2016, 141, 1874–1887. [Google Scholar] [CrossRef]
- Shimali; Chamoli, S.; Kumar, P. Current Challenges and Future Prospects of Next-Generation Microfluidics. In Next-Generation Smart Biosensing; Academic Press: Cambridge, MA, USA, 2024; ISBN 9780323988056. [Google Scholar]
- Debski, P.R.; Sklodowska, K.; Michalski, J.A.; Korczyk, P.M.; Dolata, M.; Jakiela, S. Continuous Recirculation of Microdroplets in a Closed Loop Tailored for Screening of Bacteria Cultures. Micromachines 2018, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Abrishamkar, A.; Paradinas, M.; Bailo, E.; Rodriguez-Trujillo, R.; Pfattner, R.; Rossi, R.M.; Ocal, C.; Demello, A.J.; Amabilino, D.B.; Puigmartí-Luis, J. Microfluidic Pneumatic Cages: A Novel Approach for in-Chip Crystal Trapping, Manipulation and Controlled Chemical Treatment. J. Vis. Exp. 2016, 2016, e54193. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Wang, Y.; Wei, X. Synthesis of Energetic Materials by Microfluidics. Def. Technol. 2024, in press. [Google Scholar] [CrossRef]
- Orazi, L.; Siciliani, V.; Pelaccia, R.; Oubellaouch, K.; Reggiani, B. Ultrafast Laser Micromanufacturing of Microfluidic Devices. In Proceedings of the Procedia CIRP, Lyon, France, 8–10 June 2022; Volume 110, pp. 122–127. [Google Scholar]
