In this section, we discuss the role of different process parameters to the morphology of thin films made of noble metal NP arrays, thus to their optical properties and, in turn, to their performance when used as SERS substrates. We initially focus on the determination of the amount of target material ablated by a single pulse mp, since this quantity critically affects both the expansion dynamics of the ablation plume and the development of the film nanostructure.
3.1. Determination of Ablated Mass per Pulse
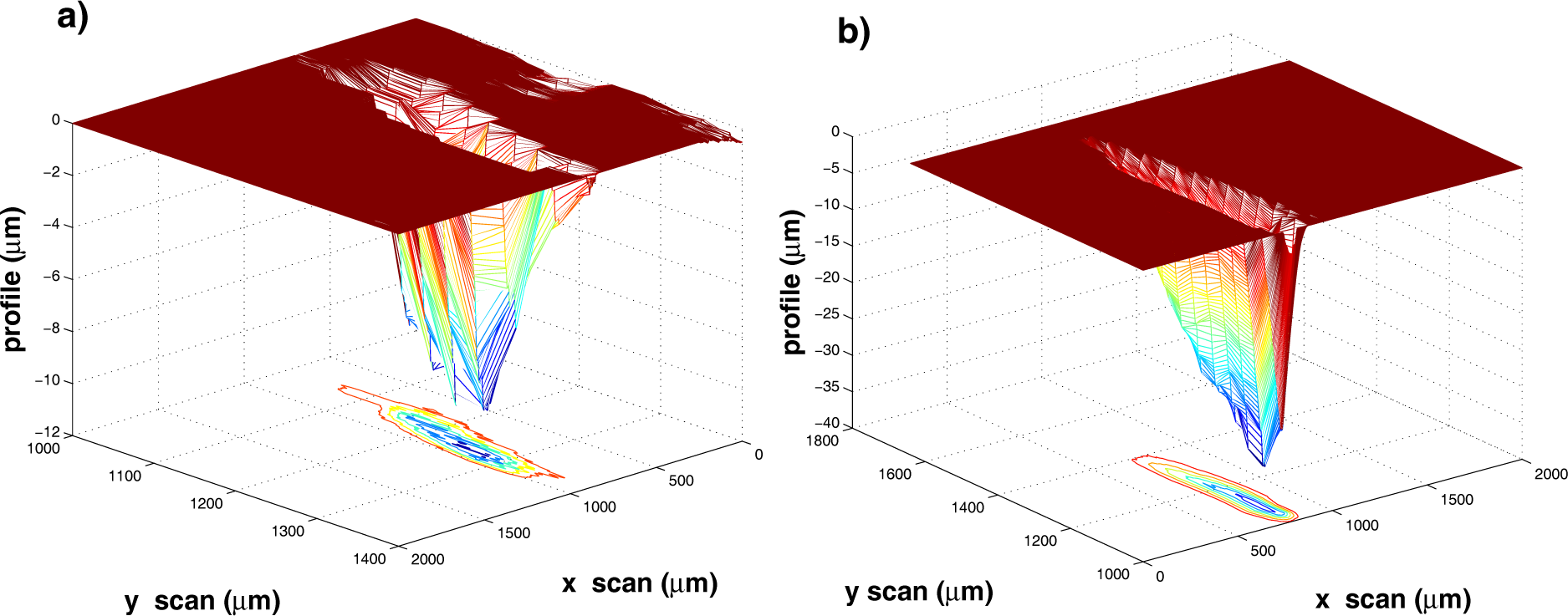
Measurements of
mp were performed by producing on the surface of a freshly polished target a number of craters using a different number of laser shots. In a typical experiment, four or more craters are produced on the target surface. Several craters of increasing depth result upon increasing the laser shot number, at fixed laser fluence. We report here as an example the ablation of a silver target at the fixed fluence of 2.0 J·cm
−2. Four craters were produced using 10, 50, 100 and 200 laser shots. The corresponding crater volumes were measured by scanning the crater area with a stylus profilometer. Two representative crater depth profiles are shown in
Figure 1. At low laser pulse number
N, craters are practically free of rims and well defined, while, at higher
N values, craters are surrounded by much redeposited material. In all cases, the aspect ratio of the crater (
i.
e., the depth-to-diameter ratio) is below unity, thus minimizing any effect of the deep-hole confinement on the estimate of the ablation rate per pulse.
