Corrosion Behavior and Biological Properties of ZK60/HA Composites Prepared by Laser Powder Bed Fusion
Abstract
:1. Introduction
2. Methodology
2.1. Preparation and Characterization of the HA/ZK60 Composites
2.2. Preparation and Characterization of the HA/ZK60 Product
2.3. Electrochemical Measurements
2.4. Immersion Experiments
3. Results and Discussion
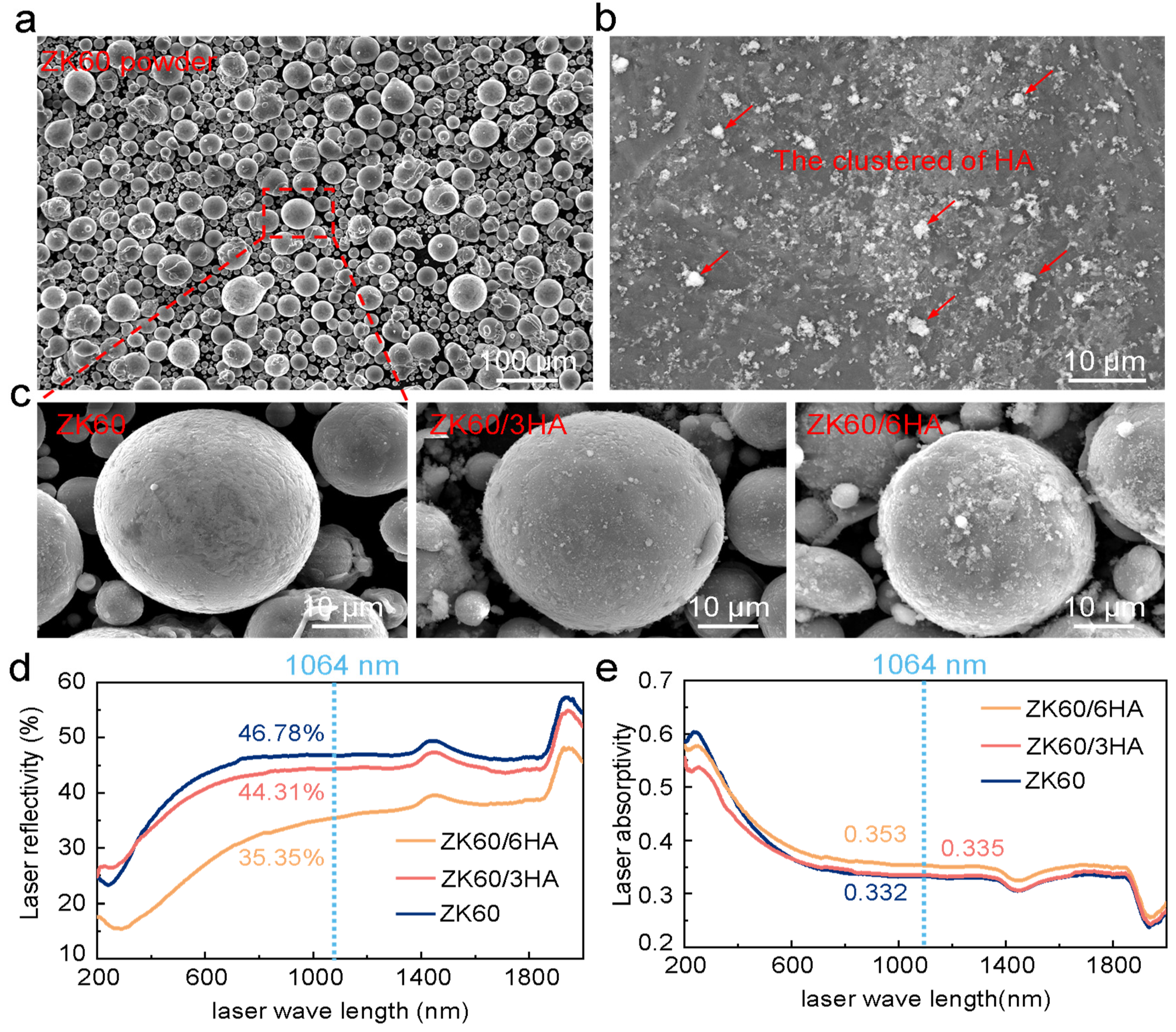
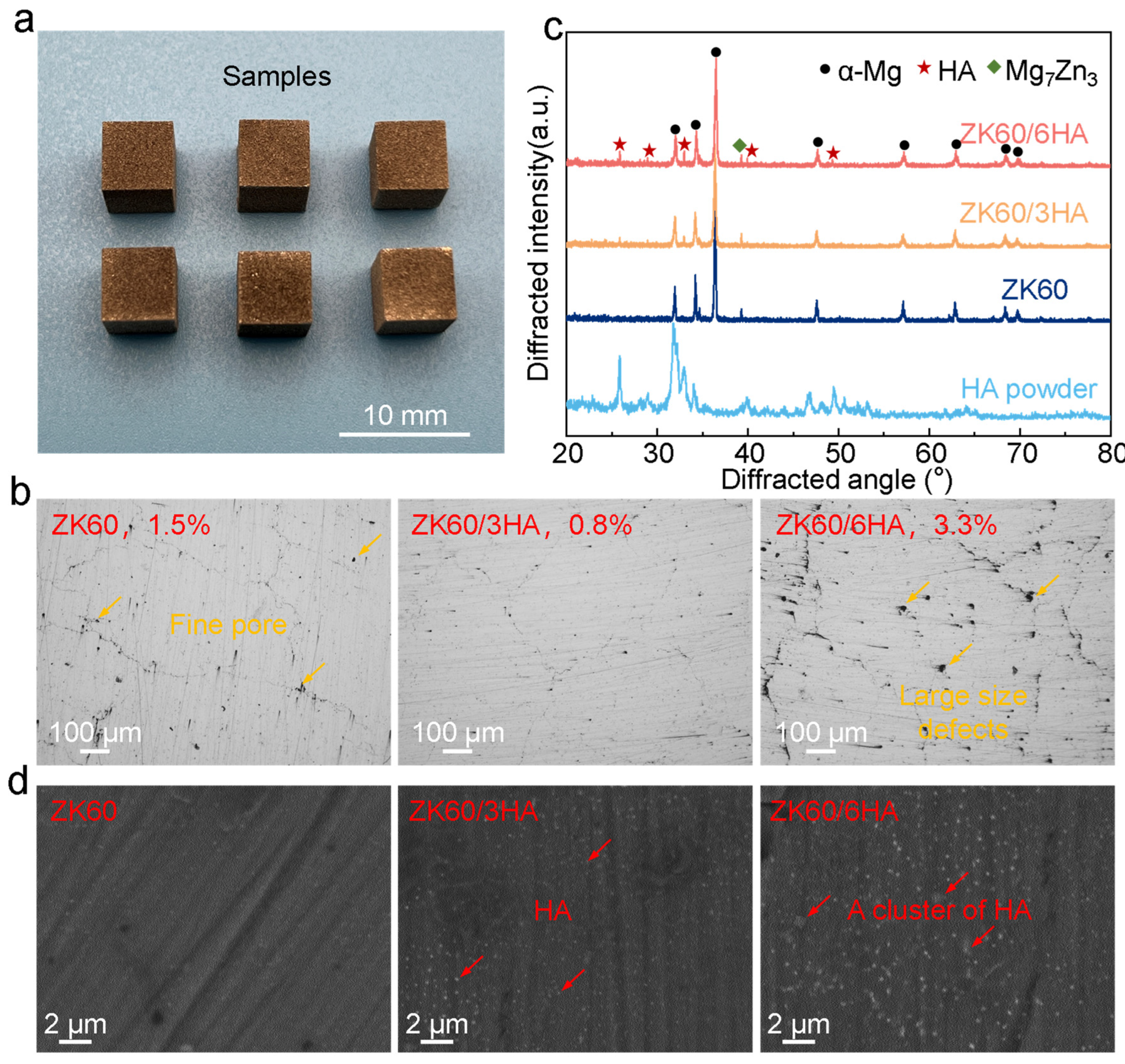
3.1. Microstructure
3.2. Electrochemical Behavior
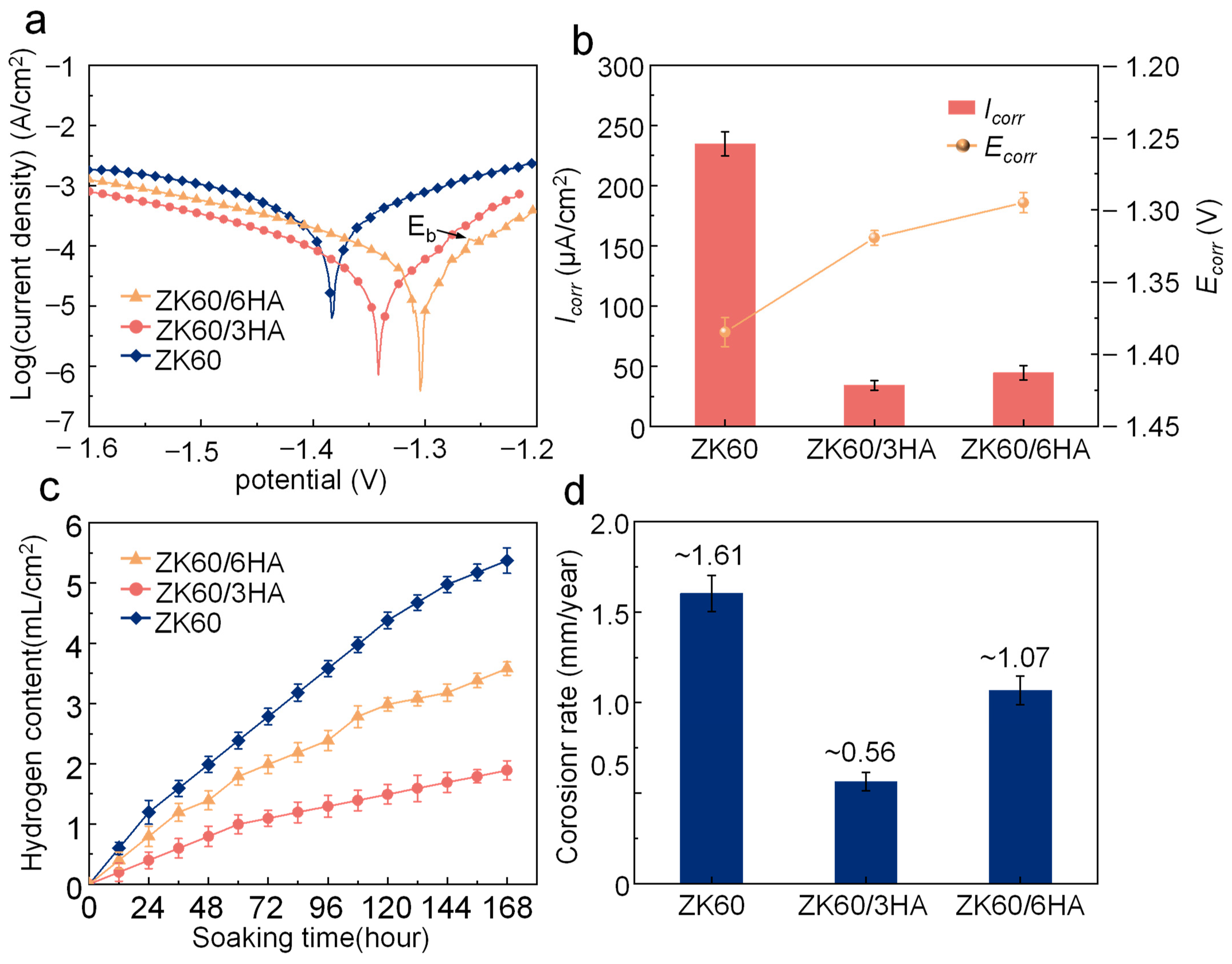
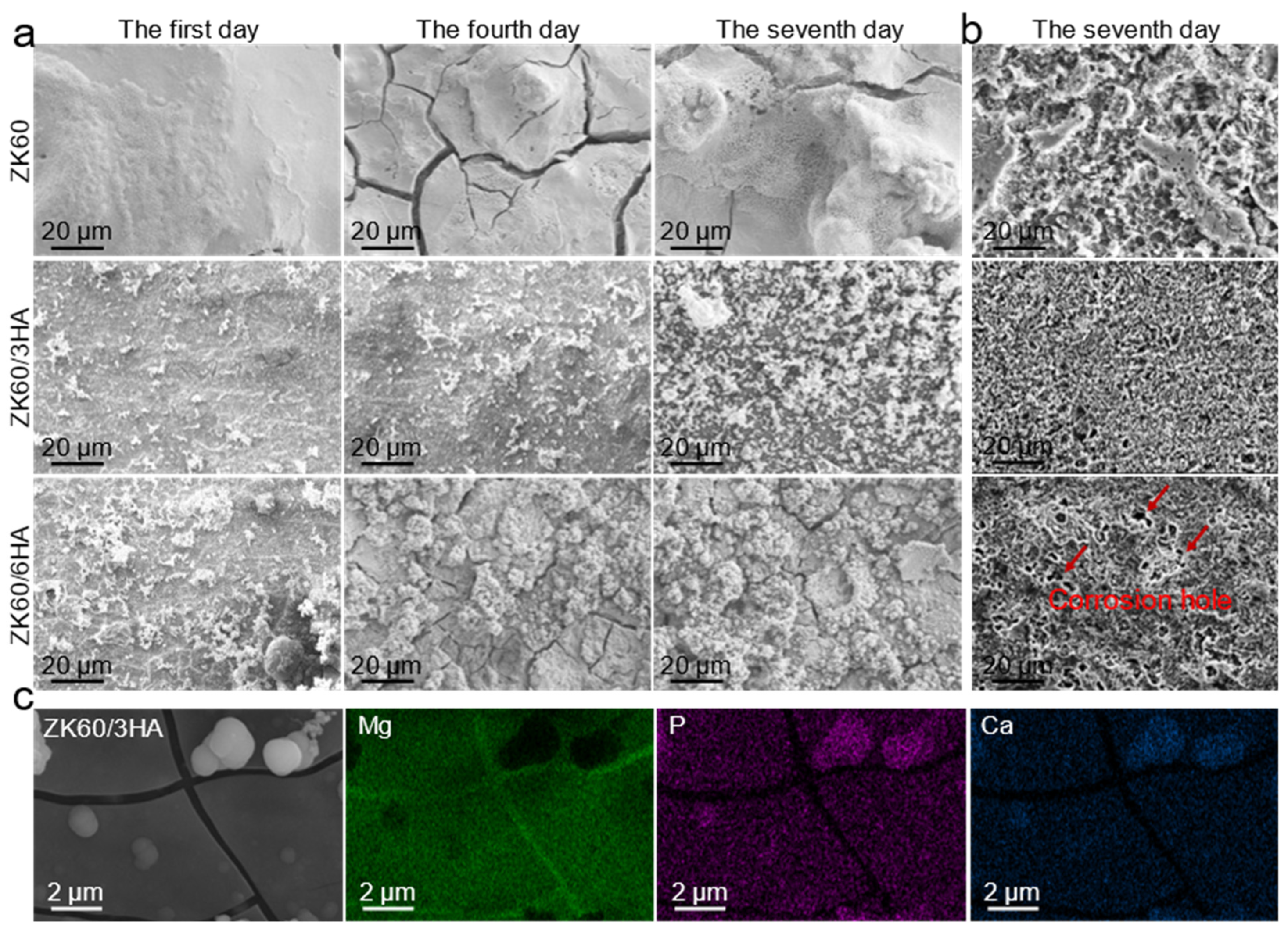
3.3. In Vitro Degradation Behavior
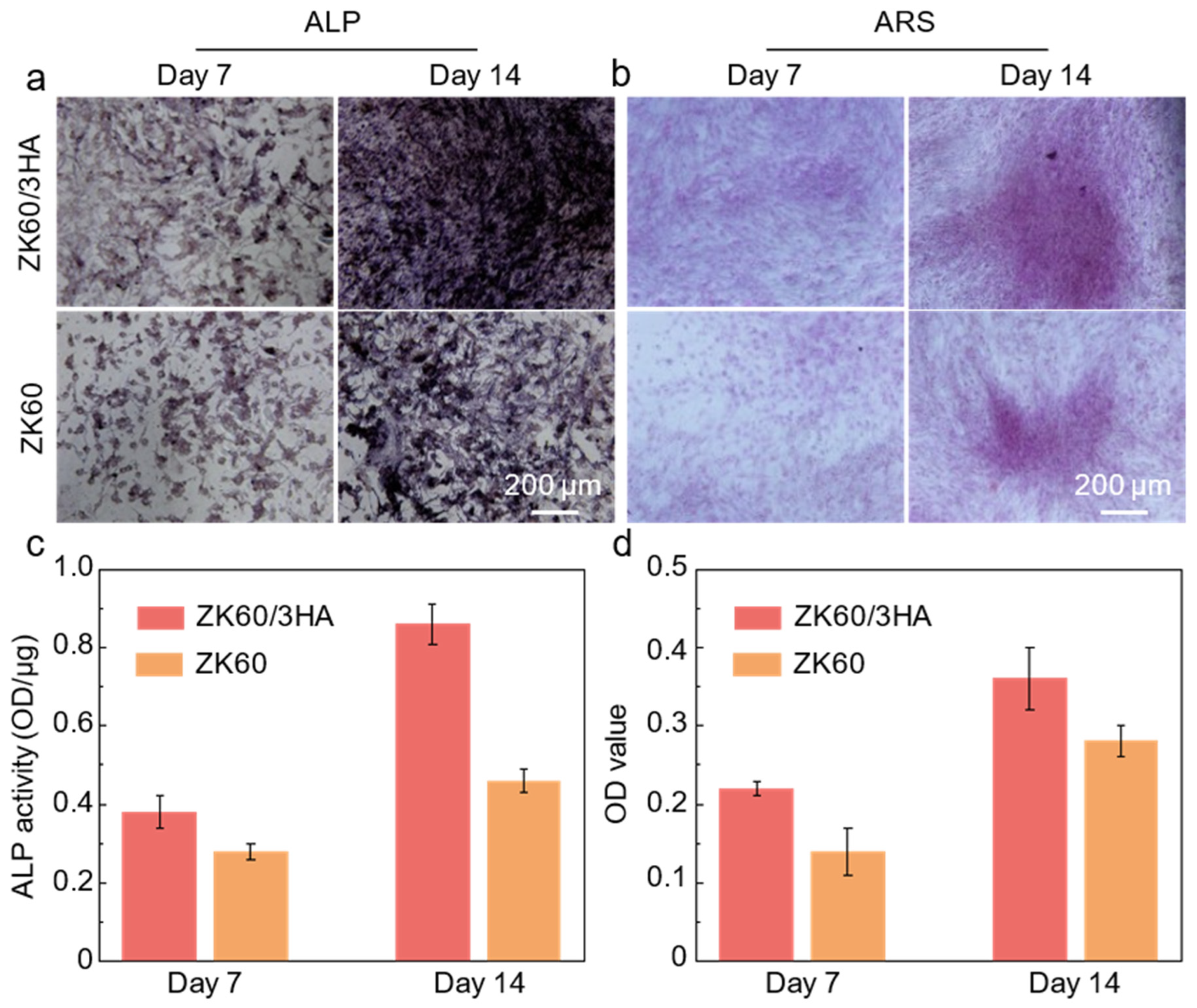
3.4. The Biological Properties of Magnesium Alloy Composites
4. Conclusions
- (1)
- The forming quality of the magnesium alloy can be evenly distributed on the surface of ZK60 spherical powder through mechanical ball grinding and adjusting the absorption and reflection of the laser. However, the introduction of excessive HA results in agglomeration on the powder surface, which is unfavorable for the formation of the magnesium alloy and instead promotes the formation of pore defects.
- (2)
- Introducing an appropriate amount of nanosized HA (3 wt.