Evaluation of Non-Faradaic Impedimetric Parameters for IL-8 Detection Using Gold Interdigitated Electrode-Based Biosensors: Towards Early Detection of Newborn Disability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biofunctionalization of IL-8 Non-Faradaic Biosensor
2.3. Non-Faradaic Detection of IL-8
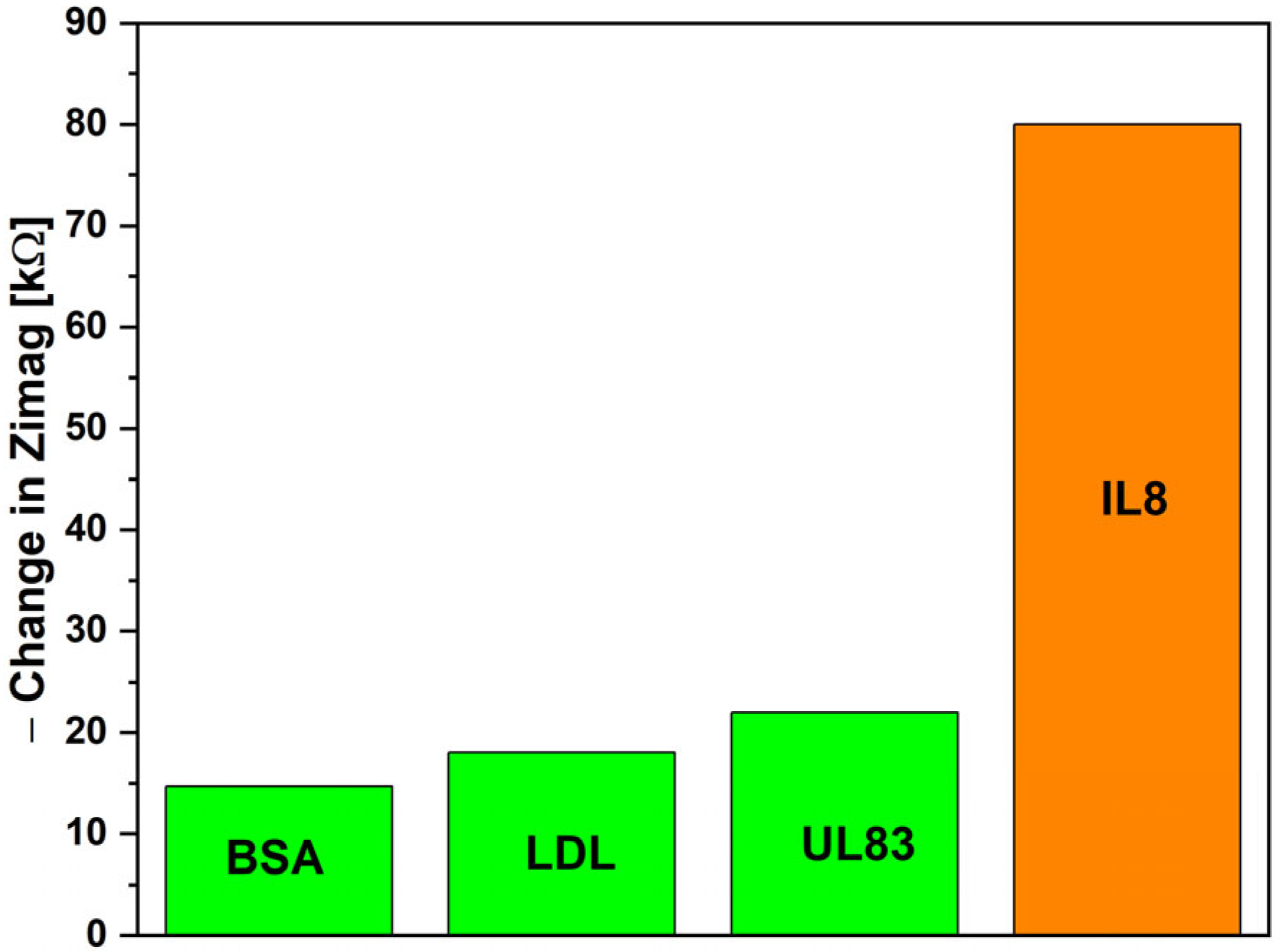
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mai, C.T.; Isenburg, J.L.; Canfield, M.A.; Meyer, R.E.; Correa, A.; Alverson, C.J.; Lupo, P.J.; Riehle-Colarusso, T.; Cho, S.J.; Aggarwal, D.; et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019, 111, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, H.; Yu, L.; Qiu, F.; Lv, Y.; Guan, J.; Gang, H.; Zuo, J.; Zheng, T.; Liu, H.; et al. Prenatal Lead Exposure, Genetic Factors, and Cognitive Developmental Delay. JAMA Netw. Open 2023, 6, e2339108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.J.; Zhao, Y.N.; Lan, X.Y.; Zhang, Y.; Zhang, R. Prenatal, perinatal and parental risk factors for autism spectrum disorder in China: A case-control study. BMC Psychiatry 2024, 24, 219. [Google Scholar] [CrossRef]
- Kohli-Lynch, M.; Tann, C.J.; Ellis, M.E. Early Intervention for Children at High Risk of Developmental Disability in Low- and Middle-Income Countries: A Narrative Review. Int. J. Environ. Res. Public Health 2019, 16, 4449. [Google Scholar] [CrossRef]
- Collins, P.Y.; Pringle, B.; Alexander, C.; Darmstadt, G.L.; Heymann, J.; Huebner, G.; Kutlesic, V.; Polk, C.; Sherr, L.; Shih, A.; et al. Global services and support for children with developmental delays and disabilities: Bridging research and policy gaps. PLoS Med. 2017, 14, e1002393. [Google Scholar] [CrossRef]
- Fajzrina, L.; Ngaisah, N.; Pratamasari, I. Analysis of Detection of Growth and Development in Gross Motor Toddlers (Case Study of Babies Aged 6 Months Cannot Pronning, Roll and Crooked). J. Early Child. Educ. 2022, 2, 206–217. [Google Scholar] [CrossRef]
- Muthusamy, S.; Wagh, D.; Tan, J.; Bulsara, M.; Rao, S. Utility of the Ages and Stages Questionnaire to Identify Developmental Delay in Children Aged 12 to 60 Months: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2022, 176, 980–989. [Google Scholar] [CrossRef]
- Sweetman, D.U.; Strickland, T.; Melo, A.M.; Kelly, L.A.; Onwuneme, C.; Watson, W.R.; Murphy, J.F.A.; Slevin, M.; Donoghue, V.; O’Neill, A.; et al. Neonatal Encephalopathy Is Associated with Altered IL-8 and GM-CSF Which Correlates with Outcomes. Front. Pediatr. 2020, 8, 556216. [Google Scholar] [CrossRef]
