Recent Advances in Microfluidics-Based Monitoring of Waterborne Pathogens: From Isolation to Detection
Abstract
:1. Introduction
2. Conventional Separation and Detection Methods
2.1. Conventional Separation Methods
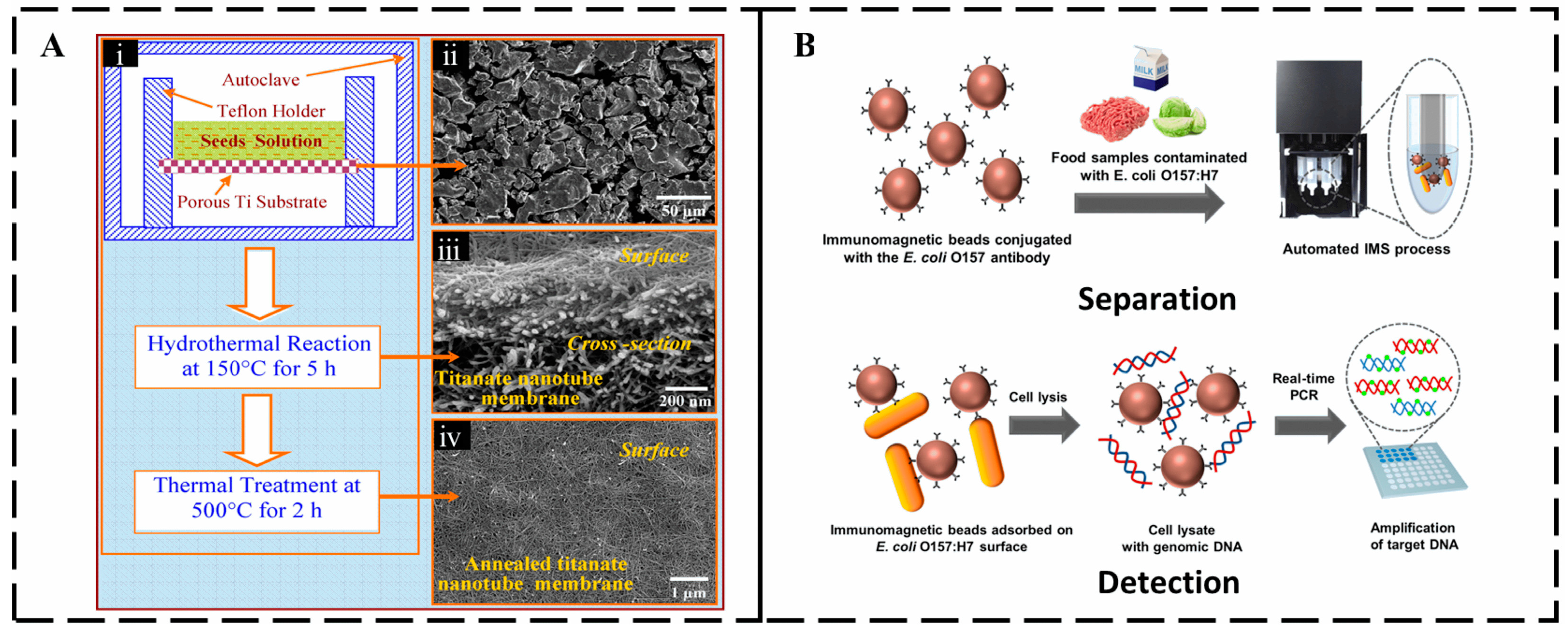
2.2. Conventional Detection Methods
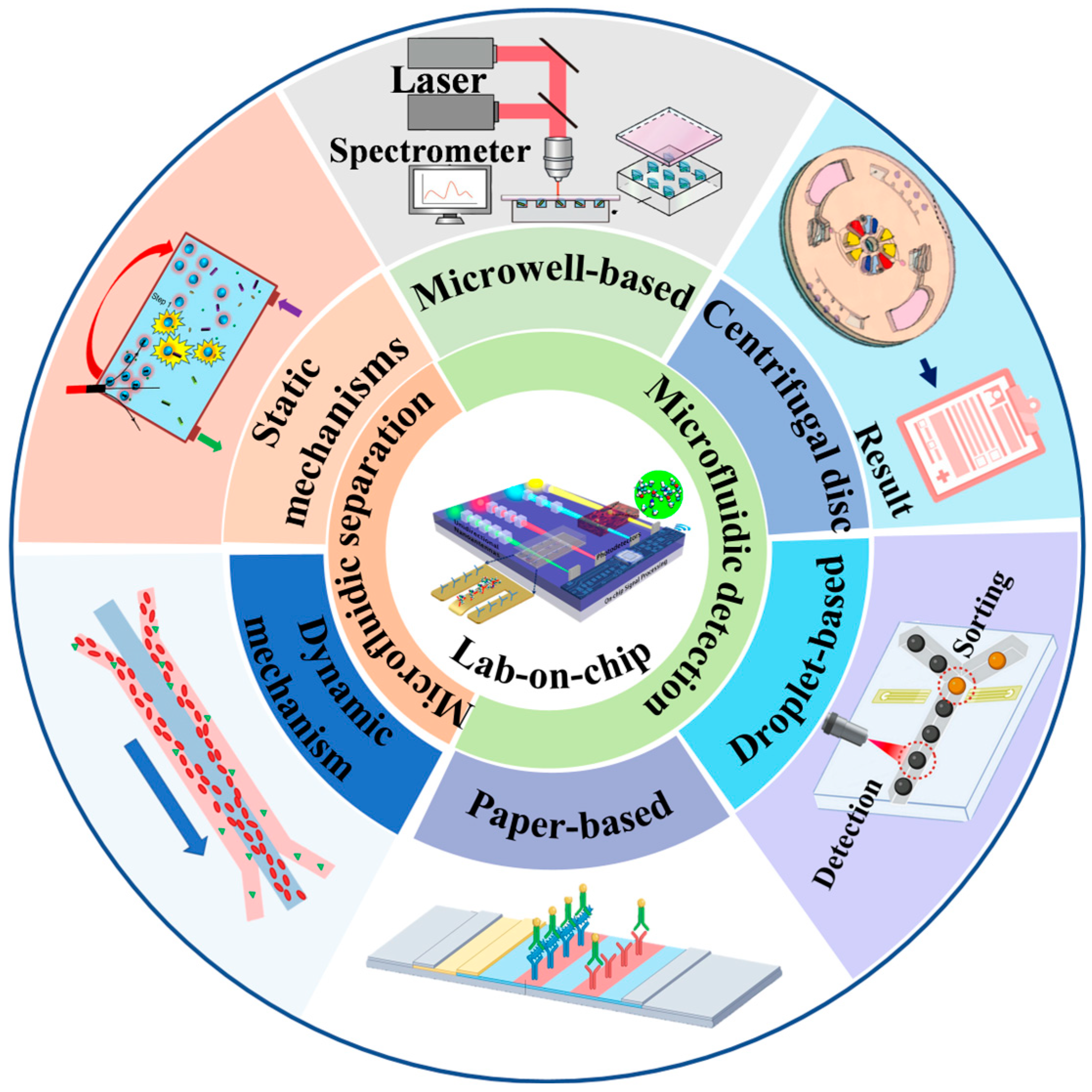
3. Microfluidics-Based Separation
3.1. Static Separation Mechanisms
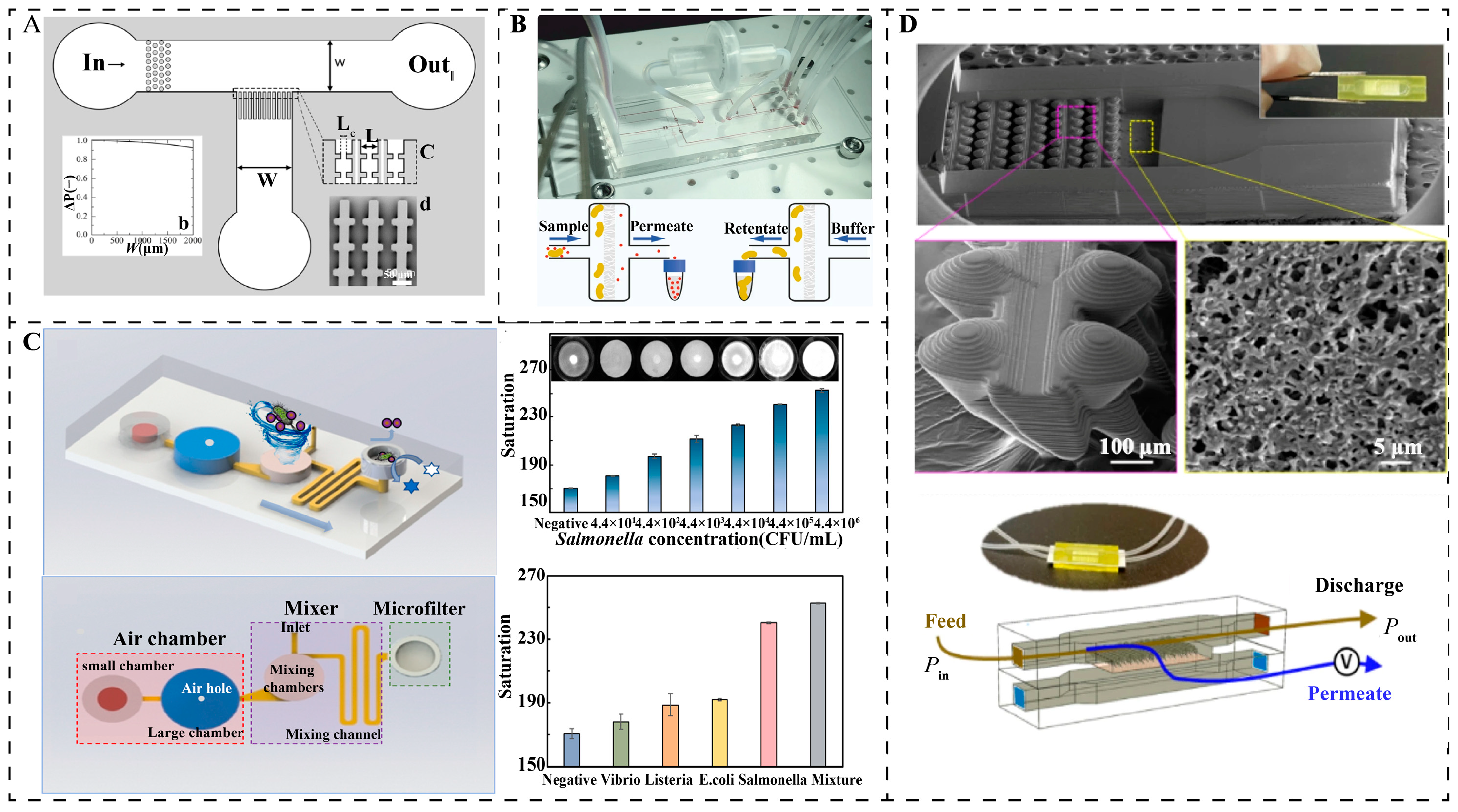
3.2. Dynamic Separation Mechanism
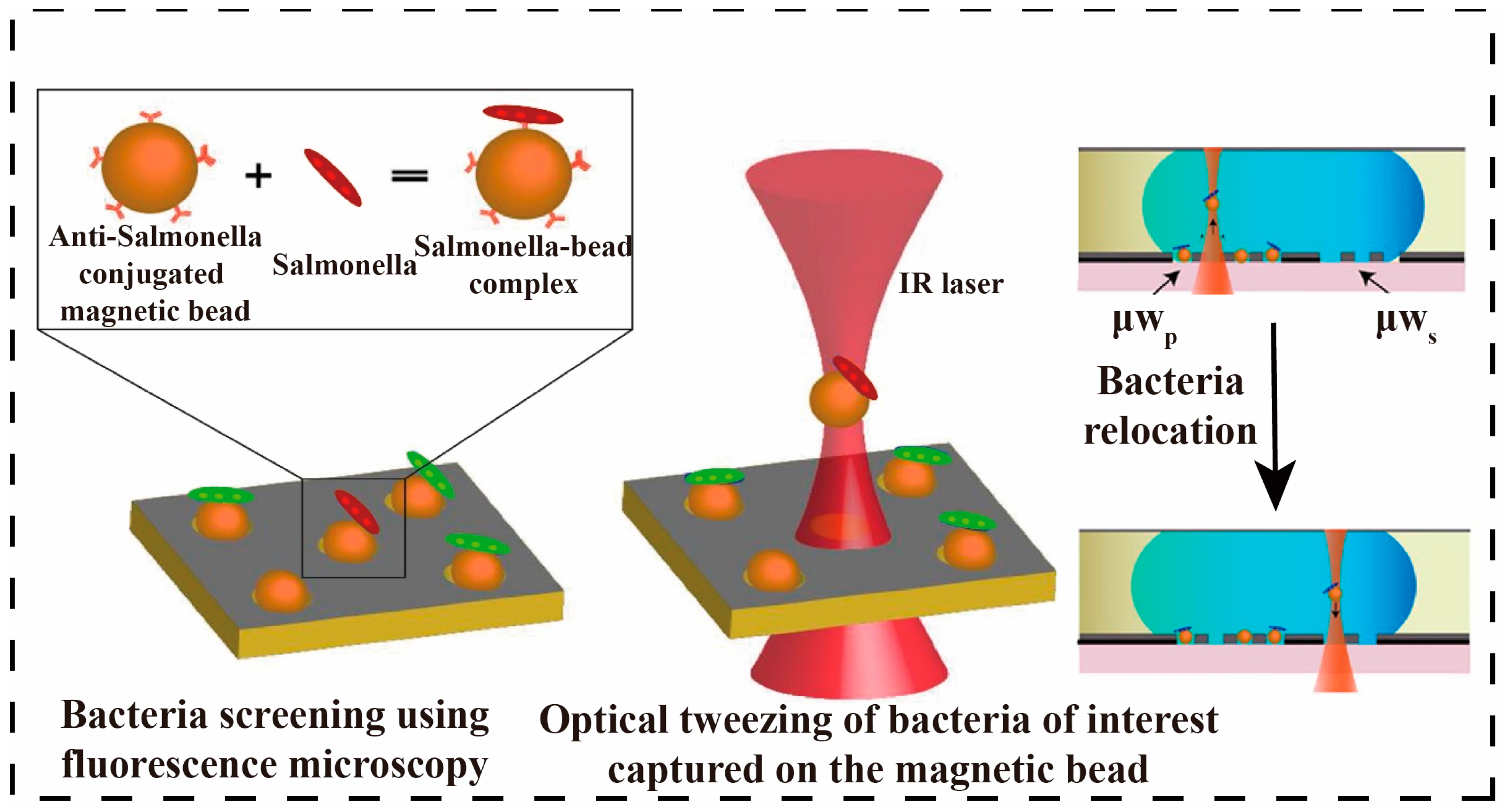
3.2.1. Optical Separation Methods
3.2.2. Electrical Separation Methods
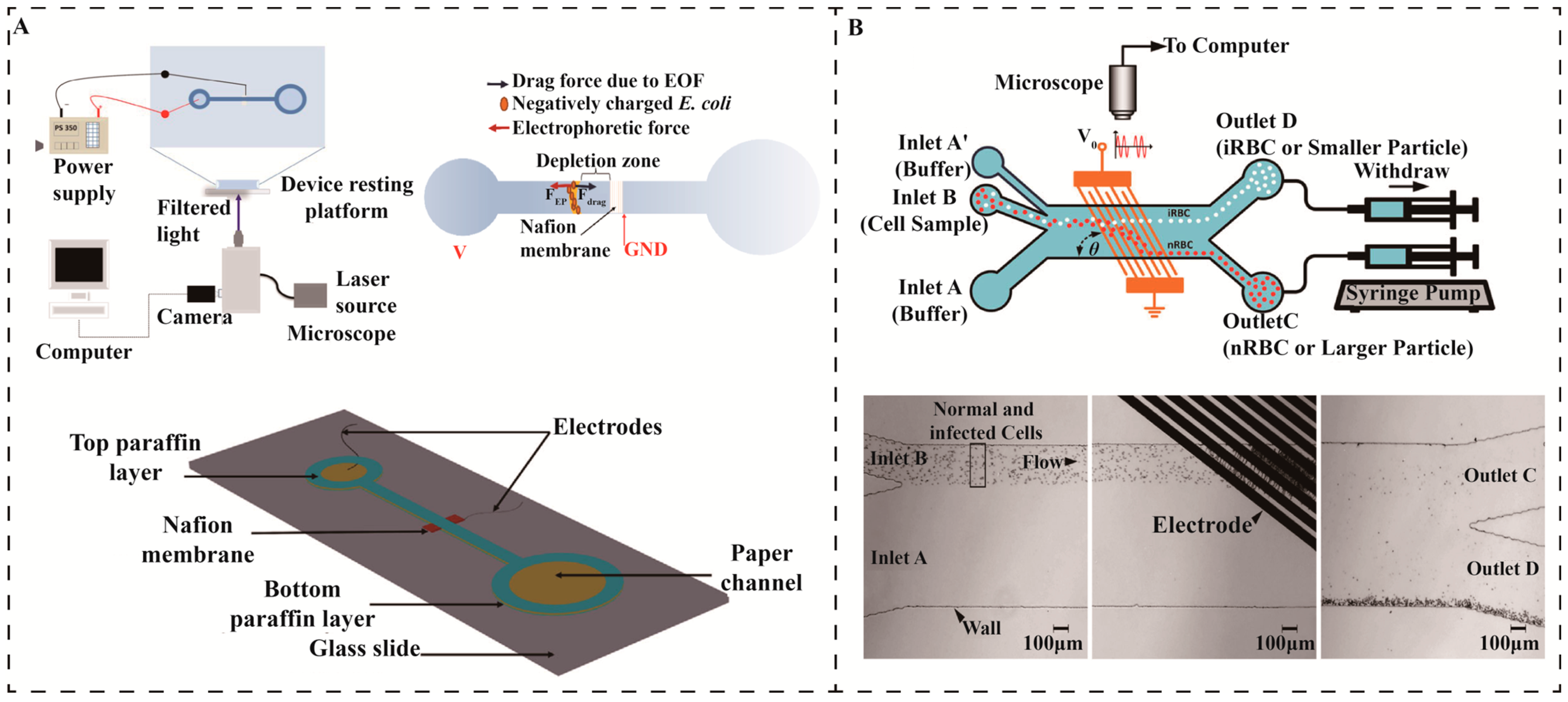
3.2.3. Fluidic Separation Methods

4. Microfluidics-Based Detection
4.1. Microwell-Based Detection of Waterborne Pathogens
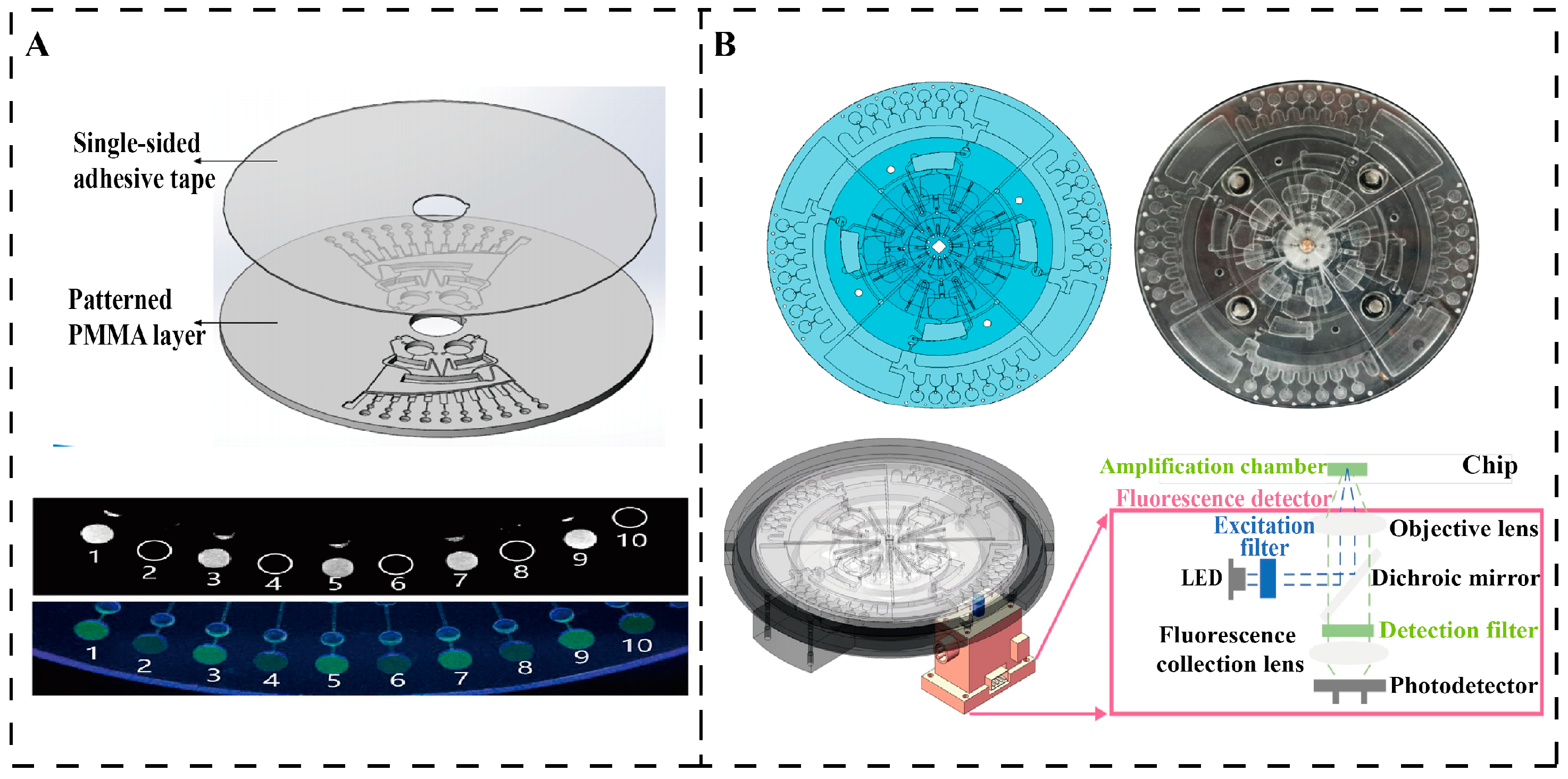
4.2. Centrifugal Microfluidic Detection of Waterborne Pathogens
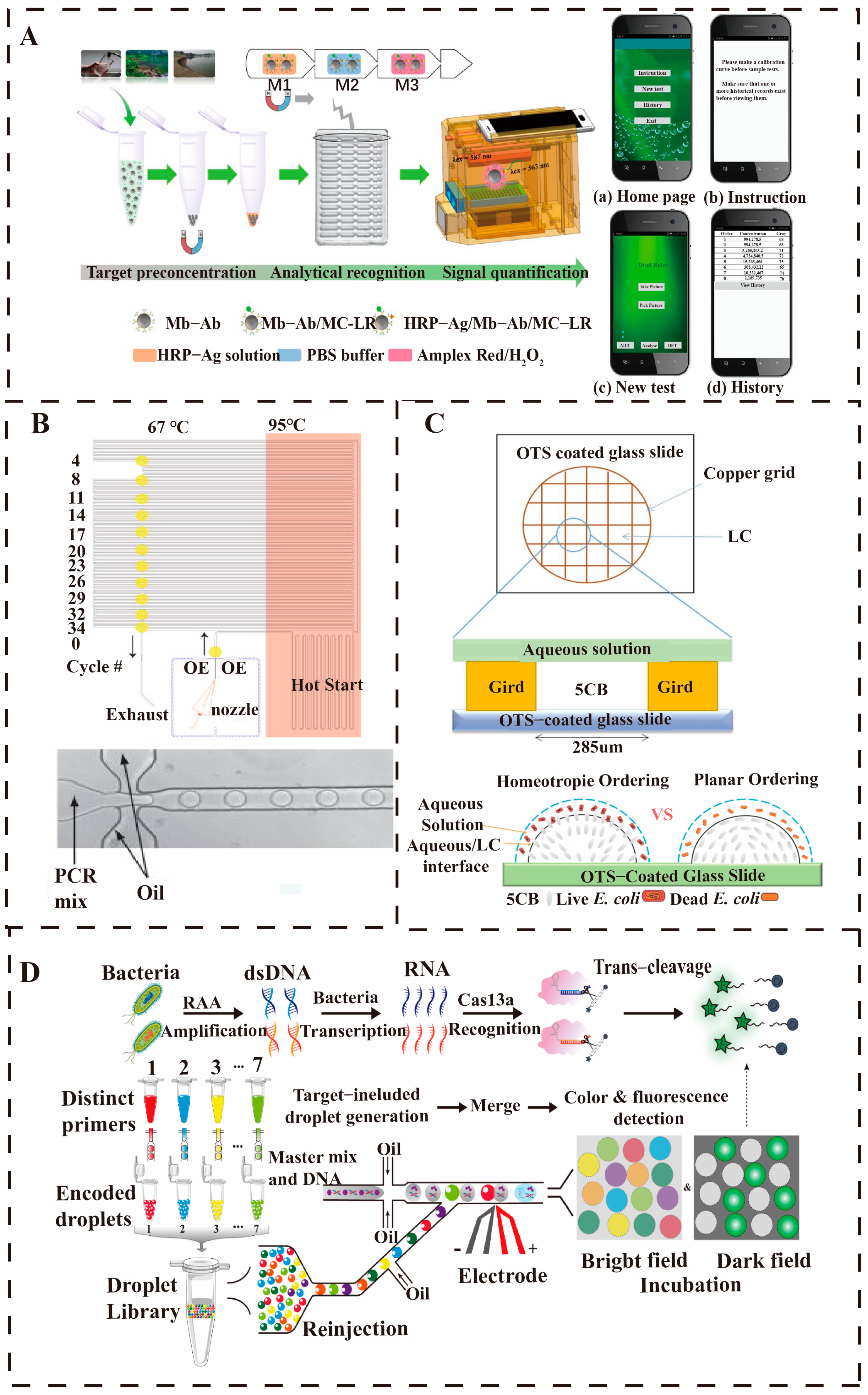
4.3. Droplet-Based Microfluidics Detection of Waterborne Pathogens
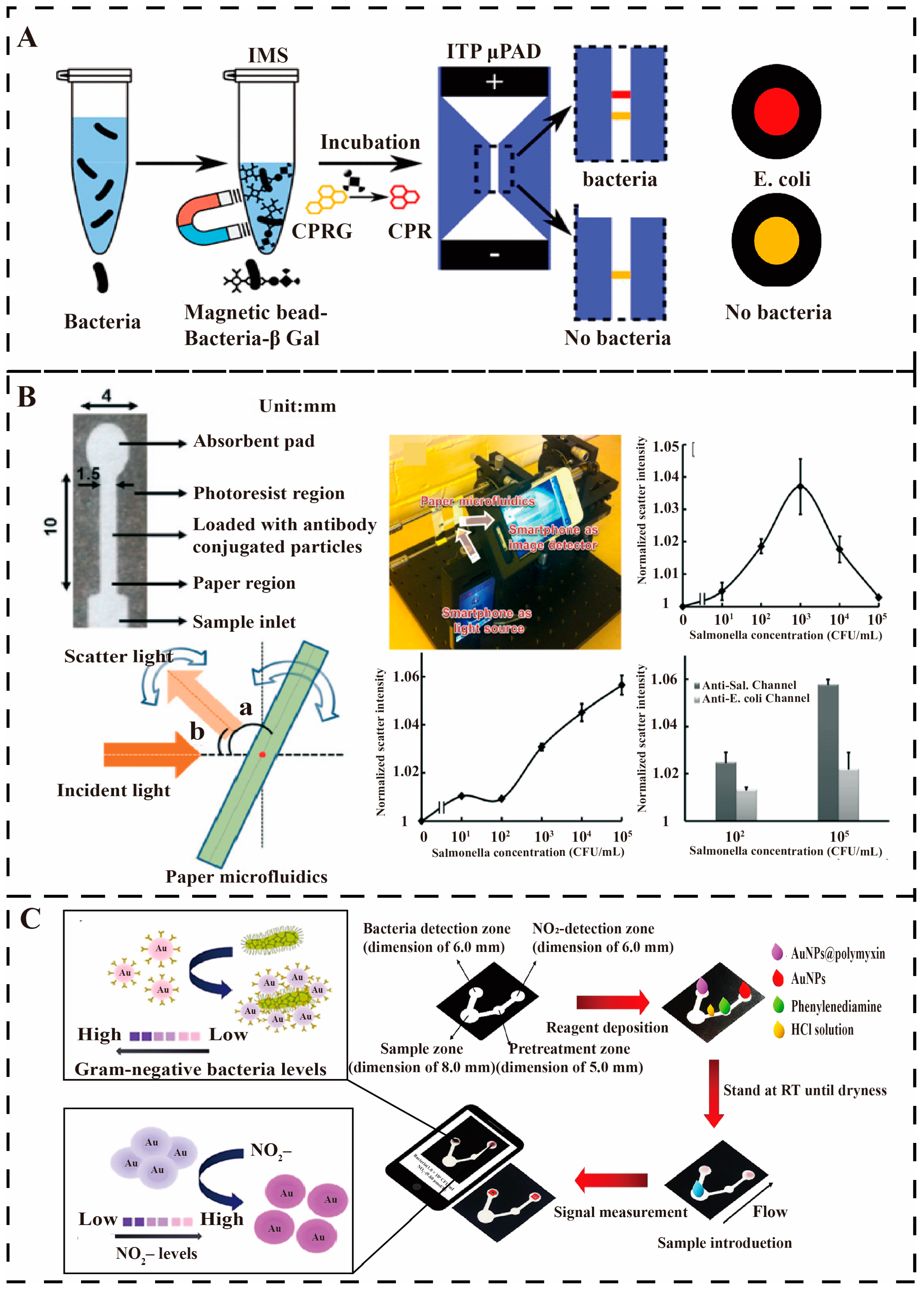
4.4. Paper-Based Microfluidics Detection of Waterborne Pathogens
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Bodirsky, B.L.; Rijneveld, R.; Beier, F.; Bak, M.P.; Batool, M.; Droppers, B.; Popp, A.; Van Vliet, M.T.H.; Strokal, M. A Triple Increase in Global River Basins with Water Scarcity Due to Future Pollution. Nat. Commun. 2024, 15, 880. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.R.; Hossain, M.B.; Chakraborty, P.A.; Mistry, S.K. Household Drinking Water E. coli Contamination and Its Associated Risk with Childhood Diarrhea in Bangladesh. Environ. Sci. Pollut. Res. 2022, 29, 32180–32189. [Google Scholar] [CrossRef]
- Al-Halhouli, A.; Doofesh, Z.; Albagdady, A.; Dietzel, A. High-Efficiency Small Sample Microparticle Fractionation on a Femtosecond Laser-Machined Microfluidic Disc. Micromachines 2020, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, X.; Wang, Q. Review of Microchip Analytical Methods for the Determination of Pathogenic Escherichia coli. Talanta 2021, 232, 122410. [Google Scholar] [CrossRef] [PubMed]
- Shukhratovich Abdullaev, S.; H Althomali, R.; Raza Khan, A.; Sanaan Jabbar, H.; Abosoda, M.; Ihsan, A.; Aggarwal, S.; Mustafa, Y.F.; Hammoud Khlewee, I.; Jabbar, A.M. Integrating of Analytical Techniques with Enzyme-Mimicking Nanomaterials for the Fabrication of Microfluidic Systems for Biomedical Analysis. Talanta 2024, 273, 125896. [Google Scholar] [CrossRef]
- Volpe, G.; Delibato, E.; Fabiani, L.; Pucci, E.; Piermarini, S.; D’Angelo, A.; Capuano, F.; De Medici, D.; Palleschi, G. Development and Evaluation of an ELIME Assay to Reveal the Presence of Salmonella in Irrigation Water: Comparison with Real-Time PCR and the Standard Culture Method. Talanta 2016, 149, 202–210. [Google Scholar] [CrossRef]
- Litvinov, J.; Moen, S.T.; Koh, C.-Y.; Singh, A.K. Centrifugal Sedimentation Immunoassays for Multiplexed Detection of Enteric Bacteria in Ground Water. Biomicrofluidics 2016, 10, 014103. [Google Scholar] [CrossRef]
- Borovic, B.; Velebit, B.; Veskovic, S.; Baltic, T.; Milijasevic, M.; Jankovic, V.; Vranic, D. Lactic Acid Bacteria Isolated from Sremska Sausage Using Molecular Methods. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012047. [Google Scholar] [CrossRef]
- Bayat, M.; Khabiri, A.; Hemati, B. Development of IgY-Based Sandwich ELISA as a Robust Tool for Rapid Detection and Discrimination of Toxigenic Vibrio cholerae. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 4032531. [Google Scholar] [CrossRef]
- Lee, S.; Khoo, V.S.L.; Medriano, C.A.D.; Lee, T.; Park, S.-Y.; Bae, S. Rapid and In-Situ Detection of Fecal Indicator Bacteria in Water Using Simple DNA Extraction and Portable Loop-Mediated Isothermal Amplification (LAMP) PCR Methods. Water Res. 2019, 160, 371–379. [Google Scholar] [CrossRef]
- Bridle, H. Overview of Waterborne Pathogens. In Waterborne Pathogens; Elsevier: Amsterdam, The Netherlands, 2014; pp. 9–40. ISBN 978-0-444-59543-0. [Google Scholar]
