Defecation Warning Monitor Based on ScAlN Piezoelectric Ultrasonic Transducer (PMUT)
Abstract
:1. Introduction
2. Working Principle
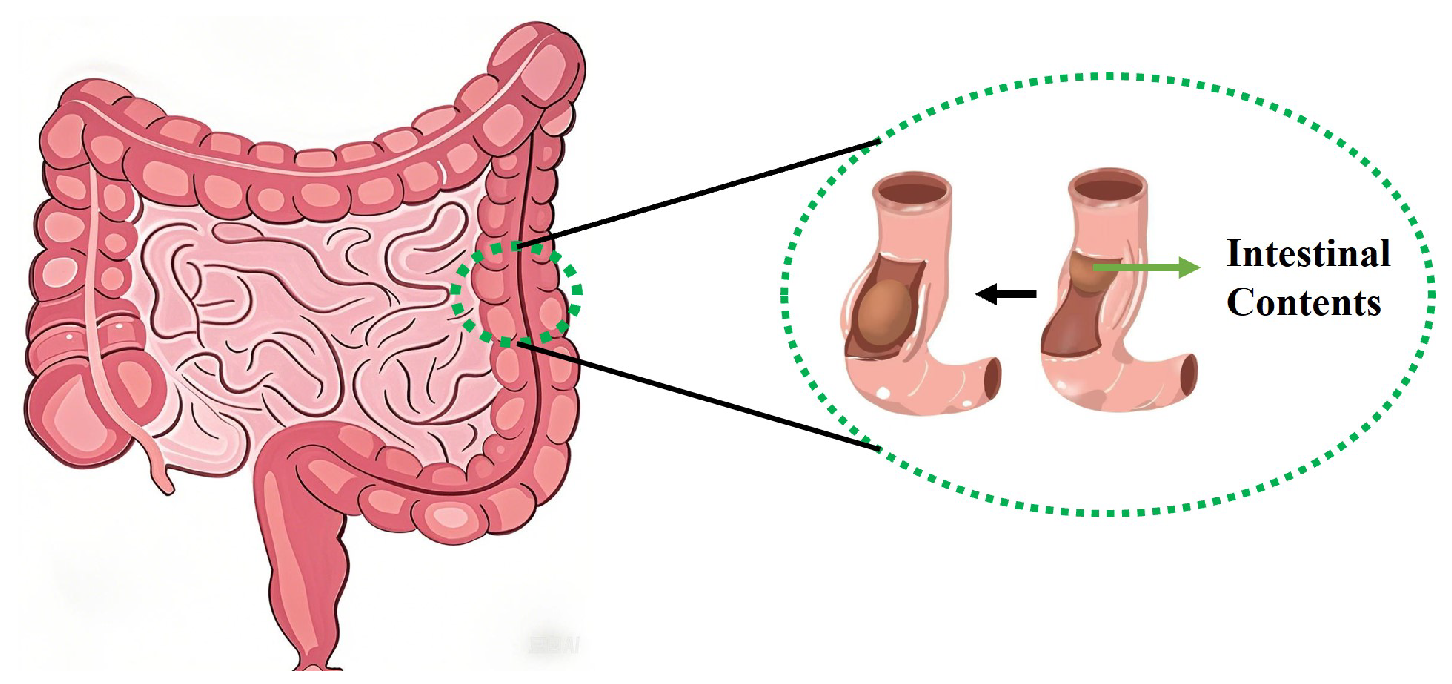
2.1. A Mathematical Model of Intestinal Sounds
2.2. Defecation Warning Monitor
3. Design and Fabrication of the Sensing Module
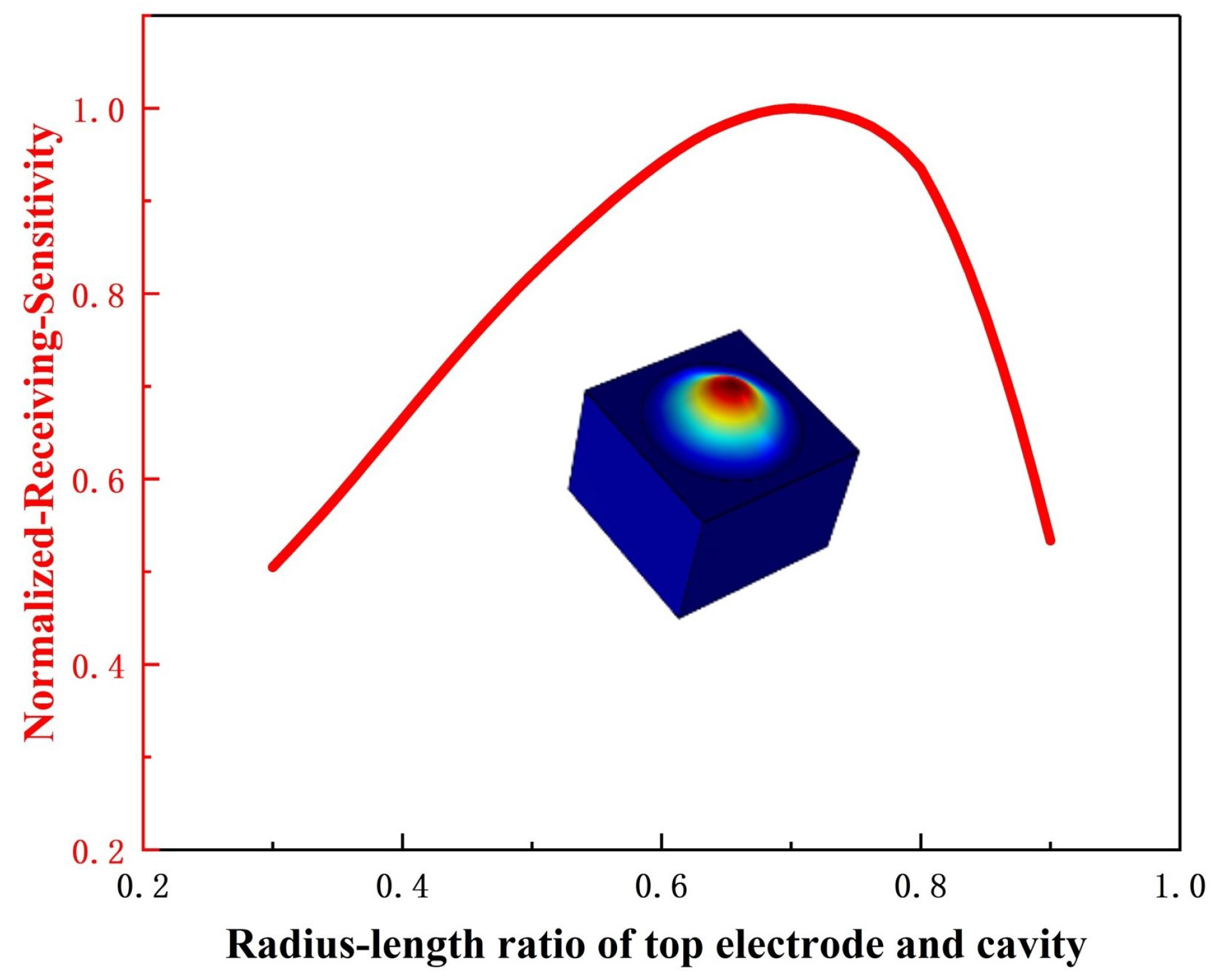
3.1. Principle and Design of PMUT
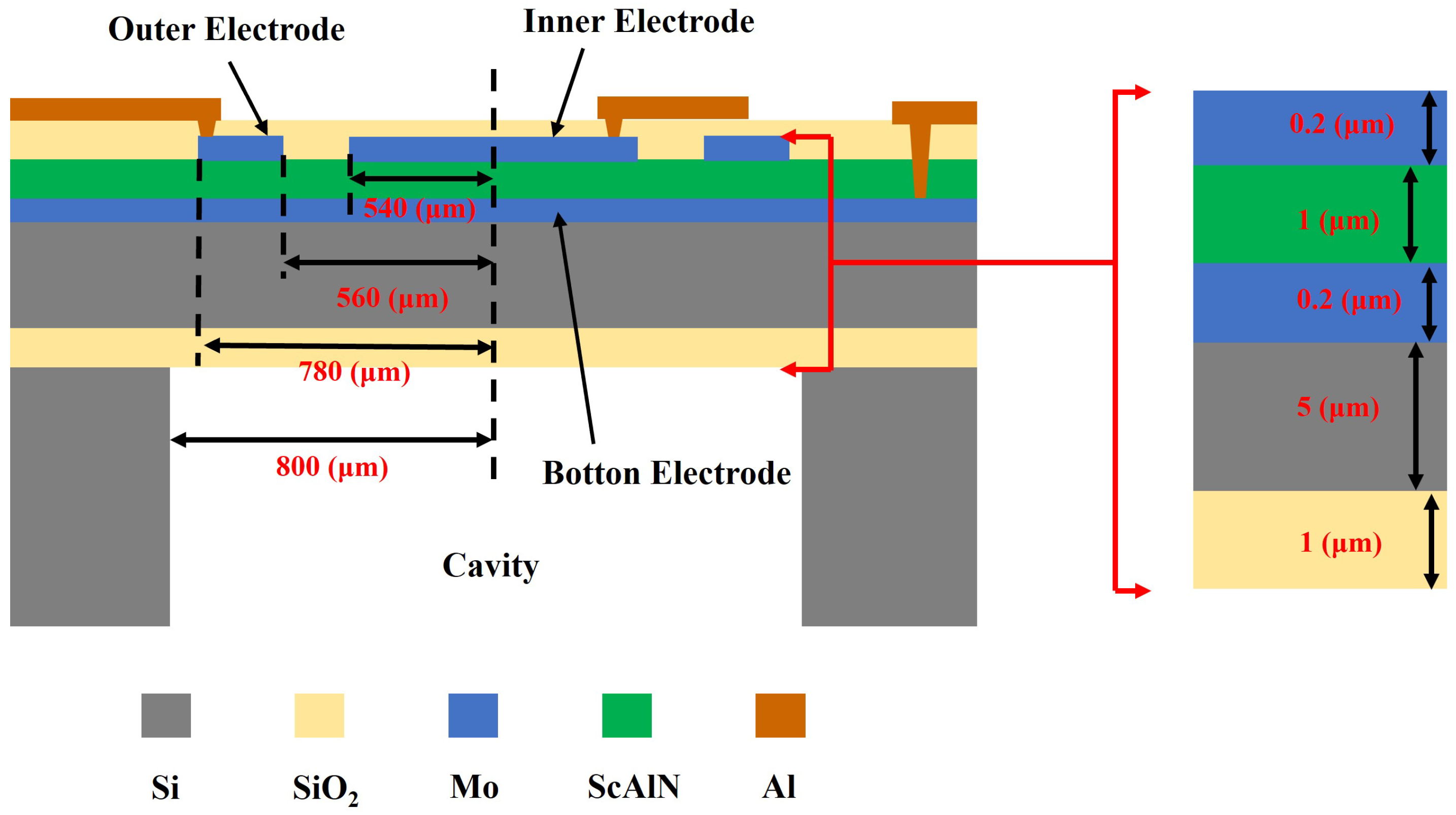
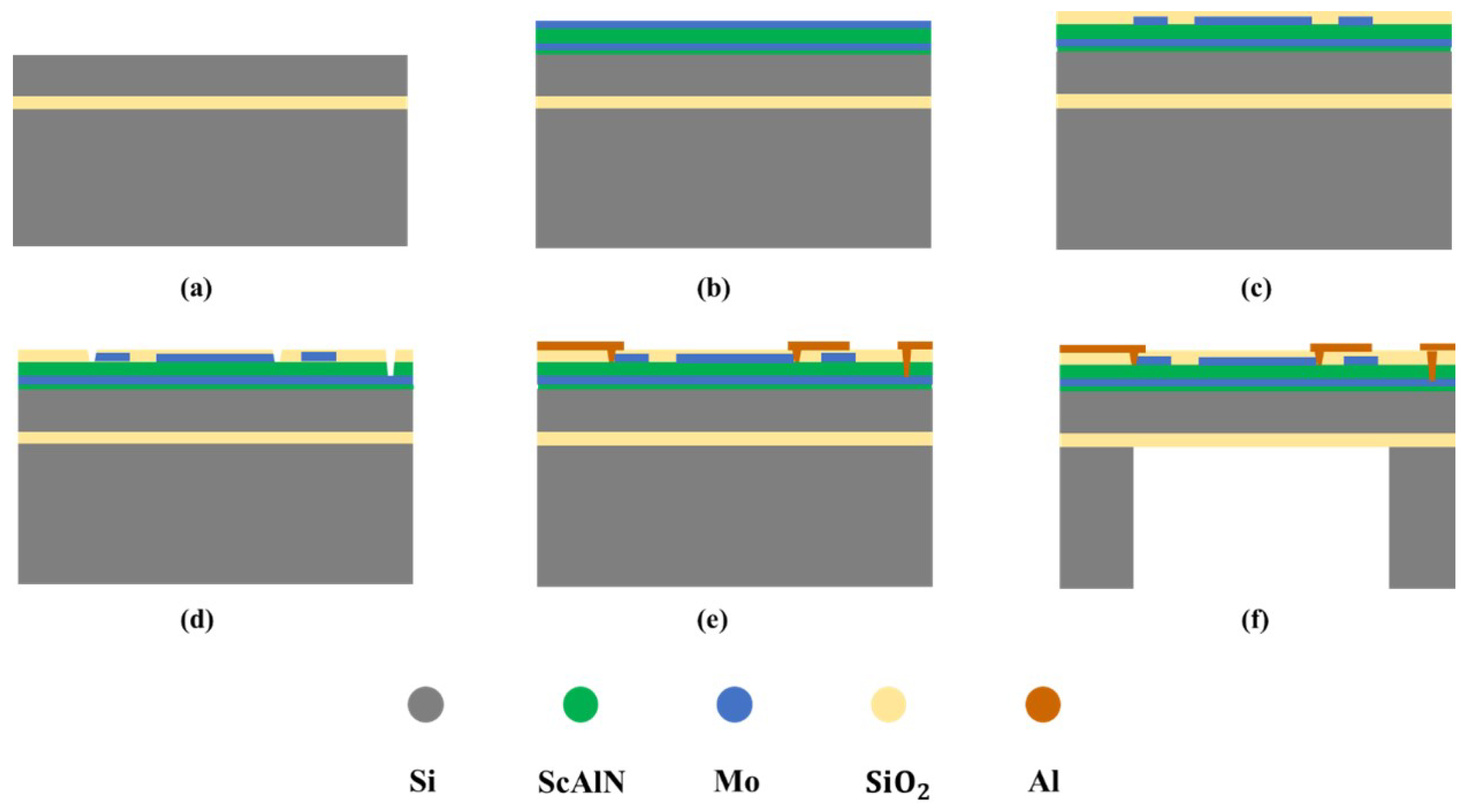
3.2. PMUT Manufacturing Industrial Process
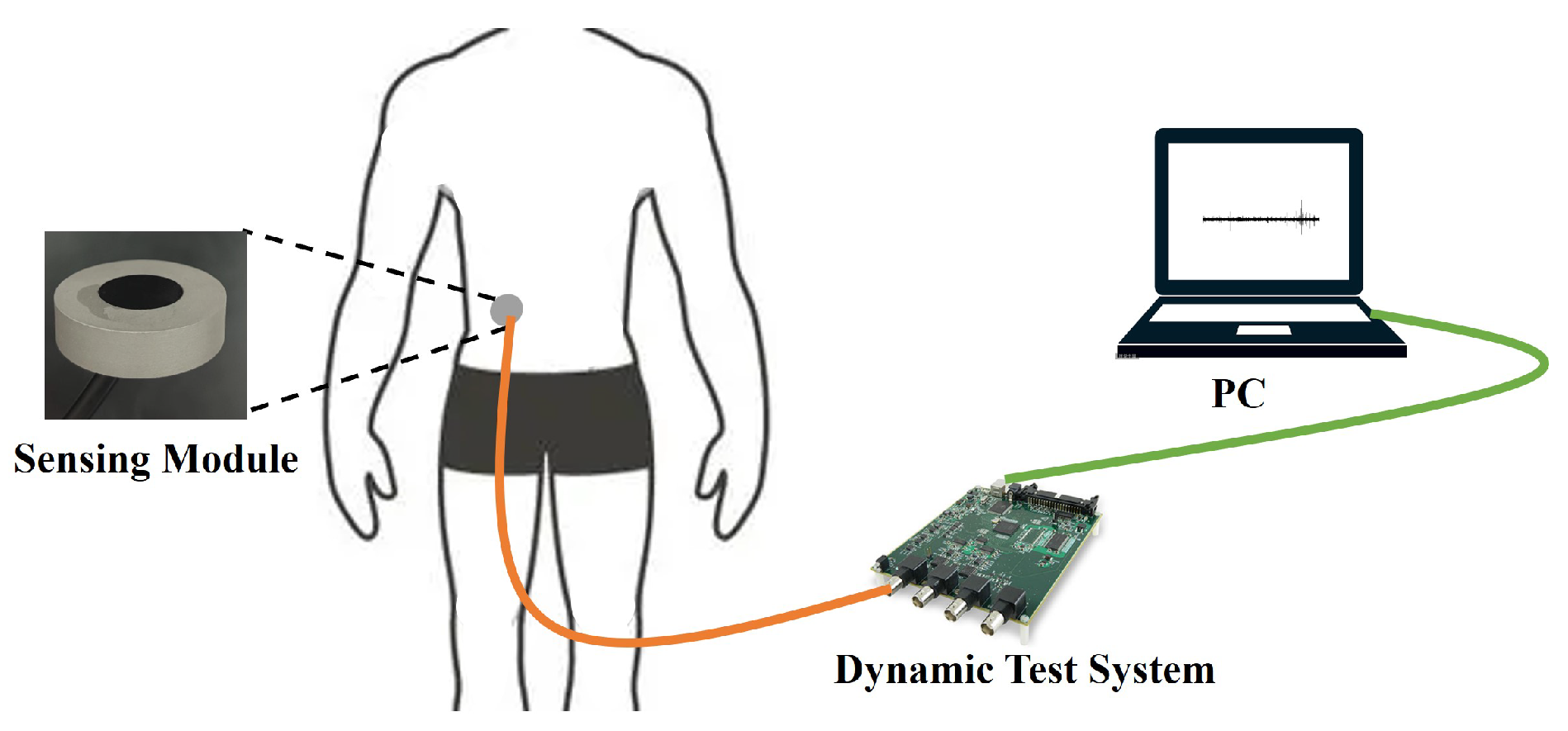
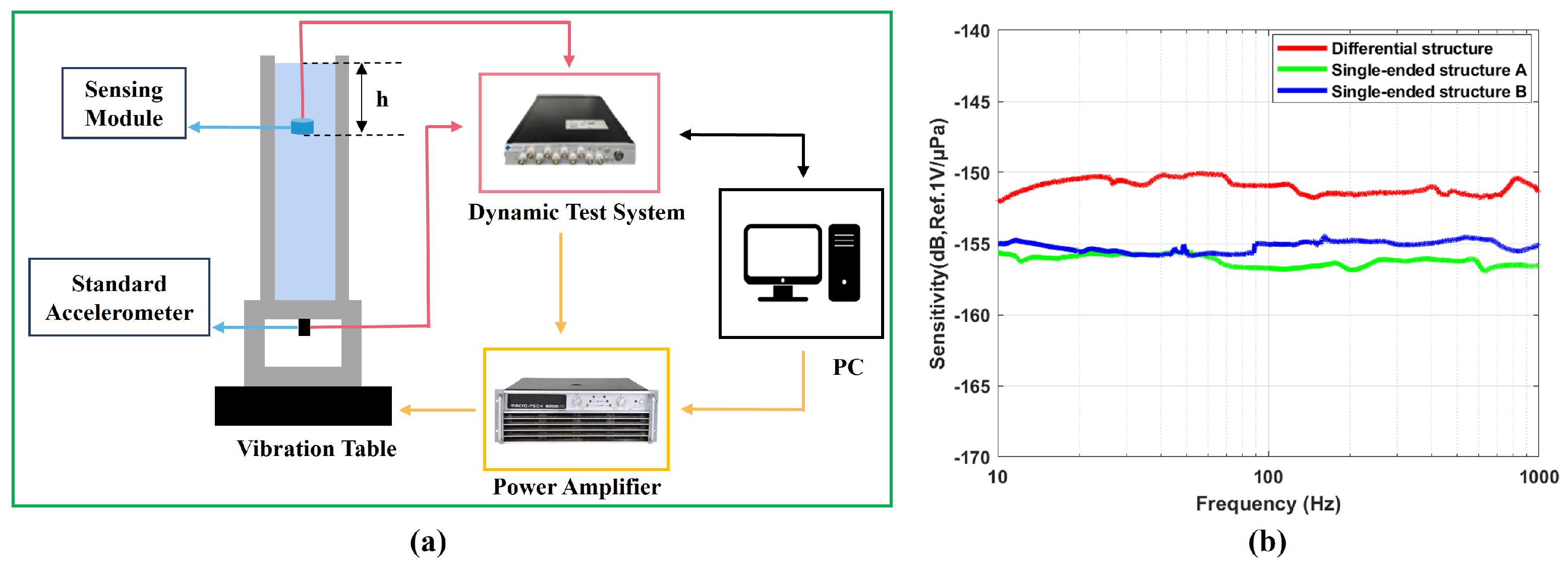
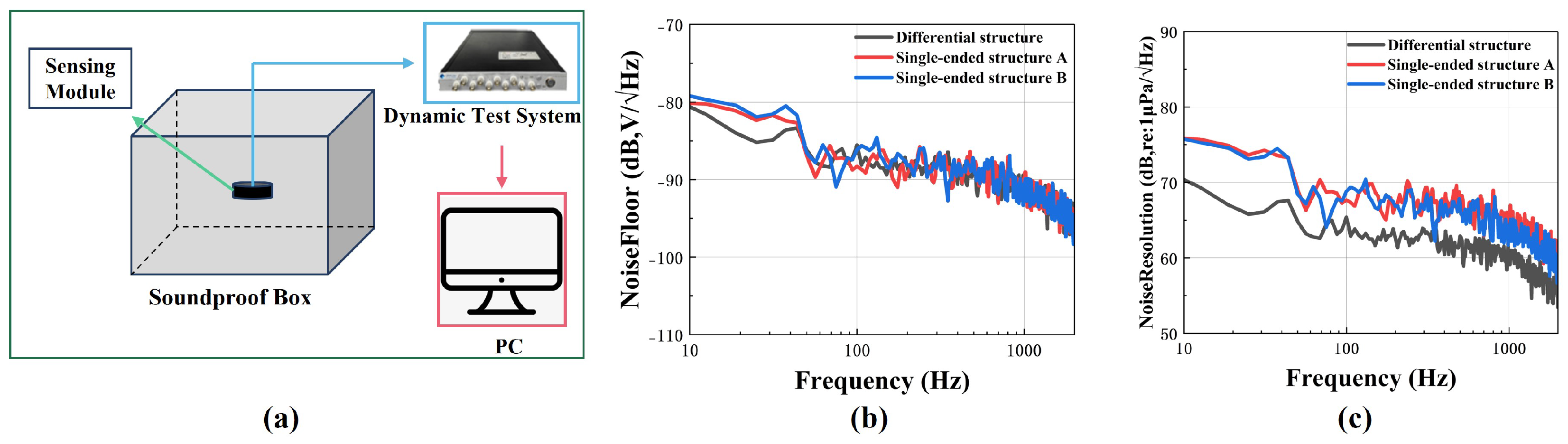
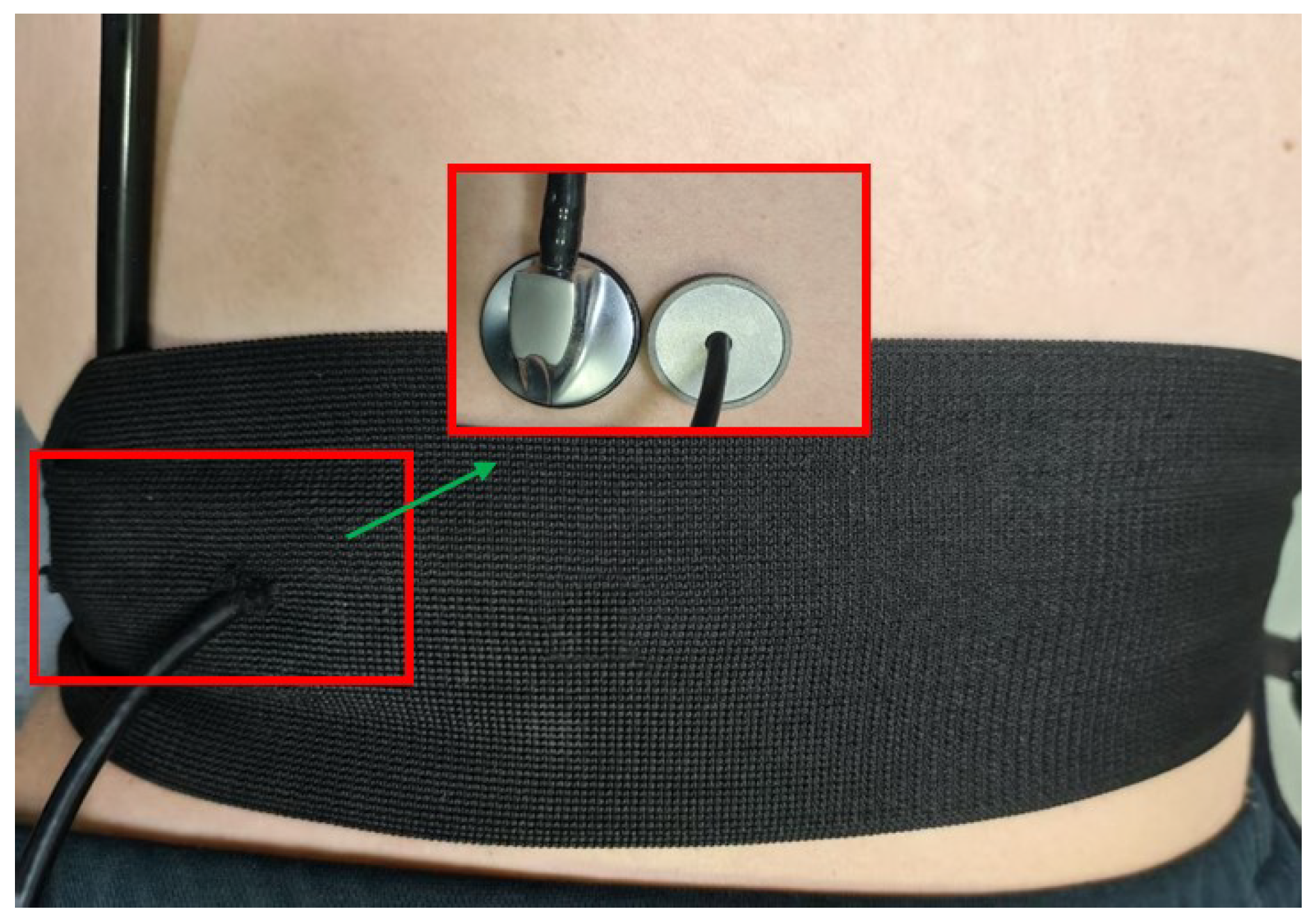
3.3. Encapsulation and Testing of the Sensing Module
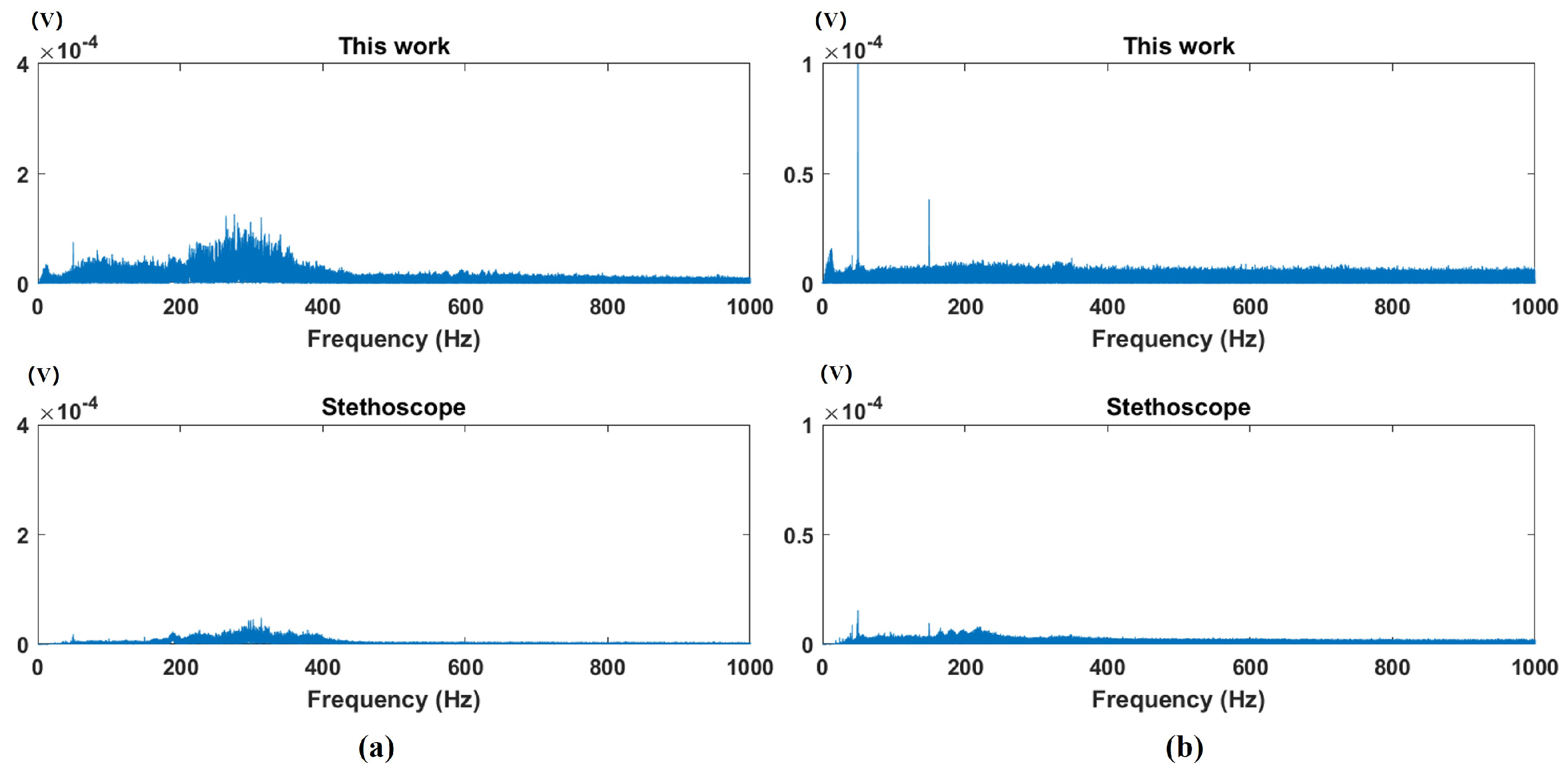
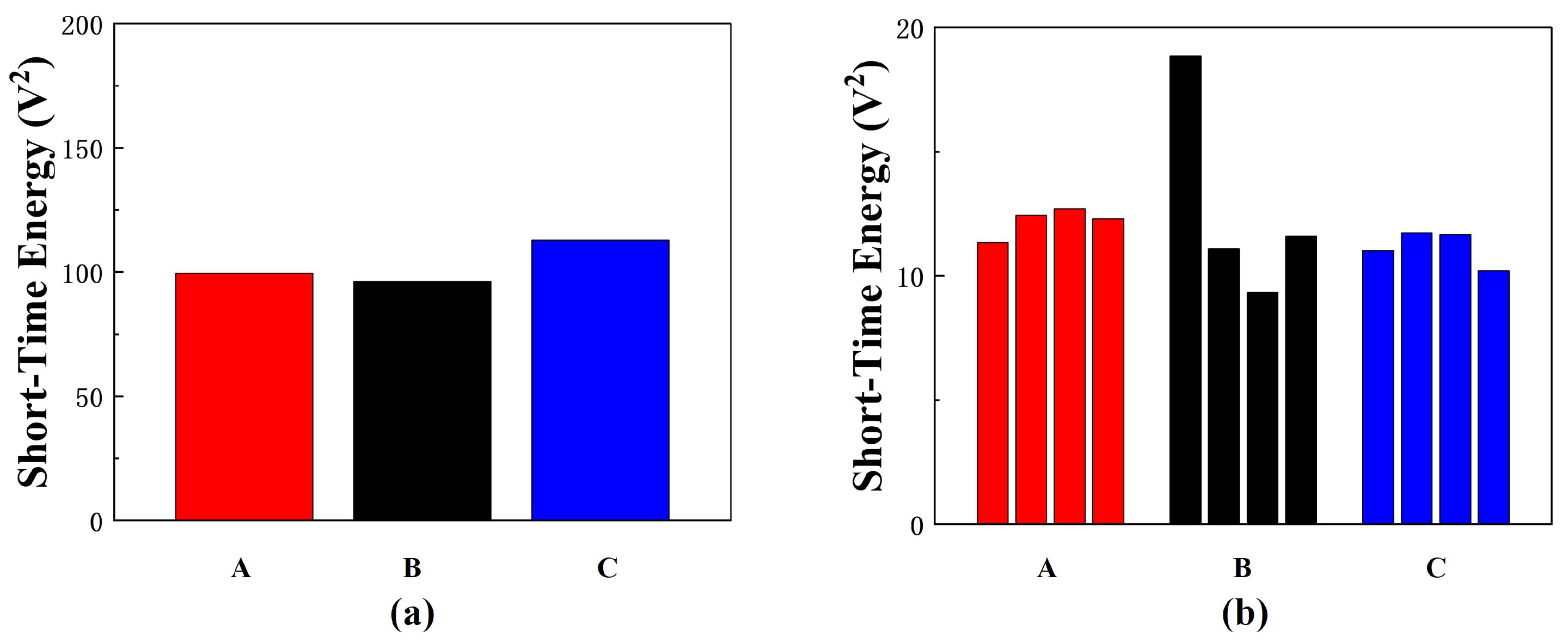
4. Experiments and Discussion
5. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Leng, M.; Guo, J.; Duan, J.; Wang, Z. The Effectiveness of Faecal Collection Devices in Preventing Incontinence-Associated Dermatitis in Critically Ill Patients