Consumer-grade thermo polymer extrusion 3D printers [
7], which are easy to operate, versatile and simple to maintain, are capable of microstructuring with resolutions in the 300–50 μm range [
6,
7] and a minimum layer height down to 25 μm [
20]. On the other hand, affordable SL 3D printers, although limited to less materials and marginally more complex to operate, also deliver resolutions in the 50-μm range [
8,
9,
10,
11]. In the particular case of the ULOC architecture presented in this work, the resolution is not the dominant factor that simplifies fabrication, minimizes materials and makes the workflow robust and reliable. Central to ULOC construction is the remarkable surface finish of the stacking plane [
9], which allows open channel sealing with regular adhesive tape, and the correlated advantage of printing open channels that enable easy removal of uncured resin and consistent dimensional features all along the layout [
10].
Figure 1a shows the spatial arrangement of the printer parts. A tank with a transparent bottom holds the printing resin in liquid form. The bottom of this tank is a 127 μm-thick Teflon film (DuPont™ Teflon
® FEP film, Dupont, Wilmington, DE, USA), stretched by contact with a quartz glass (not indicated in the figure). The printout emerges adhered to a metal base that the printer positions in the
z-axis at 50-μm resolution.
Figure 1b,c describes the 3D build-up of the printout. Initially, the base is positioned at 50 μm from the Teflon surface, leaving a gap entirely filled with liquid resin (
Figure 1b). When the resin is exposed (4 to 10 s, depending on the design), it cures through the gap and adheres to the surface of the metal base, capturing its texture in the printout, whereas the side in contact with the Teflon film does not adhere to the surface, but yet captures its surface texture. In the next step, the base is raised by 50 μm, and a new exposure occurs (
Figure 1c). In this case, the layer binds to the previous cured layer, and the process is repeated for subsequent stacking planes (
Figure 1d).
Figure 1.
(a) 3D scheme of a generic stereo lithography (SL) printer arrangement. The illuminating pattern is provided by a digital micro-mirror device (DMD), which defines the x-y resolution of the UV exposure. Liquid resin in the tank is confined between the printout base and the tank bottom, which is a transparent Teflon film. (b) Cross-section of (a) indicating the first printed layer. A 50-μm gap between the Teflon surface and the printout base is set, and upon exposure, the far side of the layer sticks to the metal surface of the printout base. Both cured layer surfaces copy the surface textures of the metal and Teflon, respectively. (c) The printout base is raised 50 μm, and the next layer is exposed, binds to the previous layer and adheres through this to the metal base. (d) The printout base is raised another 50 μm, and a third layer is exposed. The process is repeated until completing the printout, which retains in its bottom surface the Teflon texture captured in the last exposure.
![Micromachines 06 00437 g001]()
When the printout is ready, the last surface retains the Teflon texture, which, except for scratches and localized damage due to usage, typically renders a roughness under 200 nm. It is worth noticing that not only the final surface retains this surface finishing, but also any last exposed surfaces, such as the bottom of any embossed channel.
The described printing mechanism also suggests that intentionally decorating the Teflon surface with a texture is a potential alternative to introduce some level of nano-structuring on selected ULOC surfaces.
3.1. Assembly, Connectors, Transport, Mixers and Detection
Besides its simplicity, versatility and economy, the possibility to integrate specialized 3D geometries and to host established LOC configurations are other important aspects of ULOC.
Figure 2 collects demonstrated examples of such integration possibilities.
Figure 2a corresponds to a three-channel design configured for H
2O
2 fluorescence detection on a microscope stage [
10]. The 3D printed unibody solves several demanding fabrication aspects in a single procedure that takes about 10 min (depending on the printer settings). The ULOC integrates printed connectors (
Figure 2b) that can directly plug into standard silicone tubing (in this case, prepared for 1.5- mm inner diameter tubing), either for continuous flow operation or for pipetting of fixed volumes. The three-channel configuration enables one to measure an unknown sample and the two limits of the calibration range, which in that case [
10] was configured to resolve the lower limit of H
2O
2 in urine for whole body oxidative stress monitoring [
21], an indicator associated with the development of numerous serious conditions, such as cancer [
22] and heart failure [
23].
To facilitate fabrication, the functional surface was separately prepared on a large PDMS area, which was subsequently cut and attached to the unibody. To allow this type of assembly procedure, regions sealed with adhesive tape and the region sealed with the PDMS film must stay separately confined.
