Extrusion-Based 3D Printing of Pharmaceuticals—Evaluating Polymer (Sodium Alginate, HPC, HPMC)-Based Ink’s Suitability by Investigating Rheology
Abstract
1. Introduction
2. Materials and Methods
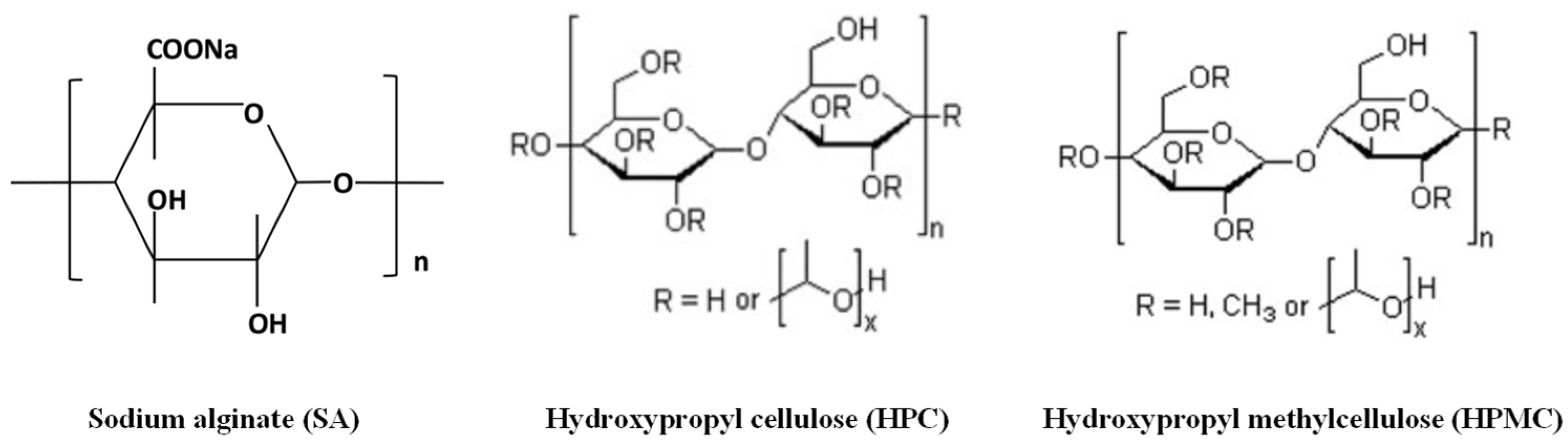
2.1. Materials
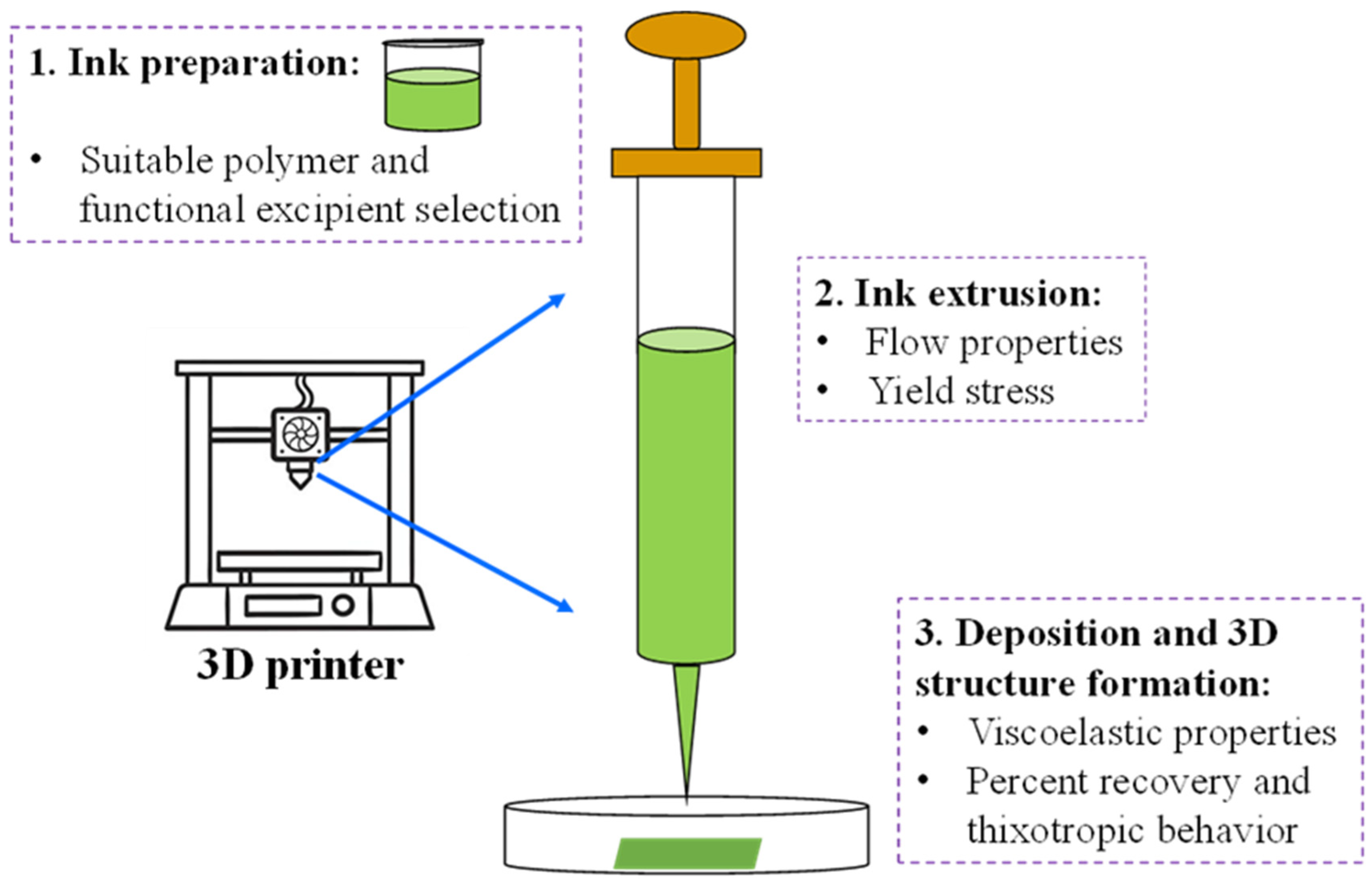
2.2. Preparation of the Ink for 3D Printing
2.2.1. Preparation of the SA Ink
2.2.2. Preparation of the HPC H Ink
2.2.3. Preparation of the HPMC (K100 or K4) Ink
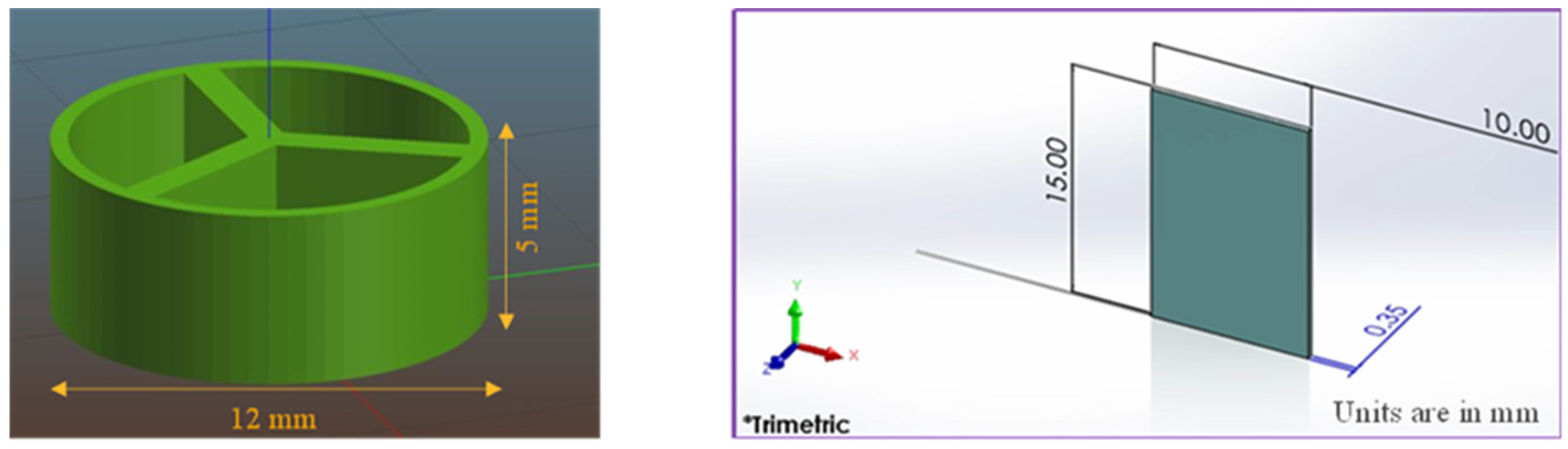
2.3. Design of the Pills and Films
2.4. Rheology
2.5. 3D Printing of the Pills and Films
3. Results and Discussion
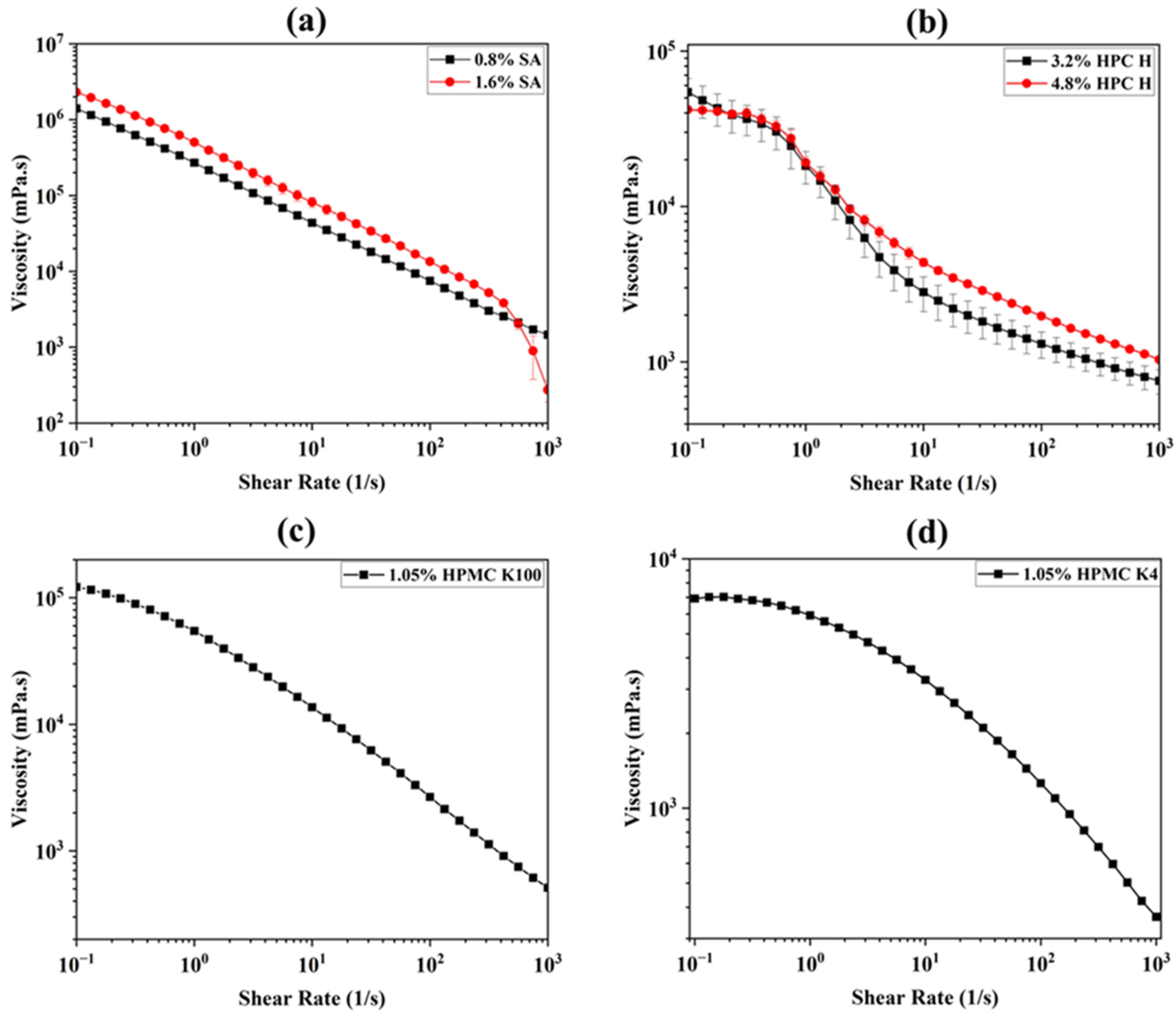
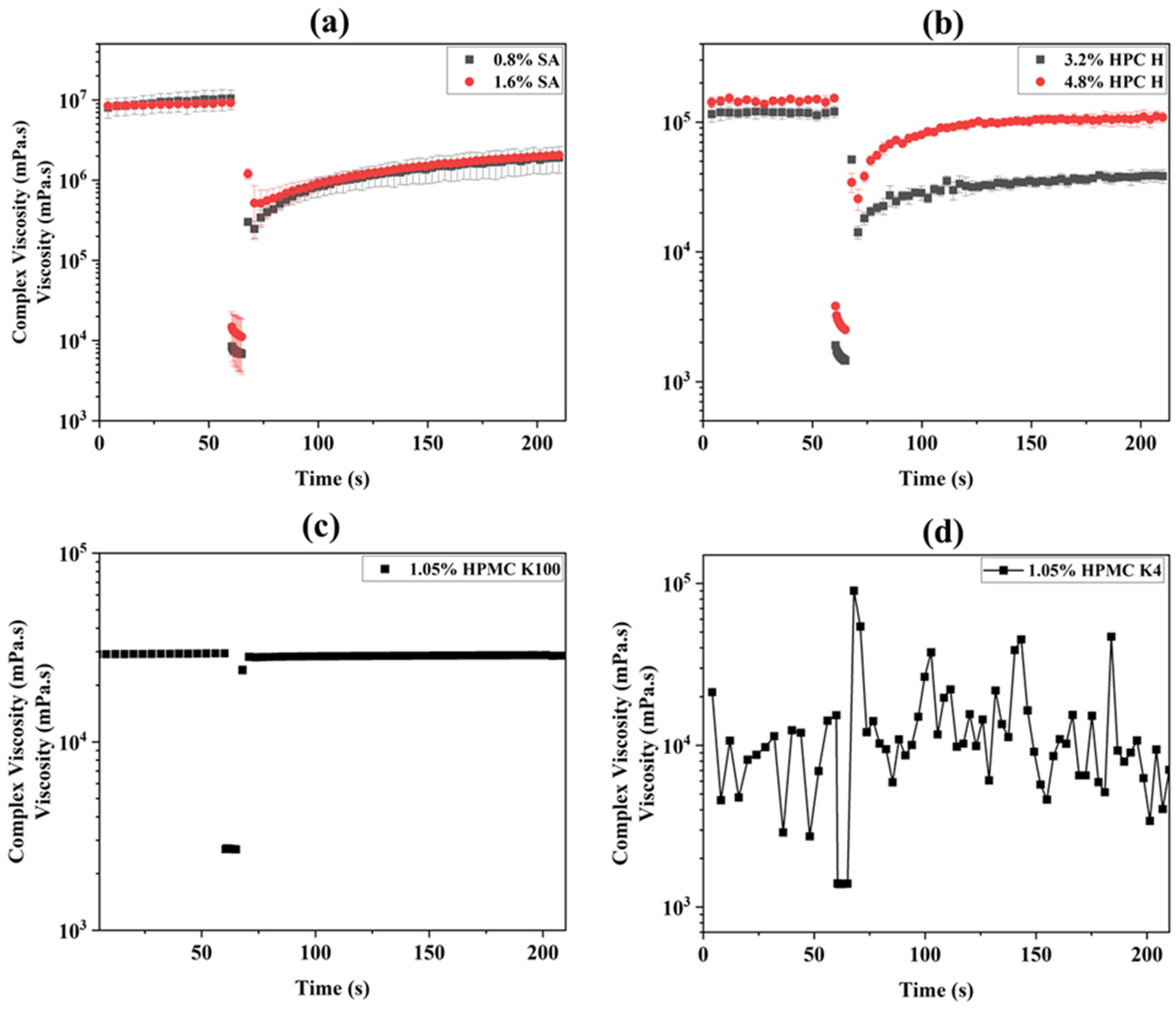
3.1. Viscosity
3.2. Amplitude Sweep
3.3. The Thixotropy Test
3.4. Comprehensive Analysis of the SA-, HPC H-, and HPMC K100/K4-Based-Inks’ Rheology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.; Jadhav, V.S. A Review on 3D Printing: An Additive Manufacturing Technology. Mater. Today Proc. 2022, 62, 2094–2099. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-Solid Extrusion 3D Printing in Drug Delivery and Biomedicine: Personalised Solutions for Healthcare Challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Sachs, E.; Cima, M.; Cornie, J. Three-Dimensional Printing: Rapid Tooling and Prototypes Directly from a CAD Model. CIRP Ann. 1990, 39, 201–204. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D Printing in Pharmaceutics: A New Tool for Designing Customized Drug Delivery Systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A New Chapter in Pharmaceutical Manufacturing: 3D-Printed Drug Products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Guvendiren, M. Current and Emerging Applications of 3D Printing in Medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef]
- Schork, N.J. Personalized Medicine: Time for One-Person Trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef]
- Giachini, P.A.G.S.; Gupta, S.S.; Wang, W.; Wood, D.; Yunusa, M.; Baharlou, E.; Sitti, M.; Menges, A. Additive Manufacturing of Cellulose-Based Materials with Continuous, Multidirectional Stiffness Gradients. Sci. Adv. 2020, 6, eaay0929. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, A.P.; Lira, J.O.d.B.; Padoin, N.; Soares, C.; Riella, H.G. Additive Manufacturing of Functional Devices for Environmental Applications: A Review. J. Environ. Chem. Eng. 2022, 10, 108049. [Google Scholar] [CrossRef]
- Outrequin, T.C.R.; Gamonpilas, C.; Siriwatwechakul, W.; Sreearunothai, P. Extrusion-Based 3D Printing of Food Biopolymers: A Highlight on the Important Rheological Parameters to Reach Printability. J. Food Eng. 2023, 342, 111371. [Google Scholar] [CrossRef]
