Overcoming Challenges in Silicon Anodes: The Role of Electrolyte Additives and Solid-State Electrolytes
Abstract
1. Introduction
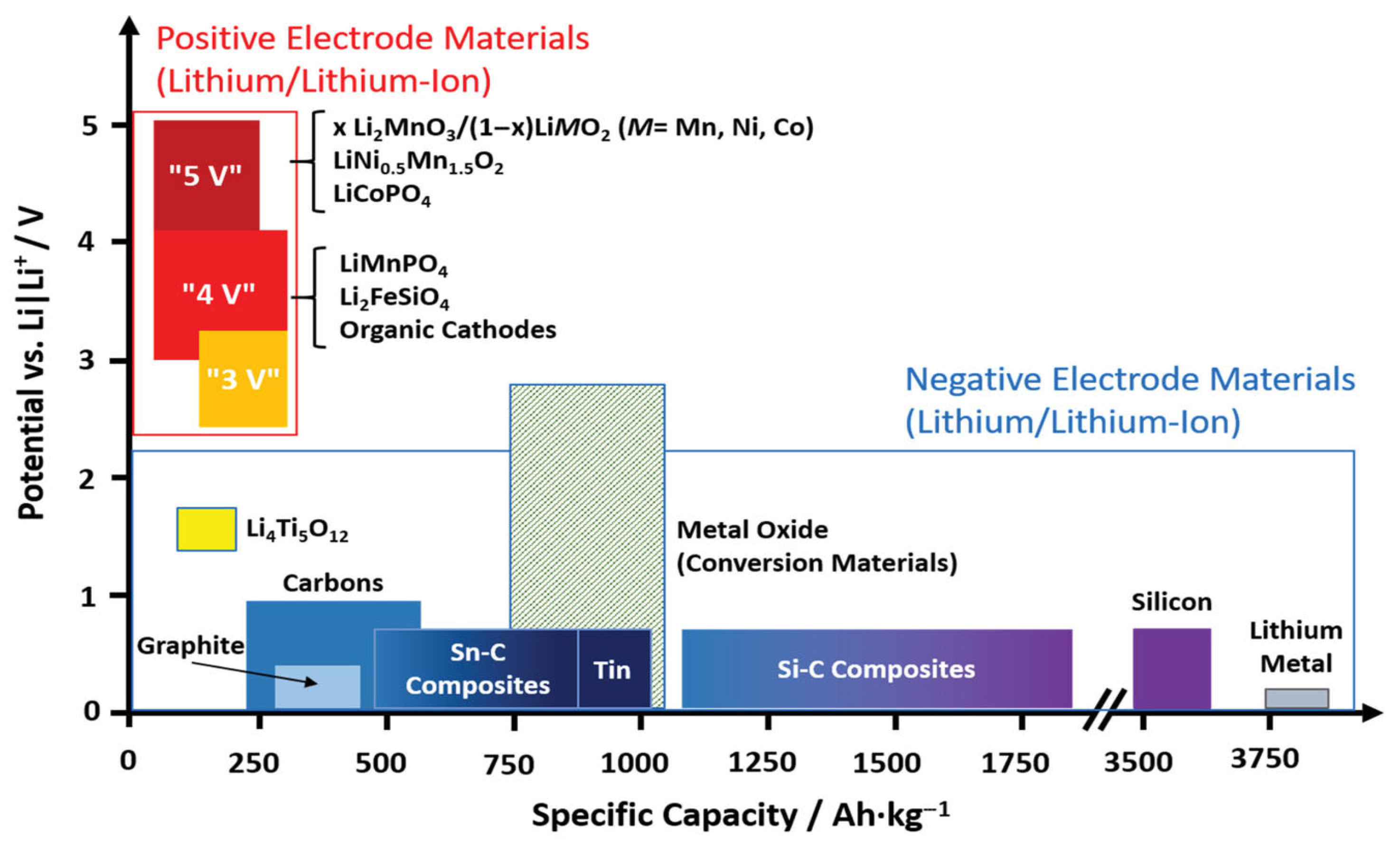
2. Si-Based Anode
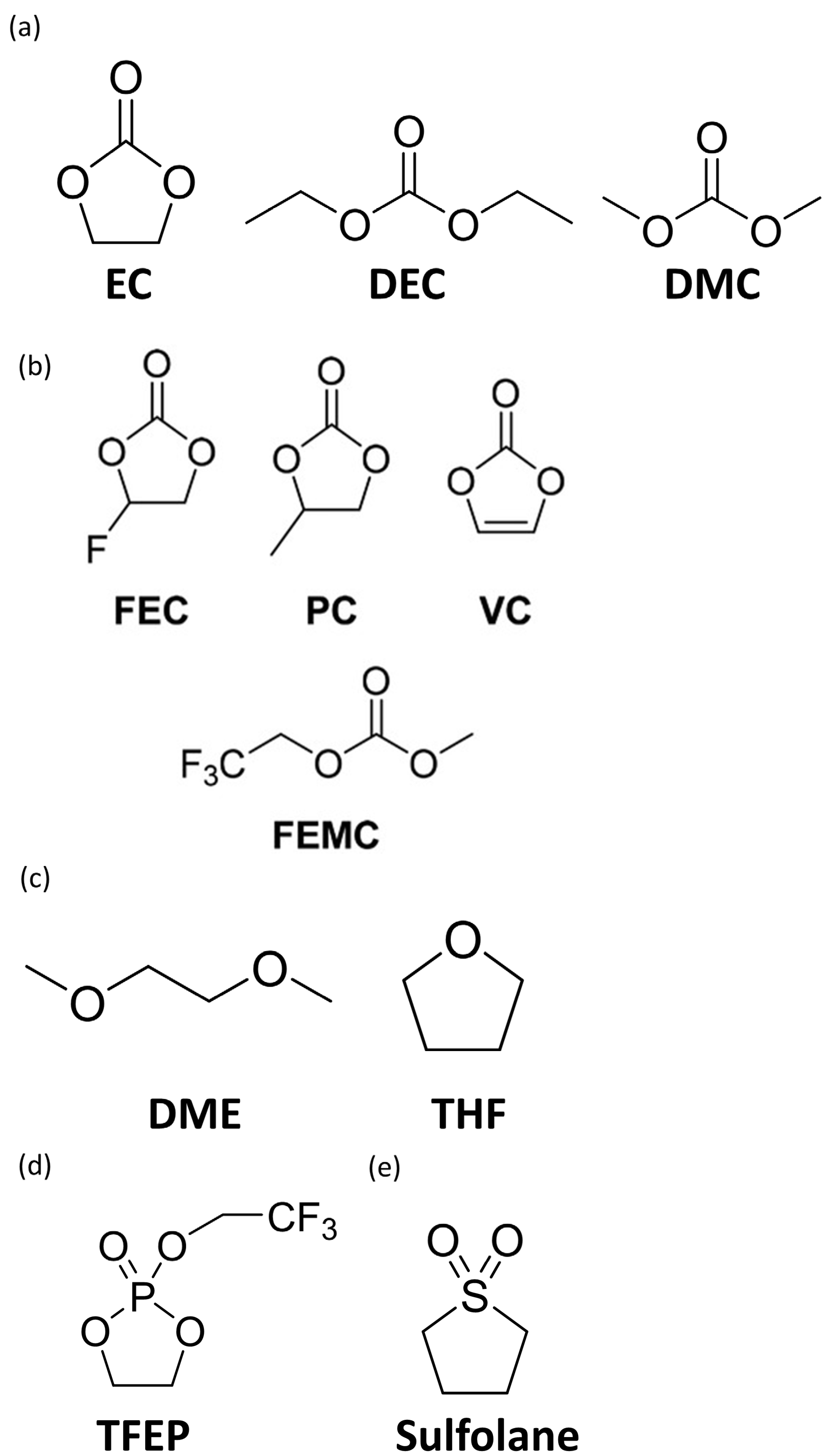
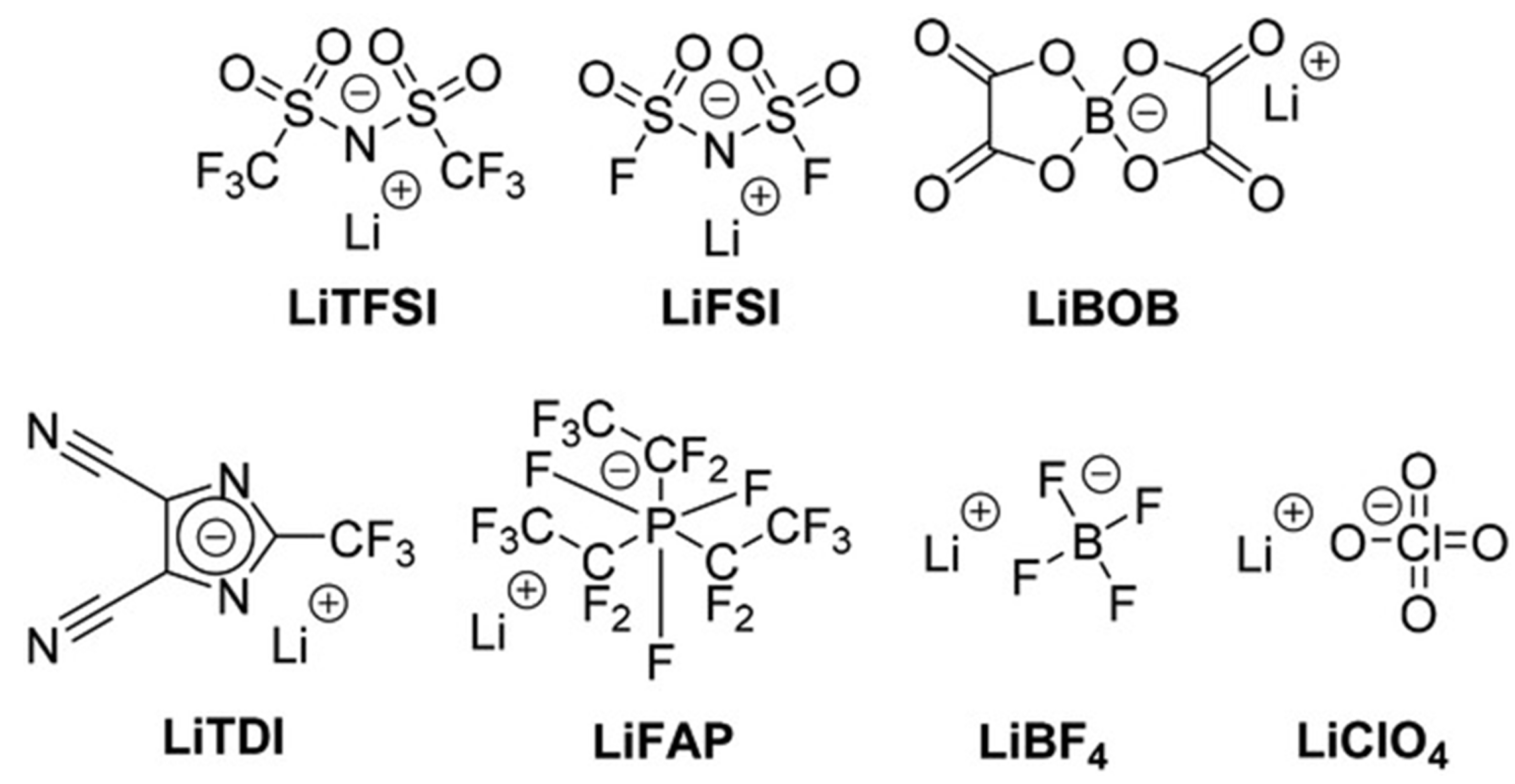
3. Electrolytes in Silicon-Based Anode
3.1. Additives
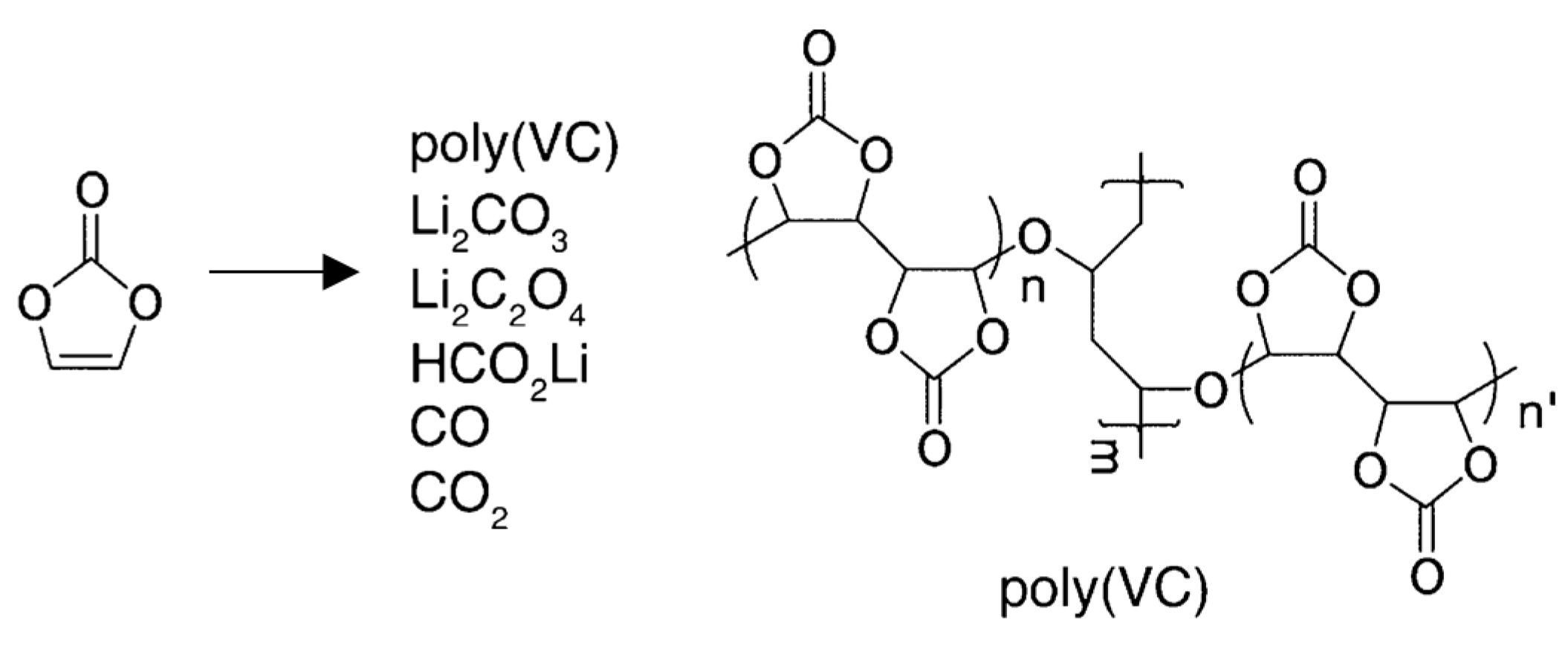
3.1.1. VC
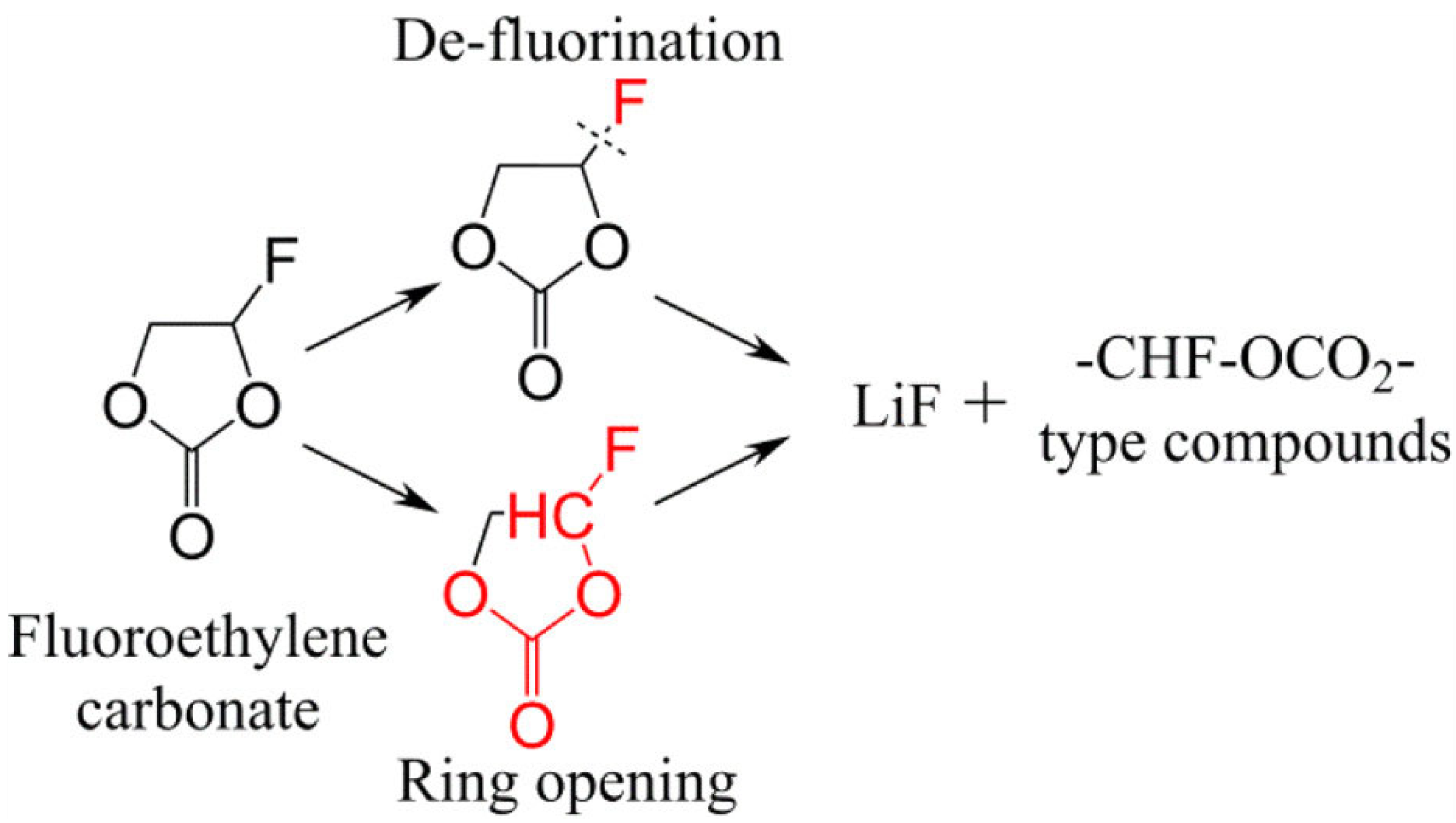
3.1.2. Fluoroethylene Carbonate
3.1.3. Silane Additives
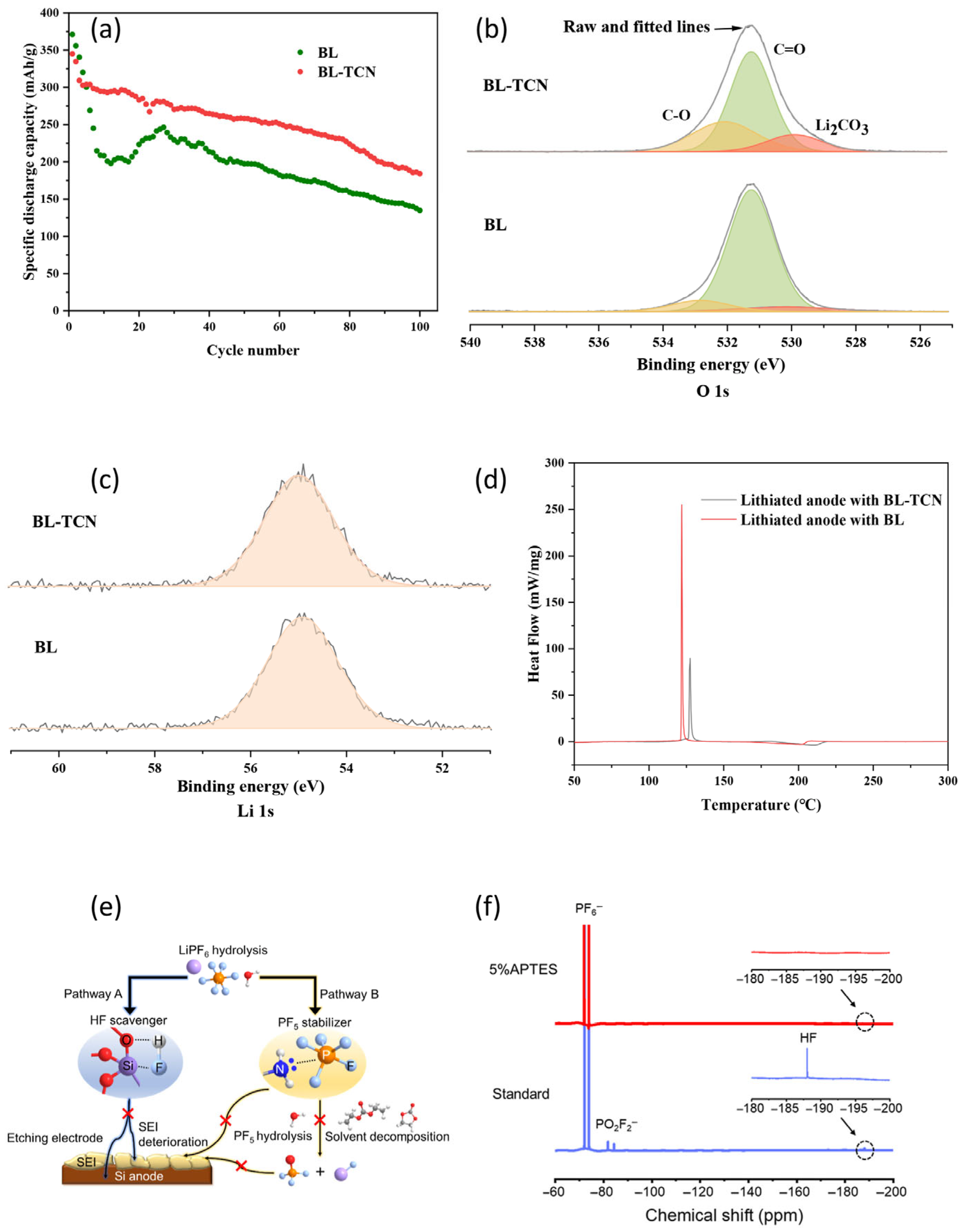
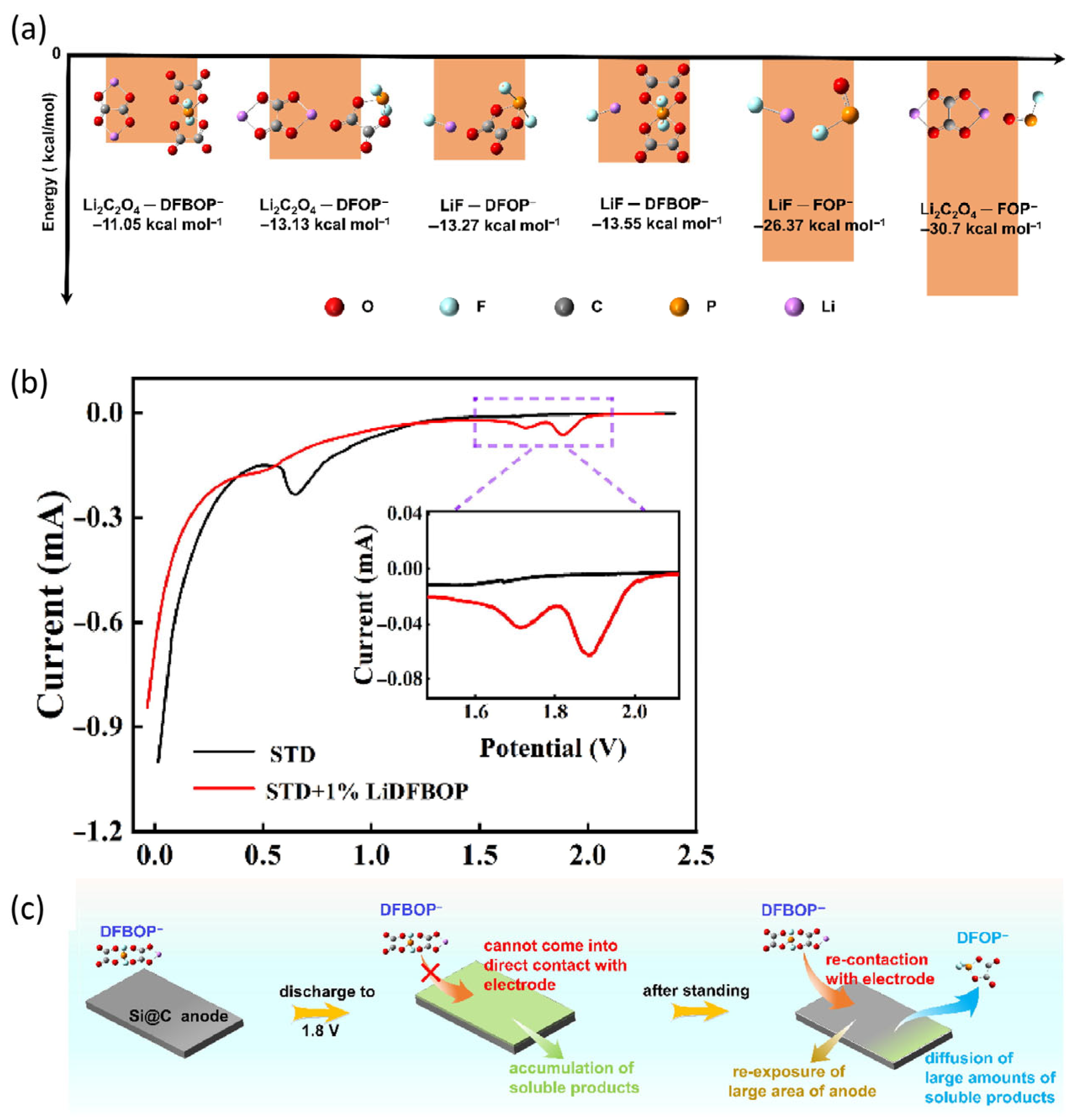
3.1.4. Other Additives
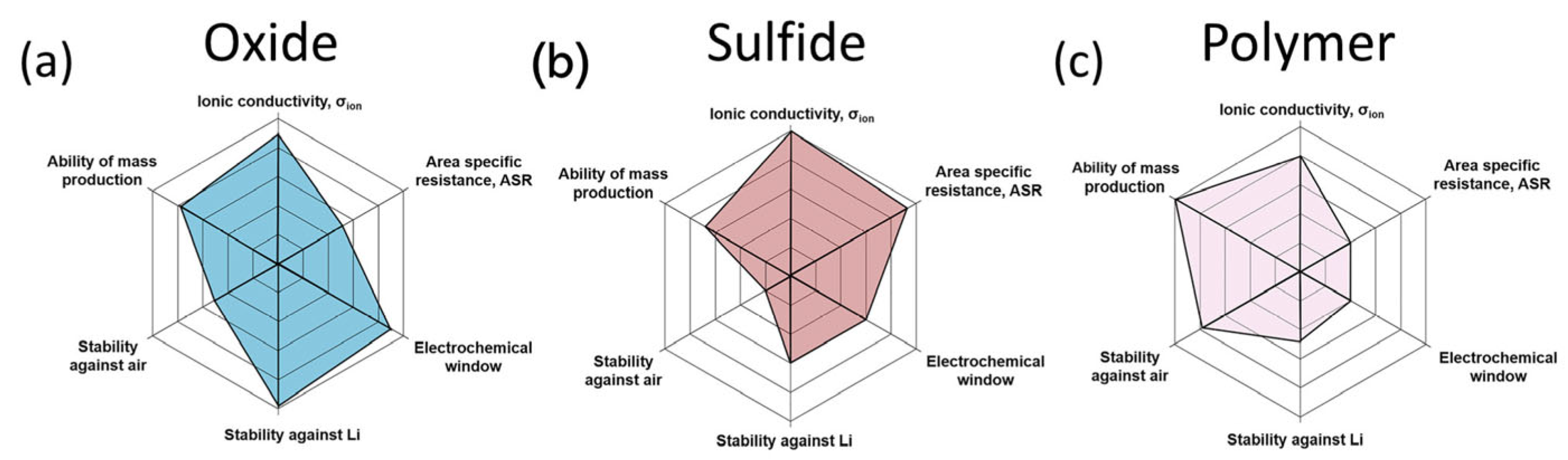
4. New Challenges in Electrolytes: Solid-State Electrolytes
4.1. Oxide-Based Solid Electrolytes
4.2. Sulfide-Based Solid Electrolytes
4.3. Polymer-Based Solid Electrolytes
4.4. Interfacial Challenges Between Solid Electrolytes and Silicon Anodes
- 1.
- Electrolyte Optimization:Quasi-solid or composite electrolytes incorporating dual lithium salts, flexible polymer matrices, or ceramic additives such as LLZTO [90] and propylene carbonate (PC) [91] have been developed to form mechanically robust and ionically conductive interfaces. These systems alleviate SEI instability, accommodate Si volume changes, and improve capacity retention and ICE [92].
- 2.
- Artificial SEI Layers:Artificial interlayers—including in situ-formed LiF-rich SEI films [93], LiAlO2 coatings [94], and LLZTO-modified surfaces [95]—have demonstrated effectiveness in suppressing electronic leakage, mitigating interfacial side reactions, and enhancing cycling stability. Both in situ and ex situ techniques have been employed to improve the structural integrity of the Si/SE interface.
- 3.
- Anode Structural Design:Nanoengineering approaches, such as the development of thin-film Si [96], columnar Si structure [97], Si@C composites [92], metal–organic framework (MOF)-derived Si@MOF architectures [98], and vertical graphene–Si hybrids [99], have been shown to effectively buffer volume changes, preserve interfacial contact, reduce interfacial resistance, and improve mechanical compliance with the electrolyte.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.; Xiao, S.; Li, D.; Liao, J.; Ji, F.; Liu, H.; Ci, L. Commercial carbon cloth: An emerging substrate for practical lithium metal batteries. Energy Storage Mater. 2022, 48, 172–190. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.-H.; Kim, N.; Sung, J.; Cho, J. Integration of graphite and silicon anodes for the commercialization of high-energy lithium-ion batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-based nanomaterials for lithium-ion batteries: A review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- He, W.; Liang, Y.; Tian, H.; Zhang, S.; Meng, Z.; Han, W.-Q. A facile in situ synthesis of nanocrystal-FeSi-embedded Si/SiOx anode for long-cycle-life lithium ion batteries. Energy Storage Mater. 2017, 8, 119–126. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Wu, J.; Shao, R.; Jiang, R.; Tie, Z.; Jin, Z. A Review on Recent Advances for Boosting Initial Coulombic Efficiency of Silicon Anodic lithium Ion batteries. Small 2022, 18, e2102894. [Google Scholar] [CrossRef]