Figure 2c illustrates the 3D printed 100 μm-thick channel roof that permits such separate sealing of contiguous sectors. Closed channels can be obviously fabricated with the 3D printer; however, the ULOC principle precludes this approach for a number of reasons. Firstly, only free surfaces retain the quality finish achievable by the Miicraft printer, whereas the free standing surface of a printed ceiling is exposed without backing on a previous layer and captures the exposure profile instead of replicating the Teflon surface. This leads to poor reproducibility, since the exposure profile will change with the curing time, which can vary from 4 to 10 s, depending on the design, and introduces spurious fluctuations in the channels cross-section. Collaterally, closed channels are more difficult for removing uncured resin, thus demanding longer sonication and rinsing cycles that erode the benefits of the fast prototyping method. Thus, the roofed channels in
Figure 2c are a compromise, which is only long enough to permit separate sealing of different sectors, and yet, short to remain easy to clean.
The open channel configuration of the unibody printouts also offers a very versatile way to integrate paper fluidics and to configure passive transport devices [
10,
12,
24]. Classical paper fluidics uses cellulose membranes to control lateral flow transport and is an established technology in diagnostics and home tests [
12]. Modern paper fluidics achieves more complex layouts by confining paper channels within hydrophobic boundaries, which can be produced by photolithography [
25] or directly printed with solid ink [
26] printers. The unibody printout offers a third alternative, to easily reconfigure a design, such as that in
Figure 2a, into a paper fluidic device, by filling the printed channels with cellulose paste, a straightforward procedure that renders well-defined paper conduits. The concept has been demonstrated on a similar design as
Figure 2b, but functionalized for colorimetric glucose detection within the clinical range [
10].
Mixing is a common preparatory stage in chemical analyses and, accordingly, another important function to integrate in ULOC. Microfluidic dimensions favor laminar flow regimes, rendering mixing a slow diffusional process; thus, designs favoring turbulence to improve the mixing performance are required [
27]. Since the bodies in ULOC designs are conceived of to accommodate the connectors, they entail a thickness of about 2.5 to 3 mm, which can be employed for fluidic purposes. In contrast with classical fabrication methods, introducing multiple depths in ULOC designs does not imply extra fabrication steps, masks or alignments, and exploiting the unibody thickness in the design permits small footprint solutions.
Figure 2d illustrates this possibility.
A localized geometric feature, such as a 1-mm diameter hole at the join of the input channels, provides a compact architecture that improves the mixing performance (
Figure 2d). In addition, this space can also accommodate glass microbeads, which can be used to further improve turbulence. The associated image illustrates the performance of the mixer hosting the microbeads and shows mixed flows at 30 μL/min in the first quarter of the serpentine, whereas a diffusional mixer (without the through hole) does not mix the flow in the complete extension of this device [
10]. The compact layout and simple fabrication of the ULOC alternative can be further appreciated by comparing with a mixer in PDMS on glass (
Figure 2e). The template was also 3D printed with the Miicraft printer [
9], which is already a significant simplification from the fabrication of templates with conventional photolithography. Here, the connectors were integrated using a template with 2 mm-high pillars, which were used as a guide for silicone tubing that was plugged into the template before pouring the PDMS. In this way, the PDMS replica integrates the silicone connectors and can be directly attached to a glass slide and used. Thick features, such as these guiding pillars, complicate classical microfabrication and add multiple fabrication steps, whereas it does not tax the fabrication of 3D printed templates, which take the same effort regardless of the number of different thicknesses involved.
The chaotic mixer design in
Figure 1e has the same general cross-section and was measured at the same flow rate as the ULOC mixer [
9,
10], but required more than 80% of the total extension to mix the streams; additionally, the motif used for the chaotic mixer must decorate the entire channel. The ULOC alternative did not involve PDMS processing, and after sonication, the unibody was simply sealed with adhesive tape and ready to operate [
10].
Figure 2.
Collection of recent unibody lab-on-a-chip (LOC) integration examples. (
a) CAD of ULOC design, integrating connectors, three channels for embedded references and sectors suitable for independent sealing. The green frame highlights the detection area. (
b) 3D detail and image of the integrated connectors, designed for tight plugging to 1.5 mm silicone tubing. (
c) Detection region showing two sectors sealed with adhesive tape (red frame) and one sector closed with a H
2O
2-functionalized PDMS film (orange frame). The cross-section along the AA’ segment shows the 100-μm roofed channels used to independently seal different sectors. For detection, the upper PDMS side is imaged with an epi-fluorescence microscope [
10]. (
d) CAD design of an ULOC micromixer and details of geometric features used to enhance the mixing performance. The 1-mm diameter hole and the joint of the input channels are a very compact feature that improves turbulence and can be used to host microbeads that further improve performance. The image shows the mixing of two solutions at a 30 μL/min flow rate with integrated beads. (
e) For comparison with ULOC mixers, a classical PDMS on glass chaotic mixer device fabricated with a 3D printer template is shown [
9]. The fabrication demands more steps, and the chaotic mixer has a larger footprint than the ULOC feature, yet lesser performance. Edited from [
9,
10], reproduced by permission of The Royal Society of Chemistry.