- Melo, A.M.; Taher, N.A.; Doherty, D.G.; Molloy, E.J. The role of lymphocytes in neonatal encephalopathy. Brain Behav. Immun. Health 2021, 18, 100380. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Alfadul, H.; Sabico, S.; Al-Daghri, N.M. The role of interleukin-1beta in type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 901616. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.P.; Liu, C.; Chiang, F.Y.; Wang, L.F.; Lee, K.W.; Chen, W.T.; Kuo, P.L.; Liang, C.H. IL-8 promotes inflammatory mediators and stimulates activation of p38 MAPK/ERK-NF-kappaB pathway and reduction of JNK in HNSCC. Oncotarget 2017, 8, 56375–56388. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hamad, K.; Al Shibitini, A.; Juma, S.; Sharifi, S.; Gould, L.; Mahmoudi, M. Investigating Inflammatory Markers in Wound Healing: Understanding Implications and Identifying Artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18–27. [Google Scholar] [CrossRef]
- Kamei, M.; Hussein, M.H.; Hattori, A.; Saleh, M.; Kakita, H.; Abdel-Hamid Daoud, G.; Ishiguro, A.; Namba, F.; Yazaki, M.; Goto, H.; et al. Oxidative and Inflammatory Markers Are Higher in Full-Term Newborns Suffering Funisitis, and Higher Oxidative Markers Are Associated with Admission. Children 2022, 9, 702. [Google Scholar] [CrossRef]
- Varner, M.W.; Marshall, N.E.; Rouse, D.J.; Jablonski, K.A.; Leveno, K.J.; Reddy, U.M.; Mercer, B.M.; Iams, J.D.; Wapner, R.J.; Sorokin, Y.; et al. The association of cord serum cytokines with neurodevelopmental outcomes. Am. J. Perinatol. 2015, 30, 115–122. [Google Scholar] [CrossRef]
- Yan, Y.; Yu, Z.; Lu, J.; Jin, P.; Tang, Z.; Hu, Y. Predictive values profiling of interleukin-2, interleukin-8, tumor necrosis factor-alpha, procalcitonin, and C-reactive protein in critical gastrointestinal cancer patients. J. Gastrointest. Oncol. 2021, 12, 1398–1406. [Google Scholar] [CrossRef]
- Pan, K.; Xu, C.; Chen, C.; Chen, S.; Zhang, Y.; Ding, X.; Xu, X.; Lv, Q. Soluble interleukin-2 receptor combined with interleukin-8 is a powerful predictor of future adverse cardiovascular events in patients with acute myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1110742. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Porzia, A.; Mainiero, F.; Costantino, C.; Bertoccini, L.; Ceccarelli, V.; Morini, S.; Baroni, M.G.; Lenzi, A.; et al. Circulating IL-8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta Diabetol. 2017, 54, 961–967. [Google Scholar] [CrossRef]