- Bilican, I.; Bahadir, T.; Bilgin, K.; Guler, M.T. Alternative Screening Method for Analyzing the Water Samples through an Electrical Microfluidics Chip with Classical Microbiological Assay Comparison of P. aeruginosa. Talanta 2020, 219, 121293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, H.; Liu, P.; Zhang, S.; Li, G. Direct Growth of Hierarchically Structured Titanate Nanotube Filtration Membrane for Removal of Waterborne Pathogens. J. Membr. Sci. 2009, 343, 212–218. [Google Scholar] [CrossRef]
- Richardson, P.; Molloy, J.; Ravenhall, R.; Holwill, I.; Hoare, M.; Dunnill, P. High Speed Centrifugal Separator for Rapid On-Line Sample Clarification in Biotechnology. J. Biotechnol. 1996, 49, 111–118. [Google Scholar] [CrossRef]
- Clark, K.D.; Purslow, J.A.; Pierson, S.A.; Nacham, O.; Anderson, J.L. Rapid Preconcentration of Viable Bacteria Using Magnetic Ionic Liquids for PCR Amplification and Culture-Based Diagnostics. Anal. Bioanal. Chem. 2017, 409, 4983–4991. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Katsube, K.; Hata, Y.; Kishi, R.; Fujiwara, S. Rapid Separation and Concentration of Food-Borne Pathogens in Food Samples Prior to Quantification by Viable-Cell Counting and Real-Time PCR. Appl. Environ. Microbiol. 2007, 73, 92–100. [Google Scholar] [CrossRef]
- Yuan, H.; Miao, Z.; Wan, C.; Wang, J.; Liu, J.; Li, Y.; Xiao, Y.; Chen, P.; Liu, B.-F. Recent Advances in Centrifugal Microfluidics for Point-of-Care Testing. Lab. Chip 2025, 25, 1015–1046. [Google Scholar] [CrossRef]
- Lee, J.; Chua, B.; Son, A. Recent Advances in Bacterial Lysis Techniques for Environmental Monitoring: A Review. Microchem. J. 2024, 207, 111865. [Google Scholar] [CrossRef]
- Liu, K.-Z.; Tian, G.; Ko, A.C.-T.; Geissler, M.; Malic, L.; Moon, B.-U.; Clime, L.; Veres, T. Microfluidic Methods for the Diagnosis of Acute Respiratory Tract Infections. Analyst 2025, 150, 9–33. [Google Scholar] [CrossRef]
- Patil, P.D.; Salokhe, S.; Karvekar, A.; Suryavanshi, P.; Phirke, A.N.; Tiwari, M.S.; Nadar, S.S. Microfluidic Based Continuous Enzyme Immobilization: A Comprehensive Review. Int. J. Biol. Macromol. 2023, 253, 127358. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.-T.; Ebrahimi Warkiani, M.; Li, W. Fundamentals and Applications of Inertial Microfluidics: A Review. Lab. Chip 2016, 16, 10–34. [Google Scholar] [CrossRef]
- Mansouri, S. Recent Developments of (Bio)-Sensors for Detection of Main Microbiological and Non-Biological Pollutants in Plastic Bottled Water Samples: A Critical Review. Talanta 2024, 274, 125962. [Google Scholar] [CrossRef] [PubMed]
- Hallam, J.M.; Rigas, E.; Charrett, T.O.H.; Tatam, R.P. 2D Spatially-Resolved Depth-Section Microfluidic Flow Velocimetry Using Dual Beam OCT. Micromachines 2020, 11, 351. [Google Scholar] [CrossRef]
- Deng, J.; Han, D.; Yang, J. Applications of Microfluidics in Liquid Crystal-Based Biosensors. Biosensors 2021, 11, 385. [Google Scholar] [CrossRef]
- Azevedo, A.S.; Fernandes, R.M.; Faria, A.R.; Silvestre, O.F.; Nieder, J.B.; Lou, C.; Wengel, J.; Almeida, C.; Azevedo, N.F. Spectral Imaging and Nucleic Acid Mimics Fluorescence in Situ Hybridization (SI-NAM-FISH) for Multiplex Detection of Clinical Pathogens. Front. Microbiol. 2022, 13, 976639. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Lee, S.H.; Song, J.; Bhattacharjee, S.; Feng, J.; Hong, S.; Song, M.; Kim, W.; Lee, J.; Bang, D.; et al. Nanophotonic Cell Lysis and Polymerase Chain Reaction with Gravity-Driven Cell Enrichment for Rapid Detection of Pathogens. ACS Nano 2019, 13, 13866–13874. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, D.; Yan, C.; Wei, C.; Ren, X.; Luo, C. Rapid and Accurate Detection of Escherichia coli O157:H7 in Beef Using Microfluidic Wax-Printed Paper-Based ELISA. Analyst 2020, 143, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Párraga-Niño, N.; Quero, S.; Ventós-Alfonso, A.; Uria, N.; Castillo-Fernandez, O.; Ezenarro, J.J.; Muñoz, F.-X.; Garcia-Nuñez, M.; Sabrià, M. New System for the Detection of Legionella Pneumophila in Water Samples. Talanta 2018, 189, 324–331. [Google Scholar] [CrossRef]