%) results in its even distribution within the matrix, providing a large number of calcium and phosphorus attachment sites and promoting the formation of an apatite protective layer. This apatite layer is dense and effectively protects the Mg matrix from further corrosion. However, excessive HA tends to cluster, and these clusters can form a local electric couple with the matrix, accelerating electrochemical corrosion.
- (3)
- ZK60/3HA not only exhibits good corrosion resistance but also provides a suitable environment for cell growth, demonstrating good biocompatibility. Furthermore, the introduction of bioactive ceramic HA enhances the release of Ca2+ and PO43−, which promotes the mineralization, proliferation, and differentiation processes of bone cells, ultimately accelerating bone healing.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloys 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Yang, M.; Chen, C.; Wang, D.; Shao, Y.; Zhou, W.; Shuai, C.; Yang, Y.; Ning, X. Biomedical rare-earth magnesium alloy: Current status and future prospects. J. Magnes. Alloys 2024, 12, 1260–1282. [Google Scholar] [CrossRef]
- Wang, J.-L.; Xu, J.-K.; Hopkins, C.; Chow, D.H.K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Qi, C.; Cai, K. Biodegradable magnesium phosphates in biomedical applications. J. Mater. Chem. B 2022, 10, 2097–2112. [Google Scholar] [CrossRef]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloys 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Kim, J.; Pan, H. Effects of magnesium alloy corrosion on biological response—Perspectives of metal-cell interaction. Prog. Mater. Sci. 2023, 133, 101039. [Google Scholar] [CrossRef]
