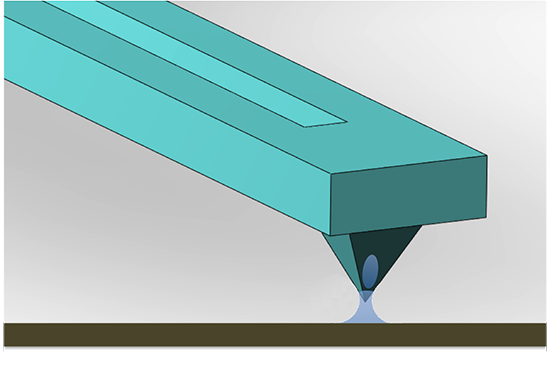
Scanning Probe Microscope-Based Fluid Dispensing
Abstract
:1. Introduction

2. Extending the Abilities of AFM for Fluid Manipulation
| SPM Based Dispensing | Schematic | Working Principle |
|---|---|---|
| (a) Dip Pen Nanolithography (DPN) [20] | 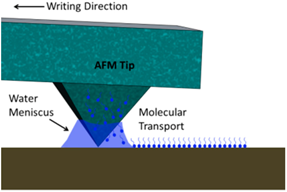 | AFM tip is dip-coated with the ink which is then transferred via water meniscus formed between tip and substrate. |
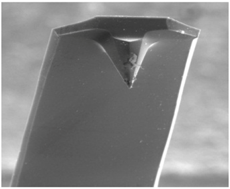 | ||
| (b) Nanoscale Dispensing (NADIS) [22] | 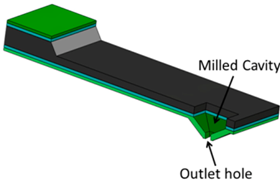 | An aperture outlet hole is milled into the hollow tip apex. The ink is placed as a large droplet into the hollow tip which serves as reservoir. |
 | ||
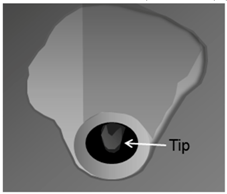
| (c) Nanofountain Probe (NFProbe) [23] | 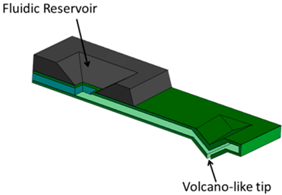 | A continuous supply of ink to the tip is achieved by on-chip reservoir and a capillary connection between them. Liquid is dispensed through a volcano-like tip. |
 | ||
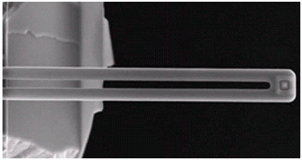
| (d) Scanning Ion Pipette (SIP) [24,25] | 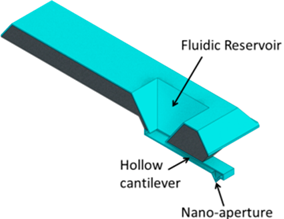 | On-chip reservoir connected to the tip through a hollow cantilever; continuous supply of ink. Liquid is dispensed through an aperture near the hollow tip. |
 |
| Dispensing Probe | Schematic | Working Principle |
|---|---|---|
| (a) Nanofountain pen (NFPen) (Nanonics) [26] | 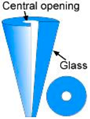 | Glass capillaries pulled into cantilevers [26]. Works similar to NFP and SIP. |
 | ||
| (b) Scanning Ion Conductance Microscope (SICM) [27,28] |  | Made of glass capillary and operated in physiological buffer. Pipette sample distance is controlled by maintaining constant ion current. |
 | ||
| (c) Bioplume (Bioforce Nanosciences) [29] | 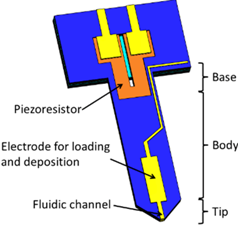 | The cantilever has an open fluidic channel and liquid transfer is achieved by direct contact of the tip and surface. Piezoresistive sensor is integrated to control theplacement. |
 |
2.1. Different Types of Probes
2.1.1. Dip Pen Nanolithography (DPN)

2.1.2. Nanoscale Dispensing (NADIS)
2.1.3. Nano Fountain Probe (NFProbe)
2.1.4. Scanning Ion Pipette (SIP)
2.1.5. Nano Fountain Pen (NFPen)
2.1.6. Scanning Ion Conductance Microscope (SICM)
2.1.7. Bioplume
2.2. Comparison of Important Characteristics of Various Probes
| SPM-Based Tool | DPN | NADIS | NFProbe | Bioplume | SIP | NFPen | SICM |
|---|---|---|---|---|---|---|---|
| Cantilever Material | Si | Si3N4 Tip; Si/SiO2/Si3N4 Cantilever | Si3N4 | Si | SiO2/ Si3N4 | Quartz | Quartz |
| Dimensions | L = 125 μm | L = 145–620 μm | L = 300–500 μm | L = 1500 μm | L = 155 μm | L = 300 μm | Nozzle Diameter = 10 nm |
| W = 30 μm | W = 110 μm | W = 20 μm | W = 120 μm | W = 6.4 μm | Diacapillary = 12 μm | ||
| T = 4 μm | T = 7–8 μm | T = 1.5 μm | T = 5 μm L | T = 4.9 μm | Tip_Height = 12 μm | ||
| Tip_height = 10–15 μm | W_Channel = 5 μm | L_Channel = 200 μm | W_Channel = 3.7 μm | Nozzle Dia = 0.1–0.5 μm | |||
| T_Channel = 0.5 μm | W_Channel =4 μm | T_Channel = 2.2 μm | |||||
| Tip_Height = 4 μm | L_Reservoir = 200 μm | Nozzle_Dia =0.1–1 μm | |||||
| W_Reservoir = 24 μm | |||||||
| Type | Contact | Contact | Contact | Contact | Contact and Non-contact | Contact | Non-contact |
| Droplet Diameter | ~15 nm (Alkanethiols on Gold) | ~70 nm (Glycerol/water mix on hydrophobic Surface) | ~40 nm (Alkanethiols on Gold) | ~ 8 μm (Glycerol/water mix on hydrophobic Si) | ~ 100 nm (Diethylcarbonate on Hydrophobic Silicon surface) | ~1 μm (Proteins on epoxy coated glass) | NA |
| Droplet Volume | ~10 zL | ~100 zL | ~100 zL | ~ 0.5 pL | ~ 100 zL | ~ 100 aL | NA |
| Environment | High humidity (33% to 57%) | Atmospheric conditions | Atmospheric conditions | Atmospheric conditions (but high boiling point liquid added) | Atmospheric conditions and liquid | Atmospheric Conditions and liquid | Liquid |
| Stored volume | None | 20 pL (on-cantilever) | ~19 nL (on-chip reservoir) | ~28 pL (on-cantilever) | ~19 nL (on-chip reservoir) | ~34 pL (inside capillary) | None |
| Possible liquids | Organic, biological | Organic, biological | Organic, biological | Organic, biological | Organic, biological | Organic, biological | Organic, biological |
| Substrate restriction | Flat | Flat | Flat | Flat | Flat | Flat | Flat |
| Read-out method | Optical | Optical | Optical | Piezoresistive | Optical or Piezoresistive | Optical | Ion-conductance |
| Pumping mechanism | None | None | External means/on-chip | None | Evaporation based, Electro-osmotic, pressure based | External | None |
| Advantage | Cheap, Simple, Massive parallelization | Electrowetting, Parallel | Parallel, Controlled dispense and Continuous flow | Parallel, Disposable, Electrowetting, Dispense and Aspirate | Imaging Capability, parallel, Transparent, Disposable, Controlled dispense and Aspirate | Simple fabrication, Dispense | Imaging Capability, Transparent, Evaporation controlled, Parallel, Disposable. |
| Disadvantage | Premature evaporation, no aspiration capability, less control on dispensing | Premature evaporation, liquid contamination, laborious alignment, without imaging capability | Premature evaporation, laborious alignment, without imaging capability, non transparent system | Premature evaporation, exposed to liquid contamination, without imaging capability, big size | Limited viscosity, laborious alignment (without piezoresistors) | Irreproducible, fabrication cannot be scaled | Limited viscosity |
2.3. Microfabrication Steps of Various Liquid Dispensing Devices