- Varshney, M.; Yang, L.; Su, X.-L.; Li, Y. Magnetic Nanoparticle-Antibody Conjugates for the Separation of Escherichia coli O157:H7 in Ground Beef. J. Food Prot. 2005, 68, 1804–1811. [Google Scholar] [CrossRef]
- Liu, Z.; Carroll, Z.S.; Long, S.C.; Roa-Espinosa, A.; Runge, T. Centrifuge Separation Effect on Bacterial Indicator Reduction in Dairy Manure. J. Environ. Manag. 2017, 191, 268–274. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, K.; Ok, G.; Chang, H.-J.; Park, T.J.; Choi, S.-W.; Lim, M.-C. Detection of Escherichia coli O157:H7 Using Automated Immunomagnetic Separation and Enzyme-Based Colorimetric Assay. Sensors 2020, 20, 1395. [Google Scholar] [CrossRef]
- Nair, G.; Jain, V. Separation of Mycobacterium smegmatis from a Mixed Culture Using the Cell Wall Binding Domain of D29 Mycobacteriophage Endolysin. Front. Microbiol. 2020, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Schaumburg, F.; Carrell, C.S.; Henry, C.S. Rapid Bacteria Detection at Low Concentrations Using Sequential Immunomagnetic Separation and Paper-Based Isotachophoresis. Anal. Chem. 2019, 91, 9623–9630. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Ramasamy, M.S.; Murugesh, S.; Murugan, A. Isolation and Screening of Marine Associated Bacteria from Tamil Nadu, Southeast Coast of India for Potential Antibacterial Activity. Ann. Microbiol. 2008, 58, 605–609. [Google Scholar] [CrossRef]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-Mediated Isothermal Amplification (LAMP): The Better Sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef]
- Truchado, P.; Hernandez, N.; Gil, M.I.; Ivanek, R.; Allende, A. Correlation between E. Coli Levels and the Presence of Foodborne Pathogens in Surface Irrigation Water: Establishment of a Sampling Program. Water Res. 2018, 128, 226–233. [Google Scholar] [CrossRef]
- Sartory, D.P.; Gu, H.; Chen, C.-M. Comparison of a Novel MPN Method against the Yeast Extract Agar (YEA) Pour Plate Method for the Enumeration of Heterotrophic Bacteria from Drinking Water. Water Res. 2008, 42, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, S.; Tawde, Y.; Chakrabarti, A.; Rudramurthy, S.M.; Kaur, H.; Shankarnarayan, S.A.; Ghosh, A. A Selective Medium for Isolation and Detection of Candida Auris, an Emerging Pathogen. J. Clin. Microbiol. 2021, 59, e00326-20. [Google Scholar] [CrossRef]
- Chaysiri, R.; Louis, G.E.; Chinviriyasit, W. Modeling the Health Impact of Water and Sanitation Service Deficits on Waterborne Disease Transmission. Adv. Differ. Equ. 2021, 2021, 405. [Google Scholar] [CrossRef]
- Nácher-Vázquez, M.; Barbosa, A.; Armelim, I.; Azevedo, A.S.; Almeida, G.N.; Pizarro, C.; Azevedo, N.F.; Almeida, C.; Cerqueira, L. Development of a Novel Peptide Nucleic Acid Probe for the Detection of Legionella spp. in Water Samples. Microorganisms 2022, 10, 1409. [Google Scholar] [CrossRef]
- Quintero-Betancourt, W.; Peele, E.R.; Rose, J.B. Cryptosporidium Parvum and Cyclospora Cayetanensis: A Review of Laboratory Methods for Detection of These Waterborne Parasites. J. Microbiol. Methods 2002, 49, 209–224. [Google Scholar] [CrossRef]
- Caradonna, T.; Marangi, M.; Del Chierico, F.; Ferrari, N.; Reddel, S.; Bracaglia, G.; Normanno, G.; Putignani, L.; Giangaspero, A. Detection and Prevalence of Protozoan Parasites in Ready-to-Eat Packaged Salads on Sale in Italy. Food Microbiol. 2017, 67, 67–75. [Google Scholar] [CrossRef]
- Tayler, S.; May, E. Detection of Specific Bacteria on Stone Using an Enzyme-Linked Immunosorbent Assay. Int. Biodeterior. Biodegrad. 1994, 34, 155–167. [Google Scholar] [CrossRef]
- Xue, J.-W.; Wang, R.; Yang, J.-Y.; Wang, L.-X.; Cao, Y.; Li, H.-D.; Yang, T.; Wang, J.-H. Sensitive Plasmonic ELISA Assay Based on Butyrylcholinesterase Catalyzed Hydrolysis for the Detection of Staphylococcus aureus. Sens. Actuators B Chem. 2022, 365, 131948. [Google Scholar] [CrossRef]