- Muñiz Castro, B.; Elbadawi, M.; Ong, J.J.; Pollard, T.; Song, Z.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. Machine Learning Predicts 3D Printing Performance of over 900 Drug Delivery Systems. J. Control. Release 2021, 337, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Ruberu, K.; Senadeera, M.; Rana, S.; Gupta, S.; Chung, J.; Yue, Z.; Venkatesh, S.; Wallace, G. Coupling Machine Learning with 3D Bioprinting to Fast Track Optimisation of Extrusion Printing. Appl. Mater. Today 2021, 22, 100914. [Google Scholar] [CrossRef]
- Zidan, A.; Alayoubi, A.; Coburn, J.; Asfari, S.; Ghammraoui, B.; Cruz, C.N.; Ashraf, M. Extrudability Analysis of Drug Loaded Pastes for 3D Printing of Modified Release Tablets. Int. J. Pharm. 2019, 554, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Su, J.; Zhou, S.; Li, J.; Zhang, K. Application of Hydrogels as Three-Dimensional Bioprinting Ink for Tissue Engineering. Gels 2023, 9, 88. [Google Scholar] [CrossRef]
- Tagami, T.; Okamura, M.; Ogawa, K.; Ozeki, T. Fabrication of Mucoadhesive Films Containing Pharmaceutical Ionic Liquid and Eudragit Polymer Using Pressure-Assisted Microsyringe-Type 3D Printer for Treating Oral Mucositis. Pharmaceutics 2022, 14, 1930. [Google Scholar] [CrossRef] [PubMed]
- Prasad, E.; Robertson, J.; Florence, A.J.; Halbert, G.W. Expanding the Pharmaceutical Formulation Space in Material Extrusion 3D Printing Applications. Addit. Manuf. 2023, 77, 103803. [Google Scholar] [CrossRef]
- Kimbell, G.; Azad, M.A. Chapter FIFTEEN—3D Printing: Bioinspired Materials for Drug Delivery. In Bioinspired and Biomimetic Materials for Drug Delivery; Nurunnabi, M., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2021; pp. 295–318. ISBN 978-0-12-821352-0. [Google Scholar]
- Danda, L.J.d.A.; Christinne Rocha de Medeiros Schver, G.; Lamartine Soares Sobrinho, J.; Lee, P.I.; Felts de La Roca Soares, M. Amorphous Solid Dispersions in High-Swelling, Low-Substituted Hydroxypropyl Cellulose for Enhancing the Delivery of Poorly Soluble Drugs. Int. J. Pharm. 2023, 642, 123122. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wu, F.; Hong, Y.; Shen, L.; Lin, X.; Zhao, L.; Feng, Y. Updates on Applications of Low-Viscosity Grade Hydroxypropyl Methylcellulose in Coprocessing for Improvement of Physical Properties of Pharmaceutical Powders. Carbohydr. Polym. 2023, 311, 120731. [Google Scholar] [CrossRef] [PubMed]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-Known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Arteaga, C.; Abdelmalek, B.; Davé, R.; Bilgili, E. Spray Drying of Drug-Swellable Dispersant Suspensions for Preparation of Fast-Dissolving, High Drug-Loaded, Surfactant-Free Nanocomposites. Drug Dev. Ind. Pharm. 2015, 41, 1617–1631. [Google Scholar] [CrossRef]