2.3.1. Nanoscale Dispenser [56]
2.3.2. Bioplume [93]
2.3.3. Scanning Ion Pipette [24]
2.3.4. Nano Fountain Probe [94]
2.4. Comparing Microfabrication Processes of NADIS, Bioplume, SIP and NFProbe
3. Handling of Fluids within the Probes
3.1. Loading of the Fluid
3.2. Pumping Mechanisms
3.2.1. Evaporation Pump


3.2.2. Thermal Pump


3.2.3. Electro-osmotic Pump

3.2.4. Syringe Pump or Pneumatic Pump

3.3. Fluid Dispensing
3.3.1. Monitoring of Fluid Handling
Dynamic Mode for Cantilever Filling and Droplet Dispensing:

Simultaneous Static and Dynamic Mode for Droplet Dispensing:

3.3.2. Physics of Dispensing
- Liquid properties: Reynolds Number, Surface tension, Capillary forces, Viscosity, Laplace pressure, and Concentration.
- Tip-Surface Interactions: Bond Number, Capillary Length, Adhesion mechanism, Molecular transport mechanism, Contact angle, Contact time, Number of contacts on the same spot and Withdrawal speed of the tip.
- Environment Conditions: Humidity, Temperature and Evaporation rate.
- Geometrical properties: Shape of the tip, Nozzle size and Nozzle position.
Reynolds Number:
Bond Number and Capillary Length:
Adhesion Mechanism:

Molecular Transport Mechanism:

Shape of the Tip:

Aperture Size and Position:


Contact Angle on the Substrate:

Laplace Pressure and Capillary Force:

Contact Angle on the Outer Tip Wall:

Contact Time:
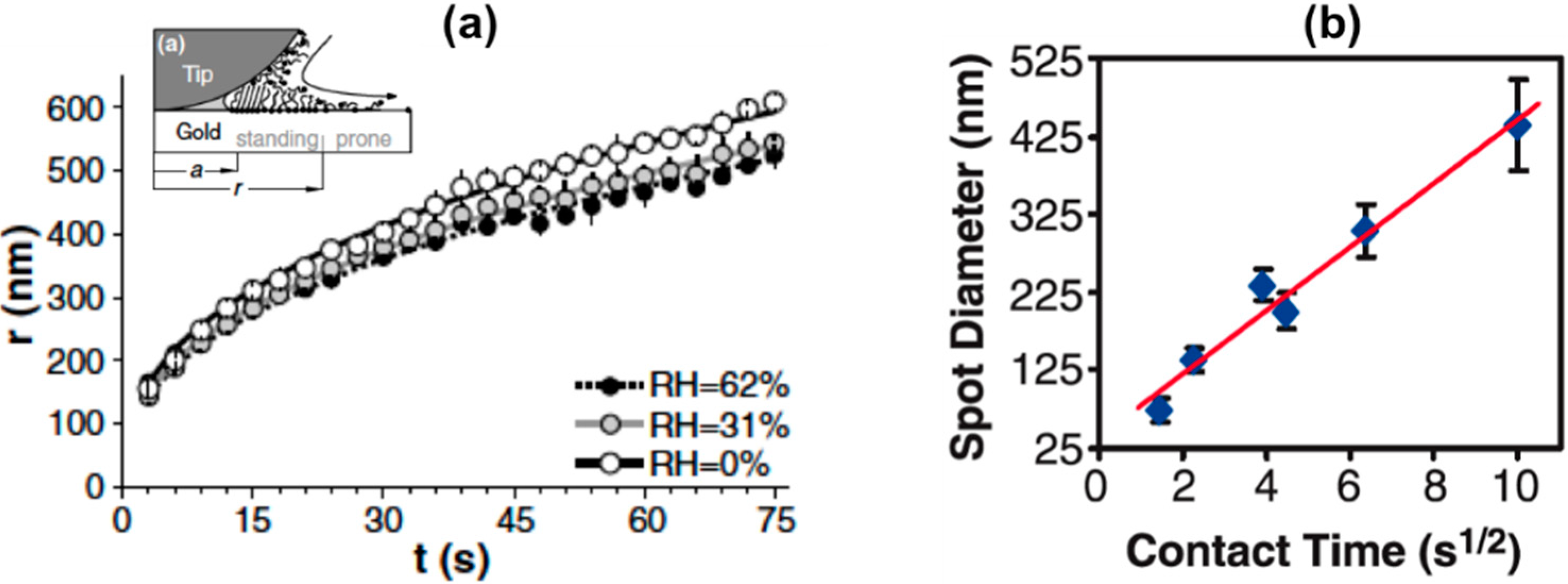
Humidity of Environment:

Effect of Temperature:

3.3.3. Droplet Characterization
Spherical Cap:
Meniscus Bridge Break-Up Distance:
3.3.4. Droplet evaporation rate


3.3.5. Imaging the Dispensed Droplets
Scanning Electron Microscope (SEM):
Atomic Force Microscope (AFM):
4. Applications
4.1. Microarray
4.2. Single Living Cell Manipulation


5. Conclusions and Future Scope

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stroscio, J.A.; Eigler, D.M. Atomic and molecular manipulation with the scanning tunneling microscope. Science 1991, 254, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- A Boy and His Atom: The World’s Smallest Movie. Available online: http://www.research.ibm.com/articles/madewithatoms.shtml (accessed on 15 October 2014).
- Wijshoff, H. The dynamics of the piezo inkjet printhead operation. Phys. Rep. 2010, 491, 77–177. [Google Scholar] [CrossRef]
- Le, H.P. Progress and trends in ink-jet printing technology. J. Imaging Sci. Techn. 1998, 42, 49–62. [Google Scholar]
- Leach, R.H. The Printing Ink Manual; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- De Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet printing of polymers: State of the art and future developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet printing-process and its applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Bietsch, A.; Hegner, M.; Lang, H.P.; Gerber, C. Inkjet deposition of alkanethiolate monolayers and DNA oligonucleotides on gold: Evaluation of spot uniformity by wet etching. Langmuir 2004, 20, 5119–5122. [Google Scholar] [CrossRef] [PubMed]
- Sutandy, F.; Qian, J.; Chen, C.S.; Zhu, H. Overview of protein microarrays. In Current Protocols in Protein Science; Wiley: Hoboken, NJ, USA, 2013; Chapter 27. [Google Scholar]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ru, C.H.; Luo, J.; Xie, S.R.; Sun, Y. A review of non-contact micro- and nano-printing technologies. J. Micromech. Microeng. 2014, 24, 053001. [Google Scholar] [CrossRef]
- Taylor, G. Disintegration of water drops in an electric field. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1964, 280, 383–397. [Google Scholar] [CrossRef]
- Wilm, M. Principles of electrospray ionization. Mol. Cell. Proteomics 2011, 10, M111.009407. [Google Scholar] [CrossRef] [PubMed]
- Galliker, P.; Schneider, J.; Eghlidi, H.; Kress, S.; Sandoghdar, V.; Poulikakos, D. Direct printing of nanostructures by electrostatic autofocussing of ink nanodroplets. Nat. Commun. 2012, 3, 890. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Schitter, G.; Steininger, J.; Heuck, F.C.A.; Staufer, U. Towards fast AFM-based nanometrology and nanomanufacturing. Int. J. Nanomanuf. 2012, 8, 392–418. [Google Scholar] [CrossRef]
- Bellido, E.; de Miguel, R.; Ruiz-Molina, D.; Lostao, A.; Maspoch, D. Controlling the number of proteins with dip-pen nanolithography. Adv. Mater. 2010, 22, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Reinhoudt, D.N.; Otto, C.; Subramaniam, V.; Velders, A.H. Strategies for patterning biomolecules with dip-pen nanolithography. Small 2011, 7, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Bellido, E.; Cardona-Serra, S.; Coronado, E.; Ruiz-Molina, D. Assisted-assembly of coordination materials into advanced nanoarchitectures by Dip Pen nanolithography. Chem. Commun. 2011, 47, 5175–5177. [Google Scholar] [CrossRef] [Green Version]
- Meister, A.; Jeney, S.; Liley, M.; Akiyama, T.; Staufer, U.; de Rooij, N.F.; Heinzelmann, H. Nanoscale dispensing of liquids through cantilevered probes. Microelectron. Eng. 2003, 67–68, 644–650. [Google Scholar] [CrossRef]
- Kim, K.H.; Moldovan, N.; Ke, C.; Espinosa, H.D. A novel AFM chip for fountain pen nanolithography—Design and microfabrication. Micro Nanosyst. 2004, 782, 267–272. [Google Scholar]
- Hug, T.; Biss, T.; de Rooij, N.; Staufer, U. Generic Fabrication Technology for Transparent and Suspended Microfluidic and Nanofluidic Channels. In Proceedings of the 13th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers’ 05), Seoul, Korea, 5–9 June 2005; pp. 1191–1194.
- Meister, A.; Gabi, M.; Behr, P.; Studer, P.; Voros, J.; Niedermann, P.; Bitterli, J.; Polesel-Maris, J.; Liley, M.; Heinzelmann, H.; Zambelli, T. FluidFM: Combining atomic force microscopy and nanofluidics in a universal liquid delivery system for single cell applications and beyond. Nano Lett. 2009, 9, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Kheifetz, Y.; Shambrodt, E.; Radko, A.; Khatchatryan, E.; Sukenik, C. Fountain pen nanochemistry: Atomic force control of chrome etching. Appl. Phys. Lett. 1999, 75, 2689–2691. [Google Scholar] [CrossRef]
- Pellegrino, M.; Orsini, P.; Pellegrini, M.; Baschieri, P.; Dinelli, F.; Petracchi, D.; Tognoni, E.; Ascoli, C. Weak hydrostatic forces in far-scanning ion conductance microscopy used to guide neuronal growth cones. Neurosci. Res. 2011, 69, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Zhou, Y.; Baker, L.A. Scanning ion conductance microscopy. Annu. Rev. Anal. Chem. 2012, 5, 207–228. [Google Scholar] [CrossRef]
- Leichle, T.; Lishchynska, M.; Mathieu, F.; Pourciel, J.B.; Saya, D.; Nicu, L. A microcantilever-based picoliter droplet dispenser with integrated force sensors and electroassisted deposition means. J. Microelectromech. Syst. 2008, 17, 1239–1253. [Google Scholar] [CrossRef]