- Tao, W.; Wang, P.; You, Y.; Park, K.; Wang, C.-Y.; Li, Y.-K.; Cao, F.-F.; Xin, S. Strategies for improving the storage performance of silicon-based anodes in lithium-ion batteries. Nano Res. 2019, 12, 1739–1749. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Choi, N.-S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.-K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. Engl. 2012, 51, 9994–10024. [Google Scholar] [CrossRef]
- Song, W.; Chae, O.B.; Ryu, J.H. Surface Nitridation of Nano-sized Anatase TiO2 using Urea and Thiourea for Enhanced Electrochemical Performance in Lithium-ion Batteries. J. Electrochem. Sci. Technol. 2024, 15, 512–520. [Google Scholar] [CrossRef]
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R. Rep. 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Kim, K.-i.; Tang, L.; Mirabedini, P.; Yokoi, A.; Muratli, J.M.; Guo, Q.; Lerner, M.M.; Gotoh, K.; Greaney, P.A.; Fang, C.; et al. [LiCl2]− Superhalide: A New Charge Carrier for Graphite Cathode of Dual-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2112709. [Google Scholar] [CrossRef]
- Yoon, M.; Dong, Y.; Huang, Y.; Wang, B.; Kim, J.; Park, J.-S.; Hwang, J.; Park, J.; Kang, S.J.; Cho, J.; et al. Eutectic salt-assisted planetary centrifugal deagglomeration for single-crystalline cathode synthesis. Nat. Energy 2023, 8, 482–491. [Google Scholar] [CrossRef]
- Weldehans, M.G.; Nguyen, T.P.; Hur, J.; Kim, I.T. Carbon-decorated hydrated V10O24 nanorods for high-performance lithium-ion battery cathodes. J. Energy Storage 2024, 101, 113888. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Kim, Y.; Stepien, D.; Moon, H.; Schönherr, K.; Schumm, B.; Kuenzel, M.; Althues, H.; Bresser, D.; Passerini, S. Artificial interphase design employing inorganic–organic components for high-energy lithium-metal batteries. ACS Appl. Mater. Interfaces 2023, 15, 20987–20997. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, I.T.; Hur, J. Highly conductive and robust telluride-carbon hybrid matrix for enhanced copper diphosphide anode in Li-ion batteries. J. Alloys Compd. 2023, 950, 169914. [Google Scholar] [CrossRef]
- Kidanu, W.G.; Hur, J.; Kim, I.T. Gallium-indium–tin eutectic as a self-healing room-temperature liquid metal anode for high-capacity lithium-ion batteries. Materials 2021, 15, 168. [Google Scholar] [CrossRef]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.S.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, J.; Li, H.; Nuli, Y.; Wang, J. Electrolytes for advanced lithium ion batteries using silicon-based anodes. J. Mater. Chem. A 2019, 7, 9432–9446. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhao, Q.; Huang, X.; Jia, S.; Ma, J.; Ren, Y. Si-based anodes: Advances and challenges in Li-ion batteries for enhanced stability. Electrochem. Energy Rev. 2024, 7, 11. [Google Scholar] [CrossRef]
- Cao, Z.; Zheng, X.; Qu, Q.; Huang, Y.; Zheng, H. Electrolyte design enabling a high-safety and high-performance Si anode with a tailored electrode–electrolyte interphase. Adv. Mater. 2021, 33, e2103178. [Google Scholar] [CrossRef]
- Park, I.; Lee, H.; Chae, O.B. Synthesis methods of Si/C composite materials for lithium-ion batteries. Batteries 2024, 10, 381. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.; Soo Jung, D.; Chul Roh, K.; Seo, J.; Kim, J.; Kim, K.; Joohyun Kim, P.; Choi, J. Synergistically enhanced LiF–rich protective layer for highly stable silicon anodes. Appl. Surf. Sci. 2024, 661, 160023. [Google Scholar] [CrossRef]