- Moreno Velasquez, I.; Gajulapuri, A.; Leander, K.; Berglund, A.; de Faire, U.; Gigante, B. Serum IL8 is not associated with cardiovascular events but with all-cause mortality. BMC Cardiovasc. Disord. 2019, 19, 34. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, N.; Mittal, R.D.; Mohindra, S.; Ghoshal, U.C. Association between pro-(IL-8) and anti-inflammatory (IL-10) cytokine variants and their serum levels and H. pylori-related gastric carcinogenesis in northern India. Meta Gene 2015, 6, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Adunphatcharaphon, S.; Elliott, C.T.; Sooksimuang, T.; Charlermroj, R.; Petchkongkaew, A.; Karoonuthaisiri, N. The evolution of multiplex detection of mycotoxins using immunoassay platform technologies. J. Hazard. Mater. 2022, 432, 128706. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and their widespread impact on human health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Shimizu, F.M.; Barros, A.d.; Braunger, M.L.; Gaal, G.; Riul, A., Jr. Information visualization and machine learning driven methods for impedimetric biosensing. TrAC Trends Anal. Chem. 2023, 165, 117115. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Assaifan, A.; Almansour, R.; Alessa, J.; Alhudaithy, S.; Fakhouri, A.; Alsaleh, A. Roles of Interdigitated Electrodes Geometry in Non-Faradaic Impedimetric Biosensors. J. Electrochem. Soc. 2024, 171, 087515. [Google Scholar] [CrossRef]
- Ibau, C.; Arshad, M.K.M.; Gopinath, S.C.B.; Nuzaihan, M.N.M.; Fathil, M.F.M.; Shamsuddin, S.A. Immunosensing prostate-specific antigen: Faradaic vs non-Faradaic electrochemical impedance spectroscopy analysis on interdigitated microelectrode device. Int. J. Biol. Macromol. 2020, 162, 1924–1936. [Google Scholar] [CrossRef]
- Assaifan, A.K.; Alqahtani, F.A.; Alnamlah, S.; Almutairi, R.; Alkhammash, H.I. Detection and Real-Time Monitoring of LDL-Cholesterol by Redox-Free Impedimetric Biosensors. BioChip J. 2022, 16, 197–206. [Google Scholar] [CrossRef]
- Kaur, J.; Preethi, M.; Srivastava, R.; Borse, V. Role of IL-6 and IL-8 biomarkers for optical and electrochemical based point-of-care detection of oral cancer. Biosens. Bioelectron. X 2022, 11, 100212. [Google Scholar] [CrossRef]
- Assaifan, A.K.; Al Habis, N.; Ahmad, I.; Alshehri, N.A.; Alharbi, H.F. Scaling-up medical technologies using flexographic printing. Talanta 2020, 219, 121236. [Google Scholar] [CrossRef]