- Jiang, Y.; De La Cruz, J.A.; Ding, L.; Wang, B.; Feng, X.; Mao, Z.; Xu, H.; Sui, X. Rheology of Regenerated Cellulose Suspension and Influence of Sodium Alginate. Int. J. Biol. Macromol. 2020, 148, 811–816. [Google Scholar] [CrossRef]
- Bari, E.; Di Gravina, G.M.; Scocozza, F.; Perteghella, S.; Frongia, B.; Tengattini, S.; Segale, L.; Torre, M.L.; Conti, M. Silk Fibroin Bioink for 3D Printing in Tissue Regeneration: Controlled Release of MSC Extracellular Vesicles. Pharmaceutics 2023, 15, 383. [Google Scholar] [CrossRef]
- Than, Y.M.; Suriyarak, S.; Titapiwatanakun, V. Rheological Investigation of Hydroxypropyl Cellulose–Based Filaments for Material Extrusion 3D Printing. Polymers 2022, 14, 1108. [Google Scholar] [CrossRef]
- Cui, M.; Pan, H.; Fang, D.; Qiao, S.; Wang, S.; Pan, W. Fabrication of High Drug Loading Levetiracetam Tablets Using Semi-Solid Extrusion 3D Printing. J. Drug Deliv. Sci. Technol. 2020, 57, 101683. [Google Scholar] [CrossRef]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Jiang, X.; Shi, X. 3D Printing of Extended-Release Tablets of Theophylline Using Hydroxypropyl Methylcellulose (HPMC) Hydrogels. Int. J. Pharm. 2020, 591, 119983. [Google Scholar] [CrossRef]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A.W. Pressure-Assisted Microsyringe 3D Printing of Oral Films Based on Pullulan and Hydroxypropyl Methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D Printing of Tablets Containing Multiple Drugs with Defined Release Profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Vélez, G.F.; Zhu, T.Z.; Linsley, C.S.; Wu, B.M. Photocurable Poly(Ethylene Glycol) as a Bioink for the Inkjet 3D Pharming of Hydrophobic Drugs. Int. J. Pharm. 2018, 546, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Knieke, C.; Azad, M.A.; To, D.; Bilgili, E.; Davé, R.N. Sub-100 Micron Fast Dissolving Nanocomposite Drug Powders. Powder Technol. 2015, 271, 49–60. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Yang, X.; Wang, F.; Feng, J.; Hua, K.; Li, Q.; Hu, Y. Demineralized Bone Matrix Carriers and Their Clinical Applications: An Overview. Orthop. Surg. 2019, 11, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Knieke, C.; To, D.; Davé, R. Preparation of Concentrated Stable Fenofibrate Suspensions via Liquid Antisolvent Precipitation. Drug Dev. Ind. Pharm. 2014, 40, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Kimbell, G. Rheology Impacts on Extrusion-Based 3D Printing of Polymeric Structures Incorporating Pharmaceuticals. Master’s Thesis, North Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2020. [Google Scholar]