- Tahvildari, R.; Beamish, E.; Briggs, K.; Chagnon-Lessard, S.; Sohi, A.N.; Han, S.; Watts, B.; Tabard-Cossa, V.; Godin, M. Manipulating Electrical and Fluidic Access in Integrated Nanopore-Microfluidic Arrays Using Microvalves. Small 2017, 13, 1602601. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J. Review of Membranes in Microfluidics. J. Chem. Tech. Biotech. 2017, 92, 271–282. [Google Scholar] [CrossRef]
- Wang, C.; Senapati, S.; Chang, H. Liquid Biopsy Technologies Based on Membrane Microfluidics: High-yield Purification and Selective Quantification of Biomarkers in Nanocarriers. Electrophoresis 2020, 41, 1878–1892. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, R.; Van De Laar, T.; Sprakel, J.; Schroën, K. From Cooperative to Uncorrelated Clogging in Cross-Flow Microfluidic Membranes. Sci. Rep. 2018, 8, 5687. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkov, V.V.; Zverev, A.V.; Echeistov, V.V.; Andronic, M.; Ryzhikov, I.A.; Budashov, I.A.; Eremenko, A.V.; Kurochkin, I.N.; Rodionov, I.A. Cyclic On-Chip Bacteria Separation and Preconcentration. Sci. Rep. 2020, 10, 21107. [Google Scholar] [CrossRef]
- Jin, N.; Xue, L.; Ding, Y.; Liu, Y.; Jiang, F.; Liao, M.; Li, Y.; Lin, J. A Microfluidic Biosensor Based on Finger-Driven Mixing and Nuclear Track Membrane Filtration for Fast and Sensitive Detection of Salmonella. Biosens. Bioelectron. 2023, 220, 114844. [Google Scholar] [CrossRef]
- Li, H.; Raza, A.; Yuan, S.; AlMarzooqi, F.; Fang, N.X.; Zhang, T. Biomimetic On-Chip Filtration Enabled by Direct Micro-3D Printing on Membrane. Sci. Rep. 2022, 12, 8178. [Google Scholar] [CrossRef]
- Jen, C.-P.; Huang, C.-T.; Shih, H.-Y. Hydrodynamic Separation of Cells Utilizing Insulator-Based Dielectrophoresis. Microsyst. Technol. 2010, 16, 1097–1104. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Bougot-Robin, K.; Cao, W.; Yeung Yeung Chau, I.; Li, W.; Wen, W. High-Throughput Particle Manipulation by Hydrodynamic, Electrokinetic, and Dielectrophoretic Effects in an Integrated Microfluidic Chip. Biomicrofluidics 2013, 7, 024106. [Google Scholar] [CrossRef] [PubMed]
- Kamuri, M.; Zainal Abidin, Z.; Yaacob, M.; Hamidon, M.; Md Yunus, N.; Kamarudin, S. Separation and Detection of Escherichia coli and Saccharomyces cerevisiae Using a Microfluidic Device Integrated with an Optical Fibre. Biosensors 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, K.H.; Lee, K.S.; Ha, B.H.; Oh, Y.S.; Sung, H.J. Optical Separation of Droplets on a Microfluidic Platform. Microfluid. Nanofluid 2014, 16, 635–644. [Google Scholar] [CrossRef]
- Locke, A.; Fitzgerald, S.; Mahadevan-Jansen, A. Advances in Optical Detection of Human-Associated Pathogenic Bacteria. Molecules 2020, 25, 5256. [Google Scholar] [CrossRef]
- Tewari Kumar, P.; Decrop, D.; Safdar, S.; Passaris, I.; Kokalj, T.; Puers, R.; Aertsen, A.; Spasic, D.; Lammertyn, J. Digital Microfluidics for Single Bacteria Capture and Selective Retrieval Using Optical Tweezers. Micromachines 2020, 11, 308. [Google Scholar] [CrossRef]
- Murata, M.; Okamoto, Y.; Park, Y.-S.; Kaji, N.; Tokeshi, M.; Baba, Y. Cell Separation by the Combination of Microfluidics and Optical Trapping Force on a Microchip. Anal. Bioanal. Chem. 2009, 394, 277–283. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Weng, Z.; Li, Y.; Shui, L.; Jiao, Z.; Chen, Y.; Luo, A.; Xing, X.; He, S. Miniaturized Optical Fiber Tweezers for Cell Separation by Optical Force. Opt. Lett. 2019, 44, 1868. [Google Scholar] [CrossRef]
- Yamini, Y.; Seidi, S.; Rezazadeh, M. Electrical Field-Induced Extraction and Separation Techniques: Promising Trends in Analytical Chemistry—A Review. Anal. Chim. Acta 2014, 814, 1–22. [Google Scholar] [CrossRef]
- Farasat, M.; Aalaei, E.; Kheirati Ronizi, S.; Bakhshi, A.; Mirhosseini, S.; Zhang, J.; Nguyen, N.-T.; Kashaninejad, N. Signal-Based Methods in Dielectrophoresis for Cell and Particle Separation. Biosensors 2022, 12, 510. [Google Scholar] [CrossRef]