- Song, W.; Chae, O.B. Surface-coating strategies of Si-negative electrode materials in lithium-ion batteries. Batteries 2024, 10, 327. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, I.; Lee, J.W.; Yoon, D.; Kim, J.H.; Lee, S.; Kim, K.; Kim, P.J.; Choi, J.; Kang, Y.C.; et al. A novel structured Si-based composite with 2D structured graphite for high-performance lithium-ion batteries. Small 2024, 20, e2405005. [Google Scholar] [CrossRef]
- Nam, H.; Song, W.; Chae, O.B. Advances in coating materials for silicon-based lithium-ion battery anodes. Energies 2024, 17, 4970. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and recent progress on silicon-based anode materials for next-generation lithium-ion batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Ko, M.; Chae, S.; Cho, J. Challenges in accommodating volume change of Si anodes for Li-ion batteries. ChemElectroChem 2015, 2, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Li, H.; Chen, X.; Han, Y.; Zhu, Z.; Chu, C. Recent research progress of silicon-based anode materials for lithium-ion batteries. ChemistrySelect 2022, 7, e202201269. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Wölke, C.; Sadeghi, B.A.; Eshetu, G.G.; Figgemeier, E.; Winter, M.; Cekic-Laskovic, I. Interfacing Si-based electrodes: Impact of liquid electrolyte and its components. Adv. Mater. Interfaces 2022, 9, 2101898. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- Raimann, P.R.; Hochgatterer, N.S.; Korepp, C.; Möller, K.C.; Winter, M.; Schröttner, H.; Hofer, F.; Besenhard, J.O. Monitoring dynamics of electrode reactions in Li-ion batteries by in situ ESEM. Ionics 2006, 12, 253–255. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Patil, P.K.; Wang, C.; Appleby, A.J.; Little, F.E.; Cocke, D.L. Electrochemical performance of lithium ion battery, nano-silicon-based, disordered carbon composite anodes with different microstructures. J. Power Sources 2004, 125, 206–213. [Google Scholar] [CrossRef]
- Hatchard, T.D.; Dahn, J.R. In situ XRD and electrochemical study of the reaction of lithium with amorphous silicon. J. Electrochem. Soc. 2004, 151, A838–A842. [Google Scholar] [CrossRef]
- Kim, J.; Chae, O.B.; Lucht, B.L. Perspective—Structure and stability of the solid electrolyte interphase on silicon anodes of lithium-ion batteries. J. Electrochem. Soc. 2021, 168, 030521. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Shao, R.; Wu, J.; Jiang, R.; Jin, Z. Recent progress and future perspective on practical silicon anode-based lithium ion batteries. Energy Storage Mater. 2022, 46, 482–502. [Google Scholar] [CrossRef]
- Nie, M.; Chalasani, D.; Abraham, D.P.; Chen, Y.; Bose, A.; Lucht, B.L. Lithium ion battery graphite solid electrolyte interphase revealed by microscopy and spectroscopy. J. Phys. Chem. C 2013, 117, 1257–1267. [Google Scholar] [CrossRef]
- Nie, M.; Abraham, D.P.; Chen, Y.; Bose, A.; Lucht, B.L. Silicon solid electrolyte interphase (SEI) of lithium ion battery characterized by microscopy and spectroscopy. J. Phys. Chem. C 2013, 117, 13403–13412. [Google Scholar] [CrossRef]