- Jaschke, M.; Butt, H.J. Deposition of organic material by the tip of a scanning force microscope. Langmuir 1995, 11, 1061–1064. [Google Scholar] [CrossRef]
- Piner, R.D.; Zhu, J.; Xu, F.; Hong, S.H.; Mirkin, C.A. “Dip-pen” nanolithography. Science 1999, 283, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Hong, S.H.; Schatz, G.C.; Ratner, M.A. Self-assembly of ink molecules in dip-pen nanolithography: A diffusion model. J. Chem. Phys. 2001, 115, 2721–2729. [Google Scholar] [CrossRef]
- Mirkin, C.A. Programming the assembly of two- and three-dimensional architectures with DNA and nanoscale inorganic building blocks. Inorg. Chem. 2000, 39, 2258–2272. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.M.; Ginger, D.S.; Park, S.J.; Li, Z.; Chung, S.W.; Mirkin, C.A. Direct patterning of modified oligonucleotides on metals and insulators by dip-pen nanolithography. Science 2002, 296, 1836–1838. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Ginger, D.S.; Morales, M.W.; Zhang, Z.F.; Chandrasekhar, V.; Ratner, M.A.; Mirkin, C.A. Top-down meets bottom-up: Dip-pen nanolithography and DNA-directed assembly of nanoscale electrical circuits. Small 2005, 1, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Park, S.J.; Mirkin, C.A.; Smith, J.C.; Mrksich, M. Protein nanoarrays generated by dip-pen nanolithography. Science 2002, 295, 1702–1705. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Ginger, D.S.; Lee, K.B.; Heo, J.; Nam, J.M.; Mirkin, C.A. Direct-write dip-pen nanolithography of proteins on modified silicon oxide surfaces. Angew. Chemie-Int. Ed. 2003, 42, 2309–2312. [Google Scholar] [CrossRef]
- Lee, K.B.; Lim, J.H.; Mirkin, C.A. Protein nanostructures formed via direct-write dip-pen nanolithography. J. Am. Chem. Soc. 2003, 125, 5588–5589. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Oh, B.K.; Sanedrin, R.G.; Salaita, K.; Fujigaya, T.; Mirkin, C.A. Biologically active protein nanoarrays generated using parallel dip-pen nanolithography. Adv. Mater. 2006, 18, 1133–1136. [Google Scholar] [CrossRef]
- Lee, K.B.; Kim, E.Y.; Mirkin, C.A.; Wolinsky, S.M. The use of nanoarrays for highly sensitive and selective detection of human immunodeficiency virus type 1 in plasma. Nano Lett. 2004, 4, 1869–1872. [Google Scholar] [CrossRef]
- Smith, J.C.; Lee, K.B.; Wang, Q.; Finn, M.G.; Johnson, J.E.; Mrksich, M.; Mirkin, C.A. Nanopatterning the chemospecific immobilization of cowpea mosaic virus capsid. Nano Lett. 2003, 3, 883–886. [Google Scholar] [CrossRef]
- Vega, R.A.; Maspoch, D.; Salaita, K.; Mirkin, C.A. Nanoarrays of single virus particles. Angew. Chemie-Int. Ed. 2005, 44, 6013–6015. [Google Scholar] [CrossRef]
- Rozhok, S.; Shen, C.K.F.; Littler, P.L.H.; Fan, Z.F.; Liu, C.; Mirkin, C.A.; Holz, R.C. Methods for fabricating microarrays of motile bacteria. Small 2005, 1, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Maynor, B.W.; Li, Y.; Liu, J. Au “ink” for AFM “dip-pen” nanolithography. Langmuir 2001, 17, 2575–2578. [Google Scholar] [CrossRef]
- Liu, X.G.; Fu, L.; Hong, S.H.; Dravid, V.P.; Mirkin, C.A. Arrays of magnetic nanoparticles patterned via “dip-pen” nanolithography. Adv. Mater. 2002, 14, 231–234. [Google Scholar] [CrossRef]
- Jung, H.; Kulkarni, R.; Collier, C.P. Dip-pen nanolithography of reactive alkoxysilanes on glass. J. Am. Chem. Soc. 2003, 125, 12096–12097. [Google Scholar] [CrossRef] [PubMed]
- Pena, D.J.; Raphael, M.P.; Byers, J.M. “Dip-Pen” nanolithography in registry with photolithography for biosensor development. Langmuir 2003, 19, 9028–9032. [Google Scholar] [CrossRef]
- Noy, A.; Miller, A.E.; Klare, J.E.; Weeks, B.L.; Woods, B.W.; DeYoreo, J.J. Fabrication of luminescent nanostructures and polymer nanowires using dip-pen nanolithography. Nano Lett. 2002, 2, 109–112. [Google Scholar] [CrossRef]
- Su, M.; Liu, X.G.; Li, S.Y.; Dravid, V.P.; Mirkin, C.A. Moving beyond molecules: Patterning solid-state features via dip-pen nanolithography with sol-based inks. J. Am. Chem. Soc. 2002, 124, 1560–1561. [Google Scholar] [CrossRef] [PubMed]
- Salaita, K.; Wang, Y.H.; Mirkin, C.A. Applications of dip-pen nanolithography. Nat. Nanotechnol. 2007, 2, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Salaita, K.; Wang, Y.H.; Fragala, J.; Vega, R.A.; Liu, C.; Mirkin, C.A. Massively parallel dip-pen nanolithography with 55000-pen two-dimensional arrays. Angew. Chemie-Int. Ed. 2006, 45, 7220–7223. [Google Scholar] [CrossRef]
- Huo, F.W.; Zheng, Z.J.; Zheng, G.F.; Giam, L.R.; Zhang, H.; Mirkin, C.A. Polymer pen lithography. Science 2008, 321, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.; Braunschweig, A.B.; Liao, X.; Chai, J.N.; Lim, J.K.; Zheng, G.F.; Mirkin, C.A. Hard-tip, soft-spring lithography. Nature 2011, 469, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Giam, L.R.; Mirkin, C.A. Cantilever-Free Scanning Probe Molecular Printing. Angew.Chemie-Int. Ed. 2011, 50, 7482–7485. [Google Scholar] [CrossRef]
- Meister, A.; Liley, M.; Brugger, J.; Pugin, R.; Heinzelmann, H. Nanodispenser for attoliter volume deposition using atomic force microscopy probes modified by focused-ion-beam milling. Appl. Phys. Lett. 2004, 85, 6260–6262. [Google Scholar] [CrossRef]
- Meister, A. Nanoscale Dispensing of Single Ultrasmall Droplets. Ph.D. Thesis, University of Basel, Basel, The Switzerland, 2005. [Google Scholar]