- Hydroxypropylcellulose, K. Physical and Chemical Properties; Ashland Inc.: Wilmington, DE, USA, 2001; pp. 17–19. [Google Scholar]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D Printing of Controlled Release Pharmaceutical Bilayer Tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef]
- Mezger, T.G. Applied Rheology: With Joe Flow on Rheology Road, 2nd ed.; Anton Paar GmbH: Sumida, Tokyo, 2018; ISBN 3-9504016-0-1. [Google Scholar]
- Nijdam, J.J.; LeCorre-Bordes, D.; Delvart, A.; Schon, B.S. A Rheological Test to Assess the Ability of Food Inks to Form Dimensionally Stable 3D Food Structures. J. Food Eng. 2021, 291, 110235. [Google Scholar] [CrossRef]
- Werner, B.; Myrseth, V.; Saasen, A. Viscoelastic Properties of Drilling Fluids and Their Influence on Cuttings Transport. J. Pet. Sci. Eng. 2017, 156, 845–851. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ettelaie, R.; Chen, J. Construction of 3D Printed Reduced-Fat Meat Analogue by Emulsion Gels. Part I: Flow Behavior, Thixotropic Feature, and Network Structure of Soy Protein-Based Inks. Food Hydrocoll. 2021, 120, 106967. [Google Scholar] [CrossRef]
- Lu, Y.; Rai, R.; Nitin, N. Image-Based Assessment and Machine Learning-Enabled Prediction of Printability of Polysaccharides-Based Food Ink for 3D Printing. Food Res. Int. 2023, 173, 113384. [Google Scholar] [CrossRef]
- Olawuni, D. Optimization of Process Parameters for Extrusion-Based 3D Printing of Polymeric Structures Incorporating Pharmaceuticals. Master’s Thesis, North Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2020. [Google Scholar]
- Azad, M.A.; Sievens-Figueroa, L.; Davé, R.N. Fast Release of Liquid Antisolvent Precipitated Fenofibrate at High Drug Loading from Biocompatible Thin Films. Adv. Powder Technol. 2018, 29, 2907–2919. [Google Scholar] [CrossRef]
- Habib, M.A.; Khoda, B. Rheological Analysis of Bio-Ink for 3D Bio-Printing Processes. J. Manuf. Process. 2022, 76, 708–718. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, W.; Bao, M.; Zhu, X.; Ning, C.; Zhang, W.; Li, Y.; Zhang, X. Process Fundamentals and Quality Investigation in Extrusion 3D Printing of Shear Thinning Materials: Extrusion Process Based on Nishihara Model. Int. J. Adv. Manuf. Technol. 2023, 124, 245–264. [Google Scholar] [CrossRef]
- Dávila, J.L.; Manzini, B.M.; d’Ávila, M.A.; da, S.J.V.L. Open-Source Syringe Extrusion Head for Shear-Thinning Materials 3D Printing. Rapid Prototyp. J. 2022, 28, 1452–1461. [Google Scholar] [CrossRef]