Considering the photo-thermal activation of the material removal in open air, the excitation energy from the laser propagates mainly within the target material due to the poor thermal contact between the target surface and the surrounding air: this results in the considerable depth of the craters as compared to their width. At higher
N, the strong heating of the target surface combined with the instabilities arising within the melted layer at the target surface heated up to the critical temperature may produce a roughening of the crater surface that we observe experimentally. The presence of rims around the craters can be also explained by the strong temperature rise of the target surface that, in turn, leads to hot and dense ablation plasmas. The latter decouple from the liquefied target surface with a large acceleration oriented normal to it, thus exerting a strong recoil pressure onto the melted layer. Some data treatment was performed leading to the crater pictures in
Figure 1: target surface leveling along the
XY plane (see the flat area in
Figure 1) and removal of any protruding features for which
Z > 0. The volume of such redeposited and then melted material removed from the crater is subtracted from the total volume of the crater. The estimated crater volumes are reported in
Table 1. We notice from
Figure 2 that the number of laser shots and the ablated mass are linearly correlated with each other. The data were fitted to the linear relation
M =
mpN,
M being the mass removed by
N laser pulses. The results of the fit are reported in
Table 1. In the considered case, about 60.0 ng, corresponding to about 3.35 × 10
14 silver atoms, are removed from the target by a single laser pulse. With an estimated error for each volume value (taking into account the proper error on
x, y and
z measurements), less than 3%, the ultimate error on
mp is about 5%, as evaluated from the fitting procedure. Determining
mp is of critical relevance when the morphologies of samples deposited by PLD in the presence of an ambient gas are investigated. Film properties, as will be discussed in the next sections, sensitively depend on the interaction between the ablation plasma and ambient gas via the interplay of plasma mass
m and gas density
ρ.
3.2. Surface Morphology
The optical properties of gold and silver nanostructured thin films are sensitively affected by their surface morphology. For isolated silver and gold NPs with a typical size ranging from a few nm up to 30 nm, the LSPR lies at about 400 and 520 nm, respectively. Moving to NP arrangements, self-organized or engineered at the nanoscale, both the position and the width of the LSPR peak depend on the size, shape, number density, mutual distance and aggregation features of the NPs [
13]. The control of these properties then results in the control of the optical properties and, ultimately, of the SERS activity of the assembly. Among the several process parameters that affect the morphology of PLD synthesized films, two appear to play a major role: laser pulse number and gas pressure. While the first controls the degree of the NP number density and aggregation on the substrate, the latter mostly influences the interaction between plasma and ambient gas. In
Figure 3, we show the surface of silver samples grown under identical conditions (
F = 2.0 J·cm
−2, P
g = 70 Pa of Ar,
dT−S = 35 mm), but changing the laser pulse number between 1000 and 3 × 10
4.
We see that, at a low pulse number (see
Figure 3a), the surface is covered by isolated, nearly spherical NPs. As
N increases, the NP number density also increases, and deviations towards non-spherical geometry, with increasing NP size, are evident at some locations, together with incipient particle coalescence. Finally, at the highest
N value (3 × 10
4; see
Figure 3c), the extensive coalescence among adjacent NPs occurs, thereby islands with an irregular shape and smooth edges form. Notably, such a surface morphology is characterized by the presence of inter-island channels with a defined average length and width. Couples or triplets of such channels meet together at junction points.