- Kaisei, K.; Satoh, N.; Kobayashi, K.; Matsushige, K.; Yamada, H. Nanoscale liquid droplet deposition using the ultrasmall aperture on a dynamic mode AFM tip. Nanotechnology 2011, 22, 175301. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Moldovan, N.; Espinosa, H.D. A nanofountain probe with sub-100 nm molecular writing resolution. Small 2005, 1, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, S.H.; Kim, Y.J.; Kim, H.M. A novel passive membrane pumping nano fountain-pen. In Proceedings of the 1st IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Zhuhai, China, 18–21 January 2006; pp. 1012–1017.
- Hwang, K.; Lee, S.H.; Kim, Y.J.; Kim, H. Control of line width with active nano fountain pen (ANFP) for nano manufacturing. J. Mech. Sci. Technol. 2008, 22, 1339–1348. [Google Scholar] [CrossRef]
- Loh, O.Y.; Ho, A.M.; Rim, J.E.; Kohli, P.; Patankar, N.A.; Espinosa, H.D. Electric field-induced direct delivery of proteins by a nanofountain probe. Proc. Natl. Acad. Sci. USA 2008, 105, 16438–16443. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, N.; Kim, K.H.; Espinosa, H.D. A multi-ink linear array of nanofountain probes. J. Micromech. Microeng. 2006, 16, 1935–1942. [Google Scholar] [CrossRef]
- Deladi, S.; Tas, N.R.; Berenschot, J.W.; Krijnen, G.J.M.; de Boer, M.J.; de Boer, J.H.; Peter, M.; Elwenspoek, M.C. Micromachined fountain pen for atomic force microscope-based nanopatterning. Appl. Phys. Lett. 2004, 85, 5361–5363. [Google Scholar] [CrossRef]
- Deladi, S.; Berenschot, J.W.; Tas, N.R.; Krijnen, G.J.M.; de Boer, J.H.; de Boer, M.J.; Elwenspoek, M.C. Fabrication of micromachined fountain pen with in situ characterization possibility of nanoscale surface modification. J. Micromech. Microeng. 2005, 15, 528–534. [Google Scholar] [CrossRef]
- Meister, A.; Polesel-Maris, J.; Niedermann, P.; Przybylska, J.; Studer, P.; Gabi, M.; Behr, P.; Zambelli, T.; Liley, M.; Voros, J.; et al. Nanoscale dispensing in liquid environment of streptavidin on a biotin-functionalized surface using hollow atomic force microscopy probes. Microelectron. Eng. 2009, 86, 1481–1484. [Google Scholar] [CrossRef]
- Geerlings, J.; Sarajlic, E.; Berenschot, J.; Sanders, R.; Abelmann, L.; Tas, N. Electrospray deposition from AFM probes with nanoscale apertures. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 100–103.
- Garza, H.; Stoute, R.; Ghatkesar, M.; Staufer, U. Self-sensing nanopipette for liquid dispensing and AFM imaging. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems, Madrid, Spain, 16–20 June 2013; pp. 2648–2651.
- Khan, M.F.; Schmid, S.; Davis, Z.J.; Dohn, S.; Boisen, A. Fabrication of resonant micro cantilevers with integrated transparent fluidic channel. Microelectron. Eng. 2011, 88, 2300–2303. [Google Scholar] [CrossRef]
- Ghatkesar, M.K.; Garza, H.H.P.; Staufer, U. Hollow AFM cantilever pipette. Microelectron. Eng. 2014, 124, 22–25. [Google Scholar] [CrossRef]
- Lovsky, Y.; Lewis, A.; Sukenik, C.; Grushka, E. Atomic-force-controlled capillary electrophoretic nanoprinting of proteins. Anal. Bioanal. Chem. 2010, 396, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Tsarfati-BarAd, I.; Sauer, U.; Preininger, C.; Gheber, L.A. Miniaturized protein arrays: Model and experiment. Biosensors Bioelectron. 2011, 26, 3774–3781. [Google Scholar] [CrossRef]
- Taha, H.; Marks, R.S.; Gheber, L.A.; Rousso, I.; Newman, J.; Sukenik, C.; Lewis, A. Protein printing with an atomic force sensing nanofountainpen. Appl. Phys. Lett. 2003, 83, 1041–1043. [Google Scholar] [CrossRef]
- Strain, K.M.; Yeshua, T.; Gromov, A.V.; Nerushev, O.; Lewis, A.; Campbell, E.E.B. Direct deposition of aligned single walled carbon nanotubes by fountain pen nanolithography. Mater. Express 2011, 1, 279–284. [Google Scholar] [CrossRef]
- Sokuler, M.; Aronov, D.; Rosenman, G.; Gheber, L.A. Tailored polymer microlenses on treated glass surfaces. Appl. Phys. Lett. 2007, 90, 203106. [Google Scholar] [CrossRef]
- Kantarovich, K.; Tsarfati, I.; Gheber, L.A.; Haupt, K.; Bar, I. Writing droplets of molecularly imprinted polymers by nano fountain pen and detecting their molecular interactions by surface-enhanced raman scattering. Anal. Chem. 2009, 81, 5686–5690. [Google Scholar] [CrossRef] [PubMed]
- Belmont, A.S.; Sokuler, M.; Haupt, K.; Gheber, L.A. Direct writing of molecularly imprinted microstructures using a nanofountain pen. Appl. Phys. Lett. 2007, 90, 193101. [Google Scholar] [CrossRef]
- Edwards, M.A.; Williams, C.G.; Whitworth, A.L.; Unwin, P.R. Scanning ion conductance microscopy: A model for experimentally realistic conditions and image interpretation. Anal. Chem. 2009, 81, 4482–4492. [Google Scholar] [CrossRef] [PubMed]
- Hansma, P.K.; Drake, B.; Marti, O.; Gould, S.A.C.; Prater, C.B. The scanning ion-conductance microscope. Science 1989, 243, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Shevchuk, A.I.; Frolenkov, G.I.; Sanchez, D.; James, P.S.; Freedman, N.; Lab, M.J.; Jones, R.; Klenerman, D.; Korchev, Y.E. Imaging proteins in membranes of living cells by high-resolution scanning ion conductance microscopy. Angew. Chemie-Int. Ed. 2006, 45, 2212–2216. [Google Scholar] [CrossRef]