- Assaifan, A.; Aljdidalmri, A.; Albrithen, H.; Alodhayb, A.; Alzahrani, K.; Alshammari, A.; Al-Gawati, M.; Aldeligan, S. Probing the Influence of Crosslinking Layer Incubation Time on the Performance of Non-Faradaic Impedimetric Biosensors. J. Electrochem. Soc. 2022, 169, 117511. [Google Scholar] [CrossRef]
- Neairat, T.; Al-Gawati, M.; Tul Ain, Q.; Assaifan, A.K.; Alshamsan, A.; Alarifi, A.; Alodhayb, A.N.; Alzahrani, K.E.; Albrithen, H. Development of a microcantilever-based biosensor for detecting Programmed Death Ligand 1. Saudi Pharm. J. 2024, 32, 102051. [Google Scholar] [CrossRef]
- Eveness, J.; Cao, L.; Kiely, J.; Luxton, R. Equivalent circuit model of a non-faradaic impedimetric ZnO nano-crystal biosensor. J. Electroanal. Chem. 2022, 906, 116003. [Google Scholar] [CrossRef]
- Tanak, A.S.; Jagannath, B.; Tamrakar, Y.; Muthukumar, S.; Prasad, S. Non-faradaic electrochemical impedimetric profiling of procalcitonin and C-reactive protein as a dual marker biosensor for early sepsis detection. Anal. Chim. Acta X 2019, 3, 100029. [Google Scholar] [CrossRef]
- Dutta, N.; Lillehoj, P.B.; Estrela, P.; Dutta, G. Electrochemical Biosensors for Cytokine Profiling: Recent Advancements and Possibilities in the Near Future. Biosensors 2021, 11, 94. [Google Scholar] [CrossRef]
- Cardona-Maya, Y.; Socorro, A.B.; Villar, I.D.; Cruz, J.L.; Corres, J.M.; Botero-Cadavid, J.F. Label-free wavelength and phase detection based SMS fiber immunosensors optimized with cladding etching. Sens. Actuators B Chem. 2018, 265, 10–19. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Gouveia, C.A.J.; Jorge, P.A.S.; Baldini, F. Towards a Uniform Metrological Assessment of Grating-Based Optical Fiber Sensors: From Refractometers to Biosensors. Biosensors 2017, 7, 23. [Google Scholar] [CrossRef]
- Sharma, R.; Deacon, S.E.; Nowak, D.; George, S.E.; Szymonik, M.P.; Tang, A.A.S.; Tomlinson, D.C.; Davies, A.G.; McPherson, M.J.; Walti, C. Label-free electrochemical impedance biosensor to detect human interleukin-8 in serum with sub-pg/ml sensitivity. Biosens. Bioelectron. 2016, 80, 607–613. [Google Scholar] [CrossRef]
- Singh, J.; Sohal, S.S.; Ahuja, K.; Lim, A.; Duncan, H.; Thachil, T.; De Ieso, P. Levels of plasma cytokine in patients undergoing neoadjuvant androgen deprivation therapy and external beam radiation therapy for adenocarcinoma of the prostate. Ann. Transl. Med. 2020, 8, 636. [Google Scholar] [CrossRef]
- Lumit® IL-8 (Human) Immunoassay. Available online: https://worldwide.promega.com/products/cell-health-assays/inflammation-assay/lumit-il-8-human-immunoassay/#resources (accessed on 26 February 2025).
