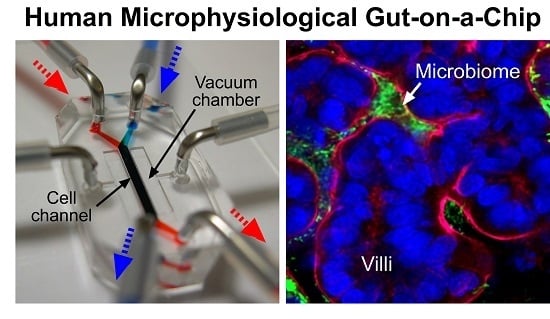
Farewell to Animal Testing: Innovations on Human Intestinal Microphysiological Systems
Abstract
Share and Cite
Kang, T.H.; Kim, H.J. Farewell to Animal Testing: Innovations on Human Intestinal Microphysiological Systems. Micromachines 2016, 7, 107. https://doi.org/10.3390/mi7070107
Kang TH, Kim HJ. Farewell to Animal Testing: Innovations on Human Intestinal Microphysiological Systems. Micromachines. 2016; 7(7):107. https://doi.org/10.3390/mi7070107
Chicago/Turabian StyleKang, Tae Hyun, and Hyun Jung Kim. 2016. "Farewell to Animal Testing: Innovations on Human Intestinal Microphysiological Systems" Micromachines 7, no. 7: 107. https://doi.org/10.3390/mi7070107
APA StyleKang, T. H., & Kim, H. J. (2016). Farewell to Animal Testing: Innovations on Human Intestinal Microphysiological Systems. Micromachines, 7(7), 107. https://doi.org/10.3390/mi7070107