Figure 2.
Collection of recent unibody lab-on-a-chip (LOC) integration examples. (
a) CAD of ULOC design, integrating connectors, three channels for embedded references and sectors suitable for independent sealing. The green frame highlights the detection area. (
b) 3D detail and image of the integrated connectors, designed for tight plugging to 1.5 mm silicone tubing. (
c) Detection region showing two sectors sealed with adhesive tape (red frame) and one sector closed with a H
2O
2-functionalized PDMS film (orange frame). The cross-section along the AA’ segment shows the 100-μm roofed channels used to independently seal different sectors. For detection, the upper PDMS side is imaged with an epi-fluorescence microscope [
10]. (
d) CAD design of an ULOC micromixer and details of geometric features used to enhance the mixing performance. The 1-mm diameter hole and the joint of the input channels are a very compact feature that improves turbulence and can be used to host microbeads that further improve performance. The image shows the mixing of two solutions at a 30 μL/min flow rate with integrated beads. (
e) For comparison with ULOC mixers, a classical PDMS on glass chaotic mixer device fabricated with a 3D printer template is shown [
9]. The fabrication demands more steps, and the chaotic mixer has a larger footprint than the ULOC feature, yet lesser performance. Edited from [
9,
10], reproduced by permission of The Royal Society of Chemistry.
![Micromachines 06 00437 g002]()
3.2. Unidirectional Valves
Benchmark target assays, such as immunosorbent assays or surface plasmon resonance measurements [
28], require sequentially delivered unidirectional flows [
15] of samples and reagents to the detection area. Robust passive check-valves suitable for integration in ULOCs are thus key components to avoid reflow, and to establish essential logic elements in fluidic circuits. Critical for field uses is also the possibility to operate within a broad range of pressures to allow simple pressurization mechanisms, such as in finger pumps [
29]. Here, we present the design of check-valves that exploit the characteristics of the ULOC principles for cost-effective fast prototyping and integration of common LOC materials, such as polydimethylsiloxane (PDMS), for the valve action.
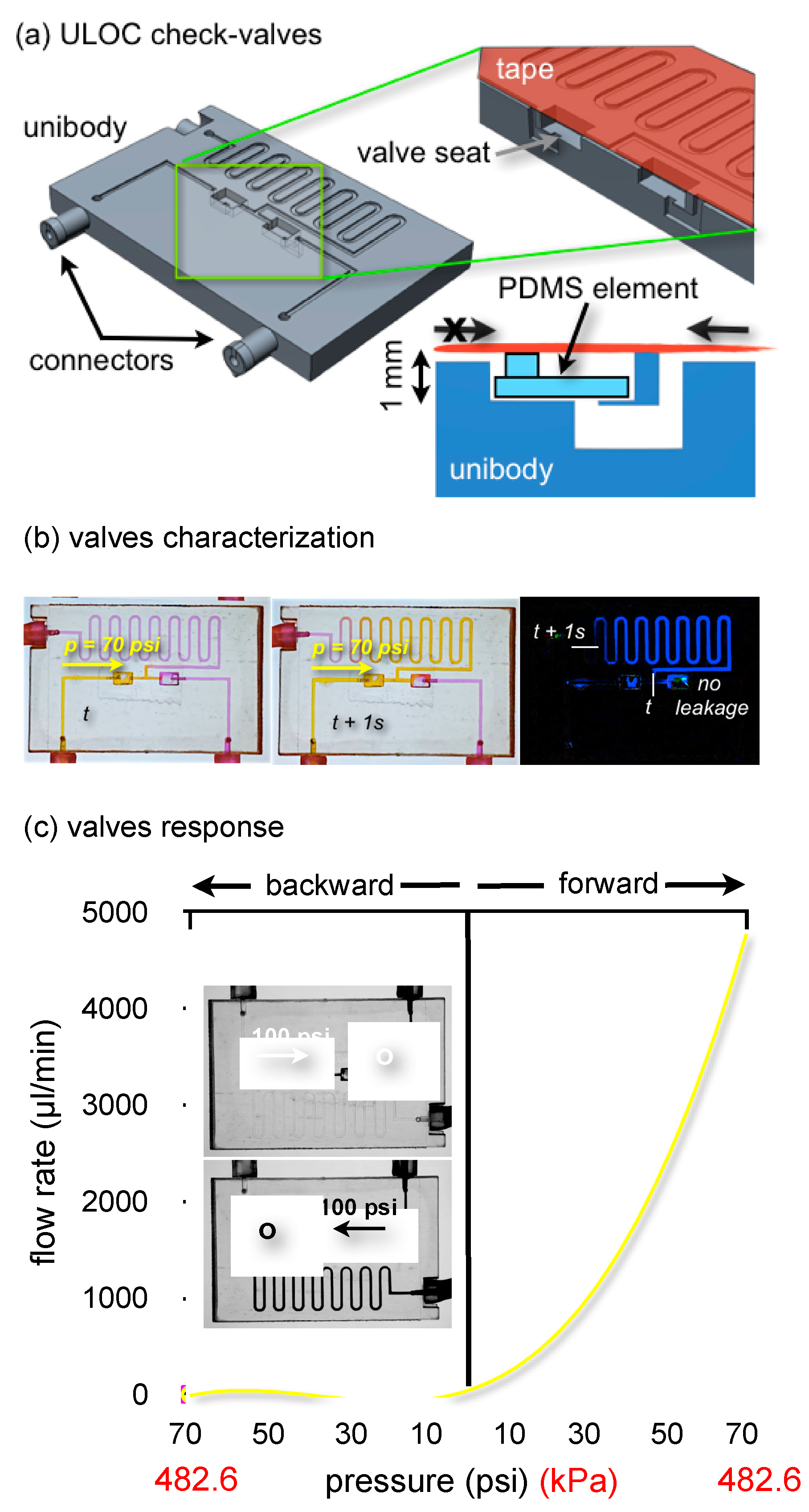
Figure 3.
Twin check-valves integrated in a ULOC test device. (a) CAD of the ULOC design. The green frame highlights the valve seats and the cross-section of one valve. The asymmetric valve seat is directionally sealed by a 200 μm-thick and ~2 × 1.5-mm2 PDMS element. (b) Example of flow rate characterization using video frame subtraction and controlled pressure pulses. (c) Twin check-valves response using 100 ms-long pressure pulses (back and forward) between 10 and 70 psi at 10-psi intervals. Inserts show zero backward flow at 100 psi.