To the different morphologies correspond different optical properties of the films. The trend of the UV-Vis absorption spectra as a function of the different morphologies is shown in
Figure 4. Increasing
N, keeping fixed all other deposition parameters, a red-shift and a broadening of the FWHM of the LSPR peak is observed. A similar trend of change of the film surface morphology and, hence, of the optical properties of the film is observed if
N is kept fixed, while the Ar pressure is changed. The effect of Ar pressure on film properties can be understood if the role of the gas on the expanding plasma is considered. Just after the end of the laser pulse, the ablated material starts to expand through the ambient gas, the expansion dynamics being driven by collisions: in the initial expansion stage, intra-plume collisions are dominant, but soon, collisions between plasma species and gas atoms become more and more effective. The collision rate depends on the gas density
ρ and increases with
ρ. The higher the collisional rate, the less the amount of material that reaches the substrate. At the same time, a higher collision rate promotes the formation and growth of NPs in the plasma. Such a complex process leads to the observed morphology dependence on
N and on ambient gas density [
14]. Thus, it is possible to tailor the optical properties of deposited films through the control of two easily adjustable deposition parameters (laser pulse number and Ar pressure), provided the relevant process parameters, in particular the laser fluence
F, are kept fixed. The latter, given by the ratio of the laser pulse energy
EL to the irradiated spot area
A, can be kept fixed by changing appropriately both
EL and
A. Fixing
F does not ensure that the ablated mass remains fixed as well. If we irradiate a larger area with a higher energy pulse, we can keep constant the fluence value, but a different
mp value results. Referring to the above discussion, for expansions through an ambient gas, the interaction with gas atoms depends on the plasma mass. We have investigated this point growing a set of samples by changing
mp, still keeping fixed
F [
15]. Two Ag samples were grown at 70 Pa of Ar, at
dT−S = 35 mm, with 10
4 laser shots,
F = 2.8 J·cm
−2. To explore the role of
mp, two focusing conditions were chosen, both corresponding to the same
F value:
E1 =10 mJ,
A = 0.011 cm
2 and
E2 =30 mJ,
A = 0.037 cm
2. For the two conditions, different
mp values result, namely
mp1 = 7.0 ng and
mp2 = 16.4 ng, as measured according to the procedure outlined in Section 3.1.
In
Figure 5 are shown the TEM pictures of the surface morphology of the two Ag films. The difference between the two morphologies is impressive. The surface of the sample deposited at lower
mp1 (7.0 ng) is characterized by the presence both of small, nearly spherical NPs and of larger ones with the shape progressively more and more irregular, up to islands that result from the coalescence of several NPs. Looking at the surface of the sample deposited at higher
mp2 (16.4 ng), we see a nearly percolated structure made of larger islands interconnected by a network of channels. In this sense,
mp has the same effect on the evolution of sample surface morphology as lowering Ar pressure or increasing the laser pulse number. It is worth outlining that two well-differentiated surface morphologies were obtained adopting the same fluence. Thus, in a report on a PLD experiment, the fluence value is not enough to make it reproducible, if laser pulse energy
EL and irradiated area
A are not reported, as well, because these two parameters determine
mp.
In
Figure 6a are displayed the UV-Vis absorption spectra of the above discussed samples. The different
mp values lead to key differences in their optical properties.
We see that the LSPR considerably red-shifts and its FWHM increases, as expected once the morphology of the two samples is taken into account. Moreover, the large FWHM increase observed in the film deposited with the higher
mp value (compare the film morphologies in
Figure 5) points out the setting up of a composite system made of the metallic film and the supporting dielectric substrate (glass). In this system, the optical transitions are detuned via dipole-dipole interactions that broaden the LSPR peak [
16,
17].
The Raman spectra acquired on the surface of the two samples after being soaked for 1 h in an aqueous solution of rhodamine 6G (R6G) at a concentration of 10
−4 M (
Figure 6b) show a dramatic difference between the SERS response of the two films. The sample deposited at the lower
mp1 shows a notably higher SERS efficiency. Thus, tuning the morphology and optical properties of nanostructured noble metal thin films is imperative to obtain highly SERS active substrates. Such a tuning cannot disregard detailed knowledge of the influence of all parameters on the deposition process, as exemplified by the study of the ablated mass per pulse.
3.3. SERS Activity of Pulsed Laser Ablated Silver and Gold Substrates
In this section, we report on some applications of Ag and Au SERS active substrates grown by PLD, whose properties were properly optimized from a parametric analysis [
18,
19]. We observed that Ag films deposited at 70 Pa of Ar, at
F ≈ 2.0 J· cm
−2 (
A = 2 ÷ 3 × 10
−3 cm
2),
dT−S = 35 mm, with 3 × 10
4 laser shots show the best SERS activity, so far. Highly SERS active Au substrates were grown under identical conditions, but the Ar pressure was 100 Pa.