- Maciel, B.; Oelschlaeger, C.; Willenbacher, N. Chain Flexibility and Dynamics of Alginate Solutions in Different Solvents. Colloid. Polym. Sci. 2020, 298, 791–801. [Google Scholar] [CrossRef]
- Hwang, J.W.; Chawla, D.; Han, G.; Eriten, M.; Henak, C.R. Effects of Solvent Osmolarity and Viscosity on Cartilage Energy Dissipation under High-Frequency Loading. J. Mech. Behav. Biomed. Mater. 2022, 126, 105014. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Fei, F.; Song, X.; Zhou, C. Analytical Study and Experimental Verification of Shear-Thinning Ink Flow in Direct Ink Writing Process. J. Manuf. Sci. Eng. 2023, 145, 071001. [Google Scholar] [CrossRef]
- Guo, Z.; Fei, F.; Song, X.; Zhou, C. Analytical Study of Shear-Thinning Fluid Flow in Direct Ink Writing Process. In Proceedings of the Volume 1: Additive Manufacturing; Biomanufacturing; Life Cycle Engineering; Manufacturing Equipment and Automation; Nano/Micro/Meso Manufacturing; American Society of Mechanical Engineers: West Lafayette, IN, USA, 2022; p. V001T01A034. [Google Scholar]
- Oh, C.M.; Heng, P.W.S.; Chan, L.W. A Study on the Impact of Hydroxypropyl Methylcellulose on the Viscosity of PEG Melt Suspensions Using Surface Plots and Principal Component Analysis. AAPS PharmSciTech 2015, 16, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, J.A.; Kausar, T.N.; Mahnashi, M.H.; Alasiri, A.; Alqahtani, A.A.; Alqahtani, T.S.; Walbi, I.A.; Alshehri, O.M.; Elnoubi, O.A.; et al. Preparation, Characterization and Evaluation of Flavonolignan Silymarin Effervescent Floating Matrix Tablets for Enhanced Oral Bioavailability. Molecules 2023, 28, 2606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Belton, P.; Yi Teoh, X.; Gleadall, A.; Bibb, R.; Qi, S. An Investigation into the Effects of Ink Formulations of Semi-Solid Extrusion 3D Printing on the Performance of Printed Solid Dosage Forms. J. Mater. Chem. B 2024, 12, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.; Marnot, A.; Ketcham, M.; Travis, C.; Brettmann, B. Direct Ink Write 3D Printing of High Solids Loading Bimodal Distributions of Particles. AIChE J. 2021, 67, e17412. [Google Scholar] [CrossRef]
- dos Santos Carvalho, J.D.; Rabelo, R.S.; Hubinger, M.D. Thermo-Rheological Properties of Chitosan Hydrogels with Hydroxypropyl Methylcellulose and Methylcellulose. Int. J. Biol. Macromol. 2022, 209, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Sun, Q.; Xu, X.; Li, M.; Xie, F. Hydroxypropyl Methylcellulose Hydrocolloid Systems: Effect of Hydroxypropy Group Content on the Phase Structure, Rheological Properties and Film Characteristics. Food Chem. 2022, 379, 132075. [Google Scholar] [CrossRef] [PubMed]