- Kumar, R.; Katyal, P. Effects of alloying elements on performance of biodegradable magnesium alloy. Mater. Today Proc. 2022, 56, 2443–2450. [Google Scholar] [CrossRef]
- Sabbaghian, M.; Mahmudi, R.; Shin, K.S. Microstructure, texture, mechanical properties and biodegradability of extruded Mg–4Zn–xMn alloys. Mater. Sci. Eng. A 2020, 792, 139828. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants–A review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in coatings on biodegradable magnesium alloys. J. Magnes. Alloys 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Ho, Y.-H.; Man, K.; Joshi, S.S.; Pantawane, M.V.; Wu, T.-C.; Yang, Y.; Dahotre, N.B. In-vitro biomineralization and biocompatibility of friction stir additively manufactured AZ31B magnesium alloy-hydroxyapatite composites. Bioact. Mater. 2020, 5, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Kasaeian-Naeini, M.; Sedighi, M.; Hashemi, R. Severe plastic deformation (SPD) of biodegradable magnesium alloys and composites: A review of developments and prospects. J. Magnes. Alloys 2022, 10, 938–955. [Google Scholar] [CrossRef]
- Yang, H.; Chen, X.; Huang, G.; Song, J.; She, J.; Tan, J.; Zheng, K.; Jin, Y.; Jiang, B.; Pan, F. Microstructures and mechanical properties of titanium-reinforced magnesium matrix composites: Review and perspective. J. Magnes. Alloys 2022, 10, 2311–2333. [Google Scholar] [CrossRef]
