Preparation and Properties Study of CsPbX3@PMMA Luminescent Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CsPbX3 Nanocrystals
2.2. Synthesis of CsPbX3@PMMA Luminescent Resin
2.3. Stability Test of CsPbX3@PMMA
3. Results and Discussion
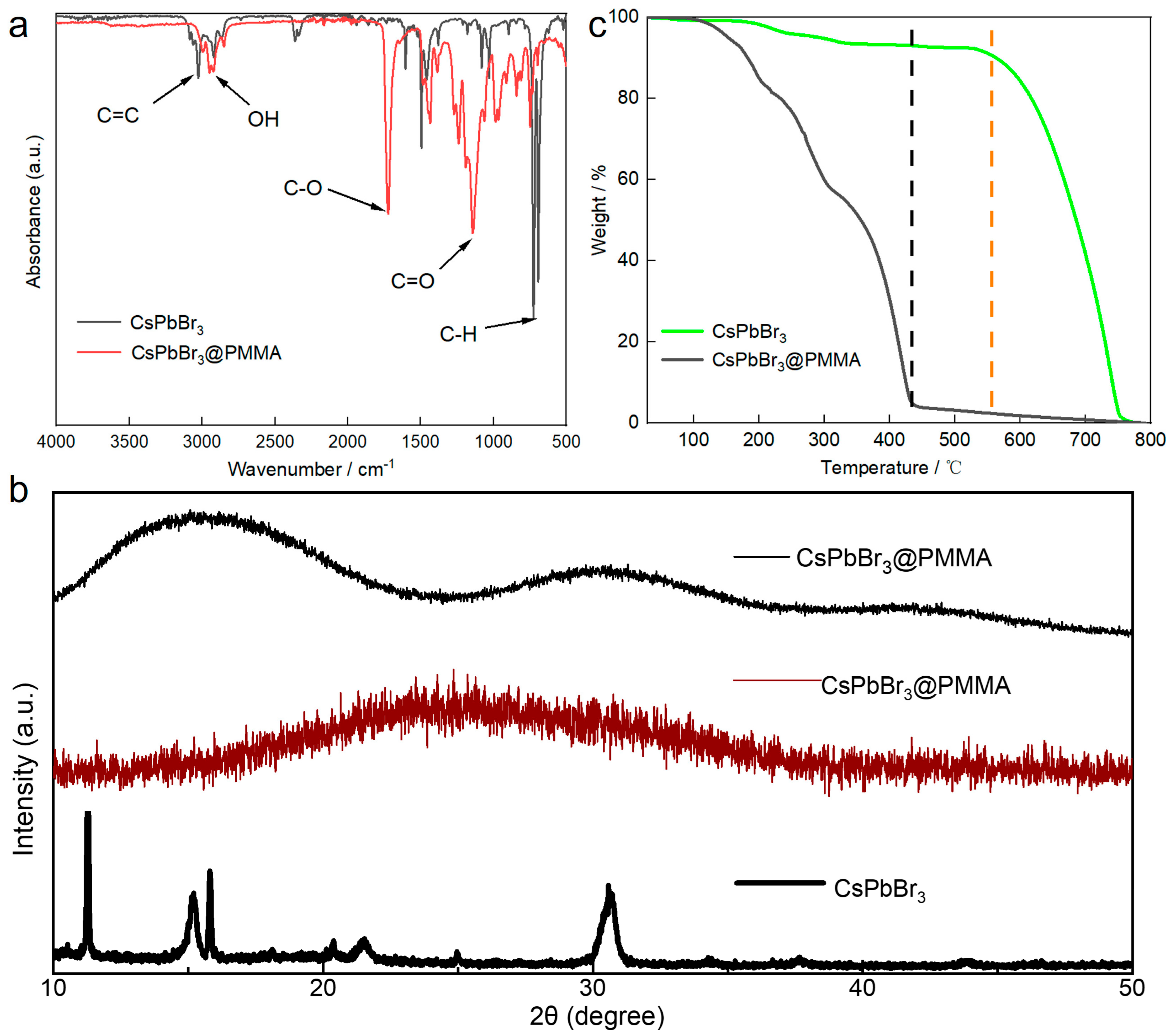
3.1. CsPbBr3@PMMA Structural Characterization
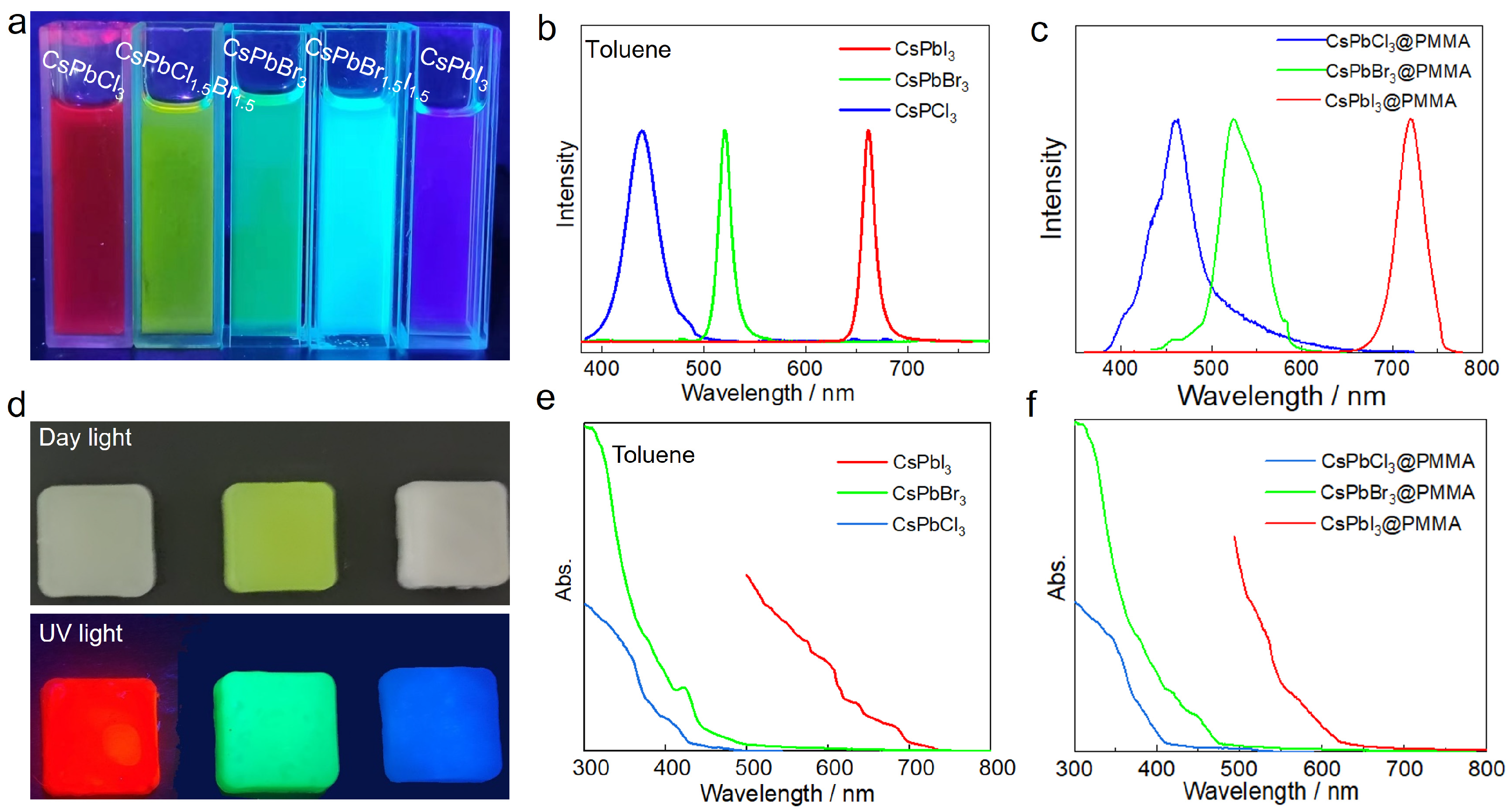
3.2. CsPbBr3@PMMA Photophysical Properties
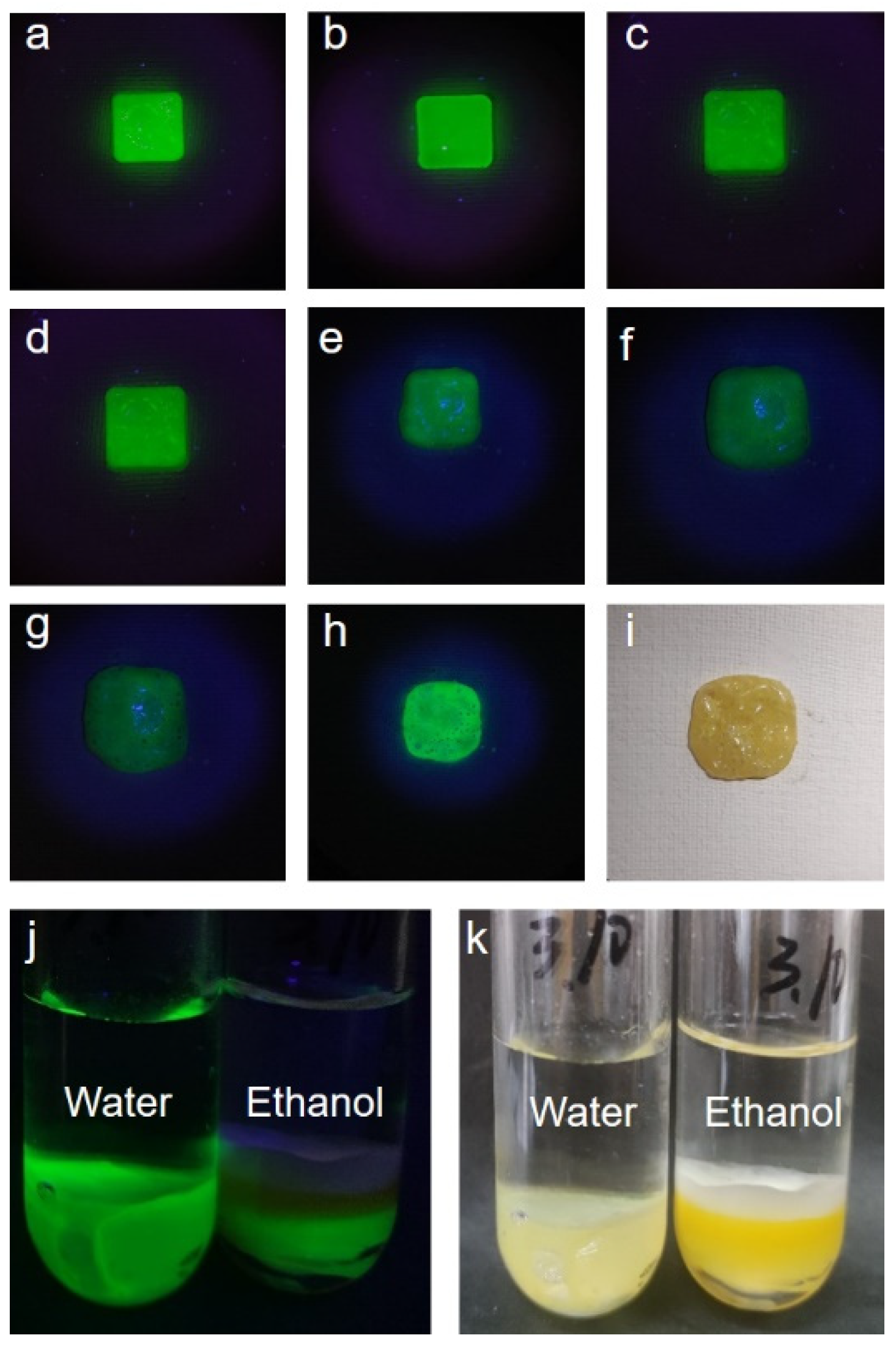
3.3. CsPbX3@PMMA Temperature Response and Stability Test
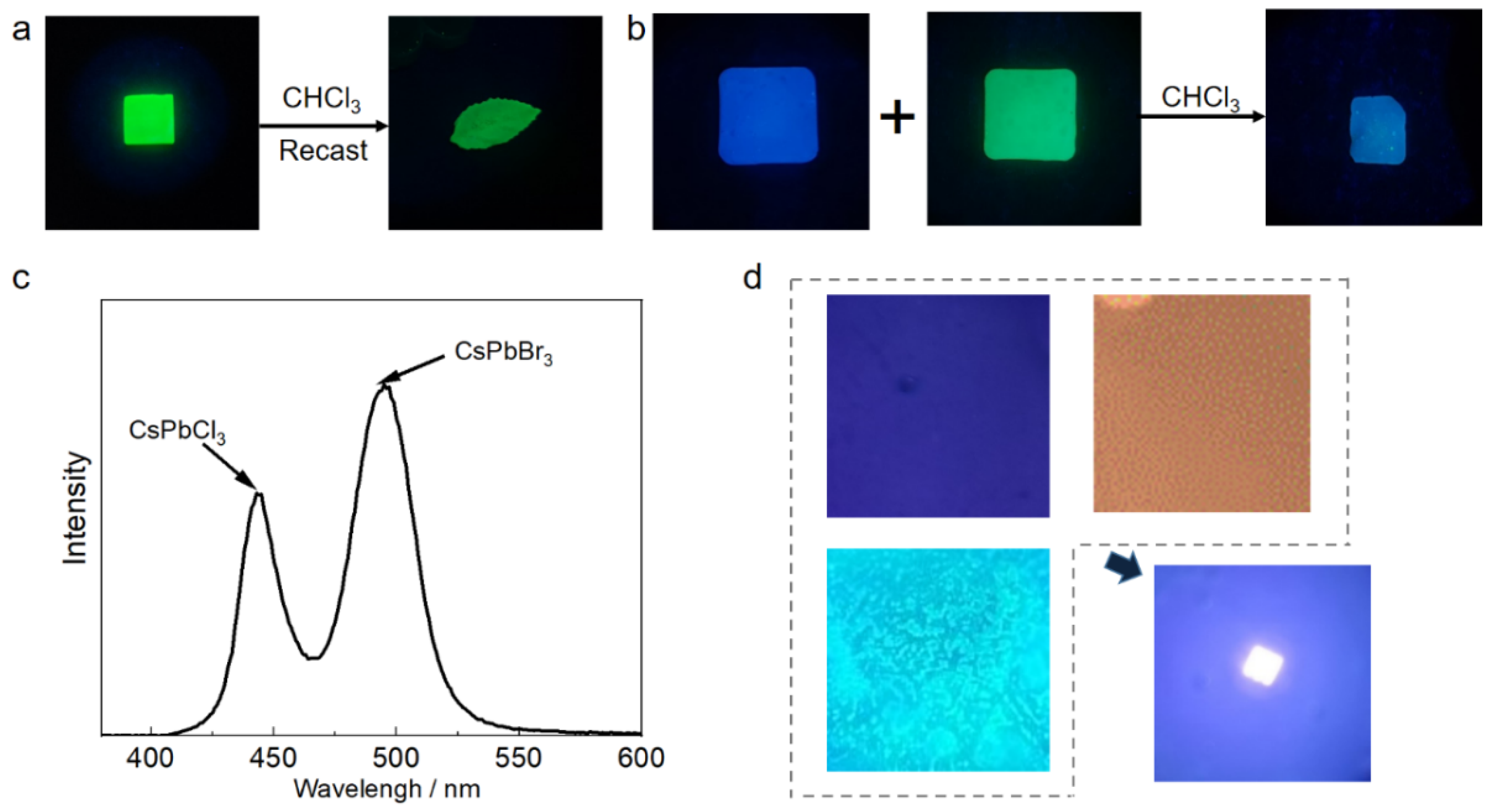
3.4. CsPbX3@PMMA Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Katz, E.A. Perovskite: Name puzzle and German-Russian odyssey of discovery. Helv. Chim. Acta 2020, 103, e2000061. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Song, J.; Li, X.; Zeng, H.; Sun, H. All-inorganic colloidal perovskite quantum dots: A new class of lasing materials with favorable characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Bai, Z.; Zhong, H. In situ fabricated perovskite nanocrystals: A revolution in optical materials. Adv. Opt. Mater. 2018, 6, 1800380. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic–inorganic perovskites: Structural versatility for functional materials design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.R.; Liao, Z.H.; Wang, F. Shape-controlled synthesis towards one-dimensional cesium lead halide perovskite nanocrystals: Methods and advances. J. Mater. Chem. C 2023, 11, 3409–3427. [Google Scholar] [CrossRef]
- Chen, L.J. Synthesis and optical properties of lead-free cesium germanium halide perovskite quantum rods. RSC Adv. 2018, 8, 18396–18399. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Hu, H.; Zou, Y.; Chen, M.; Wu, L.; Yang, D.; Yuan, X.; Fan, J.; Sun, B.; Zhang, Q. Microwave-assisted synthesis of high-quality “all-inorganic” CsPbX3 (X = Cl, Br, I) perovskite nanocrystals and their application in light emitting diodes. J. Mater. Chem. C 2017, 5, 10947–10954. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Turyanska, L.; Cao, H.; Zhao, L.; Fay, M.W.; Temperton, R.; O’Shea, J.; Thomas, N.R.; Wang, K.; Luan, W. Hybrid light emitting diodes based on stable, high brightness all-inorganic CsPbI3 perovskite nanocrystals and InGaN. Nanoscale 2019, 11, 13450–13457. [Google Scholar] [CrossRef] [PubMed]