- Happel, P.; Thatenhorst, D.; Dietzel, I.D. Scanning ion conductance microscopy for studying biological samples. Sensors 2012, 12, 14983–15008. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.A.; Actis, P.; Penfold, C.; Maalouf, M.; Vilozny, B.; Pourmand, N. Voltage controlled nano-injection system for single-cell surgery. Nanoscale 2012, 4, 5843–5846. [Google Scholar] [CrossRef] [PubMed]
- Ebejer, N.; Guell, A.G.; Lai, S.C.S.; McKelvey, K.; Snowden, M.E.; Unwin, P.R. Scanning electrochemical cell microscopy: A versatile technique for nanoscale electrochemistry and functional imaging. Annu. Rev. Anal. Chem. 2013, 6, 329–351. [Google Scholar] [CrossRef]
- Ebejer, N.; Schnippering, M.; Colburn, A.W.; Edwards, M.A.; Unwin, P.R. Localized high resolution electrochemistry and multifunctional imaging: Scanning electrochemical cell microscopy. Anal. Chem. 2010, 82, 9141–9145. [Google Scholar] [CrossRef] [PubMed]
- Snowden, M.E.; Guell, A.G.; Lai, S.C.S.; McKelvey, K.; Ebejer, N.; O’Connell, M.A.; Colburn, A.W.; Unwin, P.R. Scanning Electrochemical cell microscopy: Theory and experiment for quantitative high resolution spatially-resolved voltammetry and simultaneous ion-conductance measurements. Anal. Chem. 2012, 84, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Salazar, F.; Lesch, A.; Momotenko, D.; Busnel, J.M.; Wittstock, G.; Girault, H.H. Fountain pen for scanning electrochemical microscopy. Anal. Methods 2010, 2, 817–823. [Google Scholar] [CrossRef]
- Momotenko, D.; Qiao, L.; Cortes-Salazar, F.; Lesch, A.; Wittstock, G.; Girault, H.H. Electrochemical push-pull scanner with mass spectrometry detection. Anal. Chem. 2012, 84, 6630–6637. [Google Scholar] [CrossRef] [PubMed]
- Juncker, D.; Schmid, H.; Delamarche, E. Multipurpose microfluidic probe. Nat. Mater. 2005, 4, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Lishchynska, M.; Leiche, T.; Nicu, L. FEM assisted calibration of a MEMS-based dispensing system with integrated piezoresistive force sensors. J. Micromech. Microeng. 2008, 18, 015010. [Google Scholar] [CrossRef]
- Lishchynska, M.; Leichle, T.; Nicu, L. Semi-empirical model for longitudinal piezoresistive sensitivity of microcantilevers. Microelectron. Eng. 2008, 85, 1321–1325. [Google Scholar] [CrossRef]
- Leichle, T.; Saya, D.; Pourciel, J.B.; Mathieu, F.; Nicu, L.; Bergaud, C. Liquid loading of silicon-based cantilevers using electrowetting actuation for microspotting applications. Sens. Actuators Phys. 2006, 132, 590–596. [Google Scholar] [CrossRef]
- Berthet-Duroure, N.; Leichle, T.; Pourciel, J.B.; Martin, C.; Bausells, J.; Lora-Tamayo, E.; Perez-Murano, F.; Francois, J.M.; Trevisiol, E.; Nicu, L. Interaction of biomolecules sequentially deposited at the same location using a microcantilever-based spotter. Biomed. Microdevices 2008, 10, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Llobera, A.; Leichle, T.; Villanueva, G.; Voigt, A.; Fakhfouri, V.; Kim, J.Y.; Berthet, N.; Bausells, J.; Gruetzner, G.; et al. Novel methods to pattern polymers for microfluidics. Microelectron. Eng. 2008, 85, 972–975. [Google Scholar] [CrossRef]
- Leïchlé, T. Bioplume: A MEMS-Based Picoliter Droplet Dispenser with Electrospotting Means for Patterning Surfaces at the Micro- and the Nanometer Scales. Ph.D. Thesis, Université Paul Sabatier-Toulouse III, Toulouse, France, 5 December 2005. [Google Scholar]
- Moldovan, N.; Kim, K.H.; Espinosa, H.D. Design and fabrication of a novel microfluidic nanoprobe. J. Microelectromech. Syst. 2006, 15, 204–213. [Google Scholar] [CrossRef]
- Heuck, F.; Hug, T.; Akiyama, T.; Frederix, P.L.T.M.; Engel, A.; Meister, A.; Heinzelmann, H.; de Rooij, N.F.; Staufer, U. Evaporation based micro pump integrated into a scanning force microscope probe. Microelectron. Eng. 2008, 85, 1302–1305. [Google Scholar] [CrossRef]
- Garza, H.H.P.; Ghatkesar, M.K.; Staufer, U. Combined AFM—Nanopipette Cartridge System for Actively Dispensing Femtolitre Droplets. In Proceedings of the 2012 International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-Nano), Shanxi, China, 29 August–1 September 2012; pp. 320–325.
- Heuck, F.C.A.; Staufer, U. Low voltage electroosmotic pump for high density integration into microfabricated fluidic systems. Microfluid. Nanofluid. 2011, 10, 1317–1332. [Google Scholar] [CrossRef]
- Wang, Y.H.; Giam, L.R.; Park, M.; Lenhert, S.; Fuchs, H.; Mirkin, C.A. A self-correcting inking strategy for cantilever arrays addressed by an inkjet printer and used for dip-pen nanolithography. Small 2008, 4, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Heuck, F. Developing and Analysing Sub-10 μm Fluidic Systems with Integrated Electrodes for Pumping and Sensing in Nanotechnology Applications. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 28 October 2010. [Google Scholar]
- Janz, G.J.; Ives, D.J.G. Silver silver chloride electrodes. Ann. NY Acad. Sci. 1968, 148, 210–221. [Google Scholar] [CrossRef]
- Heuck, F.C.A.; Staufer, U. Electroless deposition and structuring of silver electrodes in closed microfluidic capillaries. J. Microelectromech. Syst. 2011, 20, 451–459. [Google Scholar] [CrossRef]
- Heuck, F.; van der Ploeg, P.; Staufer, U. Deposition and structuring of Ag/AgCl electrodes inside a closed polymeric microfluidic system for electroosmotic pumping. Microelectron. Eng. 2011, 88, 1887–1890. [Google Scholar] [CrossRef]
- Elveflow Microfluidic Flow Control System. Available online: http://www.elveflow.com/microfluidic-reviews-and-tutorials/how-to-choose-the-right-microfluidics-flow-control-system (accessed on 15 October 2014).