- Fradique, R.; Azevedo, A.M.; Chu, V.; Conde, J.P.; Aires-Barros, M.R. Microfluidic Platform for Rapid Screening of Bacterial Cell Lysis. J. Chromatogr. A 2020, 1610, 460539. [Google Scholar] [CrossRef]
- Bjork, S.M.; Joensson, H.N. Microfluidics for Cell Factory and Bioprocess Development. Curr. Opin. Biotechnol. 2019, 55, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.F.; Andrade-Pérez, V.; Vargas, M.C.; Mantilla-Orozco, A.; Osma, J.F.; Reyes, L.H.; Cruz, J.C. Breaking the Clean Room Barrier: Exploring Low-Cost Alternatives for Microfluidic Devices. Front. Bioeng. Biotechnol. 2023, 11, 1176557. [Google Scholar] [CrossRef]
- Papatheodorou, S.A.; Tsironi, T.; Giannakourou, M.; Halvatsiotis, P.; Houhoula, D. Application of Microfluidic Paper-Based Analytical Devices (ΜPADs) for Food Microbial Detection. J. Sci. Food Agric. 2023, 103, 2215. [Google Scholar] [CrossRef]
- Jiang, W.; Tang, Q.; Zhu, Y.; Gu, X.; Wu, L.; Qin, Y. Research Progress of Microfluidics-Based Food Safety Detection. Food Chem. 2024, 441, 138319. [Google Scholar] [CrossRef]
- Sridhar, A.; Kapoor, A.; Kumar, P.S.; Ponnuchamy, M.; Sivasamy, B.; Vo, D.-V.N. Lab-on-a-Chip Technologies for Food Safety, Processing, and Packaging Applications: A Review. Environ. Chem. Lett. 2022, 20, 901–927. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Šalić, A.; Valinger, D.; Jurina, T.; Benković, M.; Kljusurić, J.G.; Zelić, B. The Power of Microsystem Technology in the Food Industry—Going Small Makes It Better. Innov. Food Sci. Emerg. Technol. 2021, 68, 102613. [Google Scholar] [CrossRef]