- Aurbach, D.; Ein-Eli, Y.; Chusid (Youngman), O.; Carmeli, Y.; Babai, M.; Yamin, H. The Correlation Between the Surface Chemistry and the Performance of Li-Carbon Intercalation Anodes for Rechargeable ‘Rocking-Chair’ Type Batteries. J. Electrochem. Soc. 1994, 141, 603–611. [Google Scholar]
- Seo, D.M.; Chalasani, D.; Parimalam, B.S.; Kadam, R.; Nie, M.; Lucht, B.L. Reduction reactions of carbonate solvents for lithium ion batteries. ECS Electrochem. Lett. 2014, 3, A91–A93. [Google Scholar] [CrossRef]
- Parimalam, B.S.; MacIntosh, A.D.; Kadam, R.; Lucht, B.L. Decomposition Reactions of Anode Solid Electrolyte Interphase (SEI) Components with LiPF 6. J. Phys. Chem. C 2017, 121, 22733–22738. [Google Scholar] [CrossRef]
- Yoon, T.; Milien, M.S.; Parimalam, B.S.; Lucht, B.L. Thermal decomposition of the solid electrolyte interphase (SEI) on silicon electrodes for lithium ion batteries. Chem. Mater. 2017, 29, 3237–3245. [Google Scholar] [CrossRef]
- Cresce, A.v.; Russell, S.M.; Baker, D.R.; Gaskell, K.J.; Xu, K. In situ and quantitative characterization of solid electrolyte interphases. Nano Lett. 2014, 14, 1405–1412. [Google Scholar] [CrossRef]
- Lu, P.; Li, C.; Schneider, E.W.; Harris, S.J. Chemistry, impedance, and morphology evolution in solid electrolyte interphase films during formation in lithium ion batteries. J. Phys. Chem. C 2014, 118, 896–903. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Role of the LiPF 6 Salt for the Long-Term Stability of Silicon Electrodes in Li-ion Batteries—A Photoelectron Spectroscopy Study. Chem. Mater. 2013, 25, 394–404. [Google Scholar] [CrossRef]
- Schroder, K.W.; Dylla, A.G.; Harris, S.J.; Webb, L.J.; Stevenson, K.J. Role of surface oxides in the formation of solid–electrolyte interphases at silicon electrodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 21510–21524. [Google Scholar] [CrossRef]
- Lux, S.F.; Lucas, I.T.; Pollak, E.; Passerini, S.; Winter, M.; Kostecki, R. The mechanism of HF formation in LiPF6 based organic carbonate electrolytes. Electrochem. Commun. 2012, 14, 47–50. [Google Scholar] [CrossRef]
- Lindgren, F.; Xu, C.; Niedzicki, L.; Marcinek, M.; Gustafsson, T.; Björefors, F.; Edström, K.; Younesi, R. SEI formation and interfacial stability of a Si electrode in a LiTDI-salt based electrolyte with FEC and VC additives for Li-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 15758–15766. [Google Scholar] [CrossRef]
- Zhang, S.; He, M.; Su, C.-C.; Zhang, Z. Advanced electrolyte/additive for lithium-ion batteries with silicon anode. Curr. Opin. Chem. Eng. 2016, 13, 24–35. [Google Scholar] [CrossRef]
- Chen, L.; Wang, K.; Xie, X.; Xie, J. Effect of vinylene carbonate (VC) as electrolyte additive on electrochemical performance of Si film anode for lithium ion batteries. J. Power Sources 2007, 174, 538–543. [Google Scholar] [CrossRef]
- Feyzi, E.; Anil Kumar, M.R.; Li, X.; Deng, S.; Nanda, J.; Zaghib, K. A comprehensive review of silicon anodes for high-energy lithium-ion batteries: Challenges, latest developments, and perspectives. Next Energy 2024, 5, 100176. [Google Scholar] [CrossRef]
- Dalavi, S.; Guduru, P.; Lucht, B.L. Performance enhancing electrolyte additives for lithium ion batteries with silicon anodes. J. Electrochem. Soc. 2012, 159, A642–A646. [Google Scholar] [CrossRef]