- Dabbaghi, M.; Namjoshi, S.; Panchal, B.; Grice, J.E.; Prakash, S.; Roberts, M.S.; Mohammed, Y. Viscoelastic and Deformation Characteristics of Structurally Different Commercial Topical Systems. Pharmaceutics 2021, 13, 1351. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; Zujovic, Z.; Akbarinejad, A.; Nieuwoudt, M.; Seal, C.K.; McGillivray, D.J. The Interactions between the Two Negatively Charged Polysaccharides: Gum Arabic and Alginate. Food Hydrocoll. 2021, 112, 106343. [Google Scholar] [CrossRef]
- Hernández-Sosa, A.; Ramírez-Jiménez, R.A.; Rojo, L.; Boulmedais, F.; Aguilar, M.R.; Criado-Gonzalez, M.; Hernández, R. Optimization of the Rheological Properties of Self-Assembled Tripeptide/Alginate/Cellulose Hydrogels for 3D Printing. Polymers 2022, 14, 2229. [Google Scholar] [CrossRef] [PubMed]
- Sardelli, L.; Tunesi, M.; Briatico-Vangosa, F.; Petrini, P. 3D-Reactive Printing of Engineered Alginate Inks. Soft Matter 2021, 17, 8105–8117. [Google Scholar] [CrossRef]
- Gorroñogoitia, I.; Urtaza, U.; Zubiarrain-Laserna, A.; Alonso-Varona, A.; Zaldua, A.M. A Study of the Printability of Alginate-Based Bioinks by 3D Bioprinting for Articular Cartilage Tissue Engineering. Polymers 2022, 14, 354. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhao, J.; Chen, J.; Shimai, S.; Zhang, J.; Liu, Y.; Liu, D.; Wang, S. A Novel Experimental Approach to Quantitatively Evaluate the Printability of Inks in 3D Printing Using Two Criteria. Addit. Manuf. 2022, 55, 102846. [Google Scholar] [CrossRef]
- Wang, C.; Yan, R.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Qiu, C.; et al. Development of Emulsion-Based Edible Inks for 3D Printing Applications: Pickering Emulsion Gels. Food Hydrocoll. 2023, 138, 108482. [Google Scholar] [CrossRef]
- Glukhova, S.A.; Molchanov, V.S.; Lokshin, B.V.; Rogachev, A.V.; Tsarenko, A.A.; Patsaev, T.D.; Kamyshinsky, R.A.; Philippova, O.E. Printable Alginate Hydrogels with Embedded Network of Halloysite Nanotubes: Effect of Polymer Cross-Linking on Rheological Properties and Microstructure. Polymers 2021, 13, 4130. [Google Scholar] [CrossRef]
- Bhosale, P.S.; Berg, J.C. The Dynamics of Polymer Bridge Formation and Disruption and Its Effect on the Bulk Rheology of Suspensions. Langmuir 2012, 28, 16807–16811. [Google Scholar] [CrossRef] [PubMed]
- Macocinschi, D.; Filip, D.; Ciubotaru, B.-I.; Dumitriu, R.P.; Varganici, C.-D.; Zaltariov, M.-F. Blends of Sodium Deoxycholate-Based Poly(Ester Ether)Urethane Ionomer and Hydroxypropylcellulose with Mucosal Adhesiveness. Int. J. Biol. Macromol. 2020, 162, 1262–1275. [Google Scholar] [CrossRef]