- Delavar, H.; Mostahsan, A.J.; Ibrahim, H. Corrosion and corrosion-fatigue behavior of magnesium metal matrix composites for bio-implant applications: A review. J. Magnes. Alloys 2023, 11, 1125–1161. [Google Scholar] [CrossRef]
- Ma, C.; Du, T.; Niu, X.; Fan, Y. Biomechanics and mechanobiology of the bone matrix. Bone Res. 2022, 10, 59. [Google Scholar] [CrossRef]
- Zhang, A.-M.; Lenin, P.; Zeng, R.-C.; Kannan, M.B. Advances in hydroxyapatite coatings on biodegradable magnesium and its alloys. J. Magnes. Alloys 2022, 10, 1154–1170. [Google Scholar] [CrossRef]
- Vidal, C.; Alves, P.; Alves, M.M.; Carmezim, M.J.; Fernandes, M.H.; Grenho, L.; Inácio, P.L.; Ferreira, F.B.; Santos, T.G.; Santos, C. Fabrication of a biodegradable and cytocompatible magnesium/nanohydroxyapatite/fluorapatite composite by upward friction stir processing for biomedical applications. J. Mech. Behav. Biomed. Mater. 2022, 129, 105137. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dubey, A.; Lahiri, D. The influence of bioactive hydroxyapatite shape and size on the mechanical and biodegradation behaviour of magnesium based composite. Ceram. Int. 2020, 46, 27205–27218. [Google Scholar] [CrossRef]