- Sarker, J.C.; Hogarth, G. Dithiocarbamate complexes as single source precursors to nanoscale binary, ternary and quaternary metal sulfides. Chem. Rev. 2021, 121, 6057–6123. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Lin, C.; Guan, X.; Shahrokhi, S.; Huang, C.; Wang, Y.; He, T.; Singh, S.; Hu, L.; Retamal, J.R.D.; et al. Halide perovskites: A new era of solution-processed electronics. Adv. Mater. 2021, 33, 2005000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ding, D.; Wang, Y.; Fang, S.; Liu, Z.; Tang, J.; Li, H.; Zhong, H.; Tian, B.; Shi, Y. A scalable H2O–DMF–DMSO solvent synthesis of highly luminescent inorganic perovskite-related cesium lead bromides. Adv. Opt. Mater. 2021, 9, 2001435. [Google Scholar] [CrossRef]
- Wang, S.; Amin, A.A.Y.; Wu, L.; Cao, M.; Zhang, Q.; Ameri, T. Perovskite nanocrystals: Synthesis, stability, and optoelectronic applications. Small Struct. 2021, 2, 2000124. [Google Scholar] [CrossRef]
- Zhou, Q.; Bai, Z.; Lu, W.-G.; Wang, Y.; Zou, B.; Zhong, H. In situ fabrication of halide perovskite nanocrystal-embedded polymer composite films with enhanced photoluminescence for display backlights. Adv. Mater. 