- Pfaff, N.M.; Dijksman, J.A.; Kemperman, A.J.B.; van Loosdrecht, M.C.M.; Kleijn, J.M. Rheological Characterisation of Alginate-like Exopolymer Gels Crosslinked with Calcium. Water Res. 2021, 207, 117835. [Google Scholar] [CrossRef]
- Bercea, M. Rheology as a Tool for Fine-Tuning the Properties of Printable Bioinspired Gels. Molecules 2023, 28, 2766. [Google Scholar] [CrossRef] [PubMed]
- Barrulas, R.V.; Corvo, M.C. Rheology in Product Development: An Insight into 3D Printing of Hydrogels and Aerogels. Gels 2023, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; García-Tuñón, E. Interplay between Yielding, ‘Recovery’, and Strength of Yield Stress Fluids for Direct Ink Writing: New Insights from Oscillatory Rheology. Soft Matter 2024, 20, 7429–7447. [Google Scholar] [CrossRef] [PubMed]
- Menard, K.P.; Menard, N.R. Dynamic Mechanical Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-19030-8. [Google Scholar]
- Ebers, L.-S.; Laborie, M.-P. Direct Ink Writing of Fully Bio-Based Liquid Crystalline Lignin/Hydroxypropyl Cellulose Aqueous Inks: Optimization of Formulations and Printing Parameters. ACS Appl. Bio Mater. 2020, 3, 6897–6907. [Google Scholar] [CrossRef] [PubMed]
- Fried, J.R. Polymer Processing and Rheology. In Polymer Science and Technology; Pearson Education, Inc.: London, UK, 2014; pp. 435–482. ISBN 978-0-13-703955-5. [Google Scholar]
- Mendibil, X.; Tena, G.; Duque, A.; Uranga, N.; Campanero, M.Á.; Alonso, J. Direct Powder Extrusion of Paracetamol Loaded Mixtures for 3D Printed Pharmaceutics for Personalized Medicine via Low Temperature Thermal Processing. Pharmaceutics 2021, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]



| Polymer Name | Polymer | Drug | Functional Excipient | Solvent | |||||
|---|---|---|---|---|---|---|---|---|---|
| FNB | Mannitol | PEG | PVP | SDS | Ethanol | Water | |||
| (%, w/w) | (g) | (g) | (g) | (g) | (g) | (g) | (g) | (g) | |
| Sodium Alginate (SA) | 0.80 | 0.24 | 6.0 | 7.50 | 3 | 1.5 | - | - | 11.76 |
| 1.60 | 0.48 | 6.0 | 7.50 | 3 | 1.5 | - | - | 11.52 | |
| HPC H | 3.20 | 0.96 | 6.0 | 7.50 | 3 | 1.5 | - | - | 11.04 |
| 4.80 | 1.44 | 6.0 | 7.50 | 3 | 1.5 | - | - | 10.56 | |
| HPMC K100 | 1.05 | 0.32 | 1.5 | 7.35 | - | - | 0.15 | 5.25 | 15.43 |
| HPMC K4 | 1.05 | 0.32 | 1.5 | 7.35 | - | - | 0.15 | 5.25 | 15.43 |
| Polymer and Its Concentration (%, w/w) | Phase Shift Angle, δ | Yield Point or Stress, τy | Flow Point or Stress, τf | Flow Transition Index, τf/τy |
|---|---|---|---|---|
| (°) | (Pa) | (Pa) | ||
| 0.8% SA | 23.61 ± 2.91 | 5.77 ± 3.27 | 20.21 ± 5.70 | 4.22 ± 1.78 |