- Guo, Y.; Li, G.; Xu, Y.; Xu, Z.; Gang, M.; Sun, G.; Zhang, Z.; Yang, X.; Yu, Z.; Lian, J.; et al. The microstructure, mechanical properties, corrosion performance and biocompatibility of hydroxyapatite reinforced ZK61 magnesium-matrix biological composite. J. Mech. Behav. Biomed. Mater. 2021, 123, 104759. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium–hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Mechanical and corrosion behaviour of hydroxyapatite reinforced Mg-Sn alloy composite by squeeze casting for biomedical applications. J. Magnes. Alloys 2020, 8, 452–460. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Chen, L.; Li, L.; Hou, H.; Zhao, Y. Microstructure and properties of graphene nanoplatelets reinforced AZ91D matrix composites prepared by electromagnetic stirring casting. J. Mater. Res. Technol. 2022, 21, 214138–214150. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, W.; Li, G.; Guan, F.; Yu, Y.; Fan, Z. Microstructure and mechanical properties of SiCnp/Al6082 aluminum matrix composites prepared by squeeze casting combined with stir casting. J. Mater. Process. Technol. 2020, 283, 116699. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Khalili, V.; Moslemi, S.; Ruttert, B.; Frenzel, J.; Theisen, W.; Eggeler, G. Surface metal matrix nano-composite of magnesium/hydroxyapatite produced by stir-centrifugal casting. Surf. Coat. Technol. 2021, 406, 126654. [Google Scholar] [CrossRef]