- Diep Trinh, T.N.; Trinh, K.T.L.; Lee, N.Y. Microfluidic Advances in Food Safety Control. Food Res. Int. 2024, 176, 113799. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, J.U.; Rao, L.T.; Rewatkar, P.; Khan, H.; Dubey, S.K.; Javed, A.; Kim, G.M.; Goel, S. Single Microfluidic Fuel Cell with Three Fuels—Formic Acid, Glucose and Microbes: A Comparative Performance Investigation. J. Electrochem. Sci. Eng. 2021, 11, 305–316. [Google Scholar] [CrossRef]
- Betancur, S.; Quevedo, L.; Olmos, C.M. Microfluidic Devices, Materials, and Recent Progress for Petroleum Applications: A Review. Can. J. Chem. Eng. 2024, 102, 2583–2607. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, T.; Tao, K.; Chang, H. Generating Electricity on Chips: Microfluidic Biofuel Cells in Perspective. Ind. Eng. Chem. Res. 2018, 57, 2746–2758. [Google Scholar] [CrossRef]
- Tata Rao, L.; Rewatkar, P.; Dubey, S.K.; Javed, A.; Goel, S. Performance Optimization of Microfluidic Paper Fuel-Cell with Varying Cellulose Fiber Papers as Absorbent Pad. Int. J. Energy Res. 2020, 44, 3893–3904. [Google Scholar] [CrossRef]