Figure 3.
Twin check-valves integrated in a ULOC test device. (a) CAD of the ULOC design. The green frame highlights the valve seats and the cross-section of one valve. The asymmetric valve seat is directionally sealed by a 200 μm-thick and ~2 × 1.5-mm2 PDMS element. (b) Example of flow rate characterization using video frame subtraction and controlled pressure pulses. (c) Twin check-valves response using 100 ms-long pressure pulses (back and forward) between 10 and 70 psi at 10-psi intervals. Inserts show zero backward flow at 100 psi.
Figure 3a shows the valve design integrated in a ULOC conceived of for valve characterization. The 3D printed unibody integrates tight connectors to 1.5-mm I.D. silicone tubing and the seats for two PDMS film valves working in opposition, thus allowing the simultaneous characterization of the forward and backward behavior of each valve.
The valve seat is a 1-mm deep, 2 × 3 mm2 rectangular sector with a square 500-μm side orifice on one side. The 3D printer enables one to create this asymmetric geometry where the PDMS sits. Since the seat is a free surface, its small roughness permits a flat PDMS layer to efficiently seal the orifice when pressurized in the backwards direction, whereas it easily detaches when the flow comes from the orifice, thus offering small forward resistance. The nozzle connects to a 500 μm-wide, 200 μm-deep channel, which was sealed with regular adhesive tape.
Valves were characterized using 100-ms pressure pulses delivered by a micro-injector. Pressures were increased in steps of 10 psi, from 10 to 70 psi, and alternatively applied to the left and right valve, with the aid of two mechanical valves in line with the inputs. The entire experiment was captured in video at 30 fps, enabling the characterization of the flow rate by measuring the displacement of colored aqueous solutions with a 33.3-ms resolution. The quantification of the displacement was done by image subtraction of video frames corresponding to known time intervals (
Figure 3b). This distance was then used to compute the average forward flow rate for each pressure.
Simultaneously, the backward behavior of the opposed twin valve is captured. Recorded video frames, such as those inserted in
Figure 3c (blue channel only to make clearer the location of each substance), confirm the absence of backward flow up to 100 psi, showing that no fluorescein solution (black) has leaked into the rhodamine channel (light gray), which corresponds to zero backward flow in this direction. The same is verified for the mirrored configuration.