- Ma, C.; Ge, Q.; Yuan, L.; Gu, D.; Dai, D.; Setchi, R.; Wu, M.; Liu, Y.; Li, D.; Ma, S.; et al. The development of laser powder bed fused nano-TiC/NiTi superelastic composites with hierarchically heterogeneous microstructure and considerable tensile recoverable strain. Compos. Part B Eng. 2023, 250, 110457. [Google Scholar] [CrossRef]
- Mayer, J.M.; Abraham, J.A.; Surhigh, B.; Kinzer, B.; Chandran, R.B. Temperature-dependent diffuse reflectance measurements of ceramic powders in the near-and mid-infrared spectra. Sol. Energy 2022, 245, 193–210. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, S.; Wei, Y.; Xi, X.; Cai, K.; Pan, F. Effects of Y on the Microstructure, Mechanical and Bio-corrosion Properties of Mg–Zn–Ca Bulk Metallic Glass. J. Mater. Sci. Technol. 2014, 30, 1255–1261. [Google Scholar] [CrossRef]
- Bulina, N.V.; Makarova, S.V.; Baev, S.G.; Matvienko, A.A.; Gerasimov, K.B.; Logutenko, O.A.; Bystrov, V.S. A study of thermal stability of hydroxyapatite. Minerals 2021, 11, 1310. [Google Scholar] [CrossRef]
- Li, X.; Fang, X.; Jiang, X.; Duan, Y.; Li, Y.; Zhang, H.; Li, X.; Huang, K. Additively manufactured high-performance AZ91D magnesium alloys with excellent strength and ductility via nanoparticles reinforcement. Addit. Manuf. 2023, 69, 103550. [Google Scholar] [CrossRef]