- Wu, D.; Milutinovic, M.D.; Walt, D.R. Single molecule array (Simoa) assay with optimal antibody pairs for cytokine detection in human serum samples. Analyst 2015, 140, 6277–6282. [Google Scholar] [CrossRef]
- Congy-Jolivet, N.; Cenac, C.; Dellacasagrande, J.; Puissant-Lubrano, B.; Apoil, P.A.; Guedj, K.; Abbas, F.; Laffont, S.; Sourdet, S.; Guyonnet, S.; et al. Monocytes are the main source of STING-mediated IFN-alpha production. eBioMedicine 2022, 80, 104047. [Google Scholar] [CrossRef]
- Orlikowsky, T.W.; Neunhoeffer, F.; Goelz, R.; Eichner, M.; Henkel, C.; Zwirner, M.; Poets, C.F. Evaluation of IL-8-concentrations in plasma and lysed EDTA-blood in healthy neonates and those with suspected early onset bacterial infection. Pediatr. Res. 2004, 56, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Knapp, M.; Lang, I.; Kohler, G. Interleukin 8 (IL-8)—A universal biomarker? Int. Arch. Med. 2010, 3, 11. [Google Scholar] [CrossRef] [PubMed]

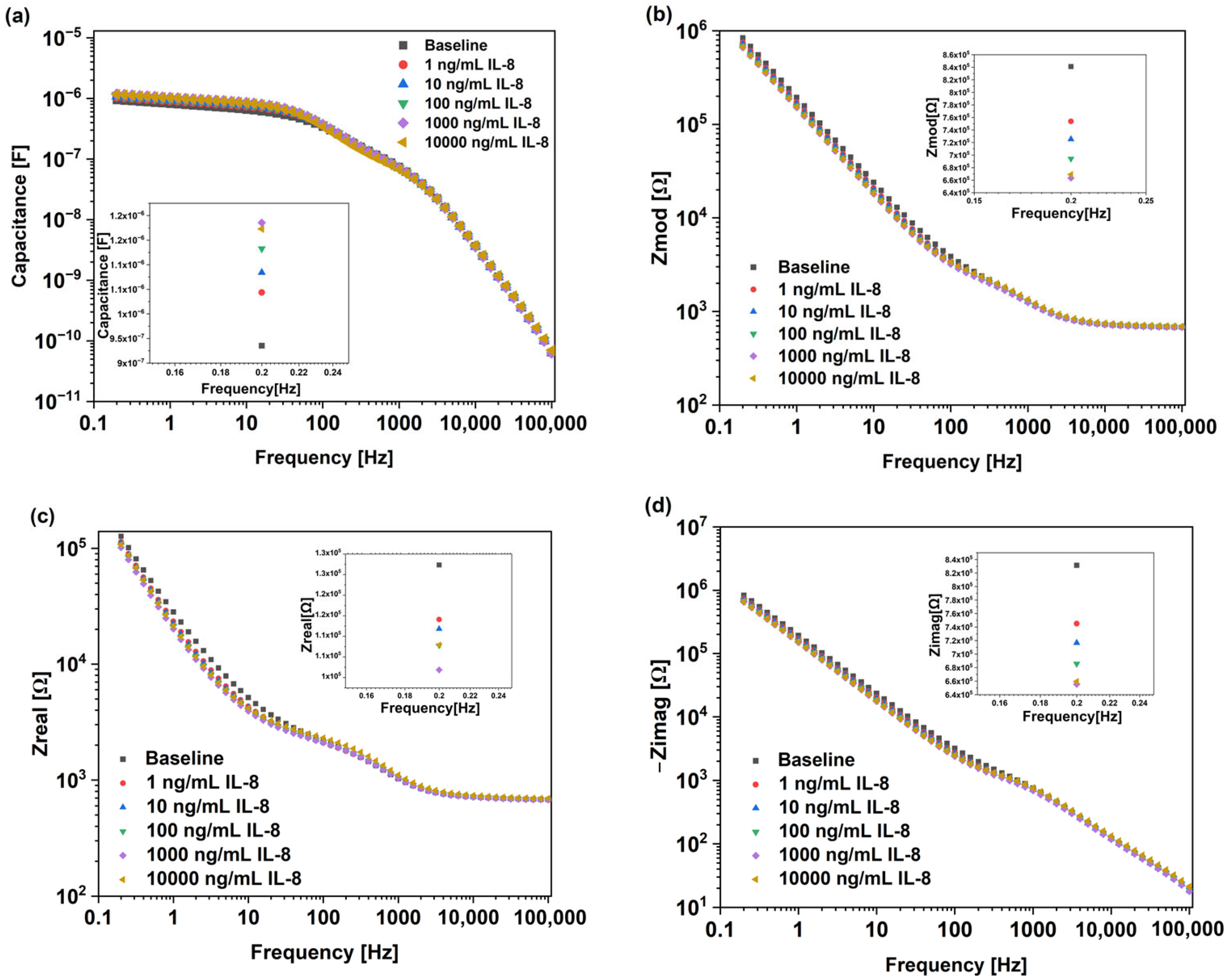

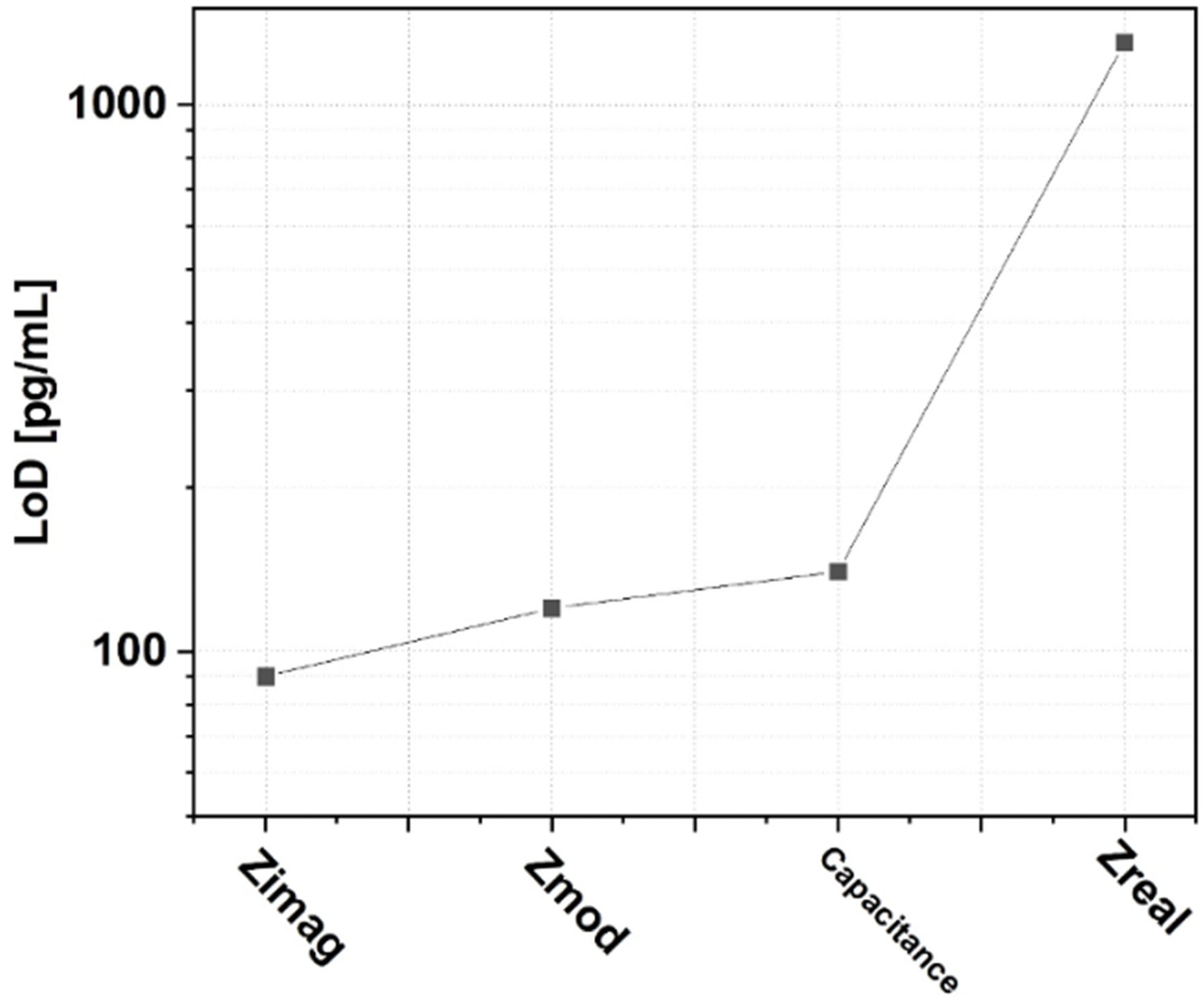
| LoD | Sensitivity | |
|---|---|---|
| Zimag | 90 pg/mL | 13.1 kΩ/log (ng/mL) |
| Zmod | 120 pg/mL | 13.2 kΩ/log (ng/mL) |
| Capacitance | 140 pg/mL | 20 nF/log (ng/mL) |
| Zreal | 1.3 ng/mL | 1.8 kΩ/log (ng/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrebaish, A.S.; Alnami, L.O.; Alshraim, J.M.; Alnghemshi, R.A.; Aljammaz, A.A.; Altinawi, A.; Alhuthali, K.K.; Alfadul, H.; Assaifan, A.K. Evaluation of Non-Faradaic Impedimetric Parameters for IL-8 Detection Using Gold Interdigitated Electrode-Based Biosensors: Towards Early Detection of Newborn Disability. Micromachines 2025, 16, 395. https://doi.org/10.3390/mi16040395
Alrebaish AS, Alnami LO, Alshraim JM, Alnghemshi RA, Aljammaz AA, Altinawi A, Alhuthali KK, Alfadul H, Assaifan AK. Evaluation of Non-Faradaic Impedimetric Parameters for IL-8 Detection Using Gold Interdigitated Electrode-Based Biosensors: Towards Early Detection of Newborn Disability. Micromachines. 2025; 16(4):395. https://doi.org/10.3390/mi16040395
Chicago/Turabian StyleAlrebaish, Abdulelah S., Layla O. Alnami, Joud M. Alshraim, Razan A. Alnghemshi, Alanoud A. Aljammaz, Amir Altinawi, Kholood K. Alhuthali, Hend Alfadul, and Abdulaziz K. Assaifan. 2025. "Evaluation of Non-Faradaic Impedimetric Parameters for IL-8 Detection Using Gold Interdigitated Electrode-Based Biosensors: Towards Early Detection of Newborn Disability" Micromachines 16, no. 4: 395. https://doi.org/10.3390/mi16040395
APA StyleAlrebaish, A. S., Alnami, L. O., Alshraim, J. M., Alnghemshi, R. A., Aljammaz, A. A., Altinawi, A., Alhuthali, K. K., Alfadul, H., & Assaifan, A. K. (2025). Evaluation of Non-Faradaic Impedimetric Parameters for IL-8 Detection Using Gold Interdigitated Electrode-Based Biosensors: Towards Early Detection of Newborn Disability. Micromachines, 16(4), 395. https://doi.org/10.3390/mi16040395