with Faecal Incontinence: A Systematic Review and Meta-Analysis. Aust. Crit. Care 2021, 34, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, Y.; Zou, Y.; Zhao, J.; Wu, S. Deep Residual Attention Network for Human Defecation Prediction Using Bowel Sounds. Multimed. Tools Appl. 2024, 83, 36097–36113. [Google Scholar] [CrossRef]
- Akasheh, F.; Myers, T.; Fraser, J.D.; Bose, S.; Bandyopadhyay, A. Development of Piezoelectric Micromachined Ultrasonic Transducers. Sens. Actuators A Phys. 2004, 111, 275–287. [Google Scholar] [CrossRef]
- Eovino, B.E.; Liang, Y.; Akhbari, S.; Lin, L. A Single-Chip Flow Sensor Based on Bimorph PMUTs with Differential Readout Capabilities. In Proceedings of the 2018 IEEE Micro Electro Mechanical Systems (MEMS), Belfast, UK, 21–25 January 2018; pp. 1084–1087. [Google Scholar] [CrossRef]
- van Neer, P.L.M.J.; Robers, T.; Volker, A.W.F. A pMUT Based Flowmeter: A Feasibility Study. In Proceedings of the 2013 IEEE International Ultrasonics Symposium (IUS), Prague, Czech Republic, 21–25 July 2013; pp. 1319–1322. [Google Scholar] [CrossRef]