- Liang, J.; Wu, S.; Li, B.; Lei, Z.; Chen, Y.; Jiang, M.; Zhang, X.; Chen, X. Microstructure and corrosion behavior of Y-modified ZK60 Mg alloy prepared by laser powder bed fusion. Corros. Sci. 2023, 211, 110895. [Google Scholar] [CrossRef]
- Chen, X.; Ning, S.; Wang, A.; Le, Q.; Liao, Q.; Jia, Y.; Cheng, C.; Li, X.; Atrens, A.; Yu, F. Microstructure, mechanical properties and corrosion behavior of quasicrystal-reinforced Mg-Zn-Y alloy subjected to dual-frequency ultrasonic field. Corros. Sci. 2020, 163, 108289. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Zhou, G.; Goshi, E.; He, Q. Micro/Nanomaterials-Augmented Hydrogen Therapy. Adv. Healthc. Mater. 2019, 8, e1900463. [Google Scholar] [CrossRef]
- Ho, Y.-H.; Joshi, S.S.; Wu, T.-C.; Hung, C.-M.; Ho, N.-J.; Dahotre, N.B. In-vitro bio-corrosion behavior of friction stir additively manufactured AZ31B magnesium alloy-hydroxyapatite composites. Mater. Sci. Eng. C 2020, 109, 110632. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Chen, J.; Zhang, X.; Kwak, M.; Wu, Y.; Fan, R.; Zhang, L.; Pei, J.; Yuan, G.; et al. A promising biodegradable magnesium alloy suitable for clinical vascular stent application. Sci. Rep. 2017, 7, 46343. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
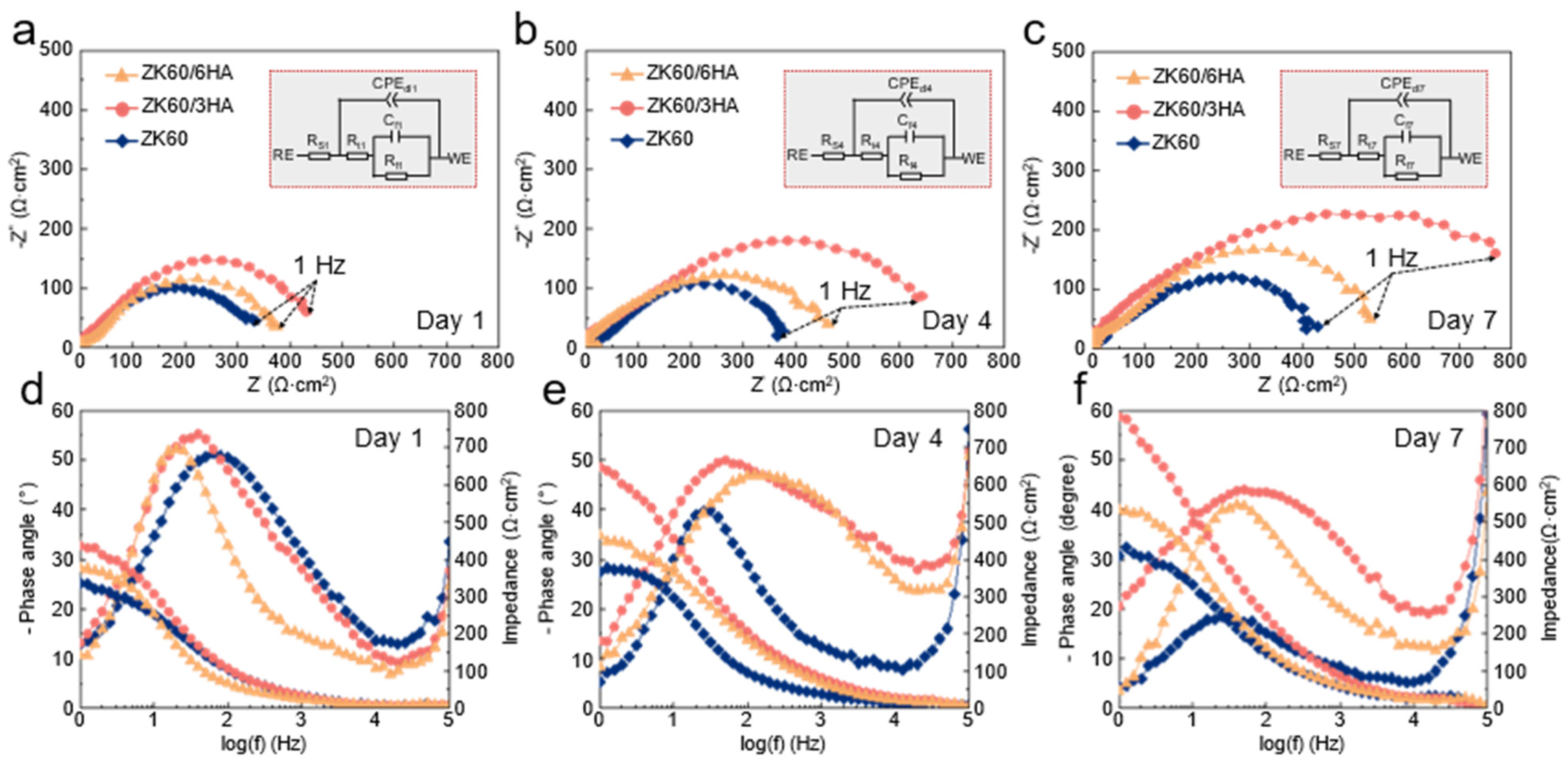

| Sample | Time | Y0 × 10−5 Ω−1 cm2sn | n | Rt (Ω·cm2) | Cf (μF/cm2) | Rf (Ω·cm2) |
|---|---|---|---|---|---|---|
| ZK60 | One day | 17.7 ± 2.3 | 0.5 ± 0.2 | 90.2 ± 3.2 | 15.7 ± 0.4 | 286.8 ± 13.2 |