- Zhang, H.; Xuan, J.; Leung, D.Y.C.; Wang, H.; Xu, H.; Zhang, L. Advanced Gas-Emission Anode Design for Microfluidic Fuel Cell Eliminating Bubble Accumulation. J. Micromechanics Microeng. 2017, 27, 105016. [Google Scholar] [CrossRef]
- Lifton, V.A. Microfluidics: An Enabling Screening Technology for Enhanced Oil Recovery (EOR). Lab. Chip 2016, 16, 1777–1796. [Google Scholar] [CrossRef]
- da Silva, A.G.P.; Santana, H.S.; Bagarolo, R.; Rodrigues, A.C.; Castilho, G.J.; Cremasco, M.A.; Taranto, O.P. Application of Raman Spectroscopy in Microfluidic Devices for On-Line Determination of Ethanol Concentration in Water and Vegetable Oil. Chem. Eng. Trans. 2019, 74, 739–744. [Google Scholar] [CrossRef]
- Kumar, R.; Chauhan, G.; Martinez-Chapa, S.O. Metal Oxides and Their Composites as Flow-through Biosensors for Biomonitoring. In Metal Oxides for Biomedical and Biosensor Applications; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128230589. [Google Scholar]
- Cheng, S.K.S.; Li, T.; Meena, S.S.; Cao, Q.; Li, B.; Kosgei, B.K.; Cheng, T.; Luo, P.; Liu, Q.; Zhu, G.; et al. Advances in Microfluidic Technologies for Energy Storage and Release Systems. Adv. Energy Sustain. Res. 2022, 3, 2200060. [Google Scholar] [CrossRef]