- Su, W.; Cook, B.S.; Fang, Y.; Tentzeris, M.M. Fully Inkjet-Printed Microfluidics: A Solution to Low-Cost Rapid Three-Dimensional Microfluidics Fabrication with Numerous Electrical and Sensing Applications. Sci. Rep. 2016, 6, 35111. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.T.K.; Pudasaini, S.; Ahmed, S.S.U.; Phan, D.; Liu, Y.; Yang, C. Rapid Pre-concentration of Escherichia coli in a Microfluidic Paper-based Device Using Ion Concentration Polarization. Electrophoresis 2020, 41, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Panklang, N.; Techaumnat, B.; Wisitsoraat, A.; Putaporntip, C.; Chotivanich, K.; Suzuki, Y. A Discrete Dielectrophoresis Device for the Separation of Malaria-infected Cells. Electrophoresis 2022, 43, 1347–1356. [Google Scholar] [CrossRef]
- Gioe, E.; Uddin, M.; Kim, J.-H.; Chen, X. Deterministic Lateral Displacement (DLD) Analysis Tool Utilizing Machine Learning towards High-Throughput Separation. Micromachines 2022, 13, 661. [Google Scholar] [CrossRef] [PubMed]
- Hochstetter, A.; Vernekar, R.; Austin, R.H.; Becker, H.; Beech, J.P.; Fedosov, D.A.; Gompper, G.; Kim, S.-C.; Smith, J.T.; Stolovitzky, G.; et al. Deterministic Lateral Displacement: Challenges and Perspectives. ACS Nano 2020, 14, 10784–10795. [Google Scholar] [CrossRef]
- Pariset, E.; Pudda, C.; Boizot, F.; Verplanck, N.; Revol-Cavalier, F.; Berthier, J.; Thuaire, A.; Agache, V. Purification of Complex Samples: Implementation of a Modular and Reconfigurable Droplet-Based Microfluidic Platform with Cascaded Deterministic Lateral Displacement Separation Modules. PLoS ONE 2018, 13, e0197629. [Google Scholar] [CrossRef]
- Wunsch, B.H.; Smith, J.T.; Gifford, S.M.; Wang, C.; Brink, M.; Bruce, R.L.; Austin, R.H.; Stolovitzky, G.; Astier, Y. Nanoscale Lateral Displacement Arrays for the Separation of Exosomes and Colloids down to 20 Nm. Nat. Nanotechnol. 2016, 11, 936–940. [Google Scholar] [CrossRef]
- Ai, Y.; Sanders, C.K.; Marrone, B.L. Separation of Escherichia coli Bacteria from Peripheral Blood Mononuclear Cells Using Standing Surface Acoustic Waves. Anal. Chem. 2013, 85, 9126–9134. [Google Scholar] [CrossRef]
- Deng, P.; Fu, C.-J.; Wu, Z. High Purity and Viability Cell Separation of a Bacterivorous Jakobid Flagellate Based on a Steep Velocity Gradient Induced Soft Inertial Force. RSC Adv. 2018, 8, 35512–35520. [Google Scholar] [CrossRef]
- Di Carlo, D. Inertial Microfluidics. Lab. Chip 2009, 9, 3038. [Google Scholar] [CrossRef]
- Seo, J.; Lean, M.H.; Kole, A. Membrane-Free Microfiltration by Asymmetric Inertial Migration. Appl. Phys. Lett. 2007, 91, 033901. [Google Scholar] [CrossRef]
- Ganz, K.R.; Clime, L.; Farber, J.M.; Corneau, N.; Veres, T.; Dixon, B.R. Enhancing the Detection of Giardia Duodenalis Cysts in Foods by Inertial Microfluidic Separation. Appl. Environ. Microbiol. 2015, 81, 3925–3933. [Google Scholar] [CrossRef]
- Nam, Y.-H.; Lee, S.; Lee, S.-K.; Kim, J.-H.; Park, J.-H. Microfluidic Chip with Integrated Separation, Mixing, and Concentration Operations for Rapid and Sensitive Bacterial Detection Utilizing Synthetic Inorganic Antibodies. Sens. Actuators B Chem. 2024, 404, 135202. [Google Scholar] [CrossRef]
- Prabhakar, A.; Jaiswar, A.; Mishra, N.; Kumar, P.; Dhwaj, A.; Nayak, P.; Verma, D. Amalgamation of Diverse Hydrodynamic Effects with Novel Triple-Sided Membrane Valves for Developing a Microfluidic Device for Filterless and Continuous Water Purification. RSC Adv. 2021, 11, 28723–28734. [Google Scholar] [CrossRef] [PubMed]
- Ogane, H.; Sato, T.-A.; Shinokawa, C.; Sawai, J. Low-Concentration Sorbic Acid Promotes the Induction of Escherichia coli into a Viable but Nonculturable State. Biocontrol Sci. 2019, 24, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, J.; Luo, N.; Chen, F.; Chen, B.; Zhao, Y. Microwell Array Chip-Based Single-Cell Analysis. Lab. Chip 2023, 23, 1066–1079. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Xie, H.; Xu, D. A Novel Method Combining Aptamer-Ag10NPs Based Microfluidic Biochip with Bright Field Imaging for Detection of KPC-2-Expressing Bacteria. Anal. Chim. Acta 2020, 1132, 20–27. [Google Scholar] [CrossRef]