- Yin, J.; Zhou, Z.; Lou, L. A Novel Nondestructive Testing Probe Using AlN-Based Piezoelectric Micromachined Ultrasonic Transducers (PMUTs). Micromachines 2024, 15, 306. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Shi, L.; Lu, Z.; Sun, C.; Wu, G. A High-Performance 9.5% Scandium-Doped Aluminum Nitride Piezoelectric MEMS Hydrophone with Honeycomb Structure. IEEE Electron Device Lett. 2021, 42, 1845–1848. [Google Scholar] [CrossRef]
- Ganji, B.A.; Nateri, M.S.; Dardel, M. Design and Modeling of a Novel High Sensitive MEMS Piezoelectric Vector Hydrophone. Microsyst. Technol. 2018, 24, 2085–2095. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Zhou, Z.; Ping, Q.; Qiu, L.; Lou, L. An AlScN Piezoelectric Micromechanical Ultrasonic Transducer-Based Power-Harvesting Device for Wireless Power Transmission. Micromachines 2024, 15, 624. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J. Wearable Apparatus for Informing the Defecation Sign of the User, Comprises a Sensor Unit Including at Least One Acoustic Sensor Measuring the Prolonged Bowel Sound Signal in the Abdomen of the User. KR 10-2019-0057771 A, 23 May 2019. [Google Scholar]
- Nakanishi, A.; Masamori, R. Apparatus for Estimating Stool Volume Collected on Patient Rectum, Has Estimation Unit That Estimates Stool Volume Which Is Collected on Rectum Based on Output of Ultrasonic Sensor Which Detects Position of Urinary Bladder Wall. WO2016178261 A1, 3 November 2016. [Google Scholar]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology; e-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Du, X.; Allwood, G.; Webberley, K.M.; Osseiran, A.; Wan, W.; Volikova, A.; Marshall, B.J. A Mathematical Model of Bowel Sound Generation. J. Acoust. Soc. Am. 2018, 144, EL485–EL491. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Q.; Chen, M.; Liu, Y.; Zhu, J.; Yang, J.; Wang, F.; Tang, Y.; Zhao, X. Multiphysics Modeling and Analysis of Sc-Doped AlN Thin Film Based Piezoelectric Micromachined Ultrasonic Transducer by Finite Element Method. Micromachines 2023, 14, 1942. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tang, H.-Y.; Fung, S.; Boser, B.E.; Horsley, D.A. Pulse-Echo Ultrasound Imaging Using an AlN Piezoelectric Micromachined Ultrasonic Transducer Array with Transmit Beam-Forming. J. Microelectromech. Syst. 2016, 25, 179–187. [Google Scholar] [CrossRef]
- Dubois, M.-A.; Muralt, P. Properties of Aluminum Nitride Thin Films for Piezoelectric Transducers and Microwave Filter Applications. Appl. Phys. Lett. 1999, 74, 3032–3034. [Google Scholar] [CrossRef]
- Mertin, S.; Heinz, B.; Rattunde, O.; Christmann, G.; Dubois, M.-A.; Nicolay, S.; Muralt, P. Piezoelectric and Structural Properties of c-Axis Textured Aluminum Scandium Nitride Thin Films up to High Scandium Content. Surf. Coat. Technol. 2018, 343, 2–6. [Google Scholar] [CrossRef]
- Shi, L.; Jia, L.; Liu, C.; Yu, H.; Sun, C.; Wu, G. Performance of Aluminum Nitride-Based Piezoelectric Micromachined Ultrasonic Transducers under Different Readout Configurations. J. Micromech. Microeng. 2022, 32, 014002. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Li, D.; Wu, P.; Zhang, J.; Dou, H.; Zhang, M.; Yang, X.; Zhang, L.; Mu, X. Airborne Rangefinding With pMUTs Array Using Differential Structure. IEEE Sens. J. 2023, 23, 22240–22247. [Google Scholar] [CrossRef]
- Jung, J.; Lee, W.; Kang, W.; Shin, E.; Ryu, J. Review of Piezoelectric Micromachined Ultrasonic Transducers and Their Applications. J. Micromech. Microeng. 2017, 27, 113001. [Google Scholar] [CrossRef]
- Tiefensee, F.; Becker-Willinger, C.; Heppe, G.; Herbeck-Engel, P.; Jakob, A. Nanocomposite Cerium Oxide Polymer Matching Layers with Adjustable Acoustic Impedance between 4 MRayl and 7 MRayl. Ultrasonics 2010, 50, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, L.; Chen, X.; Qu, M.; Zhu, K.; Ding, H.; Li, D.; Bai, Y.; Ling, J.; Xu, J.; et al. A Piezoelectric AlN MEMS Hydrophone with High Sensitivity and Low Noise Density. Sens. Actuators A 2021, 318, 112493. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Xie, Y.; Wu, G. Bone Conduction Pickup Based on Piezoelectric Micromachined Ultrasonic Transducers. In Proceedings of the 2023 IEEE 36th International Conference on Micro Electro Mechanical Systems (MEMS), Munich, Germany, 15–19 January 2023; pp. 949–952. [Google Scholar] [CrossRef]
- Jia, L.; Shi, L.; Sun, C.; Liu, S.; Wu, G. AlN Based Piezoelectric Micromachined Ultrasonic Transducers for Continuous Monitoring of the Mechano-Acoustic Cardiopulmonary Signals. In Proceedings of the 2021 IEEE 34th International Conference on Micro Electro Mechanical Systems (MEMS), Gainesville, FL, USA, 25–29 January 2021; pp. 426–429. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Cui, J.; Zhang, G.; Wang, R.; Zhang, W.; He, C.; Li, Y.; Shi, P.; Wang, S. Design and Realization of MEMS Heart Sound Sensor with Concave, Racket-Shaped Cilium. Biosensors 2022, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Fernando, S.N.; Chai, K.T.; Gu, Y. AlN-on-SOI Platform-Based Micro-Machined Hydrophone. Appl. Phys. Lett. 2016, 109, 032902. [Google Scholar] [CrossRef]
- Ranta, R.; Louis-Dorr, V.; Heinrich, C.; Wolf, D.; Guillemin, F. Digestive Activity Evaluation by Multichannel Abdominal Sounds Analysis. IEEE Trans. Biomed. Eng. 2010, 57, 1507–1519. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, T.; Zong, J.; Zhang, H.; Hou, Z.; Lou, L. Defecation Warning Monitor Based on ScAlN Piezoelectric Ultrasonic Transducer (PMUT). Micromachines 2025, 16, 498. https://doi.org/10.3390/mi16050498
Yao T, Zong J, Zhang H, Hou Z, Lou L. Defecation Warning Monitor Based on ScAlN Piezoelectric Ultrasonic Transducer (PMUT). Micromachines. 2025; 16(5):498. https://doi.org/10.3390/mi16050498
Chicago/Turabian StyleYao, Tao, Jianwei Zong, Haoyue Zhang, Zhiyuan Hou, and Liang Lou. 2025. "Defecation Warning Monitor Based on ScAlN Piezoelectric Ultrasonic Transducer (PMUT)" Micromachines 16, no. 5: 498. https://doi.org/10.3390/mi16050498
APA StyleYao, T., Zong, J., Zhang, H., Hou, Z., & Lou, L. (2025). Defecation Warning Monitor Based on ScAlN Piezoelectric Ultrasonic Transducer (PMUT). Micromachines, 16(5), 498. https://doi.org/10.3390/mi16050498