| 1.6% SA | 25.41 ± 3.42 | 2.48 ± 0.65 | 126.57 ± 19.57 | 55.95 ± 19.19 |
| 3.2% HPC H | 30.62 ± 0.80 | 0.09 ± 0.04 | 0.83 ± 0.37 | 9.78 ± 1.33 |
| 4.8% HPC H | 37.10 ± 3.19 | 0.10 ± 0.08 | 0.53 ± 0.58 | 5.44 ± 3.20 |
| 1.05% HPMC K100 | 41.74 ± 0.18 | 1.39 ± 0.05 | 45.30 ± 1.50 | 32.66 ± 0.11 |
| 1.05% HPMC K4 | 68.62 ± 3.90 | - | - | - |
| Test Name | Parameter | Polymer | |||||
|---|---|---|---|---|---|---|---|
| Sodium Alginate | HPC H | HPMC (1.05%) | |||||
| 0.8% | 1.6% | 3.2% | 4.8% | K100 | K4 | ||
| Rheological Properties (Average ±SD) | |||||||
| Flow test | Viscosity (mPa.s) at a 0.1 shear rate (1/s) | 1.40 × 106 ± 1.14 × 105 | 2.30 × 106 ± 7.88 × 104 | 5.44 × 104 ± 1.21 × 104 | 4.20 × 104 ± 1.99 × 103 | 1.22 × 105 ± 2.40 × 103 | 6.92 × 103 ± 1.20 × 102 |
| Shear thinning behavior | Linear shear thinning | Shear thinning | |||||
| Amplitude sweep | Modulus at flow point (Pa) | 1.52 × 104 ± 9.52 × 103 | 1.30 × 103 ± 1.95 × 102 | 3.43 × 102 ± 8.80 × 101 | 4.35 × 102 ± 8.42 × 101 | 1.21 × 102 ± 4.28 × 100 | - |
| Shear strain at flow point (%) | 0.27 ± 0.30 | 6.98 ± 1.11 | 0.20 ± 0.14 | 0.09 ± 0.10 | 26.5 ± 0.00 | - | |
| Ink behavior | Viscoelastic | Viscoelastic liquid | |||||
| Thixotropy | Recovery (%) | <25 | 25–80 | >80 | - | ||
| 3D printing outcomes | |||||||
| Shape fidelity | Successful | Unsuccessful | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rony, F.K.; Kimbell, G.; Serrano, T.R.; Clay, D.; Ilias, S.; Azad, M.A. Extrusion-Based 3D Printing of Pharmaceuticals—Evaluating Polymer (Sodium Alginate, HPC, HPMC)-Based Ink’s Suitability by Investigating Rheology. Micromachines 2025, 16, 163. https://doi.org/10.3390/mi16020163
Rony FK, Kimbell G, Serrano TR, Clay D, Ilias S, Azad MA. Extrusion-Based 3D Printing of Pharmaceuticals—Evaluating Polymer (Sodium Alginate, HPC, HPMC)-Based Ink’s Suitability by Investigating Rheology. Micromachines. 2025; 16(2):163. https://doi.org/10.3390/mi16020163
Chicago/Turabian StyleRony, Farzana Khan, Georgia Kimbell, Toby R. Serrano, Destinee Clay, Shamsuddin Ilias, and Mohammad A. Azad. 2025. "Extrusion-Based 3D Printing of Pharmaceuticals—Evaluating Polymer (Sodium Alginate, HPC, HPMC)-Based Ink’s Suitability by Investigating Rheology" Micromachines 16, no. 2: 163. https://doi.org/10.3390/mi16020163
APA StyleRony, F. K., Kimbell, G., Serrano, T. R., Clay, D., Ilias, S., & Azad, M. A. (2025). Extrusion-Based 3D Printing of Pharmaceuticals—Evaluating Polymer (Sodium Alginate, HPC, HPMC)-Based Ink’s Suitability by Investigating Rheology. Micromachines, 16(2), 163. https://doi.org/10.3390/mi16020163