- Huang, H.-K.; Cheng, H.-W.; Liao, C.-C.; Lin, S.-J.; Chen, Y.-Z.; Wang, J.-K.; Wang, Y.-L.; Huang, N.-T. Bacteria Encapsulation and Rapid Antibiotic Susceptibility Test Using a Microfluidic Microwell Device Integrating Surface-Enhanced Raman Scattering. Lab. Chip 2020, 20, 2520–2528. [Google Scholar] [CrossRef]
- Wu, W.; Nguyen, B.T.T.; Liu, P.Y.; Cai, G.; Feng, S.; Shi, Y.; Zhang, B.; Hong, Y.; Yu, R.; Zhou, X.; et al. Single Escherichia coli Bacteria Detection Using a Chemiluminescence Digital Microwell Array Chip. Biosens. Bioelectron. 2022, 215, 114594. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Carthy, E.; Kinahan, D.J. Biosensing on the Centrifugal Microfluidic Lab-on-a-Disc Platform. Processes 2020, 8, 1360. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, Y.; Zhang, Y.; Wang, L.; Chen, J.; Lu, Y.; Xu, Y.; Xing, W. Multiplex Detection of Bacteria on an Integrated Centrifugal Disk Using Bead-Beating Lysis and Loop-Mediated Amplification. Sci. Rep. 2017, 7, 1460. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yang, Y.; Cui, S.; Li, A.; Qian, C.; Li, X. Integrated High-Throughput Centrifugal Microfluidic Chip Device for Pathogen Detection On-Site. Biosensors 2024, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, X.; Wang, Q. Sensitive and Specific Detection of E. coli, Listeria monocytogenes, and Salmonella enterica Serovar Typhimurium in Milk by Microchip Electrophoresis Combined with Multiplex PCR Amplification. Microchem. J. 2020, 157, 104876. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Wu, J. Surface-Tension-Confined Droplet Microfluidics. Chin. Phys. B 2018, 27, 029202. [Google Scholar] [CrossRef]
- Guan, T.; He, J.; Liu, D.; Liang, Z.; Shu, B.; Chen, Y.; Liu, Y.; Shen, X.; Li, X.; Sun, Y.; et al. Open Surface Droplet Microfluidic Magnetosensor for Microcystin-LR Monitoring in Reservoir. Anal. Chem. 2020, 92, 3409–3416. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Lei, C.; Sun, X.; Zhou, Y. Ultrasensitive Detection and Quantification of E. coli O157:H7 Using a Giant Magnetoimpedance Sensor in an Open-Surface Microfluidic Cavity Covered with an Antibody-Modified Gold Surface. Microchim. Acta 2016, 183, 1831–1837. [Google Scholar] [CrossRef]
- Kiss, M.M.; Ortoleva-Donnelly, L.; Beer, N.R.; Warner, J.; Bailey, C.G.; Colston, B.W.; Rothberg, J.M.; Link, D.R.; Leamon, J.H. High-Throughput Quantitative Polymerase Chain Reaction in Picoliter Droplets. Anal. Chem. 2008, 80, 8975–8981. [Google Scholar] [CrossRef]
- Wei, Y.; Jang, C.-H. Liquid Crystal as Sensing Platforms for Determining the Effect of Graphene Oxide-Based Materials on Phospholipid Membranes and Monitoring Antibacterial Activity. Sens. Actuators B Chem. 2018, 254, 72–80. [Google Scholar] [CrossRef]
- Shang, Y.; Xing, G.; Lin, J.; Li, Y.; Lin, Y.; Chen, S.; Lin, J.-M. Multiplex Bacteria Detection Using One-Pot CRISPR/Cas13a-Based Droplet Microfluidics. Biosens. Bioelectron. 2024, 243, 115771. [Google Scholar] [CrossRef]
- Lagally, E.T.; Medintz, I.; Mathies, R.A. Single-Molecule DNA Amplification and Analysis in an Integrated Microfluidic Device. Anal. Chem. 2001, 73, 565–570. [Google Scholar] [CrossRef]
- Damit, B. Droplet-Based Microfluidics Detector for Bioaerosol Detection. Aerosol Sci. Technol. 2017, 51, 488–500. [Google Scholar] [CrossRef]
- Kaminski, T.S.; Scheler, O.; Garstecki, P. Droplet Microfluidics for Microbiology: Techniques, Applications and Challenges. Lab. Chip 2016, 16, 2168–2187. [Google Scholar] [CrossRef]