- Madukwe, D.U.P.; Mike-Ogburia, M.I.; Nduka, N.; Nzeobi, J. Smart Microfluidics: Synergy of Machine Learning and Microfluidics in the Development of Medical Diagnostics for Chronic and Emerging Infectious Diseases. Crit. Rev. Biomed. Eng. 2023, 51, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Linert, J. Applications of Microfluidics and Nanotechnologies for Point-of-Care Devices. In Proceedings of the IFAC-PapersOnLine, Prishtina, Kosovo, 26–28 October 2022; Volume 55, pp. 364–369. [Google Scholar]
- Zahed, M.A.; Kim, D.K.; Jeong, S.H.; Selim Reza, M.; Sharifuzzaman, M.; Pradhan, G.B.; Song, H.; Asaduzzaman, M.; Park, J.Y. Microfluidic-Integrated Multimodal Wearable Hybrid Patch for Wireless and Continuous Physiological Monitoring. ACS Sens. 2023, 8, 2960–2974. [Google Scholar] [CrossRef] [PubMed]
- Dorta-Gorrín, A.; Navas-Méndez, J.; Gozalo-Margüello, M.; Miralles, L.; García-Hevia, L. Detection of SARS-CoV-2 Based on Nucleic Acid Amplification Tests (NAATs) and Its Integration into Nanomedicine and Microfluidic Devices as Point-of-Care Testing (POCT). Int. J. Mol. Sci. 2023, 24, 233. [Google Scholar] [CrossRef]
- Kazemi, N.; Abdolrazzaghi, M.; Light, P.E.; Musilek, P. In–Human Testing of a Non-Invasive Continuous Low–Energy Microwave Glucose Sensor with Advanced Machine Learning Capabilities. Biosens. Bioelectron. 2023, 241, 115668. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Pan, Z.; De Bock, M.; Tan, T.X.; Wang, Y.; Shi, Y.; Yan, N.; Yetisen, A.K. A Wearable Microneedle Patch Incorporating Reversible FRET-Based Hydrogel Sensors for Continuous Glucose Monitoring. Biosens. Bioelectron. 2024, 262, 116542. [Google Scholar] [CrossRef]