- Choi, N.-S.; Yew, K.H.; Lee, K.Y.; Sung, M.; Kim, H.; Kim, S.-S. Effect of fluoroethylene carbonate additive on interfacial properties of silicon thin-film electrode. J. Power Sources 2006, 161, 1254–1259. [Google Scholar] [CrossRef]
- Domi, Y.; Usui, H.; Shimizu, M.; Miwa, K.-i.; Sakaguchi, H. Effect of film-forming additive on electrochemical performance of silicon negative-electrode in lithium-ion batteries. Int. J. Electrochem. Sci. 2015, 10, 9678–9686. [Google Scholar] [CrossRef]
- Mazouzi, D.; Delpuech, N.; Oumellal, Y.; Gauthier, M.; Cerbelaud, M.; Gaubicher, J.; Dupré, N.; Moreau, P.; Guyomard, D.; Roué, L.; et al. New insights into the silicon-based electrode’s irreversibility along cycle life through simple gravimetric method. J. Power Sources 2012, 220, 180–184. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, S.; Wang, Y.; Qu, Q.; Zheng, H. Dimethylacrylamide, a novel electrolyte additive, can improve the electrochemical performances of silicon anodes in lithium-ion batteries. RSC Adv. 2018, 9, 435–443. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Xie, L.-J.; Tang, Y.; Li, Y.; Jiang, J.-C.; Huang, A.-C. Thermal safety evaluation of Silane polymer compounds as electrolyte additives for silicon-based anode lithium-ion batteries. Processes 2022, 10, 1581. [Google Scholar] [CrossRef]
- Tan, T.; Lee, P.-K.; Marium, M.; Zettsu, N.; Yu, D.Y.W. (3-Aminopropyl)triethoxysilane as an Electrolyte Additive for Enhancing the Therl Stability of Silicon Anode in Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 11254–11262. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Y.; Quan, Y.; Zong, F.; Wang, J.; Zhao, D.; Zhang, N.; Wang, P.; Cui, X.; Li, S. Mitigating volume expansion of silicon-based anode through interfacial engineering based on intermittent discharge strategy. J. Energy Chem. 2024, 98, 680–691. [Google Scholar] [CrossRef]
- Michan, A.L.; Parimalam, B.S.; Leskes, M.; Kerber, R.N.; Yoon, T.; Grey, C.P.; Lucht, B.L. Fluoroethylene carbonate and vinylene carbonate reduction: Understanding lithium-ion battery electrolyte additives and solid electrolyte interphase formation. Chem. Mater. 2016, 28, 8149–8159. [Google Scholar] [CrossRef]
- Leveau, L.; Laïk, B.; Pereira-Ramos, J.-P.; Gohier, A.; Tran-Van, P.; Cojocaru, C.-S. Cycling strategies for optimizing silicon nanowires performance as negative electrode for lithium battery. Electrochim. Acta 2015, 157, 218–224. [Google Scholar] [CrossRef]
- Jaumann, T.; Balach, J.; Langklotz, U.; Sauchuk, V.; Fritsch, M.; Michaelis, A.; Teltevskij, V.; Mikhailova, D.; Oswald, S.; Klose, M.; et al. Lifetime vs. rate capability: Understanding the role of FEC and VC in high-energy Li-ion batteries with nano-silicon anodes. Energy Storage Mater. 2017, 6, 26–35. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Allouche, J.; Lindgren, F.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Nanosilicon electrodes for lithium-ion batteries: Interfacial mechanisms studied by hard and soft X-ray photoelectron spectroscopy. Chem. Mater. 2012, 24, 1107–1115. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Improved performances of nanosilicon electrodes using the salt LiFSI: A photoelectron spectroscopy study. J. Am. Chem. Soc. 2013, 135, 9829–9842. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Vegunta, S.S.S.; Flake, J.C. Surface-modified silicon nanowire anodes for lithium-ion batteries. J. Power Sources 2011, 196, 8583–8589. [Google Scholar] [CrossRef]