- Suo, Y.; Yin, W.; Wu, W.; Cao, W.; Zhu, Q.; Mu, Y. A Large-Scale Pico-Droplet Array for Viable Bacteria Digital Counting and Dynamic Tracking Based on a Thermosetting Oil. Analyst 2022, 147, 3305–3314. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Gao, Z.; Sekine, S.; You, Q.; Zhuang, S.; Zhang, D.; Feng, S.; Yamaguchi, Y. Lower Fluidic Resistance of Double-Layer Droplet Continuous Flow PCR Microfluidic Chip for Rapid Detection of Bacteria. Anal. Chim. Acta 2023, 1251, 340995. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Pan, M.; Patil, S.; Wang, J.-H.; Matin, A.C.; Andrews, J.R.; Tang, S.K.Y. Phenotyping Antibiotic Resistance with Single-Cell Resolution for the Detection of Heteroresistance. Sens. Actuators B Chem. 2018, 270, 396–404. [Google Scholar] [CrossRef]
- Lan, F.; Demaree, B.; Ahmed, N.; Abate, A.R. Single-Cell Genome Sequencing at Ultra-High-Throughput with Microfluidic Droplet Barcoding. Nat. Biotechnol. 2017, 35, 640–646. [Google Scholar] [CrossRef]
- Li, W.; Ma, X.; Yong, Y.-C.; Liu, G.; Yang, Z. Review of Paper-Based Microfluidic Analytical Devices for in-Field Testing of Pathogens. Anal. Chim. Acta 2023, 1278, 341614. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Li, W.; McCracken, K.E.; Yoon, J.-Y. Smartphone Quantifies Salmonella from Paper Microfluidics. Lab. Chip 2013, 13, 4832. [Google Scholar] [CrossRef]
- Khachornsakkul, K.; Del-Rio-Ruiz, R.; Creasey, H.; Widmer, G.; Sonkusale, S.R. Gold Nanomaterial-Based Microfluidic Paper Analytical Device for Simultaneous Quantification of Gram-Negative Bacteria and Nitrite Ions in Water Samples. ACS Sens. 2023, 8, 4364–4373. [Google Scholar] [CrossRef]
- Pelton, R. Bioactive Paper Provides a Low-Cost Platform for Diagnostics. Trac Trends Anal. Chem. 2009, 28, 925–942. [Google Scholar] [CrossRef]
- Mao, K.; Min, X.; Zhang, H.; Zhang, K.; Cao, H.; Guo, Y.; Yang, Z. Paper-Based Microfluidics for Rapid Diagnostics and Drug Delivery. J. Control. Release 2020, 322, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. Compact 3D Microfluidic Channel Structures Embedded in Glass Fabricated by Femtosecond Laser Direct Writing. J. Laser Micro Nanoeng. 2013, 8, 170–174. [Google Scholar] [CrossRef]
- Abdul Rasheed, P.; Sandhyarani, N. Attomolar Detection of BRCA1 Gene Based on Gold Nanoparticle Assisted Signal Amplification. Biosens. Bioelectron. 2015, 65, 333–340. [Google Scholar] [CrossRef]
- Somvanshi, S.B.; Ulloa, A.M.; Zhao, M.; Liang, Q.; Barui, A.K.; Lucas, A.; Jadhav, K.M.; Allebach, J.P.; Stanciu, L.A. Microfluidic Paper-Based Aptasensor Devices for Multiplexed Detection of Pathogenic Bacteria. Biosens. Bioelectron. 2022, 207, 114214. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhao, Z.; Lian, K.; Yin, L.; Wang, J.; Man, S.; Liu, G.; Ma, L. SERS-Based CRISPR/Cas Assay on Microfluidic Paper Analytical Devices for Supersensitive Detection of Pathogenic Bacteria in Foods. Biosens. Bioelectron. 2022, 207, 114167. [Google Scholar] [CrossRef]
- Aglietti, C.; Meinecke, C.D.; Ghelardini, L.; Barnes, I.; Van Der Nest, A.; Villari, C. Rapid Detection of Pine Pathogens Lecanosticta acicola, Dothistroma pini and D. septosporum on Needles by Probe-Based LAMP Assays. Forests 2021, 12, 479. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.Y.; Shu, M.; Zhang, T.T.; Bi, Y.; Gao, Y.Y.; Wu, G.P. Detection and Evaluation of Viable but Non-Culturable Escherichia coli O157:H7 Induced by Low Temperature with a BCAC-EMA-Rti-LAMP Assay in Chicken Without Enrichment. Food Anal. Methods 2019, 12, 458–468. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Shi, Y.; Wang, L.; Zhang, M.; Wu, J.; Chen, H. Carrying out Pseudo Dual Nucleic Acid Detection from Sample to Visual Result in a Polypropylene Bag with CRISPR/Cas12a. Biosens. Bioelectron. 2021, 178, 113001. [Google Scholar] [CrossRef]
- Bai, S.; Song, J.; Pu, H.; Yu, Y.; Song, W.; Chen, Z.; Wang, M.; Campbell-Valois, F.-X.; Wong, W.-L.; Cai, Q.; et al. Chemical Biology Approach to Reveal the Importance of Precise Subcellular Targeting for Intracellular Staphylococcus aureus Eradication. J. Am. Chem. Soc. 2023, 145, 23372–23384. [Google Scholar] [CrossRef]