2016, 28, 9163–9168. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, R.; Ma, W.; Zhang, H.; Yang, L. One-step spray coating strategy toward a highly uniform large-area CsPbBr 3@ PMMA composite film for backlit display. Opt. Express 2022, 30, 20241–20249. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.K.; Maddalena, F.; Kowal, D.; Makowski, M.; Mahato, S.; Jȩdrzejewski, R.; Bhattarai, R.; Witkowski, M.E.; Drozdowski, K.J.; Drozdowski, W.; et al. Effect of Dual-Organic Cations on the Structure and Properties of 2D Hybrid Perovskites as Scintillators. ACS Appl. Mater. Interfaces 2024, 16, 25529–25539. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, T.; Jin, Z.; Li, M.; Zhang, D.; Duan, L.; Zhao, Z.; Wang, C. Tough, stable and self-healing luminescent perovskite-polymer matrix applicable to all harsh aquatic environments. Nat. Commun. 2022, 13, 1338. [Google Scholar] [CrossRef] [PubMed]
- Ganewatta, M.S.; Wang, Z.; Tang, C. Chemical syntheses of bioinspired and biomimetic polymers toward biobased materials. Nat. Rev. Chem. 2021, 5, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yang, W. Surface chemoselective phototransformation of C–H bonds on organic polymeric materials and related high-tech applications. Chem. Rev. 2013, 113, 5547–5594. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, Z.-j.; Wang, J.; Huang, C.-h.; Liu, L.-z.; Qiao, X.-z.; Liu, C.; Song, Q.; Li, Y.-c. Post treatment preparation of hybrid metal halide perovskite nanocrystal-embedded polymethylmethacrylate blends with enhanced stablity. J. Meas. Sci. Instrum. 2017, 8, 103. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Fan, S.; Yang, W.; Wei, J.; Wang, X.; Ni, J.; Cheng, W.; Zhang, Q. Preparation and Properties Study of CsPbX3@PMMA Luminescent Resin. Micromachines 2024, 15, 1150. https://doi.org/10.3390/mi15091150
Ma X, Fan S, Yang W, Wei J, Wang X, Ni J, Cheng W, Zhang Q. Preparation and Properties Study of CsPbX3@PMMA Luminescent Resin. Micromachines. 2024; 15(9):1150. https://doi.org/10.3390/mi15091150
Chicago/Turabian StyleMa, Xinqiang, Shengying Fan, Wenwen Yang, Jiajie Wei, Xiaolei Wang, Jincheng Ni, Wei Cheng, and Qinhe Zhang. 2024. "Preparation and Properties Study of CsPbX3@PMMA Luminescent Resin" Micromachines 15, no. 9: 1150. https://doi.org/10.3390/mi15091150