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of fluoroethylene carbonate (FEC) on the performance and surface chemistry of Si-nanowire Li-ion battery anodes. Langmuir 2012, 28, 965–976. [Google Scholar] [CrossRef]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Björefors, F.; Edström, K.; Gustafsson, T. Improved performance of the silicon anode for Li-ion batteries: Understanding the surface modification mechanism of fluoroethylene carbonate as an effective electrolyte additive. Chem. Mater. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Nakai, H.; Kubota, T.; Kita, A.; Kawashima, A. Investigation of the solid electrolyte interphase formed by fluoroethylene carbonate on Si electrodes. J. Electrochem. Soc. 2011, 158, A798–A801. [Google Scholar] [CrossRef]
- Ryu, Y.-G.; Lee, S.; Mah, S.; Lee, D.J.; Kwon, K.; Hwang, S.; Doo, S. Electrochemical behaviors of silicon electrode in lithium salt solution containing alkoxy Silane additives. J. Electrochem. Soc. 2008, 155, A583–A589. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Zhang, H. Effects of electrolyte additive on the electrochemical performance of Si/C anode for lithium-ion batteries. Ionics 2018, 24, 3691–3698. [Google Scholar] [CrossRef]
- Jo, H.; Kim, J.; Nguyen, D.-T.; Kang, K.K.; Jeon, D.-M.; Yang, A.-R.; Song, S.-W. Stabilizing the solid electrolyte interphase layer and cycling performance of silicon–graphite battery anode by using a binary additive of fluorinated carbonates. J. Phys. Chem. C 2016, 120, 22466–22475. [Google Scholar] [CrossRef]
- Han, J.-G.; Lee, J.B.; Cha, A.; Lee, T.K.; Cho, W.; Chae, S.; Kang, S.J.; Kwak, S.K.; Cho, J.; Hong, S.Y.; et al. Unsymmetrical fluorinated malonatoborate as an amphoteric additive for high-energy-density lithium-ion batteries. Energy Environ. Sci. 2018, 11, 1552–1562. [Google Scholar] [CrossRef]
- Zhao, N.; Khokhar, W.; Bi, Z.; Shi, C.; Guo, X.; Fan, L.-Z.; Nan, C.-W. Solid garnet batteries. Joule 2019, 3, 1190–1199. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Chen, C.; Li, Q.; Li, Y.; Cui, Z.; Guo, X.; Li, H. Sustainable interfaces between Si anodes and garnet electrolytes for room-temperature solid-state batteries. ACS Appl. Mater. Interfaces 2018, 10, 2185–2190. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Chen, Y.-T.; Yang, H.; Bao, W.; Sreenarayanan, B.; Doux, J.-M.; Li, W.; Lu, B.; Ham, S.-Y.; Sayahpour, B.; et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 2021, 373, 1494–1499. [Google Scholar] [CrossRef]
- Si, Q.; Kawakubo, M.; Matsui, M.; Horiba, T.; Yamamoto, O.; Takeda, Y.; Seki, N.; Imanishi, N. Silicon–carbon composite dispersed in a carbon paper substrate for solid polymer lithium-ion batteries. J. Power Sources 2014, 248, 1275–1280. [Google Scholar] [CrossRef]
- Chen, F.; Li, J.; Huang, Z.; Yang, Y.; Shen, Q.; Zhang, L. Origin of the phase transition in lithium garnets. J. Phys. Chem. C 2018, 122, 1963–1972. [Google Scholar] [CrossRef]