- Xu, K.-X.; Chen, X.-L.; Li, D.-C.; Yu, H.-X. Minimally Invasive Continuous Blood Glucose Monitor Based on Microfluidic and Enzyme Colorimetric Technologies. Guangxue Jingmi Gongcheng/Opt. Precis. Eng. 2018, 26, 2615–2622. [Google Scholar] [CrossRef]
- Annese, V.F.; Hu, C. Integrating Microfluidics and Electronics in Point-of-Care Diagnostics: Current and Future Challenges. Micromachines 2022, 13, 1923. [Google Scholar] [CrossRef]
- Roy, N.; Jaiswal, S.; Dhwaj, A.; Verma, D.; Prabhakar, A. Nanobiosensor-Based Microfluidic Point-of-Care Platforms: Fabrication, Characterization, and Applications; Springer: Singapore, 2023; ISBN 9789811951411. [Google Scholar]
- Boyd, M.; Woolley, T. Point of Care Testing. Surgery 2016, 34, 91–93. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Saberi, Z.; Kazemifard, N. Functionalized Nanomaterial-Based Medical Sensors for Point-of-Care Applications: An Overview. In Functionalized Nanomaterial-Based Electrochemical Sensors; Woodhead Publishing: Sawston, UK, 2022; ISBN 9780128237885. [Google Scholar]
- Kumar, S.; Nehra, M.; Khurana, S.; Dilbaghi, N.; Kumar, V.; Kaushik, A.; Kim, K.-H. Aspects of Point-of-Care Diagnostics for Personalized Health Wellness. Int. J. Nanomed. 2021, 16, 383–402. [Google Scholar] [CrossRef]