Only the PDMS seat chamber is flooded with fluorescein solution, thus defining a dead volume of about 3 μL in this device. The motivation for the size of the PDMS elements relates to the smallest elements that can be easily hand cut and assembled. Using a laser cutter or a 3D printed template for PDMS could significantly reduce the dimension of these valves and the consequent possibility to reduce the dead volume; however, we speculate that would require a different design to facilitate the assembly.
Although the long exit channel implies that essentially the 70 psi are applied to the valve in the backward configuration, an additional experiment was run with the exit channel blocked, which confirmed zero leakage in the backward direction, up to 100 psi.
Figure 3c collects the characterization of both valves under forward and backward pressures up to 70 psi. Above 70 psi, the forward behavior departed from the exponential trend, whereas the back-flow of both valves remained identically zero up to 100 psi. Observed blistering of the tape seal at these pressures precluded operation beyond the 70-psi interval for regular tape.
The different forward response depends on the dimensions of the PDMS element, which in one case was 500 μm wider and behaved according to similar valve designs [
16]. The ability of this simple feature to introduce differences in forward response is important to configure pressure-regulated sequential injection in a compact and robust way. As mentioned before, the hand-cut PDMS element is the least accurate part of this device, and to reduce variability, it could be fabricated in a different way; however, that will depend on the application, and for manually-actuated systems, the present performance would be sufficient.
Cured resin used in ULOC devices supports contact to several organic solvents [
8], eliminating one of the limitations of many of the other materials available for 3D printers; however, cell viability studies are necessary to assess if ULOC is toxic for cell growth. For biocompatibility analysis, a cell viability test using the Almar Blue assay was performed.
Since ULOCs entail the printed unibody and adhesive tape, it is necessary to address the biocompatibility of both components. Cells may be affected by proximity to these materials or because they fail to grow in contact with them. Cured resin and tape were placed in the bottom and the walls of a microtiter plate and tested with four different cell concentrations in triplicates. Active cells change the medium color from blue to magenta, and by calibration with control cells, the color response can be used to quantify cell viability.
Figure 4 shows the results of the microplate reader quantification. The viability assay showed that cells died when in the presence of the cured unibody resin for 48 h. Additionally, a slow acidification of the growth medium could be detected due to the fact that the medium, which contains the pH indicator phenol red, turned orange (indicating pH of ~7) from its pink-red color at normal conditions (
i.e., pH 7.4), after 48 h of incubation.
Figure 4.
ULOC cell viability test assessing the toxicity of cured Miicraft resin close to cells (resin wall) and in contact with cells (resin bottom). An equivalent evaluation for cells with respect to adhesive tape was performed.
Figure 4.
ULOC cell viability test assessing the toxicity of cured Miicraft resin close to cells (resin wall) and in contact with cells (resin bottom). An equivalent evaluation for cells with respect to adhesive tape was performed.
The adhesive tape, however, which usually is used to seal channels rather than for cell substrates, had very little effect on cell viability, especially when surrounding the wall of the growth wells. Its presence on the bottom of the well was not as well tolerated, most likely due to the texture of the adhesive coating, which compromised cell adhesion.
Accordingly, although the default resin is not biocompatible, the tape as normally used in ULOC is largely biocompatible. On the other hand, ISO 10993 biocompatible resins of similar cost as the Miicraft exist for more expensive printers that use laser illumination in the 325- and 355-nm range [
30], whereas affordable Bio™ Mold (Asiga, Anaheim Hills, CA, USA) resins that can be used in contact with skin are suitable with printers using LED sources in 405- and 385-nm ranges, such as Miicraft [
31].
The advantages of direct additive printing are exploited in ULOC to fabricate, in a single step, the geometries required to support the device function, but tailored to facilitate all of the other aspects of fabrication, such as material usage, printing time and assembly simplicity. Thus, the unibody design optimizes additive prototyping by minimizing material and by exploiting the planar dimension, where the printer can deliver sub-micrometric surface roughness.
The considered examples indicate the ULOC’s ability to integrate established principles and migrate classical LOC designs to a method that demands less resources and initial investment than classical microfabrication. ULOC can also integrate essential components as check-valves, the dimensions of which are compatible with manual configuration and assembly. The check-valves demonstrated here are an important contribution to ULOCs’ capabilities, which must be complemented by other valve configurations to offer the complete range of LOC possibilities.
The default material for the Miicraft printer is robust to handling common organic solvents and support chemical sensing applications. Although the default resin is not biocompatible, the tape can be used for its regular purpose in ULOC, whereas biocompatible formulations are commercially available.