| Separation Method | Detection Method | Bacteria | Separation Efficiency | Detection Limit | Reference |
|---|---|---|---|---|---|
| Staggered Flow Microfiltration Membrane | Microscopic Imaging | E. coli | 90% | / | [48] |
| Periodic Fluid Drive + Membrane Filtration | Fluorescence Microscopy | E. coli | 100 times | 102 CFU/mL | [49] |
| Membrane | Electrochemical Sensing | Salmonella | 95% | 102 CFU/mL | [50] |
| 3D Printed Bionic Membrane | Optical Microscopy | E. coli | 85% | / | [51] |
| Photofluidics | High-Speed Microscopy | E. coli | 90% | 103 CFU/mL | [5] |
| DMF combined with optical tweezers | Fluorescence Microscopy | E. coli | 70% | 10 CFU/mL | [57] |
| Infrared Optical Tweezers | Fluorescent Labeling | Saccharomyces cerevisiae | 95% | Single Bacteria | [58] |
| Miniaturized Fiber Optic Tweezers | Quantum Dot Labeling | E. coli | 85% | Single Bacteria | [59] |
| Hydrodynamic Focusing | Fiber Optic Transmission Spectroscopy | E. coli | 90% | 102 CFU/mL | [54] |
| DEP | Fluorescence Microscopy | Lactobacillus | 96% | Single Bacteria | [61] |
| ICP | Smartphone Fluorescence Imaging | E. coli | / | 102 CFU/mL | [63] |
| Inertial Microfluidics | High-speed microimaging | Saccharomyces cerevisiae | 95% | / | [71] |
| Inertial Microfluidics | Fluorescent Antibody Labeling | Giardia duodenalis | 78% | 1 cyst/g | [73] |
| Inertial Microfluidics | Fluorescent labeling | Jakobid flagellates | 90% | / | [70] |
| DLD | Electrochemical Impedance Sensing | E. coli | 92% | 10 CFU/mL | [74] |
| SAW | qPCR | E. coli | 88% | 102 CFU/mL | [69] |
| / | Flow Cytometry | E. coli | / | 103 CFU/mL | [76] |
| Microporous Array Aptamer-Ag10NPs | Brightfield imaging analysis | Klebsiella pneumoniae | 85% | 103 CFU/mL | [78] |
| SERS | SERS Spectroscopy | Staphylococcus aureus | 80% | Single Bacteria | [79] |
| Chemiluminescent Digital Microtiter Array | High-Sensitivity CCD Imaging | E. coli | 95% | 1 CFU/mL | [80] |
| Centrifugal sorting | LAMP Amplification | Listeria monocytogenes | >90% | 102 CFU/mL | [82] |
| Electromagnetic Field Assisted Magnetic Beads | Real-time fluorescence LAMP | E. coli | / | 102 CFU/mL | [83] |
| Antibody-Modified Magnetic Beads | Fluorescence Microscopy | Shigella | / | 103 CFU/mL | [7] |
| / | Multiplex PCR | E. coli, Listeria monocytogenes, Salmonella typhimurium | / | 102 CFU/mL | [84] |
| / | Fluorescent Labeling | Saccharomyces cerevisiae | / | / | [85] |
| / | GMI | E. coli O157:H7 | 85% | 10 CFU/mL | [87] |
| Droplet Encapsulation | CRISPR/Cas13a | Staphylococcus aureus | / | 10 CFU/mL | [90] |
| Aerosol particles | Flow-through Fluorescence | Bacillus | / | 1 CFU/mL | [92] |
| Centrifugation | Smartphone Camera | Salmonella | 85% | 103 CFU/mL | [99] |
| CRISPR-Cas12a targeted shearing | SERS | Staphylococcus aureus | / | 1 CFU/mL | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Cai, G.; Liang, L.; Cheng, J.; Song, L.; Sun, R.; Shen, F.; Liu, B.; Feng, S.; Zhang, J. Recent Advances in Microfluidics-Based Monitoring of Waterborne Pathogens: From Isolation to Detection. Micromachines 2025, 16, 462. https://doi.org/10.3390/mi16040462
Xu G, Cai G, Liang L, Cheng J, Song L, Sun R, Shen F, Liu B, Feng S, Zhang J. Recent Advances in Microfluidics-Based Monitoring of Waterborne Pathogens: From Isolation to Detection. Micromachines. 2025; 16(4):462. https://doi.org/10.3390/mi16040462
Chicago/Turabian StyleXu, Guohao, Gaozhe Cai, Lijuan Liang, Jianxin Cheng, Lujie Song, Rui Sun, Feng Shen, Bo Liu, Shilun Feng, and Jin Zhang. 2025. "Recent Advances in Microfluidics-Based Monitoring of Waterborne Pathogens: From Isolation to Detection" Micromachines 16, no. 4: 462. https://doi.org/10.3390/mi16040462
APA StyleXu, G., Cai, G., Liang, L., Cheng, J., Song, L., Sun, R., Shen, F., Liu, B., Feng, S., & Zhang, J. (2025). Recent Advances in Microfluidics-Based Monitoring of Waterborne Pathogens: From Isolation to Detection. Micromachines, 16(4), 462. https://doi.org/10.3390/mi16040462