- Ayatollahi, H. Point-of-Care Diagnostics with Smartphone. In Smartphone-Based Detection Devices; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128236963. [Google Scholar]
- Borriello, M.; Tarabella, G.; D’Angelo, P.; Liboà, A.; Barra, M.; Vurro, D.; Lombari, P.; Coppola, A.; Mazzella, E.; Perna, A.F.; et al. Lab on a Chip Device for Diagnostic Evaluation and Management in Chronic Renal Disease: A Change Promoting Approach in the Patients’ Follow Up. Biosensors 2023, 13, 373. [Google Scholar] [CrossRef]
- Mondal, S.; Narasimhan, R.; Yathirajula, R.B.; Medhi, I.; Li, L.; Wang, S.; Iyer, P.K. Emerging Technology for Point-of-Care Diagnostics: Recent. Developments. In Advanced Nanomaterials for Point of Care Diagnosis and Therapy; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323857253. [Google Scholar]
- Bissonnette, L.; Bergeron, M.G. Portable Devices and Mobile Instruments for Infectious Diseases Point-of-Care Testing. Expert. Rev. Mol. Diagn. 2017, 17, 471–494. [Google Scholar] [CrossRef]
- Wu, A.H.B. “On Vivo” and Wearable Clinical Laboratory Testing Devices for Emergency and Critical Care Laboratory Testing. J. Appl. Lab. Med. 2019, 4, 254–263. [Google Scholar] [CrossRef]

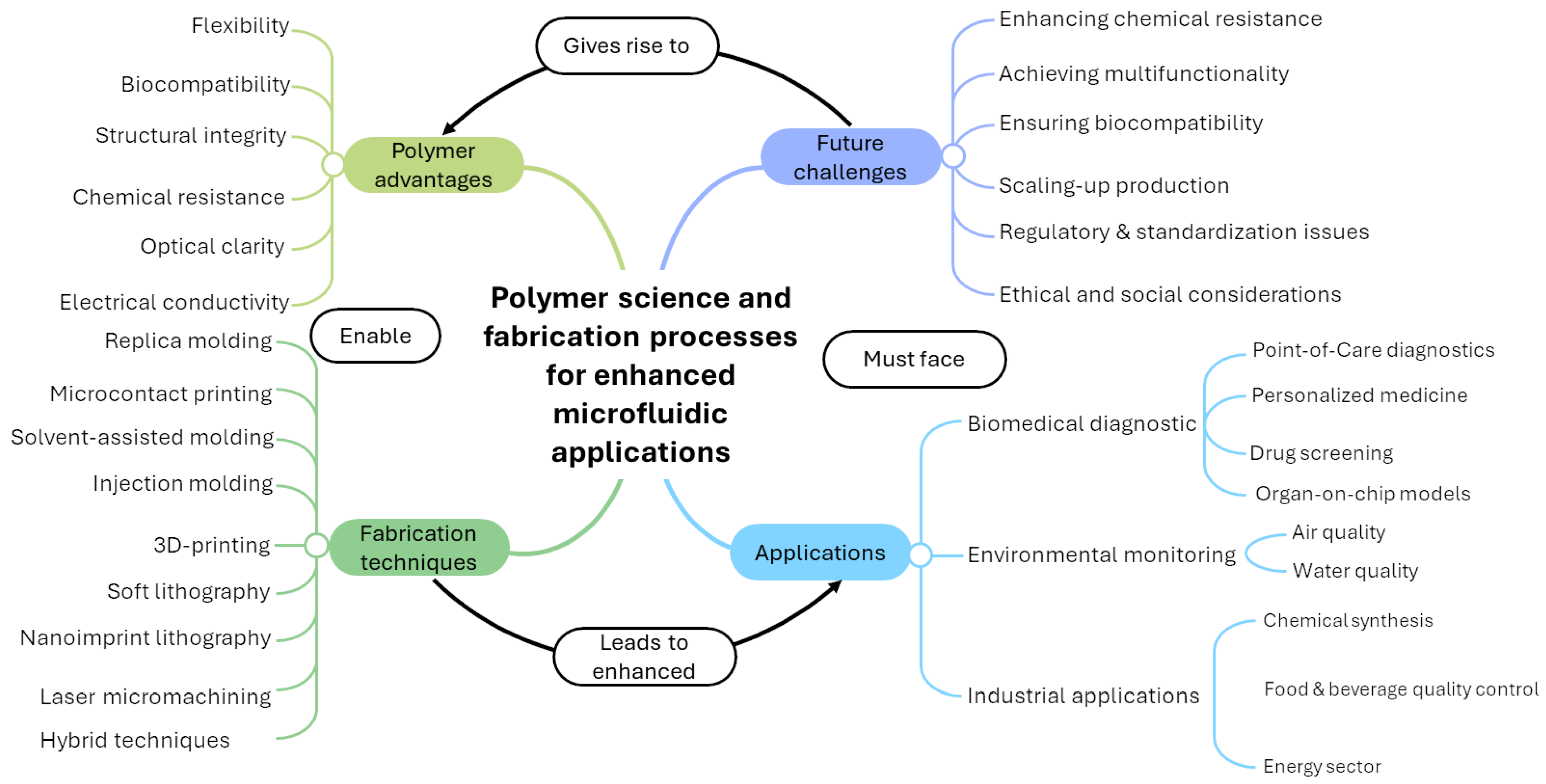

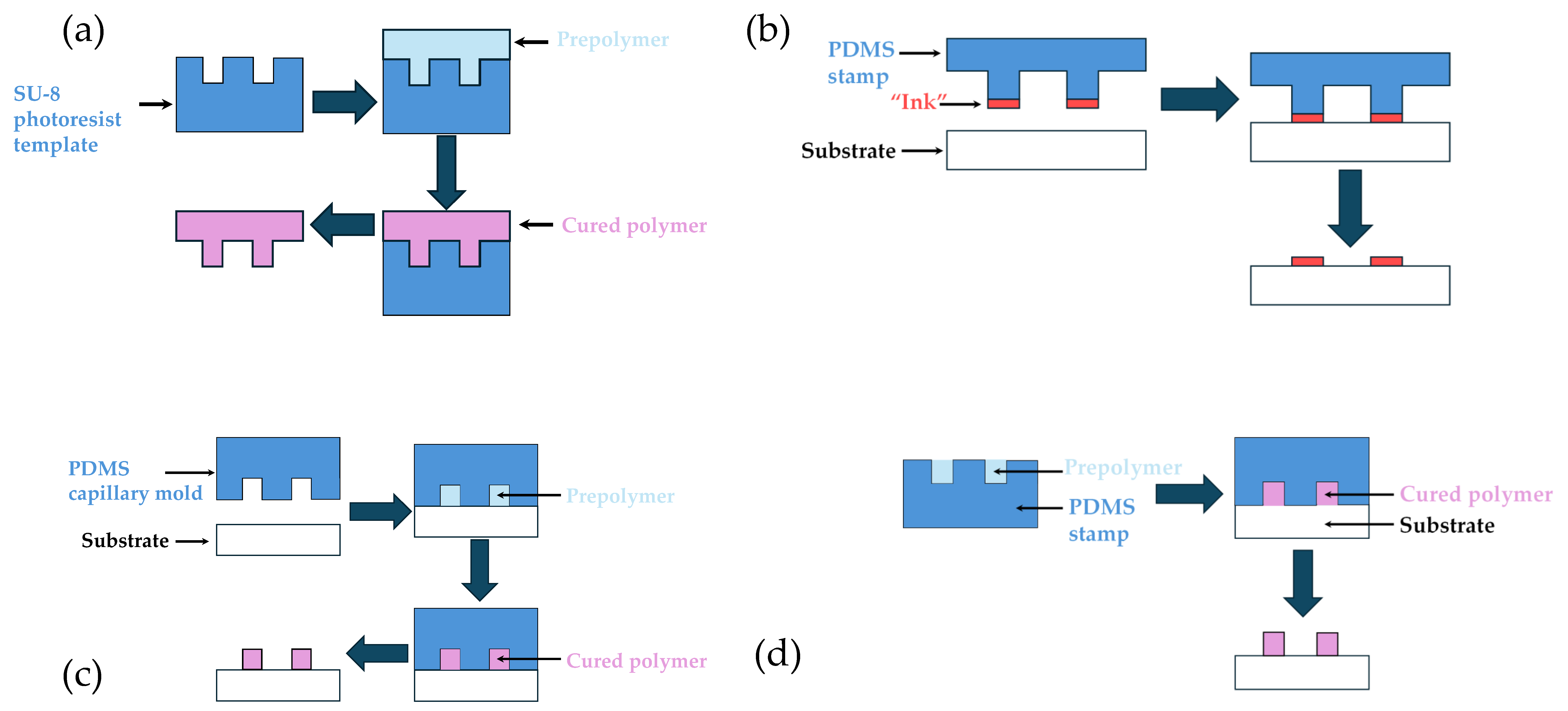