| ZK60/3HA | 15.4 ± 1.6 | 0.4 ± 0.1 | 125.2 ± 8.1 | 9.8 ± 1.2 | 393.3 ± 16.2 | |
| ZK60/6HA | 14.9 ± 2.1 | 0.5 ± 0.1 | 103.9 ± 4.7 | 12.9 ± 2.3 | 306.6 ± 14.7 | |
| ZK60 | Four days | 12.2 ± 2.1 | 0.6 ± 0.2 | 98.8 ± 6.1 | 13.7 ± 2.3 | 323.6 ± 12.5 |
| ZK60/3HA | 26.6 ± 3.2 | 0.4 ± 0.1 | 228.7 ± 1.6 | 8.1 ± 0.02 | 604 ± 26.8 | |
| ZK60/6HA | 19.2 ± 2.6 | 0.5 ± 0.2 | 141.3 ± 8.7 | 10.8 ± 0.02 | 436 ± 13.5 | |
| ZK60 | Seven days | 11.6 ± 1.5 | 0.5 ± 0.2 | 141.8 ± 16.1 | 4.4 ± 1.1 | 355 ± 15.4 |
| ZK60/3HA | 22.7 ± 5.1 | 0.3 ± 0.1 | 303.3 ± 4.1 | 2.2 ± 1.3 | 673 ± 34.5 | |
| ZK60/6HA | 17.8 ± 5.1 | 0.4 ± 0.1 | 201.4 ± 3.2 | 3.8 ± 1.2 | 489 ± 32.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, C.; Chen, C.; Zhao, Z.; Yang, Y. Corrosion Behavior and Biological Properties of ZK60/HA Composites Prepared by Laser Powder Bed Fusion. Micromachines 2024, 15, 1156. https://doi.org/10.3390/mi15091156
Shuai C, Chen C, Zhao Z, Yang Y. Corrosion Behavior and Biological Properties of ZK60/HA Composites Prepared by Laser Powder Bed Fusion. Micromachines. 2024; 15(9):1156. https://doi.org/10.3390/mi15091156
Chicago/Turabian StyleShuai, Cijun, Cheng Chen, Zhenyu Zhao, and Youwen Yang. 2024. "Corrosion Behavior and Biological Properties of ZK60/HA Composites Prepared by Laser Powder Bed Fusion" Micromachines 15, no. 9: 1156. https://doi.org/10.3390/mi15091156