- Bernstein, N.; Johannes, M.D.; Hoang, K. Origin of the structural phase transition in Li7La3Zr2O12. Phys. Rev. Lett. 2012, 109, 205702. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-F.; Pang, W.K.; Peterson, V.K.; Wei, L.; Guo, X. Garnet-type fast Li-ion conductors with high ionic conductivities for all-solid-state batteries. ACS Appl. Mater. Interfaces 2017, 9, 12461–12468. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, J.; Li, Y.; Nie, X.; Dai, L.; Ci, L. Li metal-free rechargeable all-solid-state Li2S/Si battery based on Li7P3S11 electrolyte. J. Solid State Electrochem. 2019, 23, 3145–3151. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wei, H.; Fu, J.; Huang, Y.; Tang, X. Novel PEO-based solid composite polymer electrolytes with inorganic–organic hybrid polyphosphazene microspheres as fillers. J. Appl. Electrochem. 2010, 40, 1475–1481. [Google Scholar] [CrossRef]
- Okuno, R.; Yamamoto, M.; Kato, A.; Takahashi, M. Performance improvement of nanoporous Si composite anodes in all-solid-state lithium-ion batteries by using acetylene black as a conductive additive. Electrochem. Commun. 2022, 138, 107288. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Ye, Y.; Liu, Z.; Cheng, F.; Li, H. A durable gel polymer electrolyte with excellent cycling and rate performance for enhanced lithium storage. ACS Appl. Energy Mater. 2020, 3, 4906–4913. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L.; Wang, M.; Zhang, Y.; Liu, T.; Bi, J.; Wu, B.; Su, Y.; Wu, F. The interface compatibility between solid-state electrolytes and lithium/silicon anodes: Challenges, recent progress and perspectives. J. Energy Storage 2024, 101, 113774. [Google Scholar] [CrossRef]
- Huo, H.; Sun, J.; Chen, C.; Meng, X.; He, M.; Zhao, N.; Guo, X. Flexible interfaces between Si anodes and composite electrolytes consisting of poly(propylene carbonates) and garnets for solid-state batteries. J. Power Sources 2018, 383, 150–156. [Google Scholar] [CrossRef]
- Pan, J.; Peng, H.; Yan, Y.; Bai, Y.; Yang, J.; Wang, N.; Dou, S.; Huang, F. Solid-state batteries designed with high ion conductive composite polymer electrolyte and silicon anode. Energy Storage Mater. 2021, 43, 165–171. [Google Scholar] [CrossRef]
- Zhao, E.; Luo, S.; Hu, A.; Liao, Z.; Huang, C.; Akihiro, O.; Jiang, P.; Yang, L. Rational design of an in-build quasi-solid-state electrolyte for high-performance lithium-ion batteries with the silicon-based anode. Chem. Eng. J. 2023, 463, 142306. [Google Scholar] [CrossRef]
- Han, X.; Gu, L.; Sun, Z.; Chen, M.; Zhang, Y.; Luo, L.; Xu, M.; Chen, S.; Liu, H.; Wan, J.; et al. Manipulating charge-transfer kinetics and a flow-domain LiF-rich interphase to enable high-performance microsized silicon–silver–carbon composite anodes for solid-state batteries. Energy Environ. Sci. 2023, 16, 5395–5408. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Q.; Li, Y.; Ji, F.; Cheng, J.; Zhang, H.; Zeng, Z.; Rao, Y.; Liu, H.; Li, D.; et al. Nano Silicon Anode without Electrolyte Adding for Sulfide-Based All-Solid-State Lithium-Ion Batteries. Small 2023, 19, 2302934. [Google Scholar] [CrossRef]
- Zeng, B.; Gu, Q.; Zhang, Y.; Wang, M.; Gao, J.; Fan, C.; Tang, W. Engineering electrode–electrolyte interface for ultrastable Si-based solid-state batteries. Surf. Interfaces 2024, 44, 103687. [Google Scholar] [CrossRef]
- Ping, W.; Yang, C.; Bao, Y.; Wang, C.; Xie, H.; Hitz, E.; Cheng, J.; Li, T.; Hu, L. A silicon anode for garnet-based all-solid-state batteries: Interfaces and nanomechanics. Energy Storage Mater. 2019, 21, 246–252. [Google Scholar] [CrossRef]