In
Figure 7, we report the SERS spectra acquired on Ag and Au samples soaked in aqueous solutions of R6G, at the lowest concentrations we tested. The corresponding laser excitations were 632.8 nm for Ag and 785 nm for Au to achieve the maximum EF by matching the corresponding SPR absorption peak positions, observed near 550 and 800 nm, respectively [
20,
21].
The R6G peaks lying at 1189, 1314, 1366 and 1513 cm−1 assigned to the stretching modes of aromatic C bonds are clearly visible. Typical integration times varied between a few seconds and 240 s, pointing out that detection might be possible for this reference analyte at even lower concentration levels.
We believe that the detection at such a low concentration level is due to the formation of hot spots, whose number density strictly depends on film surface morphology, in turn controlled by an easily accessible deposition parameter,
i.
e., the laser pulse number (see
Figure 2.). It is noteworthy that compared to other deposition methods, like thermal evaporation and sputtering, PLD allows synthesizing films without any post-deposition thermal treatment. Moreover, the process is performed at room temperature, thus making it possible to use every kind of substrate material. Finally, PLD is a physical deposition technique that does not require any chemical precursor, different from routes based on the chemical reduction of silver and gold salts to obtain NPs: these have undesired chemical residuals, often introducing disturbing features in SERS spectra.
The observed SERS enhancement was so high, since we choose the excitation wavelength (632.8 nm), such that the surface plasmon absorption band (see
Figure 4) lies between the wavelengths of the exciting and the Raman scattered light; indeed, according to the electromagnetic theory, the optical properties of the metallic NPs set the choice of the excitation wavelength in SERS.
Raman spectroscopy is one of the most employed techniques in the cultural heritage field. Identification of dyes is a relevant issue in the field from the historical, conservation and restoration view points. Raman scattering presents some advantages with respect to other techniques, like X-ray fluorescence, UV-Vis absorption and Fourier transform infrared spectroscopy. The most relevant are its non-destructive character and its selectivity. With the advent of portable Raman microscopy apparatuses, measurements can be performed
in situ on micrometer-sized areas of the artwork. SERS applied to this kind of investigation opens the way to the detection of exiguous amounts of substances employed in the realization of the work of art [
22]. In
Figure 8, we report as an example SERS measurements performed on a silver substrate soaked in a 10
−4 M concentrated aqueous solution of alizarine. Alizarine, with its hydrolyzed counterpart, purpurine, are the two chromophores that characterize the organic garanza lake dye extracted from the root of the
Rubia tinctorium plant. Red lake has been used since ancient Egyptian times until today. Its detection and the relative concentration of the two chromophores can allow identifying the origin and authenticity of the work of art or the presence of restoration works.
All the alizarine Raman features were detected at 658, 823, 900, 1065, 1156, 1186, 1211, 1269, 1293, 1320, and 1425 cm
−1. One of the most important requirements for a SERS substrate is the spatial homogeneity; indeed, Raman spectra should be reproducible over micron-sized areas with intensity variations within 20%. In
Figure 8b, we report the results of a Raman mapping experiment performed over an area of 10 × 12
μm
2 on an Ag substrate soaked in an aqueous solution of alizarine at a 10
−4 M concentration. Raman spectra were acquired with a spatial resolution of about 1
μm, both along
x and along y. In the Raman map (see
Figure 8b), we report the intensity of the Raman peak at 900 cm
−1 as a function of the position. We chose this peak because it is well separated from other alizarine features, thus making it easier to evaluate its intensity over the background. The mean intensity of the peak was of 2003 counts with a standard deviation of 318 counts, corresponding to a variation of about 15%. A companion experiment was performed on a gold substrate soaked in a purpurine aqueous solution at the same 10
−4 M concentration. The results are reported in
Figure 9. Purpurine Raman features are located at 620, 650, 820, 904, 970, 1065, 1313, 1440 and 1470 cm
−1. The investigated area to test the substrate spatial homogeneity was 10 × 8
μm
2 and the intensity fluctuations of the peak at 1065 cm
−1 as a function of the position on the considered surface was about 11% of the average peak value.