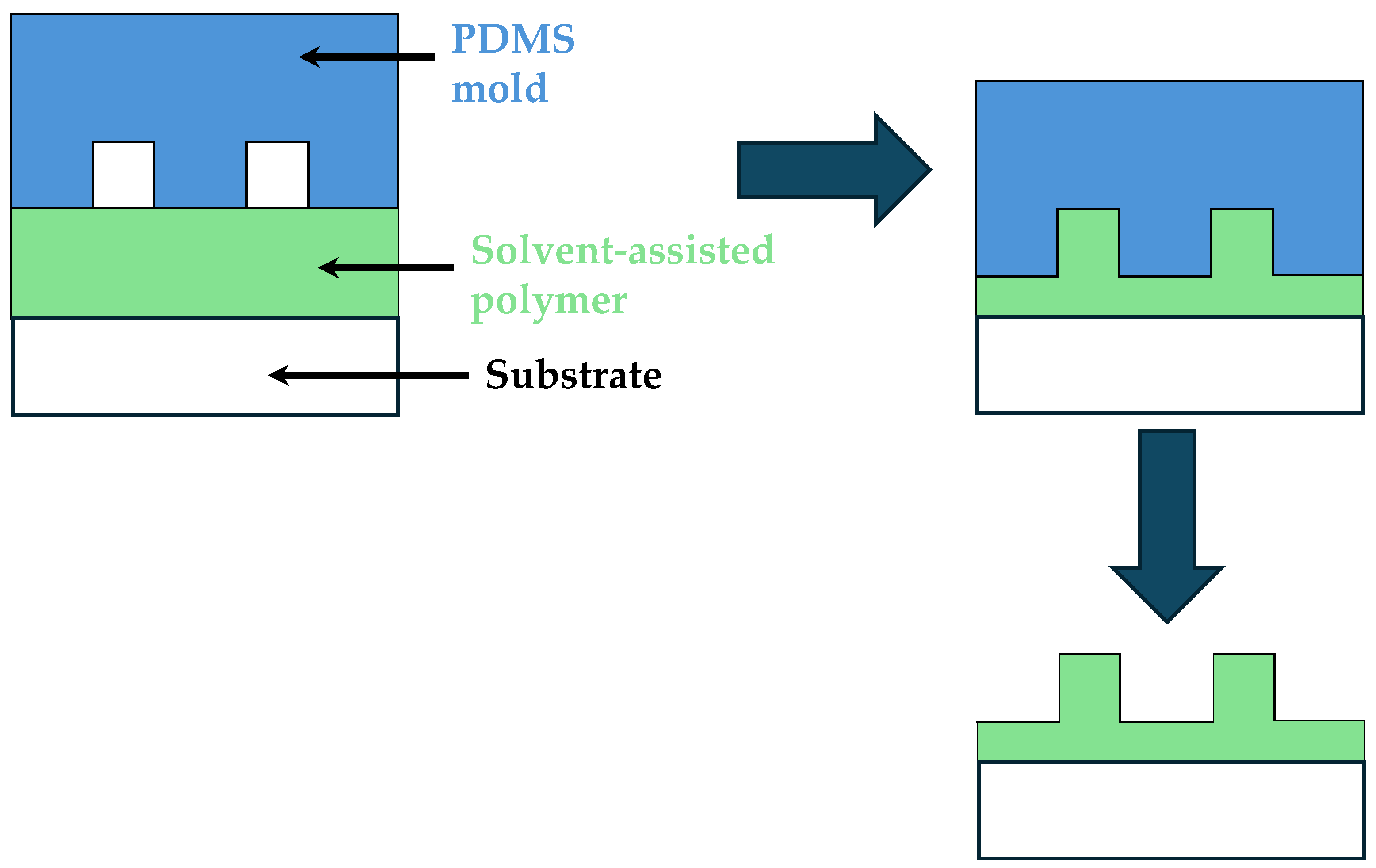
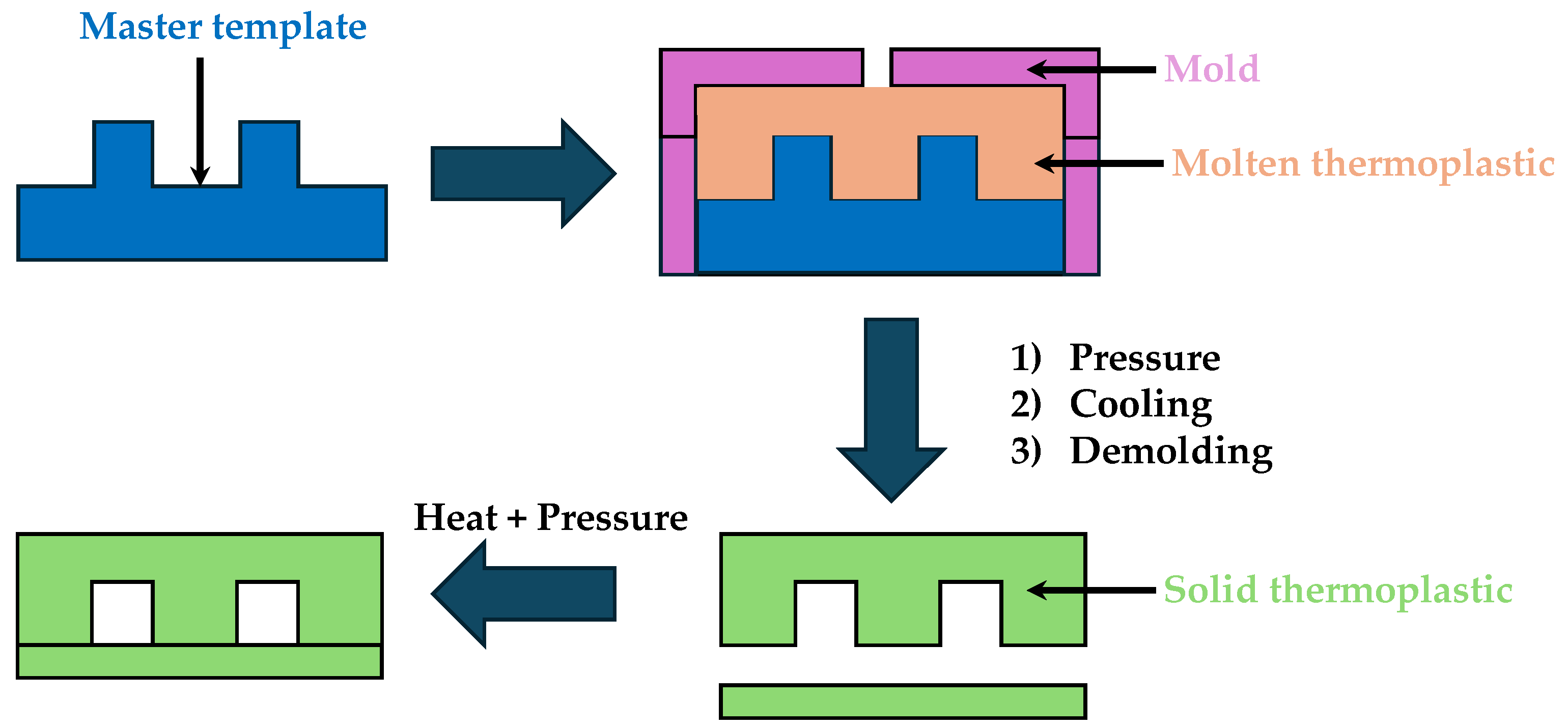
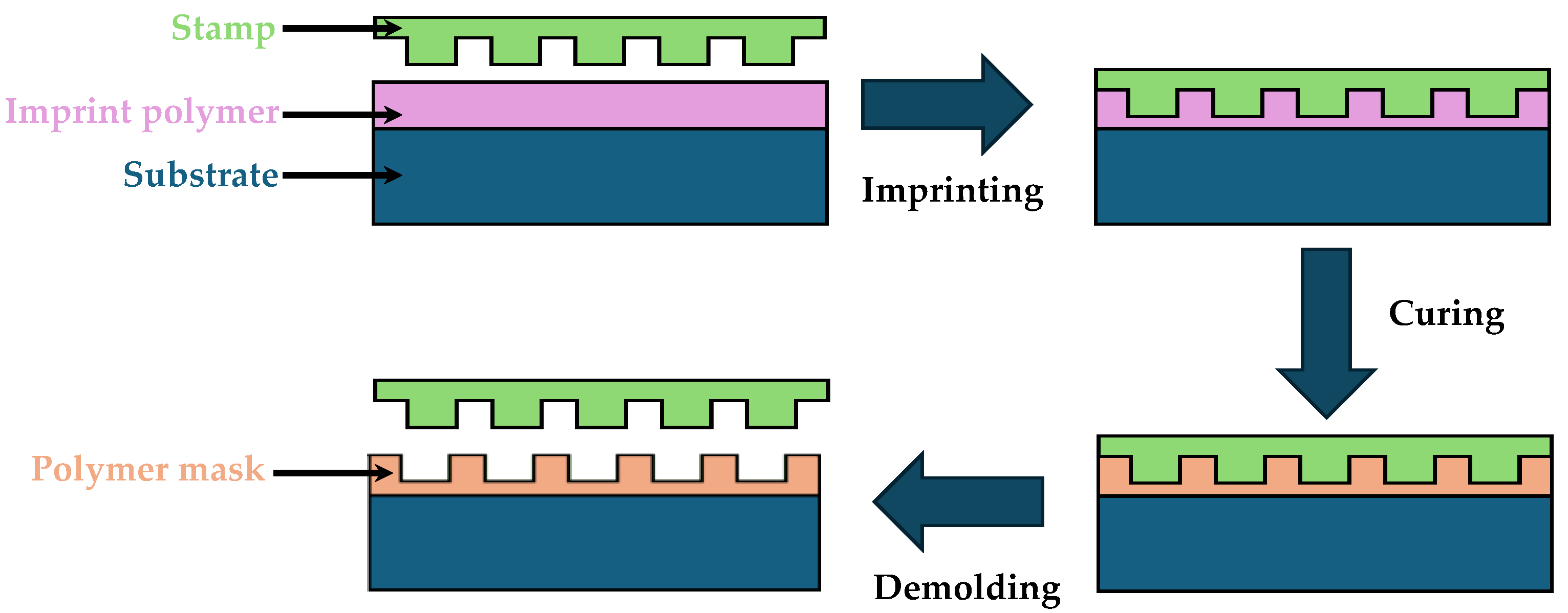
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandre-Franco, M.F.; Kouider, R.; Kassir Al-Karany, R.; Cuerda-Correa, E.M.; Al-Kassir, A. Recent Advances in Polymer Science and Fabrication Processes for Enhanced Microfluidic Applications: An Overview. Micromachines 2024, 15, 1137. https://doi.org/10.3390/mi15091137
Alexandre-Franco MF, Kouider R, Kassir Al-Karany R, Cuerda-Correa EM, Al-Kassir A. Recent Advances in Polymer Science and Fabrication Processes for Enhanced Microfluidic Applications: An Overview. Micromachines. 2024; 15(9):1137. https://doi.org/10.3390/mi15091137
Chicago/Turabian StyleAlexandre-Franco, María F., Rahmani Kouider, Raúl Kassir Al-Karany, Eduardo M. Cuerda-Correa, and Awf Al-Kassir. 2024. "Recent Advances in Polymer Science and Fabrication Processes for Enhanced Microfluidic Applications: An Overview" Micromachines 15, no. 9: 1137. https://doi.org/10.3390/mi15091137
APA StyleAlexandre-Franco, M. F., Kouider, R., Kassir Al-Karany, R., Cuerda-Correa, E. M., & Al-Kassir, A. (2024). Recent Advances in Polymer Science and Fabrication Processes for Enhanced Microfluidic Applications: An Overview. Micromachines, 15(9), 1137. https://doi.org/10.3390/mi15091137