- Cangaz, S.; Hippauf, F.; Reuter, F.S.; Doerfler, S.; Abendroth, T.; Althues, H.; Kaskel, S. Enabling High-Energy Solid-State Batteries with Stable Anode Interphase by the Use of Columnar Silicon Anodes. Adv. Energy Mater. 2020, 10, 2001320. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.; Peng, X.; Wu, M.; Zhao, T. A High-Capacity Polyethylene Oxide-Based All-Solid-State Battery Using a Metal–Organic Framework Hosted Silicon Anode. ACS Appl. Mater. Interfaces 2022, 14, 24798–24805. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, P.; Zhang, Y.; Zhao, X.; Yu, J. Vertical Graphene Sheet-Encapsulated Silicon Nanoparticles for Anodes of Polymer-Based All-Solid-State Batteries. ACS Appl. Energy Mater. 2023, 7, 726–734. [Google Scholar] [CrossRef]



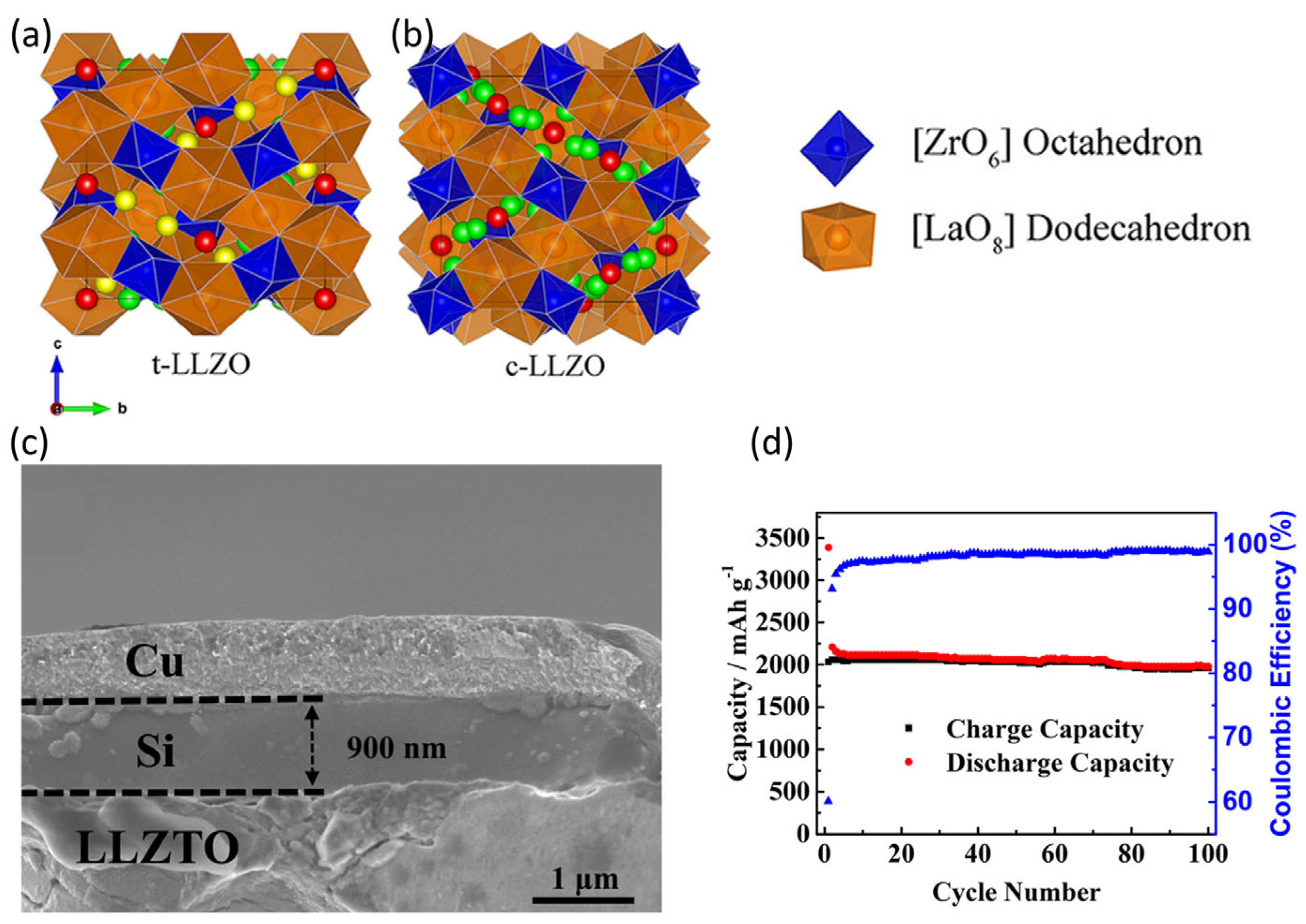
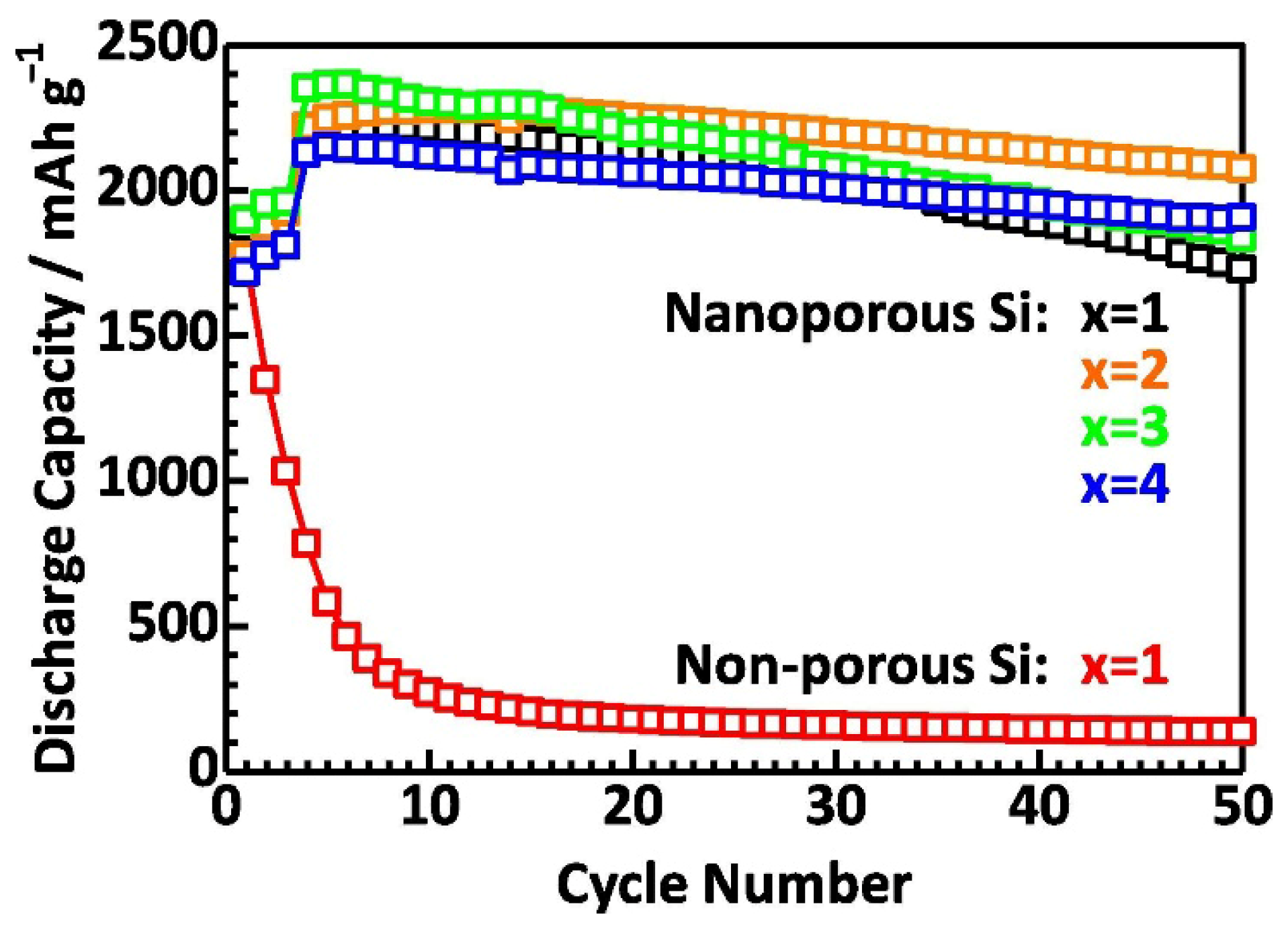
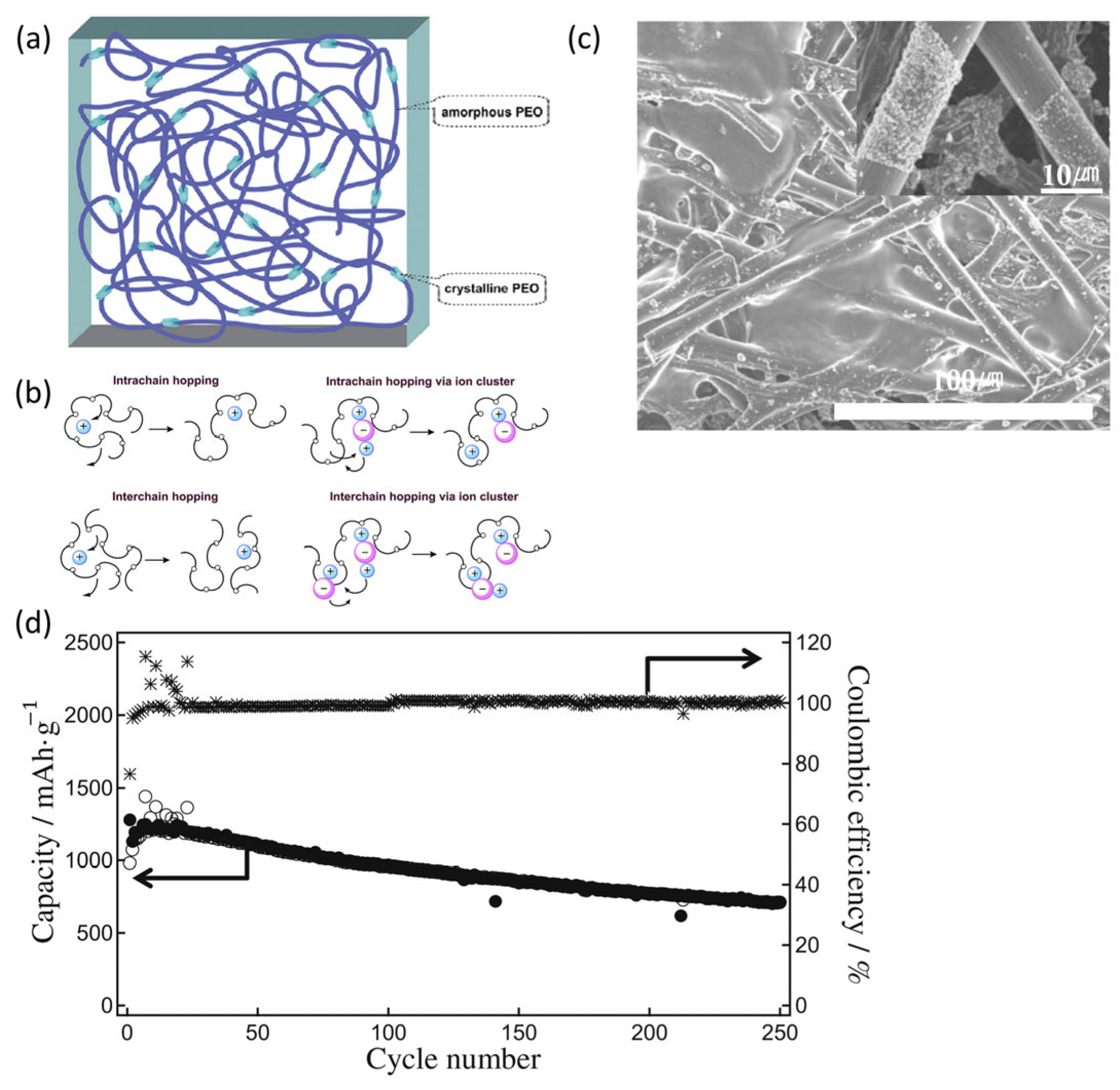
| Additive | SEI Stability | ICE (%) | Cycling Performance | Estimated Cost | References |
|---|---|---|---|---|---|
| VC | Impermeable SEI with low impedance growth during cycling | 67.9 → 72.5 | ~2000 mAh g−1 (200 cycles), >500 mAh g−1 (500 cycles) | Low | [54,55] |
| FEC | Forms LiF-rich SEI; mechanically robust | 88.7 | Maintains > 90% capacity; >99% efficiency over extended cycles at high rate | Low | [56,57,58,59] |
| DMAA | Forms dense, stable SEI and suppresses side reactions | ~80.2 | >80% retention after 500 cycles | Low | [60] |
| TCN | table SEI rich in Li2CO3 | - | Reduced capacity fade and improved stability | Midium | [61] |
| APTES | Forms protective layer with SiO2-rich SEI | - | Enhances cycling stability with 5 wt% addition | Midium | [62] |
| LiDFBOP | Forms LiF/Li2C2O4 SEI; reduces volume expansion | - | Improves Li+ diffusion and preserves structural integrity | High | [63] |
| SE Type | Ionic Conductivity (S/cm) | Mechanical Stability | Interfacial Compatibility with Si | Estimated Cost | References |
|---|---|---|---|---|---|
| Oxide (LLZO) | ~3 × 10−4 | High (brittle) | Moderate (requires doping/coating) | Medium | [78,79] |
| Sulfide (Li6PS5Cl) | ~10−3~10−2 | Moderate (ductile, sensitive) | Good (enhanced with coatings) | High | [80] |
| Polymer (PEO) | ~10−6 at RT; better at 60 °C | Low (soft, flexible) | Excellent (good interface contact) | Low | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, J.; Lee, H.; Chae, O.B. Overcoming Challenges in Silicon Anodes: The Role of Electrolyte Additives and Solid-State Electrolytes. Micromachines 2025, 16, 800. https://doi.org/10.3390/mi16070800
Nam J, Lee H, Chae OB. Overcoming Challenges in Silicon Anodes: The Role of Electrolyte Additives and Solid-State Electrolytes. Micromachines. 2025; 16(7):800. https://doi.org/10.3390/mi16070800
Chicago/Turabian StyleNam, Jinsik, Hanbyeol Lee, and Oh B. Chae. 2025. "Overcoming Challenges in Silicon Anodes: The Role of Electrolyte Additives and Solid-State Electrolytes" Micromachines 16, no. 7: 800. https://doi.org/10.3390/mi16070800
APA StyleNam, J., Lee, H., & Chae, O. B. (2025). Overcoming Challenges in Silicon Anodes: The Role of Electrolyte Additives and Solid-State Electrolytes. Micromachines, 16(7), 800. https://doi.org/10.3390/mi16070800