- Oh, K.W.; Lee, K.; Ahn, B.; Furlani, E.P. Design of pressure-driven microfluidic networks using electric circuit analogy. Lab. Chip 2012, 12, 515–545. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Hirtz, M.; Fuchs, H. Measurement of Mass Transfer during Dip-Pen Nanolithography with Phospholipids. Small 2011, 7, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, J.; Dujardin, E.; Rius, G.; Perez-Murano, F.; Ondarcuhu, T. Evaporation of femtoliter sessile droplets monitored with nanomechanical mass sensors. J. Phys. Chem. B 2007, 111, 13020–13027. [Google Scholar] [CrossRef] [PubMed]
- Burg, T.P.; Godin, M.; Knudsen, S.M.; Shen, W.; Carlson, G.; Foster, J.S.; Babcock, K.; Manalis, S.R. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 2007, 446, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Ghatkesar, M.K.; Braun, T.; Barwich, V.; Ramseyer, J.P.; Gerber, C.; Hegner, M.; Lang, H.P. Resonating modes of vibrating microcantilevers in liquid. Appl. Phys. Lett. 2008, 92, 043106. [Google Scholar] [CrossRef]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Annu. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Gunther, A.; Jensen, K.F. Multiphase microfluidics: from flow characteristics to chemical and materials synthesis. Lab. Chip 2006, 6, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Hager, W.H. Wilfrid Noel Bond and the Bond number. J. Hydraul Res. 2012, 50, 3–9. [Google Scholar] [CrossRef]
- Rozhok, S.; Sun, P.; Piner, R.; Lieberman, M.; Mirkin, C.A. AFM study of water meniscus formation between an AFM tip and NaCl substrate. J. Phys. Chem. B 2004, 108, 7814–7819. [Google Scholar] [CrossRef]
- Sirghi, L. Transport mechanisms in capillary condensation of water at a single-asperity nanoscopic contact. Langmuir 2012, 28, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.E.; Whitman, L.J. Thiol diffusion and the role of humidity in “dip pen nanolithography”. Phys. Rev. Lett. 2002, 88, 156104. [Google Scholar] [CrossRef] [PubMed]
- Rozhok, S.; Piner, R.; Mirkin, C.A. Dip-pen nanolithography: What controls ink transport? J. Phys. Chem. B 2003, 107, 751–757. [Google Scholar] [CrossRef]
- Ho, A.M.; Espinosa, H.D. Scanning Probes for the Life Sciences. In Applied Scanning Probe Methods VIII; Bhushan, B., Fuchs, H., Eds.; Springer: Berlin Heidelberg, Germany, 2008; pp. 183–217. [Google Scholar]
- Espinosa, H.D.; Moldovan, N.; Kim, K.-H. Novel AFM nanoprobes. Appl. Scanning Probe Methods VII 2007, 77–134. [Google Scholar]
- Fang, A.P.; Dujardin, E.; Ondarcuhu, T. Control of droplet size in liquid nanodispensing. Nano Lett. 2006, 6, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Nakamura, K.; Horiike, S.; Nagai, M.; Kawashima, T.; Mineta, T.; Makino, E. Fabrication and characterization of bioprobe integrated with a hollow nanoneedle for novel AFM applications in cellular function analysis. Microelectron. Eng. 2013, 111, 325–331. [Google Scholar] [CrossRef]
- Good, R.J. Contact-angle, wetting, and adhesion—A critical review. J. Adhes. Sci. Technol. 1992, 6, 1269–1302. [Google Scholar] [CrossRef]
- Derjagui, B.V.; Churaev, N.V. Structural component of disjoining pressure. J. Coll. Interface Sci. 1974, 49, 249–255. [Google Scholar]
- Michael Nosonovsky, B.B. Capillary Adhesion and Nanoscale Properties of Water; Springer: Berlin, Germany, 2011. [Google Scholar]
- Israelachvili, J.N. Intermolecular and Surface Forces; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Butt, H.J.; Kappl, M. Normal capillary forces. Adv. Coll. Interface 2009, 146, 48–60. [Google Scholar] [CrossRef]
- Gao, C. Theory of menisci and its applications. Appl. Phys. Lett. 1997, 71, 1801–1803. [Google Scholar] [CrossRef]
- Hotta, K.; Takeda, K.; Iinoya, K. Capillary binding force of a liquid bridge. Powder Technol. 1974, 10, 231–242. [Google Scholar] [CrossRef]
- Fabie, L.; Durou, H.; Ondarcuhu, T. Capillary forces during liquid nanodispensing. Langmuir 2010, 26, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Hampton, J.R.; Dameron, A.A.; Weiss, P.S. Double-ink dip-pen nanolithography studies elucidate molecular transport. J. Am. Chem. Soc. 2006, 128, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Fabie, L.; Ondarcuhu, T. Writing with liquid using a nanodispenser: Spreading dynamics at the sub-micron scale. Soft Matter 2012, 8, 4995–5001. [Google Scholar] [CrossRef]
- van Honschoten, J.W.; Brunets, N.; Tas, N.R. Capillarity at the nanoscale. Chem. Soc. Rev. 2010, 39, 1096–1114. [Google Scholar]
- Chung, S.; Felts, J.R.; Wang, D.; King, W.P.; De Yoreo, J.J. Temperature-dependence of ink transport during thermal dip-pen nanolithography. Appl. Phys. Lett. 2011, 99, 193101. [Google Scholar] [CrossRef]
- Andrienko, D.; Patricio, P.; Vinogradova, O.I. Capillary bridging and long-range attractive forces in a mean-field approach. J. Chem. Phys. 2004, 121, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
- Sirghi, L.; Szoszkiewicz, R.; Riedo, E. Volume of a nanoscale water bridge. Langmuir 2006, 22, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- O’connell, C.D.; Higgins, M.J.; Marusic, D.; Moulton, S.E.; Wallace, G.G. Liquid ink deposition from an atomic force microscope tip: Deposition monitoring and control of feature size. Langmuir 2014, 30, 2712–2721. [Google Scholar] [CrossRef]
- Bonaccurso, E.; Golovko, D.S.; Bonanno, P.; Raiteri, R.; Haschke, T.; Wiechert, W.; Butt, H.-J. Atomic Force Microscope Cantilevers Used as Sensors for Monitoring Microdrop Evaporation. In Applied Scanning Probe Methods XI; Springer: Berlin, Germany, 2009; pp. 17–38. [Google Scholar]
- Butt, H.J.; Golovko, D.S.; Bonaccurso, E. On the derivation of Young’s equation for sessile drops: Nonequilibrium effects due to evaporation. J. Phys. Chem. B 2007, 111, 5277–5283. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, F.; Graf, K.; Hardt, S.; Butt, H.J. Evaporation dynamics of sessile liquid drops in still air with constant contact radius. Int. J. Heat Mass Transf. 2008, 51, 3696–3699. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Golding, J.; Forsyth, S.; Forsyth, M.; Deacon, G.B. Low viscosity ionic liquids based on organic salts of the dicyanamide anion. Chem. Commun. 2001, 1430–1431. [Google Scholar]
- Heller, M.J. DNA microarray technology: Devices, systems, and applications. Annu. Rev. Biomed. Eng. 2002, 4, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Stoughton, R.B. Applications of DNA microarrays in biology. Annu. Rev. Biochem. 2005, 74, 53–82. [Google Scholar] [CrossRef] [PubMed]
- Gruter, R.R.; Voros, J.; Zambelli, T. FluidFM as a lithography tool in liquid: Spatially controlled deposition of fluorescent nanoparticles. Nanoscale 2013, 5, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.M.; Yavari, F.; Minary-Jolandan, M.; Giraldo-Vela, J.P.; Safi, A.; McNaughton, R.L.; Parpoil, V.; Espinosa, H.D. Nanofountain probe electroporation (NFP-E) of single cells. Nano Lett. 2013, 13, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.M.; McNaughton, R.L.; Yavari, F.; Minary-Jolandan, M.; Safi, A.; Espinosa, H.D. Microfluidic parallel patterning and cellular delivery of molecules with a nanofountain probe. Jala 2014, 19, 100–109. [Google Scholar] [PubMed]
- Actis, P.; Maalouf, M.M.; Kim, H.J.; Lohith, A.; Vilozny, B.; Seger, R.A.; Pourmand, N. Compartmental genomics in living cells revealed by single-cell nanobiopsy. ACS Nano 2014, 8, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.A.; Actis, P.; Vilozny, B.; Pourmand, N. Single cell sensing and manipulation by scanning nanopore microscopy. Biophys. J. 2011, 100, 183–183. [Google Scholar] [CrossRef] [PubMed]
- Guillaume-Gentil, O.; Potthoff, E.; Ossola, D.; Franz, C.M.; Zambelli, T.; Vorholt, J.A. Force-controlled manipulation of single cells: from AFM to FluidFM. Trends Biotechnol. 2014, 32, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Cong, P.J.; Doolen, R.D.; Fan, Q.; Giaquinta, D.M.; Guan, S.H.; McFarland, E.W.; Poojary, D.M.; Self, K.; Turner, H.W.; Weinberg, W.H. High-throughput synthesis and screening of combinatorial heterogeneous catalyst libraries. Angew. Chemie-Int. Ed. 1999, 38, 484–488. [Google Scholar] [CrossRef]
- Jambunathan, K.; Hillier, A.C. Measuring electrocatalytic activity on a local scale with scanning differential electrochemical mass spectrometry. J. Electrochem. Soc. 2003, 150, E312–E320. [Google Scholar] [CrossRef]
- Ghatkesar, M.K.; Barwich, V.; Braun, T.; Ramseyer, J.P.; Gerber, C.; Hegner, M.; Lang, H.P.; Drechsler, U.; Despont, M. Higher modes of vibration increase mass sensitivity in nanomechanical microcantilevers. Nanotechnology 2007, 18, 445502. [Google Scholar] [CrossRef]
- Burg, T.P.; Manalis, S.R. Suspended microchannel resonators for biomolecular detection. Appl. Phys. Lett. 2003, 83, 2698–2700. [Google Scholar]
- Woolley, A.T.; Lao, K.Q.; Glazer, A.N.; Mathies, R.A. Capillary electrophoresis chips with integrated electrochemical detection. Anal. Chem. 1998, 70, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.S.; Walsh, K.M.; Crain, M.M.; Roussel, T.J.; Jackson, D.J.; Baldwin, R.P.; Keynton, R.S.; Naber, J.F. Fully integrated three-dimensional electrodes for electrochemical detection in microchips: Fabrication, characterization, and applications. Anal. Chem. 2009, 81, 4762–4769. [Google Scholar] [CrossRef] [PubMed]
- Bratten, C.D.T.; Cobbold, P.H.; Cooper, J.M. Micromachining sensors for electrochemical measurement in subnanoliter volumes. Anal. Chem. 1997, 69, 253–258. [Google Scholar] [CrossRef]
- Korchev, Y.E.; Negulyaev, Y.A.; Edwards, C.R.W.; Vodyanoy, I.; Lab, M.J. Functional localization of single active ion channels can the surface of a living cell. Nat. Cell Biol. 2000, 2, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflug. Arch. Eur. J. Physiol. 1981, 391, 85–100. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghatkesar, M.K.; Garza, H.H.P.; Heuck, F.; Staufer, U. Scanning Probe Microscope-Based Fluid Dispensing. Micromachines 2014, 5, 954-1001. https://doi.org/10.3390/mi5040954
Ghatkesar MK, Garza HHP, Heuck F, Staufer U. Scanning Probe Microscope-Based Fluid Dispensing. Micromachines. 2014; 5(4):954-1001. https://doi.org/10.3390/mi5040954
Chicago/Turabian StyleGhatkesar, Murali Krishna, Hector Hugo Perez Garza, Friedjof Heuck, and Urs Staufer. 2014. "Scanning Probe Microscope-Based Fluid Dispensing" Micromachines 5, no. 4: 954-1001. https://doi.org/10.3390/mi5040954
APA StyleGhatkesar, M. K., Garza, H. H. P., Heuck, F., & Staufer, U. (2014). Scanning Probe Microscope-Based Fluid Dispensing. Micromachines, 5(4), 954-1001. https://doi.org/10.3390/mi5040954